Abstract
While a doctor-diagnosis of asthma is associated with an increased risk of pain and acute chest syndrome (ACS) in children with sickle cell anemia (SCA), little is known about the relationship between specific asthma characteristics and clinical factors and future morbidity in children with SCA. We evaluated the relationship between 1) asthma risk factors at the time of a clinical visit (respiratory symptoms, maternal history of asthma, allergy skin tests, spirometry results) and 2) the known risk factor of ACS early in life, on prospective pain and ACS episodes in a cohort of 159 children with SCA followed from birth to a median of 14.7 years. An ACS episode prior to 4 years of age, (incidence rate ratio (IRR) =2.84; p<0.001), female gender (IRR=1.80; p=0.009), and wheezing causing shortness of breath (IRR=1.68; p=0.042) were associated with future ACS rates. We subsequently added spirometry results (obstruction defined as FEV1/FVC less than the lower limits of normal; and bronchodilator response, FEV1 ≥12%) and prick skin test responses to the model. Only ≥2 positive skin tests had a significant effect (IRR 1.87; p = 0.01). Thus, early in life ACS events, wheezing causing shortness of breath, and ≥2 positive skin tests predict future ACS events.
Keywords: sickle cell anemia, acute chest syndrome, asthma
Introduction
Asthma is associated with an increased rate of pain and acute chest syndrome (ACS) in sickle cell anemia (SCA). However, the diagnosis of asthma in SCA is particularly challenging because clinical features commonly associated with asthma, such as wheezing[1], an elevated IgE level[2], increased response to bronchodilators[3, 4], and airway hyper-responsiveness[4–6] can occur in children with SCA with or without a diagnosis of asthma.
Well-established challenges are present in making a consistent diagnosis of asthma in children[7]. Thus, as part of the primary aim of the NHLBI funded prospective observational cohort study in SCA, the Sickle Asthma Cohort (SAC) study, we tested the following hypothesis: in children with SCA, respiratory symptoms and other risk factors for asthma are associated with an increased incidence of hospitalizations for pain and ACS. If criteria, other than a physician diagnosis of asthma[8], or early onset of ACS[9], could be identified, these risk factors could be used as prognostic markers for children at greater risk for severe pain episodes and ACS events. This would facilitate stratification of management targeting those features that increase risks of ACS or pain.
Methods
Institutional approval was obtained from participating sites, Washington University School of Medicine in St. Louis, Missouri; Case Western Reserve in Cleveland, Ohio; and University College London in London, United Kingdom. Three pediatric hematology centers participated in London via the National Health Service Research Ethics Committee approval, Guys and St. Thomas NHS Foundation Trust, Imperial College and Imperial College Healthcare NHS Foundation Trust and North Middlesex University Hospital NHS Trust. Institutional approval was also obtained from the Coordinating Center at Vanderbilt School of Medicine in Nashville Tennessee.
In 2005, we initiated our prospective observational cohort study of children age 4–18 years with SCA (Hgb SS [homozygous for the βs-globin mutation] or sickle-betaº-thalassemia [Hgb Sβº]). Children were excluded from enrolling in the observation study if they: 1) were on regular blood transfusion therapy; 2) in a clinical trial evaluating hydroxyurea or oxygen therapy; or 3) had any additional co-morbidity associated with lung disease other than asthma.
Definition of Outcomes
A vaso-occlusive pain episode was defined as an episode directly associated with SCA, which required hospitalization, and was treated with opioids. Headaches that required admission to the hospital and were treated with opioids were not considered a vaso-occlusive pain episode. Acute chest syndrome (ACS) was defined as an episode of acute respiratory distress associated with at least one new radiodensity on chest roentgenogram, temperature greater than 38° Celsius and increased respiratory effort with decreased oxygen saturation or increased respiratory rate documented in the medical record. A diagnosis of pneumonia was considered an ACS episode.
Clinical Features Associated with Asthma: Atopy, Lung Function, and Respiratory Symptoms: Allergy skin tests were performed by technicians who were centrally trained and certified using the prick puncture technique (MulitTestII device, Lincoln Diagnostics, Decatur, IL) to nine aeroallergens (Aspergillus and Alternaria molds, cat, dog, dust mite, and cockroach, and site specific tree, grass, and weed pollens). Each wheal was outlined with a skin-marking pen. Each outline was transferred with transparent tape to the boxed areas on the skin test results form. After removing the tape from the child’s back or arm, use a Paper Mate Gel Writer pen to draw line “a” to represent the longest length of the wheal. Line “b” was drawn perpendicular to “a” at the widest width. Line “b” will not necessarily be the midpoint of line “a”. The length of both lines was taken inside the ink outline. These measurements in millimeters were recorded on a skin test result sheet. A testing sessions was considered valid if the histamine positive control had a mean wheal size of at least 3 mm greater than the measurement from the saline control. The result of an individual test for an aeroallergen was considered positive if the mean wheal size of at least 3 mm greater than the saline control. Results were re-measured by a single investigator to ensure reliability. Two or more positive tests versus less than 2 positive tests were considered positive results. While atopy is often defined as 1 positive result,two positive skin tests were used as the indicator of atopy to give assurance of clear evidence of atopic status, as data from the Childhood Asthma Management Program research group has found that more than 1 positive skin test better correlated with methacholine responsiveness[10], hospitalization[11], and nocturnal awakenings[12].
Spirometry was performed before and after bronchodilator, administered as 4 inhalations of albuterol, 90 mcg/inhalation, via an aerochamber; all results were over-read by a single certified pulmonary function technologist using ATS criteria appropriately modified for children[13] with only those meeting these criteria being used in subsequent analyses. Spirometric results were adjusted for age, sex, height and ethnicity using the GLI-2012 all age-multi-ethnic reference equations for Black subjects[14]. Airway obstruction was defined as the forced expired volume in 1 second/forced vital capacity (FEV1/FVC) < lower limit of normal (LLN) according to these equations[14] and a significant bronchodilator response was defined as an increase in FEV1 ≥ 12%.
During the initial evaluation, all parents of the participants were asked to complete the American Thoracic Society Division of Lung Disease (ATS/DLD) questionnaire to ascertain the presence of respiratory symptoms[15].
Data Quality
To ensure a uniform definition of pain and ACS episodes in this multi-center study, the charts of all patients diagnosed with ACS or a vaso-occlusive pain episode requiring hospitalization for pain in the chest, extremities or other areas of the body, were reviewed by a single investigator at each of the participating sites after training by the PI, who reviewed all the hospital visits at St Louis and the de-identified clinical documentation for 100% of hospital visits for the first four years of the study at the Cleveland and London sites. When concerns about the assignment of the diagnosis were raised, clarity of the assessment was discussed with the site investigators.
Construction of SAC Cohort
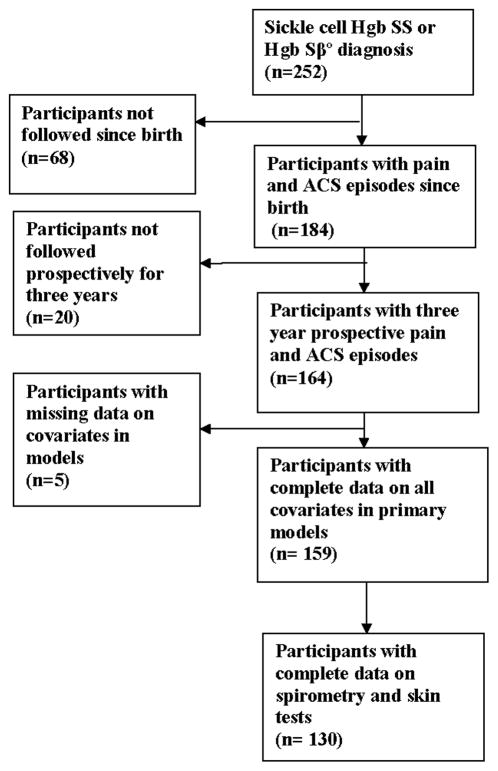
All patients included in the analysis had been followed from birth and for at least three years subsequent to enrolling in the study at one of the three sites. Follow-up from birth was deemed necessary because we have previously demonstrated that an ACS episode prior to four years of age was associated with a doctor diagnosis of asthma[16]. Specifically for this analysis, we wanted to determine whether an early event of pain or ACS was associated with future ACS and pain events. Figure 1 describes the flow diagram for study participants who were included in these analyses. All patients in the cohort were prospectively followed from the date of informed consent until death, start of regular blood transfusion therapy, or last date of follow up, whichever came first.
Figure 1.
Flow diagram of patients from the Sleep and Asthma Cohort included in the analysis to determine whether asthma risk factors are associated with acute chest syndrome and vaso-occlusive pain episodes.
Validation of Cohort Findings Obtained in the SAC cohort
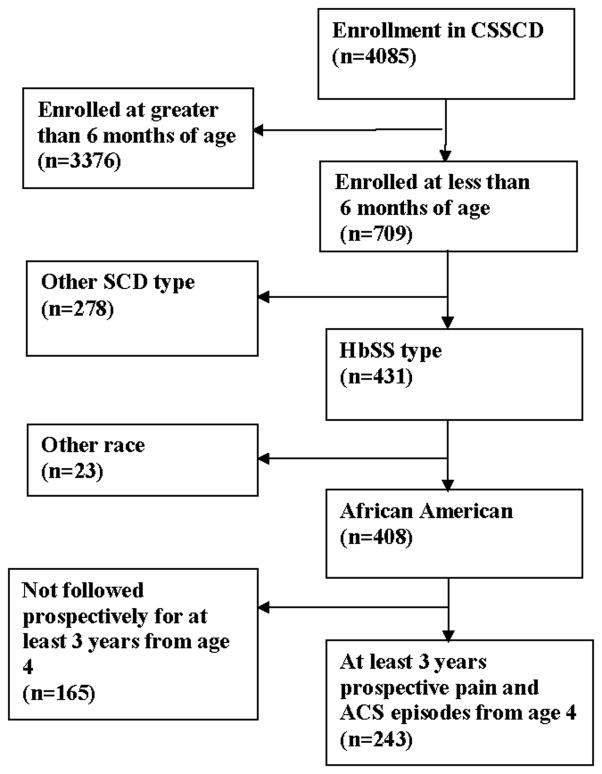
Data from the Cooperative Study for Sickle Cell Disease (CSSCD) infant cohort data set were used to confirm the primary results of the study. For all practical purposes, the infant CSSCD cohort was a birth cohort due to the average age of enrollment of less than 6 months, so we modeled the prospective pain and ACS events after 4 years of age to match the age of entry of the SAC study participants. An ACS episode was defined as a new pulmonary infiltrate demonstrable on chest radiograph. Figure 2 describes the flow diagram of the CSSCD study participants selected for this analysis. In the CSSCD cohort, unlike the SAC cohort, asthma predictors included only, but not spirometry evaluation or the ATS/DLD questionnaire.
Figure 2.
Flow diagram of patients from the Cooperative Study of Sickle Cell Anemia cohort included in the analysis to determine whether asthma risk factors are associated with acute chest syndrome and vaso-occlusive pain episodes
Statistical Analysis
Analyses were conducted using Stata statistical software (Version 12, College Station, TX: StataCorp LP) and IBM SPSS Statistics (Version 22, Chicago, IL, IBM). Continuous data were analyzed using t-tests for variables with a normal distribution and the Wilcoxon-Mann-Whitney test for variables with non-normal distributions. Categorical data were analyzed using chi-square tests. Rates of missing data for the primary analysis in each covariate ranged from 0 to 3.0%. Multivariable negative binomial regressions were performed using characteristics postulated to be relevant to predicting rates of pain or ACS, including the previously documented association between an early episode of ACS and subsequent risk of ACS[9]. Regression was conducted in two steps. First, a model was constructed with all the relevant predictors and a significance of p<0.20 was used to screen for inclusion in a reduced model. Then a second model was run with the screened set of characteristics. Collinearity was assessed with a criterion variance inflation factor < 3.0.
Duration of hydroxyurea therapy ranged from 0.10 to 13.92 years (mean 3.59 years, median 2.50 years). The incidence rates of pain or ACS were not decreased by use of hydroxyurea (n=52) during the study period (Table 1), we therefore did not include hydroxyurea medication as a covariate in the multivariable analysis.
Table 1.
Pain and ACS Rates for time on and off Hydroxyurea in the SAC cohort.#
| Type of Event | On | Off | P-value* |
|---|---|---|---|
| Hydroxyurea | Hydroxyurea | ||
| Pain rate (events/patient year) | 1.02 (2.41) § | 0.56 (0.99) | 0.003 |
| ACS rate (events/patient year) | 0.18 (0.71) | 0.25 (0.40) | 0.891 |
Wilcoxon signed rank test
Includes only those followed since birth and administered hydroxyurea; n=52
Median (IQR)
Duration of hydroxyurea therapy ranges from 0.10 to 13.92 years. The mean is 3.59 years, median is 2.50 years.
The measures of spirometry evaluation and skin testing were added to the model with asthma symptoms and clinical risk factors in a separate regression. We use this modeling strategy because it more closely mimics clinical practice, as clinicians would be expected to take a history and ask a series of questions about asthma symptoms and clinical risk factors before ordering tests to evaluate the presence of additional asthma risk factors (spirometry results and skin testing).
The minimum age of the cohort eligibility, four years, decreased the number of children with evaluable spirometry (n=144 of 159 possible participants). Some members of our cohort do not have skin test data (n=18) because several parents did not agree to the skin testing and some patients had negative histamine controls making their results uninterpretable. Consequently, the expanded regression model included 130 participants with complete data on bronchodilator response, skin test, and lower airway obstruction and not the 159 participants in the asthma symptoms regression model. There were few differences between those patients with missing data who were excluded from the expanded model and those with complete data (Table 2).
Table 2.
Comparison of patients in the SAC study missing data on covariates in models including positive skin tests and spirometry to those patients with complete data.
| Characteristic | No Missing Data (n=130) | Missing Data (n=29) | P Value† |
|---|---|---|---|
| Sex, (% Males) | 53.8 | 41.4 | 0.224 |
| Age (yrs.) § | 10.00 | 8.00 | 0.031 |
| Prospective Follow-up* (yrs.) § | 5.46 | 4.49 | 0.004 |
| Pain rate, lifetime (events/patient year) § | 0.38 | 0.30 | 0.623 |
| Pain rate, prospective (events/patient year) § | 0.44 | 0.50 | 0.862 |
| Pain event before age 4, (%) | 53.1 | 51.7 | 0.895 |
| ACS rate, lifetime (events/patient year) § | 0.16 | 0.08 | 0.041 |
| ACS rate, prospective (events/patient year) § | 0.17 | 0.00 | 0.363 |
| ACS event before age 4, (%) | 42.3 | 20.7 | 0.030 |
| Hemoglobin (g/dl)# | 8.21 | 8.41 | 0.440 |
| White Blood Cells, 109/L# | 12.13 | 12.09 | 0.962 |
| Mother has asthma, (%) | 11.5 | 20.7 | 0.188 |
| Wheezing cause shortness of breath, (%) | 25.4 | 31.0 | 0.533 |
| Wheezing after playing hard, (%) | 35.4 | 24.1 | 0.245 |
| Wheezing without having a cold, (%) | 22.3 | 13.8 | 0.307 |
| Cough without having a cold, (%) | 36.2 | 44.8 | 0.384 |
| Congested or phlegm without a cold, (%) | 13.1 | 17.2 | 0.557 |
| Two or more positive skin tests, (%)‡ | 27.7 | 18.2 | 0.727 |
| Bronchodilator response ≥ 12%, (%)‡ | 22.3 | 14.3 | 0.735 |
| Lower airway obstruction, (%)‡ | 20.8 | 7.1 | 0.305 |
Prospective follow-up is from date of entry in SAC; Minimum follow-up of three years
Chi-square test for categorical variables; t-test for variables with mean reported; Mann-Whitney U test for variables with median reported
Mean (std dev.) for continuous variables with a normal distribution
Median (IQR) for continuous variables with a non-normal distribution
18 cases with data in missing group for two or more positive skin tests; 15 cases in missing group for bronchodilator response and lower airway obstruction, respectively.
Results
Demographics
A total of 159 participants with SCA from the SAC cohort were included in the primary analysis. All participants had been followed from birth in their respective clinical center. The median age of the cohort at entry was 9.0 years, (range 4–18 years); 51.6 % were male. They were followed prospectively after entry into SAC for a median of 5.2 years (range 3.0 to 7.2) until a median age of 14.7 years (range 7.2 to 23.8. The replication analysis included 243 participants from the CSSCD cohort. The median age at entry into the cohort was 2.8 months (range 0 to 6 months), with 51.4% male, all with a diagnosis of SCA. The CSSCD cohort was followed prospectively for a median of 8.9 (range 3.2 to 15.8) years. Table 3 includes the clinical features of both SAC and CSSCD participants.
Table 3.
Demographic and clinical characteristics of participants evaluated in the Sleep and Asthma Cohort (SAC) Study and the pediatric component of the Cooperative Study of Sickle Cell Disease (CSSCD) cohort.
| Characteristic | SAC Study (n=159) | CSSCD (n=243) |
|---|---|---|
| Sex, n (% Males) | 82 (51.6) | 125 (51.4) |
| Age of first contact (yrs.)§ | 9.00 (6.0) | 0.23 (0.19) |
| Prospective Follow-up* (yrs.) § | 5.23 (1.71) | 8.93 (4.65) |
| Pain rate, lifetime (events/patient year)§ | 0.37 (0.70) | 0.35 (0.79) |
| Pain rate, prospective (events/patient year)§ | 0.46 (1.17) | 0.40 (0.89) |
| Pain event before age 4, n (%) | 84 (52.8) | 103 (42.4) |
| ACS rate, lifetime (events/patient year)§ | 0.14 (0.32) | 0.14 (0.34) |
| ACS rate, prospective (events/patient year)§ | 0.16 (0.35) | 0.12 (0.32) |
| ACS event before age 4, n (%) | 38.4% | 48% |
| Hemoglobin (g/dl)# | 8.25 ± 1.20 | 8.60 ± 1.00 |
| White Blood Cells, 109/L# | 12.12 ± 3.88 | 12.70 ± 2.75 |
| Mother has asthma, n (%) | 21 (13.2) | 17 (9.1) |
| Wheezing causing shortness of breath, n (%) | 42 (26.4) | Not Evaluated |
| Wheezing after playing hard, n (%) | 53 (33.3) | Not Evaluated |
| Wheezing without having a cold, n (%) | 33 (20.8) | Not Evaluated |
| Cough without having a cold, n (%) | 60 (37.7) | Not Evaluated |
| Congested or phlegm without a cold, n (%) | 22 (13.8) | Not Evaluated |
| Two or more positive skin tests, n (%)** | 38 (27.0) | Not Evaluated |
| Bronchodilator response ≥ 12%, n (%)** | 31 (21.5) | Not Evaluated |
| Lower airway obstruction, n (%)** | 28 (19.4) | Not Evaluated |
Prospective follow-up is from date of entry in SAC and from the age of 4 in CSSCD; Minimum follow-up of three years
Mean (std dev.) for continuous variables with a normal distribution
Median (IQR) for continuous variables with a non-normal distribution
141 patients with non-missing data in SAC for skin tests, 144 patients with non-missing data in SAC for bronchodilator response, and 144 patients with non-missing data in SAC for lower airway obstruction, defined as the forced expired volume in 1 second/forced vital capacity (FEV1/FVC) < lower limit of normal (LLN) according these equations provided by Quanjer et al11.
Asthma features to predict future ACS episodes after enrollment into SAC
Initial evaluation of factors potentially associated with incidence of ACS included asthma features (the ATS/DLD questions and the mother’s asthma status), a history of hospital admission for ACS before age 4 years, gender, age, white blood cell count, and hemoglobin. In the initial screening model, gender, early ACS episode, wheezing causing shortness of breath, and wheezing after exercise all passed the screening criterion of p<0.20.
In the reduced model (Table 4), increased prospective ACS events were associated with gender (female IRR=1.80; 95% CI 1.16–2.79; p=0.009), the presence of at least one ACS episode < 4 years of age (IRR=2.84; 95% CI 1.84–4.37; p<0.001), and history of wheezing causing shortness of breath (IRR=1.68; 95% CI 1.02–2.77 p=0.042).
Table 4.
Final multiple variable negative binomial regression model for prospective ACS and pain events in the Sleep and Asthma Cohort Study for children identified after 4 years of age and up to 17 years of age. Participants with complete data on all covariates in primary models (n= 159).#
| Outcome | Covariate* | Incidence Rate Ratio | 95% Conf. Interval | p |
|---|---|---|---|---|
| Prospective ACS events§ | Gender (female) | 1.80 | (1.16, 2.79) | 0.009 |
| ACS episode before age 4 | 2.84 | (1.84, 4.37) | <0.001 | |
| Wheezing causing shortness of breath | 1.68 | (1.02, 2.77) | 0.042 | |
| Wheezing after exercise | 1.41 | (0.87, 2.28) | 0.163 | |
| Prospective Pain events§ | Age of first contact | 1.04 | (0.99, 1.10) | 0.119 |
| Hemoglobin (g/dl)# | 1.20 | (1.01, 1.42) | 0.035 | |
| Mother has asthma | 1.82 | (0.98, 3.38) | 0.056 |
Negative Binomial regression model with adjustment for over dispersion. Two-tailed significance values.
Patients had to be followed for at least 3 years after enrollment in study. Participants (n=159) and risk factors were identified during their first study visit and followed for a median of 5.2 years.
Models were constructed in two steps. All covariates of interest were entered in step 1. Those with p < 0.20 in the first model were selected for the second, final model displayed here.
Asthma features to predict future pain episodes after enrollment into SAC
Asthma symptoms and clinical features did not predict future pain episodes that required hospitalizations. Evaluation of asthma features included the same covariates as for the model for future ACS episodes, including the ATS/DLD questions, the mother’s asthma status, and a history of hospital admission for ACS before age 4 years. In the screening model, only age, hemoglobin, and mother’s asthma status passed the screening criterion of p<0.20, but not early ACS. In the reduced model (Table 4), increased prospective pain events were associated with increased hemoglobin (IRR=1.20; 95% CI 1.01–1.42, p=0.035).
Ability of spirometry results and allergy skin testing to improve prediction of future pain and ACS episodes
When spirometry results (airway obstruction and bronchodilator responsiveness) and positive allergy skin testing were added to the regression model to predict ACS events, only two or more or more positive skin tests were associated with an increased incidence of ACS (IRR=1.87; CI 1.16–3.00; p=0.010). An ACS episode when less than 4 years of age and wheezing causing shortness of breath remained significant in this model (Table 5). When these additional covariates were added to the regression model for pain events, only hemoglobin level was associated with an increased incidence of pain.
Table 5.
Final multiple variable negative binomial regression models for prospective pain and ACS events in the Sleep and Asthma Cohort Study Cohort Study when spirometry results and skin testing are included in the regression model. The minimum age of the cohort, four years, decreased the number of children with evaluable spirometry and skin testing results. Participants with complete data on spirometry and skin tests (n= 130).
| Outcome | Covariate* | Incidence Rate Ratio | 95% Conf. Interval | P** |
|---|---|---|---|---|
| Prospective ACS§ | Gender (female) | 1.54 | (0.94, 2.53) | 0.089 |
| ACS episode before age 4 | 2.56 | (1.59, 4.12) | <0.001 | |
| Wheezing caused shortness of breath | 1.78 | (1.02, 3.09) | 0.042 | |
| Wheezing after exercise | 1.31 | (0.78, 2.22) | 0.312 | |
| Two or more positive skin tests | 1.87 | (1.16, 3.00) | 0.010 | |
| FEV1/FVC <LLN | 0.86 | (0.48, 1.53) | 0.606 | |
| Bronchodilator response ≥ 12% | 1.18 | (0.66, 2.11) | 0.581 | |
| Prospective Pain§ | Age of first contact | 1.05 | (0.99, 1.12) | 0.116 |
| Hemoglobin (g/dl)# | 1.32 | (1.09, 1.60) | 0.004 | |
| Mother has asthma | 1.50 | (0.73, 3.09) | 0.273 | |
| Two or more positive skin tests | 1.14 | 0.68, 1.92) | 0.628 | |
| FEV1/FVC <LLN | 0.76 | (0.42, 1.39) | 0.377 | |
| Bronchodilator response ≥ 12% | 0.89 | (0.48, 1.66) | 0.720 |
Negative Binomial regression model with adjustment for over dispersion. Two-tailed significance values.
130 patients with non-missing data in SAC cohort with skin tests, bronchodilator response, and lower airway obstruction, defined as the forced expired volume in 1 second/forced vital capacity (FEV1/FVC) < lower limit of normal (LLN) according these equations provided by Quanjer et al.14
Replication of effects of early ACS episodes on prospective ACS events in children enrolled in the CSSCD
Given the association between early onset of ACS and increased rate of prospective ACS events in the SAC cohort, we elected to replicate this analysis in a second prospective cohort, the CSSCD. To validate the model we chose only the covarites that were statistically significant in predicting ACS episodes in the SAC cohort and that could be evaluated in the CSSCD, gender and ACS before the age of 4 years. Initially, the analysis in the SAC cohort was repeated using only those variables available in the CSSCD cohort to provide true replication. In the repeat SAC analysis, both female gender and ACS under the age of 4 years were significant at predicting ACS episodes after enrollment in SAC at age 9.0 years through age 14.7 years (IRR=1.6; 95% CI 1.01–2.46; p=0.46 and IRR=3.05;95% CI 1.95–4.76; p<0.0001, respectively). In this CSSCD analysis, ACS episodes before the age of 4 years were a significant predictor of ACS episodes after the age of 4 years (IRR=2.9; 95% CI 2.1–4.0; p<0.001), but female gender was not (IRR=0.81; 95% CI 0.59–1.10; p=0.18).
Discussion
A doctor-diagnosis of asthma in children with SCD is associated with increased SCD related morbidity[2, 8, 16, 17] and mortality[18]. In the SAC cohort specifically, asthma diagnosis was associated statistically with ACS occurring after entry into the study[8]. However, no comprehensive evaluations of specific asthma signs and symptoms have been conducted to determine whether they are associated with SCD morbidity. A priori, we elected not to include a physician diagnosis of asthma in our study design because not only has the diagnosis of asthma in children with SCA proven to be challenging given the overlap in asthma symptoms that are common in children with SCA that do not have asthma[19, 20], but there is considerable variability in general in making an asthma diagnosis[7]. We instead focused our primary analysis on asthma symptoms and risk factors, which we believed would make our results more generalizable. We elected to include ACS events before 4 years of age in our analyses, as Boyd et al. demonstrated that those with asthma were younger at the time of the first ACS episode than those without an asthma diagnosis[16] and Quinn et al. found that early ACS events predicted subsequent ACS events[9]. The results of our prospective study indicate that at least one early life ACS episode was a significant predictor of later ACS events, and this finding was replicated in a second cohort.
One asthma symptom and one asthma risk factor were associated with future ACS events, specifically history of wheeze causing shortness of breath and presence of two or more positive allergy skin tests. As the children were studied when well and at least one month since a respiratory illness, it is not surprising that airway obstruction or bronchodilator response were not significant covariates in the final multiple variable models.
The mechanism for the association of wheezing and aeroallergen sensitivity to future ACS events is unknown, but two experimental studies undertaken in the transgenic SCD mouse models and two clinical studies completed in children by our team provide a basis to postulate. First, transgenic SCD mice have increased airway lability when compared to hemoglobin A mice[21] at baseline. Second, when sensitized to an inflammatory stimulus, ovalbumin, SCD mice have an additionally exaggerated inflammatory airway response, as demonstrated by increased levels of peri-alveolar eosinophils, plasma IgE, and bronchoalveolar lavage fluid IL-1β, IL-4, IL-6, and monocyte chemotactic protein, when compared to the hemoglobin A mice[21]. Supporting the findings that children with SCA have an increased sensitivity to inflammatory stimuli, two separate studies documented that in children with SCA, total and specific IgE levels, were dramatically elevated when compared to expected ranges in children without SCA[2, 22].
Based on pre-clinical and clinical studies demonstrating a propensity for increased inflammatory response to stimuli, we postulate that similar to the SCD mouse model, external inflammatory stimuli result in an exaggerated response in children with SCD and lead to increased pre-disposition to ACS. For children less than 4 years of age, the age group most susceptible to multiple viral respiratory infections, we posit that these acute respiratory viral infections predispose the young child to development of increased airway inflammation in the near-term and asthma in the long-term. These changes would then predispose to additional SCD events in the near- and long-term. Only future basic science and translational research in this area will ultimately confirm whether acute viral respiratory infections predispose children to future lung disease. The well-established evidence regarding acute infections of respiratory syncytial virus [23–25] or acute rhinovirus infections[26–28] and their associated predisposition for future obstructive lung disease, coupled with current results, makes the study of early viral infection, an important area for investigating the etiology and potential treatment of lung disease in SCA.
We did not identify an association between asthma symptoms or asthma risk factors and pain; although, all of the effects were in the expected direction in the initial logistic model. The lack of a significant association may be due to either a true or false negative association. The false negative association might be related to the relatively small number of patient-years of follow-up, particularly when compared to other studies that have showed an association[2, 16]. Evidence to support this possibility is the observation that the current study had less than 1,000 patient years accumulated in the analysis, while the two largest cohort studies had approximately 3,000[2] and 4,000 patient years[16], respectively, with both demonstrating a statistically significant relationship between an asthma diagnosis and an increased incidence of both ACS and pain episodes when compared to children without an asthma diagnosis. Based on these studies, asthma symptoms and asthma risk factors are more strongly related to the increased incidence of ACS than pain events.
This study had several limitations. The SAC cohort consisted of participants from three academic medical centers with a high level of sickle cell disease and asthma expertise. Thus the patient population cannot be considered generalizable to all children with SCA. However, there was no pre-selection of children at the three sites for lung disease, the prevalence of asthma (28% in the SAC cohort used in the models) was similar to the figure of 27% (140 of 521) in the largest study to date of children with SCA to assess the prevalence of asthma[2]. Furthermore, our primary results were replicated in a cohort followed from early infancy where children were not selected for severity of disease[29] and in addition are similar to findings in another cohort followed from birth[9]. In the CSSCD cohort, we were unable to confirm whether affirmative answers to the ATS/DLD questions was associated with future ACS or pain episodes, as these data were not collected. However, a history of recurrent severe wheezing was associated with future ACS events in a prospective study of adults[30] and the presence of wheezing was associated with an increased incidence of pain and ACS presentation to the emergency department in a retrospective study in children and adults with SCA[31]. Taken together the results from this study and the other two studies indicate that history of wheezing is a significant predictor for ACS in children and adults with SCA.
Based on the results of this study, health care providers can now begin to identify children with SCA at risk for future ACS events with a simple questions about the presence of wheezing causing shortness of breath coupled with obtaining a history of an ACS episode prior to four years of age. Future research is needed to better understand the reasons underlying the peak incidence of ACS in SCA in children between 1 and 4 years of age, and how this leads to an increased incidence rate of future ACS events as clearly documented in this study.
Supplementary Material
Acknowledgments
This work was supported by the NHLBI, R01-HL079937, MRD and RCS
Footnotes
Disclosure of Conflicts of Interest: The authors declare no competing financial interests.
References
- 1.Vichinsky EP, Styles LA, Colangelo LH, et al. Acute chest syndrome in sickle cell disease: clinical presentation and course. Cooperative Study of Sickle Cell Disease. Blood. 1997;89:1787–1792. [PubMed] [Google Scholar]
- 2.An P, Barron-Casella EA, Strunk RC, et al. Elevation of IgE in children with sickle cell disease is associated with doctor diagnosis of asthma and increased morbidity. J Allergy Clin Immunol. 2011;127:1440–1446. doi: 10.1016/j.jaci.2010.12.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koumbourlis AC, Zar HJ, Hurlet-Jensen A, et al. Prevalence and reversibility of lower airway obstruction in children with sickle cell disease. The Journal of pediatrics. 2001;138:188–192. doi: 10.1067/mpd.2001.111824. [DOI] [PubMed] [Google Scholar]
- 4.Field JJ, Stocks J, Kirkham FJ, et al. Airway hyperresponsiveness in children with sickle cell anemia. Chest. 2011;139:563–568. doi: 10.1378/chest.10-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ozbek OY, Malbora B, Sen N, et al. Airway hyperreactivity detected by methacholine challenge in children with sickle cell disease. Pediatric pulmonology. 2007;42:1187–1192. doi: 10.1002/ppul.20716. [DOI] [PubMed] [Google Scholar]
- 6.Sylvester KP, Patey RA, Rafferty GF, et al. Airway hyperresponsiveness and acute chest syndrome in children with sickle cell anemia. Pediatric pulmonology. 2007;42:272–276. doi: 10.1002/ppul.20571. [DOI] [PubMed] [Google Scholar]
- 7.Van Sickle D, Magzamen S, Maenner MJ, et al. Variability in the labeling of asthma among pediatricians. PloS one. 2013;8:e62398. doi: 10.1371/journal.pone.0062398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strunk RC, Cohen RT, Cooper BP, et al. Wheezing symptoms and parental asthma are associated with a physician diagnosis of asthma in children with sickle cell anemia. The Journal of pediatrics. 2014;164:821–826. e821. doi: 10.1016/j.jpeds.2013.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quinn CT, Shull EP, Ahmad N, et al. Prognostic significance of early vaso-occlusive complications in children with sickle cell anemia. Blood. 2007;109:40–45. doi: 10.1182/blood-2006-02-005082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson HS, Szefler SJ, Jacobs J, et al. The relationships among environmental allergen sensitization, allergen exposure, pulmonary function, and bronchial hyperresponsiveness in the Childhood Asthma Management Program. J Allergy Clin Immunol. 1999;104:775–785. doi: 10.1016/s0091-6749(99)70287-3. [DOI] [PubMed] [Google Scholar]
- 11.Bacharier LB, Dawson C, Bloomberg GR, et al. Hospitalization for asthma: atopic, pulmonary function, and psychological correlates among participants in the Childhood Asthma Management Program. Pediatrics. 2003;112:e85–92. doi: 10.1542/peds.112.2.e85. [DOI] [PubMed] [Google Scholar]
- 12.Strunk RC, Sternberg AL, Bacharier LB, et al. Nocturnal awakening caused by asthma in children with mild-to-moderate asthma in the childhood asthma management program. J Allergy Clin Immunol. 2002;110:395–403. doi: 10.1067/mai.2002.127433. [DOI] [PubMed] [Google Scholar]
- 13.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 14.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferris BG. Epidemiology Standardization Project (American Thoracic Society) The American review of respiratory disease. 1978;118:1–120. [PubMed] [Google Scholar]
- 16.Boyd JH, Macklin EA, Strunk RC, et al. Asthma is associated with acute chest syndrome and pain in children with sickle cell anemia. Blood. 2006;108:2923–2927. doi: 10.1182/blood-2006-01-011072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knight-Madden JM, Forrester TS, Lewis NA, et al. Asthma in children with sickle cell disease and its association with acute chest syndrome. Thorax. 2005;60:206–210. doi: 10.1136/thx.2004.029165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernaudin F, Strunk RC, Kamdem A, et al. Asthma is associated with acute chest syndrome, but not with an increased rate of hospitalization for pain among children in France with sickle cell anemia: a retrospective cohort study. Haematologica. 2008;93:1917–1918. doi: 10.3324/haematol.13090. [DOI] [PubMed] [Google Scholar]
- 19.Field JJ, DeBaun MR. Asthma and sickle cell disease: two distinct diseases or part of the same process? Hematology / the Education Program of the American Society of Hematology American Society of Hematology Education Program. 2009:45–53. doi: 10.1182/asheducation-2009.1.45. [DOI] [PubMed] [Google Scholar]
- 20.Anim SO, Strunk RC, DeBaun MR. Asthma morbidity and treatment in children with sickle cell disease. Expert review of respiratory medicine. 2011;5:635–645. doi: 10.1586/ers.11.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pritchard KA, Jr, Feroah TR, Nandedkar SD, et al. Effects of experimental asthma on inflammation and lung mechanics in sickle cell mice. Am J Respir Cell Mol Biol. 2012;46:389–396. doi: 10.1165/rcmb.2011-0097OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howie J. Portraits from memory. 22--Dr Samuel Tertius Cowan (1905–76) British medical journal. 1987;295:1114–1115. doi: 10.1136/bmj.295.6606.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sigurs N, Gustafsson PM, Bjarnason R, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. American journal of respiratory and critical care medicine. 2005;171:137–141. doi: 10.1164/rccm.200406-730OC. [DOI] [PubMed] [Google Scholar]
- 24.Sly PD, Hibbert ME. Childhood asthma following hospitalization with acute viral bronchiolitis in infancy. Pediatric pulmonology. 1989;7:153–158. doi: 10.1002/ppul.1950070307. [DOI] [PubMed] [Google Scholar]
- 25.Stein RT, Sherrill D, Morgan WJ, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541–545. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 26.Jackson DJ, Gangnon RE, Evans MD, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. American journal of respiratory and critical care medicine. 2008;178:667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lemanske RF, Jr, Jackson DJ, Gangnon RE, et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116:571–577. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 28.Jackson DJ, Lemanske RF., Jr The role of respiratory virus infections in childhood asthma inception. Immunology and allergy clinics of North America. 2010;30:513–522. vi. doi: 10.1016/j.iac.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaston M, Smith J, Gallagher D, et al. Recruitment in the Cooperative Study of Sickle Cell Disease (CSSCD) Controlled clinical trials. 1987;8:131S–140S. doi: 10.1016/0197-2456(87)90016-x. [DOI] [PubMed] [Google Scholar]
- 30.Cohen RT, Madadi A, Blinder MA, et al. Recurrent, severe wheezing is associated with morbidity and mortality in adults with sickle cell disease. Am J Hematol. 2011;86:756–761. doi: 10.1002/ajh.22098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glassberg JA, Chow A, Wisnivesky J, et al. Wheezing and asthma are independent risk factors for increased sickle cell disease morbidity. Br J Haematol. 2012;159:472–479. doi: 10.1111/bjh.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.