Abstract
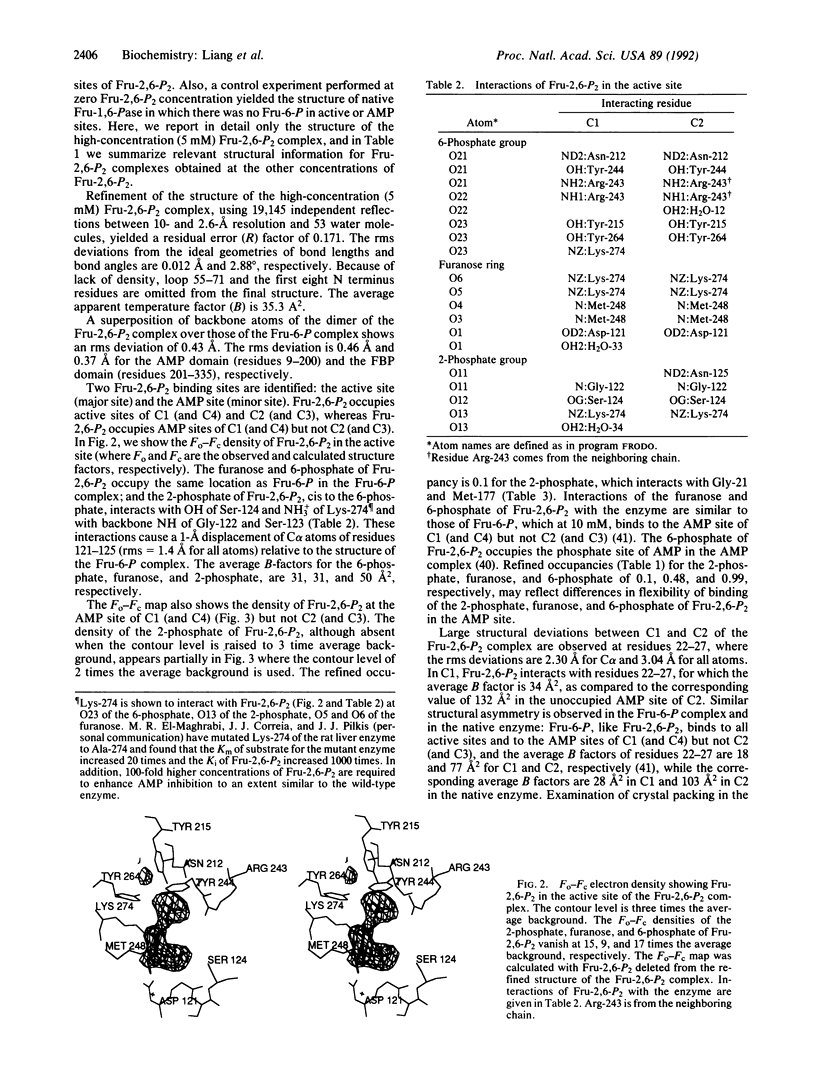
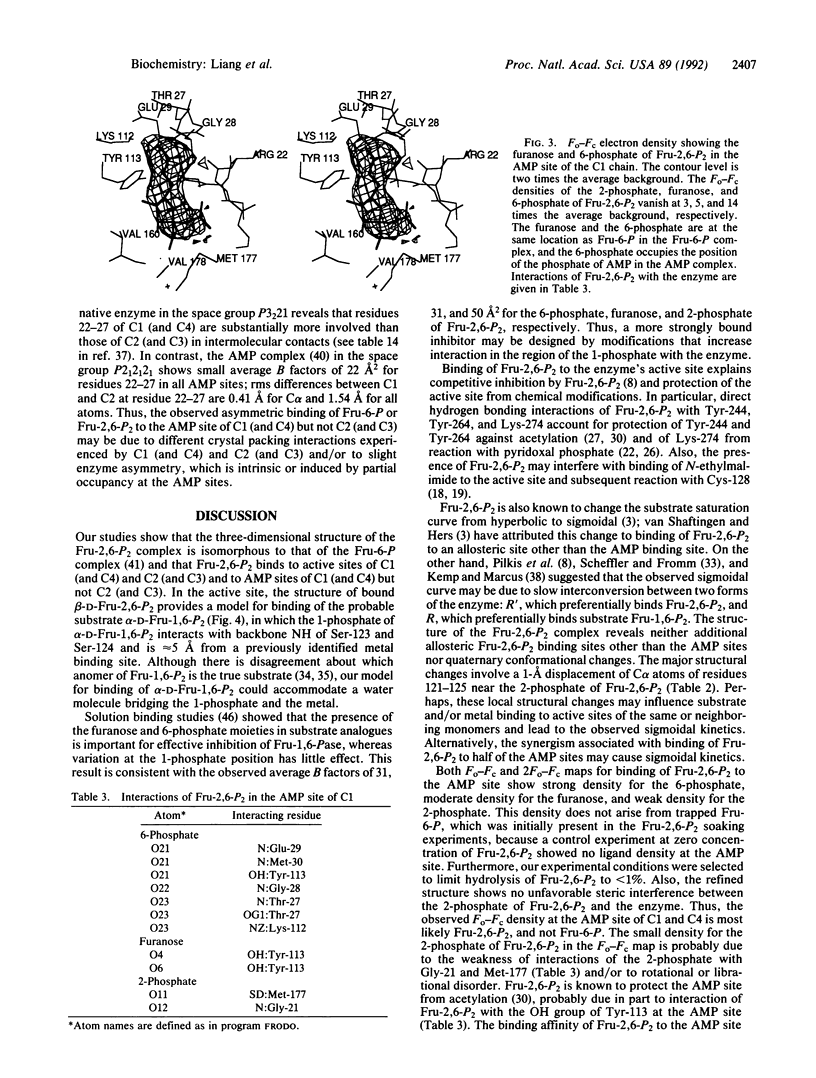
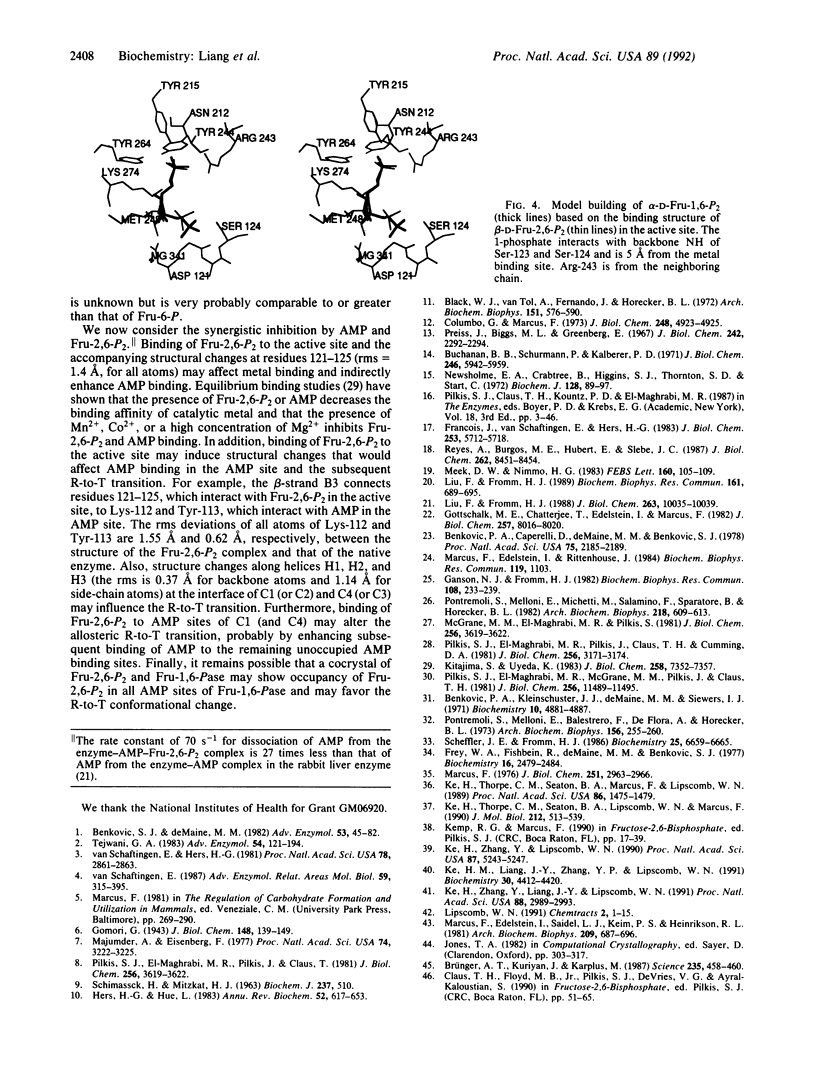
The three-dimensional structure of the complex between fructose 1,6-bisphosphatase (EC 3.1.3.11) and the physiological inhibitor beta-D-fructose 2,6-bisphosphate (Fru-2,6-P2), an analogue of the substrate (fructose 1,6-bisphosphate), has been refined at 2.6-A resolution to a residual error (R) factor of 0.171. The rms deviations are 0.012 A and 2.88 degrees from ideal geometries of bond lengths and angles, respectively. The Fru-2,6-P2 occupies the active sites of both polypeptides C1 and C2 in the crystallographic asymmetric unit in the space group P3(2)21. The furanose and 6-phosphate of Fru-2,6-P2 are located at the fructose 6-phosphate site established earlier, and the 2-phosphate binds to the OH of Ser-124, the NH3+ of Lys-274, and the backbone NH of Gly-122 and Ser-123. Backbone displacements of 1 A occur for residues from Asp-121 to Asn-125. Model building of substrate alpha-D-Fru-1,6-P2 based on the binding structure of Fru-2,6-P2 in the active site shows interactions of the 1-phosphate with the backbone NH of Ser-123 and Ser-124. In the AMP sites, density peaks attributed to Fru-2,6-P2 are seen in C1 (and C4) but not in C2 (and C3). This minor binding of Fru-2,6-P2 to AMP sites partially explains the synergistic interaction between AMP and Fru-2,6-P2 and the protection of the AMP site from acetylation in the presence of Fru-2,6-P2. In the synergistic interaction between AMP and Fru-2,6-P2, inhibition of catalytic metal binding by the presence of Fru-2,6-P2 at the active site, and propagation of structural changes over some 28 A along beta-strand B3 from residues 121 to 125 in the active site to Lys-112 and Tyr-113 in the AMP site, as well as movement of helices across the interdimeric interfaces, may affect AMP binding and the subsequent R-to-T transition. In addition, occupancy of Fru-2,6-P2 at the AMP sites of C1 and C4 may favor binding of AMP to the remaining unoccupied AMP sites and thus promote the accompanying quaternary conformational changes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benkovic P. A., Caperelli C. A., de Maine M., Benkovic S. J. Binding and kinetic data for rabbit liver fructose-1,6-bisphosphatase with Zn2+ as cofactor. Proc Natl Acad Sci U S A. 1978 May;75(5):2185–2189. doi: 10.1073/pnas.75.5.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkovic S. J., Kleinschuster J. J., DeMaine M. M., Siewers I. J. On the mechanism of action of fructose 1,6-diphosphatase. Inhibition by structural analogs of fructose 1,6-diphosphate. Biochemistry. 1971 Dec 21;10(26):4881–4887. doi: 10.1021/bi00802a008. [DOI] [PubMed] [Google Scholar]
- Benkovic S. J., deMaine M. M. Mechanism of action of fructose 1,6-bisphosphatase. Adv Enzymol Relat Areas Mol Biol. 1982;53:45–82. doi: 10.1002/9780470122983.ch2. [DOI] [PubMed] [Google Scholar]
- Black W. J., Van Tol A., Fernando J., Horecker B. L. Isolation of ahighly active fructose diphosphatase from rabit muscle: its subunit structure and activation by monovalent cations. Arch Biochem Biophys. 1972 Aug;151(2):576–590. doi: 10.1016/0003-9861(72)90535-8. [DOI] [PubMed] [Google Scholar]
- Brünger A. T., Kuriyan J., Karplus M. Crystallographic R factor refinement by molecular dynamics. Science. 1987 Jan 23;235(4787):458–460. doi: 10.1126/science.235.4787.458. [DOI] [PubMed] [Google Scholar]
- Buchanan B. B., Schürmann P., Kalberer P. P. Ferredoxin-activated fructose diphosphatase of spinach chloroplasts. Resolution of the system, properties of the alkaline fructose diphosphatase component, and physiological significance of the ferredoxin-linked activation. J Biol Chem. 1971 Oct 10;246(19):5952–5959. [PubMed] [Google Scholar]
- Frey W. A., Fishbein R., de Maine M. M., Benkovic S. J. Substrate form of D-frutose 1,6-bisphosphate utilized by fructose 1,6-bisphosphatase. Biochemistry. 1977 May 31;16(11):2479–2484. doi: 10.1021/bi00630a025. [DOI] [PubMed] [Google Scholar]
- Ganson N. J., Fromm H. J. The effect of fructose 2,6-bisphosphate on the reverse reaction kinetics of fructose 1,6-bisphosphatase from bovine liver. Biochem Biophys Res Commun. 1982 Sep 16;108(1):233–239. doi: 10.1016/0006-291x(82)91856-3. [DOI] [PubMed] [Google Scholar]
- Gottschalk M. E., Chatterjee T., Edelstein I., Marcus F. Studies on the mechanism of interaction of fructose 2,6-bisphosphate with fructose-1,6-bisphosphatase. J Biol Chem. 1982 Jul 25;257(14):8016–8020. [PubMed] [Google Scholar]
- Hers H. G., Hue L. Gluconeogenesis and related aspects of glycolysis. Annu Rev Biochem. 1983;52:617–653. doi: 10.1146/annurev.bi.52.070183.003153. [DOI] [PubMed] [Google Scholar]
- Ke H. M., Liang J. Y., Zhang Y. P., Lipscomb W. N. Conformational transition of fructose-1,6-bisphosphatase: structure comparison between the AMP complex (T form) and the fructose 6-phosphate complex (R form). Biochemistry. 1991 May 7;30(18):4412–4420. doi: 10.1021/bi00232a007. [DOI] [PubMed] [Google Scholar]
- Ke H. M., Thorpe C. M., Seaton B. a., Lipscomb W. N., Marcus F. Structure refinement of fructose-1,6-bisphosphatase and its fructose 2,6-bisphosphate complex at 2.8 A resolution. J Mol Biol. 1990 Apr 5;212(3):513–539. doi: 10.1016/0022-2836(90)90329-k. [DOI] [PubMed] [Google Scholar]
- Ke H. M., Zhang Y. P., Liang J. Y., Lipscomb W. N. Crystal structure of the neutral form of fructose-1,6-bisphosphatase complexed with the product fructose 6-phosphate at 2.1-A resolution. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):2989–2993. doi: 10.1073/pnas.88.8.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke H. M., Zhang Y. P., Lipscomb W. N. Crystal structure of fructose-1,6-bisphosphatase complexed with fructose 6-phosphate, AMP, and magnesium. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5243–5247. doi: 10.1073/pnas.87.14.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke H., Thorpe C. M., Seaton B. A., Marcus F., Lipscomb W. N. Molecular structure of fructose-1,6-bisphosphatase at 2.8-A resolution. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1475–1479. doi: 10.1073/pnas.86.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima S., Uyeda K. A binding study of the interaction of beta-D-fructose 2,6-bisphosphate with phosphofructokinase and fructose-1,6-bisphosphatase. J Biol Chem. 1983 Jun 25;258(12):7352–7357. [PubMed] [Google Scholar]
- Liu F., Fromm H. J. Relationship between thiol group modification and the binding site for fructose 2,6-bisphosphate on rabbit liver fructose-1,6-bisphosphatase. J Biol Chem. 1988 Jul 15;263(20):10035–10039. [PubMed] [Google Scholar]
- Liu F., Roy M., Fromm H. J. The site of substrate and fructose 2,6-bisphosphate binding to rabbit liver fructose-1,6-bisphosphatase. Biochem Biophys Res Commun. 1989 Jun 15;161(2):689–695. doi: 10.1016/0006-291x(89)92654-5. [DOI] [PubMed] [Google Scholar]
- Majumder A. L., Eisenberg F., Jr Unequivocal demonstration of fructose-1,6-bisphosphatase in mammalian brain. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3222–3225. doi: 10.1073/pnas.74.8.3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus C. J. Inhibition of bovine hepatic fructose-1,6-diphosphatase by substrate analogs. J Biol Chem. 1976 May 25;251(10):2963–2966. [PubMed] [Google Scholar]
- Marcus F., Edelstein I., Rittenhouse J. Inhibition of Escherichia coli fructose-1,6-bisphosphatase by fructose 2,6-bisphosphate. Biochem Biophys Res Commun. 1984 Mar 30;119(3):1103–1108. doi: 10.1016/0006-291x(84)90888-x. [DOI] [PubMed] [Google Scholar]
- Marcus F., Edelstein I., Saidel L. J., Keim P. S., Heinrikson R. L. The covalent structure of pig kidney fructose 1,6-bisphosphatase: sequence of the 60-residue NH2-terminal peptide produced by digestion with subtilisin. Arch Biochem Biophys. 1981 Jul;209(2):687–696. doi: 10.1016/0003-9861(81)90330-1. [DOI] [PubMed] [Google Scholar]
- Meek D. W., Nimmo H. G. The interaction of fructose 2,6-bisphosphate with an allosteric site of rat liver fructose 1,6-bisphosphatase. FEBS Lett. 1983 Aug 22;160(1-2):105–109. doi: 10.1016/0014-5793(83)80946-6. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Crabtree B., Higgins S. J., Thornton S. D., Start C. The activities of fructose diphosphatase in flight muscles from the bumble-bee and the role of this enzyme in heat generation. Biochem J. 1972 Jun;128(1):89–97. doi: 10.1042/bj1280089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilkis S. J., El-Maghrabi M. R., McGrane M. M., Pilkis J., Claus T. H. The role of fructose 2,6-bisphosphate in regulation of fructose-1,6-bisphosphatase. J Biol Chem. 1981 Nov 25;256(22):11489–11495. [PubMed] [Google Scholar]
- Pilkis S. J., El-Maghrabi M. R., Pilkis J., Claus T. H., Cumming D. A. Fructose 2,6-bisphosphate. A new activator of phosphofructokinase. J Biol Chem. 1981 Apr 10;256(7):3171–3174. [PubMed] [Google Scholar]
- Pilkis S. J., El-Maghrabi M. R., Pilkis J., Claus T. Inhibition of fructose-1,6-bisphosphatase by fructose 2,6-bisphosphate. J Biol Chem. 1981 Apr 25;256(8):3619–3622. [PubMed] [Google Scholar]
- Pilkis S. J., El-Maghrabi M. R., Pilkis J., Claus T. Inhibition of fructose-1,6-bisphosphatase by fructose 2,6-bisphosphate. J Biol Chem. 1981 Apr 25;256(8):3619–3622. [PubMed] [Google Scholar]
- Pontremoli S., Melloni E., Balestrero F., De Flora A., Horecker B. L. Ligand-induced conformational states of rabbit liver fructose 1,6-bisphosphatase as revealed by digestion with subtilisin. Arch Biochem Biophys. 1973 May;156(1):255–260. doi: 10.1016/0003-9861(73)90363-9. [DOI] [PubMed] [Google Scholar]
- Pontremoli S., Melloni E., Michetti M., Salamino F., Sparatore B., Horecker B. L. On the mechanism of inhibition of fructose 1,6-bisphosphatase by fructose 2,6-bisphosphate. Arch Biochem Biophys. 1982 Oct 15;218(2):609–613. doi: 10.1016/0003-9861(82)90386-1. [DOI] [PubMed] [Google Scholar]
- Preiss J., Biggs M. L., Greenberg E. The effect of magnesium ion concentration on the pH optimum of the spinach leaf alkaline fructose diphosphatase. J Biol Chem. 1967 May 10;242(9):2292–2294. [PubMed] [Google Scholar]
- Reyes A., Burgos M. E., Hubert E., Slebe J. C. Selective thiol group modification renders fructose-1,6-bisphosphatase insensitive to fructose 2,6-bisphosphate inhibition. J Biol Chem. 1987 Jun 25;262(18):8451–8454. [PubMed] [Google Scholar]
- SCHIMASSEK H., MITZKAT H. J. UBER EINE SPEZIFISCHE WIRKUNG DES GLUCAGON AUF DIE EMBDEN-MEYERHOF-KETTE IN DER LEBER. VERSUCHE AN DER ISOLIERT PERFUNDIERTEN RATTENLEBER. Biochem Z. 1963 Aug 14;337:510–518. [PubMed] [Google Scholar]
- Scheffler J. E., Fromm H. J. Regulation of rabbit liver fructose-1,6-bisphosphatase by metals, nucleotides, and fructose 2,6-bisphosphate as determined from fluorescence studies. Biochemistry. 1986 Oct 21;25(21):6659–6665. doi: 10.1021/bi00369a050. [DOI] [PubMed] [Google Scholar]
- Tejwani G. A. Regulation of fructose-bisphosphatase activity. Adv Enzymol Relat Areas Mol Biol. 1983;54:121–194. doi: 10.1002/9780470122990.ch3. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E. Fructose 2,6-bisphosphate. Adv Enzymol Relat Areas Mol Biol. 1987;59:315–395. doi: 10.1002/9780470123058.ch7. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E., Hers H. G. Inhibition of fructose-1,6-bisphosphatase by fructose 2,6-biphosphate. Proc Natl Acad Sci U S A. 1981 May;78(5):2861–2863. doi: 10.1073/pnas.78.5.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]



