Abstract
To study how the interaction between orbitofrontal (OFC) and rhinal (Rh) cortices influences the judgment of reward size, we reversibly disconnected these regions using the hM4Di-DREADD (Designer Receptor Exclusively Activated by Designer Drug). Repeated inactivation reduced sensitivity to differences in reward size in two monkeys. Results suggest that retrieval of relative stimulus values from memory appears to depend on interaction between Rh and OFC.
Interrupting the flow of information by disconnecting brain regions from one another has been, and remains, a powerful means to investigate interactions between putative components of neural circuits. Previous work from this laboratory has demonstrated that functionally disconnecting rhinal cortex (Rh) and orbitofrontal cortex (OFC) leads to altered estimates of expected value through reward-stimulus association1. In that earlier study, the disconnection was achieved by removing Rh in one hemisphere, and OFC in the contralateral hemisphere, presumably disrupting the intrahemispheric flow of information between them. One intriguing possible mechanism for the loss of reward sensitivity in the Rh-OFC disconnection is that the monkeys are unable to retrieve information about the relative values of the rewards. To test for a failure in retrieval requires that the testing be instituted in a manner that allows repeated periods of disconnection after the initial acquisition. Here, a disconnection was accomplished by implementing the DREADD chemogenetic technique in monkeys.
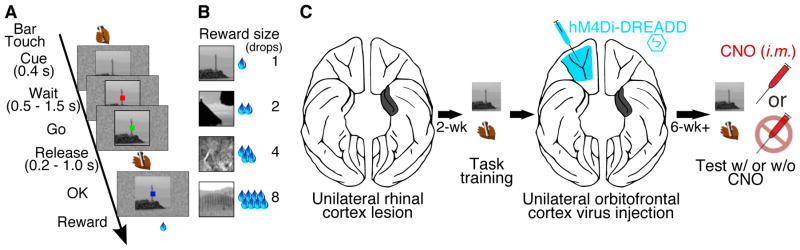
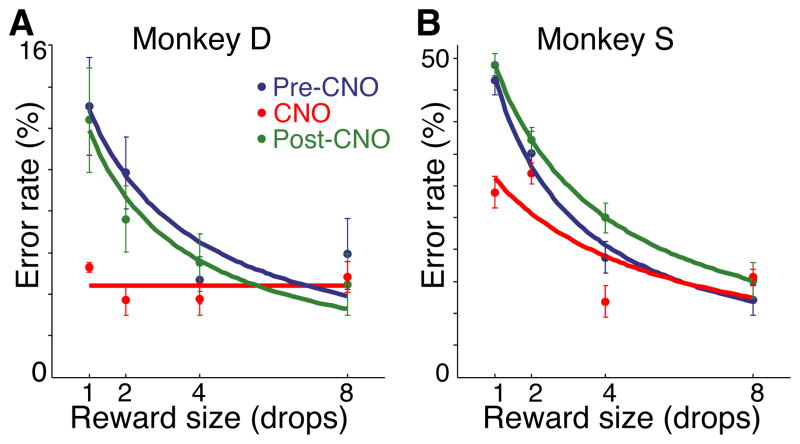
We performed the study as follows: two naïve monkeys (1) received unilateral removal of Rh cortex followed by a recovery period, (2) were trained to perform a stimulus-reward association task, (3) received unilateral viral vector injections into OFC contralateral to the Rh removal, and (4) were tested on the stimulus-reward association task with or without CNO (Fig. 1). Monkeys were trained on the association task as described in figure 1A. For the viral vector injections, a lentiviral vector expressing an hM4Di-CFP (DREADD) fusion protein under a neuron-specific promoter was generated (method in supplemental text – ‘DNA constructs’). When the lenitviral vector was used to express hM4Di in murine cells in vitro, binding of the systemic inducer, CNO, induced silencing of neuronal activity (Fig. S1A–D). Injections of the lentiviral vector (46 for monkey D; 71 for monkey S) were placed in OFC in an open surgery, in the hemisphere contralateral to the Rh ablation. Animals recovered without incident. After the injections there was a 6-week minimum waiting period for vector expression to become fully established before testing resumed on the task. Both monkeys began discriminating between the cues within the first testing session. The error rates of the monkeys decreased with increasing drop size (LME; reward size, z = 12.74, p < 10−15), with performance indistinguishable from unoperated controls 2. Because sensory and motor demands were trial invariant, we interpret the differences in performance across reward size as reflecting the subjective valuation of the expected reward by the monkey. Monkeys were tested on 5-day cycles. On days 1,2, 4 and 5 there was no drug treatment. On day 3 the monkeys were given an intramuscular CNO injection (10 mg/kg). The experimental cycle was repeated 4 times for monkey D and 5 times for monkey S. CNO treatment induced a reduction in overall error rate (main effect of treatment) and the pattern of errors no longer reflected the reward size (treatment x reward size interaction); there was a reduction or loss in discrimination among expected reward sizes, for both monkeys (Fig. 2)(linear mixed effects model with binomial link function (LME) - CNO vs. baseline: Monkey D; treatment, z = 4.62, p < 10−4, treatment x reward size, z = −3.87, p < 10−3; Monkey S; treatment, z = 7.12, p < 10−11, treatment x reward size, z = −2.93, p < 0.005). Performance returned to baseline level the day after CNO induction of neuronal silencing (LME - baseline vs. treatment day +1: Monkey D; main effect, z = −0.5, p > 0.05, interaction with reward size, z = −0.002, p > 0.05; Monkey S; main effect, z = 1.29, p > 0.05, interaction with reward size, z = −0.17, p > 0.05). CNO injections did not alter reaction times, total trials completed or total reward earned (Fig. S2). Vehicle injections in monkey D, and CNO administered prior to virus injection in monkey S were without effect (Fig. S3). All behavioral testing was conducted during a period in which CNO concentration in the cerebrospinal fluid (CSF) was well above baseline level (Fig. S1)
Fig. 1.
Task and experimental design. (A) A trial in the reward size task - at the beginning of each trial, a visual cue signaled the amount of reward (1, 2, 4 or 8 drops) that would be delivered after a correct red-green color discrimination. After each correct trial, a new cue-reward size pair was picked from the set of 4 at random. (B) Relationship between cues and reward sizes. (C) Experimental design and timeline: unilateral Rh lesion (black) paired with contralateral injection of hM4Di vector in OFC (cyan) to produce functional disconnection when hM4Di receptors are activated with CNO.
Fig. 2.
Behavioral effects of CNO (10 mg/kg, i.m.) (A) Monkey D and (B) Monkey S, after injection of OFC with hM4Di vector. Error rates as a function of reward size are shown. Pre-CNO: two days prior to CNO treatment. CNO: treatment day. Post-CNO: two days after CNO treatment.
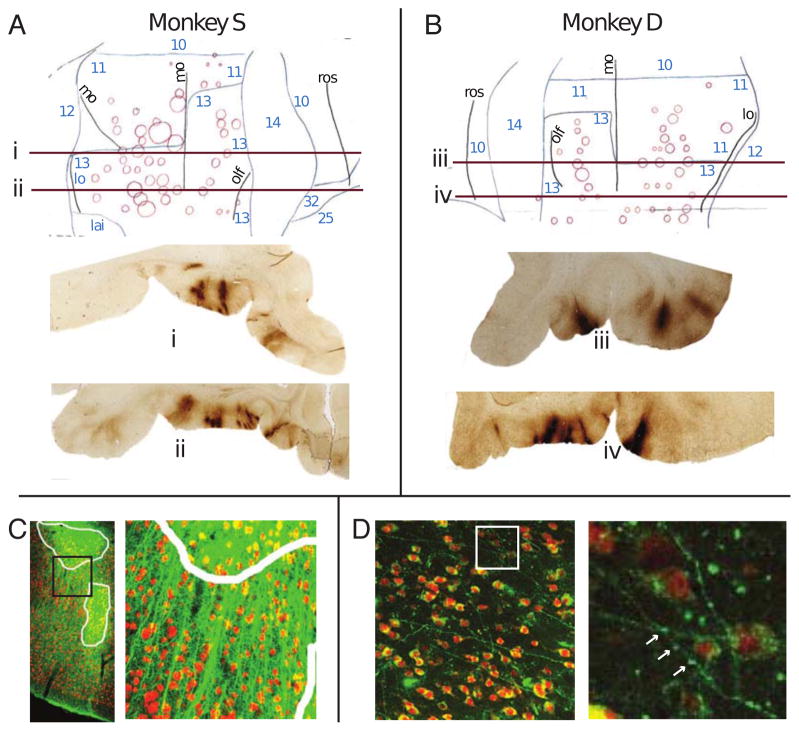
The extent and specificity of receptor expression was analyzed in post-mortem tissue. GFP antibody treated tissue was stained with diamino-benzidine (DAB) and examined using conventional light microscopy. Standard flat maps were constructed 3. In Brodmann area 13 there was 6.8% tissue coverage in monkey D and 12.2 % in monkey S; coverage of area 11 was lower; 4.5% for monkey D and 9.1% for monkey S (Fig. 3A–B). Using confocal fluorescent detection of regions with DREADD and neuronal cell marker NeuN overlap, in area 13 cell expression was 6.8% in monkey D and 6.7 % in monkey S, and in area 11 there was 3.0% for monkey D and 3.4% for monkey S (Fig 3C&D); expression was distributed throughout all layers of the cortex with a somewhat higher coverage in the middle layers (Fig. S4). Neuronal penetrance was as high as 100% within a 100μm radius from the center of some expression areas (Fig. S4G).
Fig. 3.
Histology analysis of hM4Di-CFP DREADD expression. (A and B) Flatmap reconstructions of DREADD expression from DAB-stained sections – e.g. sections i, ii, iii, iv presented below flatmaps. Brodmann regions indicated by blue numbers. Sulci: mo – medial orbital, lo – lateral orbital, olf – olfactory, rs – retrospenial. (C–D) Confocal fluorescent images of DREADD expression (green) and somata (red). (C) DREADD expression in OFC. Cellular expression areas are outlined in white. Black square in left panel indicates area enlarged in the right panel. (D) DREADD-expressing axonal projections in Rh. White square in left panel indicates area enlarged in the right panel. Synaptic boutons are visible along projections (white arrows).
In the current study we have shown that when Rh removal is combined with CNO-induced chemogenetic silencing of the contralateral OFC, sensitivity to differences in reward size decreases during the silencing, and recovers afterwards. We believe that the functional disconnection produces a specific effect on cognitive processing rather than a general modulation of motivation or attention, because the CNO-induced silencing had no effect on reaction times, total trials completed in a session or total reward earned. The deficit we observe with CNO-induced silencing disappeared completely within 24 hours after a single parenteral dose of CNO. Because the monkeys are more sensitive to differences among expected rewards on the days both before and after the CNO, it seems likely that the information about the relationship between stimuli and rewards has been stored, and the deficit that arises during the period of disconnection (during the DREADD activation with CNO) arises because the monkeys are impaired in retrieving or using information about the relative values of stimulus-reward associations. The present experiments do not address the issue of whether the Rh-OFC connection is required for acquisition or consolidation.
There are several advantages to DREADDs for studying the influence of local circuitry on behavior. Because the inactivation is temporary, the chance for adaptation is small. In contrast to aspiration lesions, there is no risk of damaging fibers of passage. For experiments of a few hours duration, silencing requires only a single systemic injection of the activating drug in each testing session. There is no potentially damaging instrumentation of the tissue as would be needed in conventional pharmacology where local injections must be repeated, optogenetics where instruments to deliver light must be present, or cooling where the cooling device must be present4–6. The location, spread, and density of receptor expression can be estimated in vivo with a PET ligand targeting the receptor (Takafumi Minamimoto, personal communication). At the conclusion of the experiment, post-mortem histochemical analysis can be used to identify the neurons expressing the DREADDs.
Disadvantages of the CNO-DREADD include a slow onset and slower recovery. For detailed studies into the dynamics of neural circuits the slow onset and disappearance of effect is a disadvantage. For some types of behavioral experiments, such as here, the relatively long duration of action can be seen as an advantage. During screening (for details see Online methods), two monkeys were affected when treated with CNO alone, that is, before receiving any viral construct. This sensitivity could limit the utility of the CNO-DREADD system for future studies and makes clear the importance of examining CNO sensitivity before injection of virus.
The relative sparseness of affected neurons was surprising. Nonetheless, we observed a repeatable change in behavior with inhibitory DREADD activation. Thus it appears that inactivation of even a limited number of neurons in OFC is sufficient to disrupt the functional connection between OFC and Rh. Factors that may contribute to the effectiveness in modifying behavior despite the apparent sparseness of transduction are: first, the penetrance of receptor expression was relatively high (as high as 100% of cells within a 100-μm radius at some expression sites – cf. Fig. S4); second, there is extensive local arborization of fibers, covering most of OFC, and diffuse projections to structures such as Rh and medial striatum7; and third, based on in vivo PET data, 10 mg/kg CNO would produce near-maximal occupancy of hM4Di receptors (Takafumi Minamimoto, personal communication). Irrespective of mechanism, the results described here raise the possibility that the encoding of information within OFC is so delicately balanced that disruption of 10% or fewer of neurons is sufficient to produce a marked change in behavior.
The current study, in which OFC-Rh interaction was temporarily and reversibly disrupted, demonstrates that the DREADD system can be used to disrupt cortical information processing reversibly and repeatedly. Using the DREADD we show that the Rh-OFC interaction is required for monkeys to retrieve information about the relative values of sets of stimulus-reward associations, a result that would have been difficult to obtain without this technique.
Online methods
DNA constructs
To create the Lenti-hSyn::hM4Di-CFP plasmid, we replaced the mCherry open reading frame with the sequence for the cyan fluorescent protein, cerulean 8, in the hM4Di-mCherry sequence 9, synthesized the resulting hM4Di-CFP gene (Geneart – lifetechnologies.com) and cloned it into the Lenti-syn::GFP vector 10 by replacing the GFP sequence.
Virus preparation and storage
Lentivirus was produced at titers >10E9 using methods similar to those described by Han et al. 11. In short, 293T cells (Lenti-X Invitrogen 632180, Life Technologies) were transfected with the Lenti-backbone plasmid and packaging plasmids (Addgene, Cambridge, MA, USA). Supernatant was replaced after 24 h with Ultraculture medium (Invitrogen). Lentivirus was harvested by collecting the supernatant 48 and 72 h after transfection and spinning it at 22000 r.p.m (Beckman S28 rotor) through a 20% sucrose cushion in phosphate-buffered saline (PBS). Virus pellet was suspended by incubation in ice-cold PBS for 1-h, carefully mixed, aliquoted and frozen on dry ice for storage at −80°C. Titers were determined by preparing genomic DNA from 293T cells transduced with the Lentivirus and using quantitative PCR to compare amplification of a 150 bp lentivirus fragment with amplification of a comparable fragment of the endogenous human vasopressin receptor gene (hVAR1).
Subjects
Subjects were 17 adult monkeys (Macaca mulatta). One 9-year old male (monkey D) and one 14-year old female (monkey S), both experimentally naïve, and weighing 9 and 6 kg, respectively, were used for behavioral testing. Monkey D received a unilateral Rh lesion (including perirhinal and entorhinal cortex) in the left hemisphere, and 46 virus injections into contralateral OFC (areas 11 and 13). Monkey S received a Rh lesion to the right hemisphere, and 71 virus injections to contralateral OFC (areas 11, 13 and 14). The Rh lesions were as intended, and consistent with previous descriptions 12–14, with near-complete unilateral removal of Rh (Fig. S5). A third subject, an 8-year old female (monkey G), weighing 7 kg, was used for the time series CSF study (see below). The remaining 14 monkeys received a single acute cistern tap, and blood draw from the femoral artery, for the collection of samples 60 minutes after CNO injection, for metabolite analysis. Monkeys were screened for sensitivity to CNO (10 mg/kg i.m.) prior to viral injections. If CNO sensitivity was evinced - manifested as a reduction in trials performed and slowed reaction times in the stimulus-reward association task - animals were not entered in this study (this resulted in the exclusion of 2 animals). Monkeys were pair-housed and maintained on a 12-hour light-dark cycle. All experimental procedures conformed to the Institute of Medicine (IOM) Guide for the Care and Use of Laboratory Animals and were carried out under an animal study protocol approved by the National Institute of Mental Health Animal Care and Use Committee.
Surgeries
Surgeries were performed under aseptic conditions in a fully equipped operating suite with veterinary supervision. Before surgery, animals were sedated with ketamine hydrochloride (10 mg/kg, intramuscular); a surgical level of anesthesia was then induced and maintained with isoflurane gas (1–4% to effect). Body temperature, heart rate, blood pressure, SpO2 and expired CO2 were monitored throughout all surgical procedures. For both aspiration lesions and virus injections, the cortex was exposed by removing a bone flap and reflecting the dura mater. Aspiration lesions of Rh have been described in detail previously 12–14. Handheld injections into the OFC were performed in a similar manner to Rh injections that have been described in detail previously 15. All virus aliquots were allowed to thaw on ice for at least 15 min. If necessary, the liquid was spun to the bottom of the tube with a small bench-top centrifuge. Virus was then carefully taken up into a 10 μL Hamilton syringe by manually raising the plunger. The syringe, fitted with a cemented 30 gauge needle, was inserted into the intended area of injection by one person and a second person pressed the plunger to expel approximately 1μL, after which the needle was removed and inserted into the next location. Injections were placed approximately 1–2 mm apart. Injections of the lentiviral vector (46 for monkey D; 71 for monkey S) were placed in OFC in an open surgery, in the hemisphere contralateral to the Rh ablation. The area to be injected was bounded medially by the olfactory sulcus, laterally by the lateral orbital sulcus, rostrally by an imaginary line between the rostral tips of the medial and lateral orbital sulci, and caudally by line between the caudal tips of the medial and lateral orbital sulci, i.e. the intended area to be injected included Brodmann areas 11 and 13. Following surgery, a minimum period of 6 weeks elapsed before behavioral testing commenced, in order to allow sufficient time for viral expression to asymptote (Takafumi Minamimoto, personal communication).
For lumbar catheterization, a laminotomy was performed in vertebra L5 to provide access to the thecal sac. A small incision was made in the sac to permit catheter insertion. Blunt dissection was used in the area of the upper thigh to create a conventional port pocket to accommodate the port head. The catheter was tunneled subcutaneously from the lumbar region to the upper thigh. The CSF access port was connected to the catheter and then secured to the musculature of the thigh.
Behavior
The tasks and training have been described in detail previously 2. Monkeys sat in a primate chair inside a darkened, sound-attenuated testing chamber. They were positioned 57 cm from a computer monitor (Samsung 2233RZ - 16) subtending 40 × 30 degrees of visual angle. Task timing and visual stimulus presentation were under the control of networked computers running, respectively, custom written (Real-time Experimentation and Control—REX 17) and commercially available software (Presentation, Neurobehavioral Systems) for the design and control of behavioral experiments. Monkeys were initially trained to grasp and release a touch sensitive bar to earn fluid rewards. After this initial shaping, a red - green color discrimination task was introduced18. Red - green trials began with a bar press, 100 ms later a small red target square (0.5°) was presented at the center of the display (over-laying a white noise background). Animals were required to continue grasping the touch bar until the color of the target square changed from red to green. Color changes occurred randomly between 500 and 1,500 ms after bar touch. Rewards were delivered if the bar was released between 200 and 1,000 ms after the color change; releases occurring either before or after this epoch were counted as errors. All correct responses were followed by visual feedback (target square color changed to blue) after bar release and reward delivery 200 – 400 ms after visual feedback. After an animal reached criterion in the red - green task (2 consecutive sessions with >85% correct performance) a visually cued reward size task was introduced (Fig. 1A). Each trial began when animals grasped the touch bar, bar press was now initially followed (after 100 ms) by the presentation of a cue image (gray scale natural images, 10° × 10°, superimposed on a white noise background). Each cue signaled which of four different reward sizes (1, 2, 4, or 8 drops, random draw) the animal would earn upon successful completion of the trial. Four hundred milliseconds after cue presentation a red target square appeared, now centered on the cue. Animals were once more required to hold the touch bar until the target square color changed from red to green (500 –1,500 ms). Releases that occurred outside of the 200 – 1,000 ms interval following the color change were counted as errors; error trials were repeated until completed correctly. Successful bar releases were signaled via visual feedback (blue square). Reward delivery followed feedback by 200 – 400 ms and lasted for between 150 and 2,500 ms (200 ms inter-drop interval). Monkeys were tested in the visually-cued reward size task for 90 minutes each session, 1 session per day, 5–7 days per week; the same set of four cue images were used throughout the study.
Drugs
CNO (RTI international, North Carolina) was dissolved at 66 mg/ml in 100% DMSO, then diluted with phosphate-buffered saline (PBS) to produce a final concentration of 10 mg/ml CNO in 15% DMSO.
Data analysis
Modeling and statistical methods were implemented in Matlab (MathWorks) and R (Team, 2004). Linear mixed effects models (LME) were used to evaluate whether the dependent variable, reward size, or the factors, session number, CNO treatment, treatment cycle, monkey, had a significant effect on performance, quantified as error rates. This approach assumes that variation in repeated measures data is due to both fixed (e.g. reward size) and random (e.g. monkey) effects, allows independent variables to be treated as continuous (e.g. reward size) or categorical (e.g. CNO treatment), and allows for non-normal dependent measures (i.e. error rates) 19. A binomial link function was used to relate the mean of the error rates to the linear predictors in the model.
ANOVA models were used to analyze reaction times and total rewards earned per session.
The behavioral data were fit with the function: E = c / (b + R), where the error rates, E, are well approximated by an inverse function of reward size, R, with c and b constant for each monkey 20. For parameter and R2 values see supplementary Table S1.
Histology
After the completion of behavioral testing, each monkey was deeply anaesthetized with Beuthanasia solution and perfused with 1 L of normal saline solution, followed by 3 L of 4% paraformaldehyde in 0.1 M PBS. The brains were removed and cryoprotected through a series of glycerols in 0.1 M PBS. The brains were blocked in the coronal plane and then quickly frozen in −80°C isopentane. The brain was sectioned into 40-μm slices in the coronal plan and the sections collected in 10 series. For hM4Di-CFP histochemistry and immunofluorescence, sections were blocked in 5% normal goat serum and 0.3% Triton-X 100 in tris-buffered saline (TBS), incubated with rabbit anti GFP primary antibody (1:10,000 - Abcam AB290) in blocking buffer, washed and incubated with a biotinylated goat anti-rabbit IgG antibody (1:500 - Vector Laboratories BA-1000). The signal was amplified with the VECTASTAIN ABC System (Vector Laboratories) according to specifications. For immunohistochemistry expression was visualized with a 3,3′-diaminobenzidine (DAB) reaction and optionally counterstained with thionine. For immunofluorescence expression was visualized with Streptavidin, Alexa Fluor 488 conjugate (LifeTechnologies). For NeuN immunofluorescence, sections were blocked as above, incubated with mouse anti NeuN primary antibody (1:1,000 Chemicon MAB377), washed and detected with Alexa Fluor 555 goat anti-mouse IgG (1:500 Lifetechnologies A-21422). DAB stained sections were examined using a Zeiss Axiovert x 200 microscope connected to a Neurolucida stage imaging software (MBF Bioscience). Confocal imaging was performed using and LSM 780 inverted scanning confocal instrument and analyzed with the Zeiss Zen software. Counts of cells expressing CFP as a proportion of all NeuN stained cells present were made by eye using an overlaid 100-μm2 grid. All cell counts were performed on optical confocal slices to ensure co-localization of fluorescent staining.
Flat map and lesion reconstructions
Following the procedure of Van Essen and Maunsell 3 a two-dimensional reconstruction (flattened map) of the orbital frontal cortex was completed for each case. Tracings of layer IV were made from projections of thionine-DAB stained coronal sections, 40 μm thick, taken at 800 μm intervals through the frontal lobe. On each stained section the cytoarchitectonic borders, the fundus of each sulcus, and the extent of each viral injection site were marked. The area of effective viral penetration was defined as the area in which the cortical laminar pattern was obscured. The cytoarchitectonic divisions of the frontal cortex were adapted from the early descriptions of Walker 21 and later revised by Pandya and colleagues 22, 23 and Carmichael and Price 24.
Photographs of thionine-stained coronal sections taken at 400 μm intervals were used to reconstruct the Rh lesions in both subjects. The extent of the damage in each case was plotted on drawings of a standard rhesus monkey brain at ~1-mm intervals, and the lesions were reconstructed (Fig. S5).
In-vitro experiments
Whole brain neurons were dissociated and isolated from embryos of B6J mice (embryonic day 14.5) using ‘Dissociation Kit’ and ‘Neuron Isolation Kit’ (Miltenyi Biotec, Auburn, CA, USA; cat. no. 130-094-802 and 130-098-752, respectively). Dissociated neurons were diluted at a density of 1 × 106 cells/mL in a neuron culture medium. A 20μL aliquot of each cell suspension was plated on a poly-L-lysine (Sigma-Aldrich), and Matrigel (BD, Becton, Dickinson and co.)-coated MED64 Probe Multi-electrode Dish (MED-P515A, Alpha MED Scientific, Osaka, Japan), which consisted of 64 electrodes in an 8 × 8 grid with inter-electrode spacing of 150 μm. The neurons were maintained in culture medium (DMEM/F12 (Invitrogen) supplemented with 10% Knockout Serum Replacement (KSR; Invitrogen), N2 (Invitrogen), B-27 Vitamin A (Invitrogen), 10 ng/mL BDNF, 10 ng/mL GDNF, 10 ng/mL NT-3) and incubated at 37°C in a humidified atmosphere with 5% CO2. At 0 or 7 days of culture, the neurons were infected by Lenti-hSyn:: hM4Di-CFP vector or Lenti-hSyn::GFP vector for 8–48 h. At 22–24 day of culture, the extracellular action potentials of neurons were recorded by a 64-channel recording system (MED64 system, Alpha MED Science) with a sampling rate of 20 kHz for 60s. The recordings were performed as following conditions, baseline, CNO and washout. At CNO condition, recording was started at 20 min after additional CNO (1 μM) into culture medium. At washout condition, medium was replaced with fresh medium without CNO and incubated for 20 min for washout of CNO, and then the activity was recorded. Extracellular spikes were determined as events when their amplitude exceeded a pre-specified threshold (30μV). Six single-unit activities that firing rate was less than 25Hz were used for the analysis from each condition.
Cerebrospinal fluid and serum analysis
Prior to the beginning of CSF sample collection for time course analysis, the monkey was habituated to being restrained within the home cage. The monkey was then restrained to allow 0.5 mL of CSF to be sampled from the subcutaneously implanted port (see ‘Surgeries’ above) using a Huber needle, at various time-points after CNO administration. For acute CSF and serum sample collection, monkeys were anaesthetized following CNO injection, and an acute cistern tap, and blood draw from the femoral artery, were performed 60 minutes after CNO injection.
CSF samples were diluted 1:20 in a 50% acetonitrile, 50% ddH2O solution containing 0.1 μM Chlorpropamide (internal standard). Diluted samples were vortexed briefly, then ultracentrifuged at 14000 g, at 4°C, for 15 minutes. 150 μL of supernatant from each sample was transferred to a well in an ultra performance liquid crystalography (UPLC) plate. Serum samples were diluted 1:10 in a 100% acetonitrile solution containing 0.1 μM Chlorpropamide. Diluted samples were vortexed briefly, then ultracentrifuged at 14000 g, at 4°C, for 20 minutes. Crashed serum was diluted 1:10 in a 50% acetonitrile, 50% ddH2O solution containing 0.1 μM Chlorpropamide (internal standard), for a final dilution of 1:100. 200 μL of each each final solution was transferred to a well in a UPLC plate. The use of 100% acetonitrile in the preparation of serum is necessary to ensure the removal of lipid and protein matter.
Quantification of clozapine and its N-Oxide was performed by multiple reaction monitoring (MRM) using a Waters UPLC Acquity I-class system coupled to a Waters Xevo TQ-S Triple Quadrupole Mass Spectrometer operated by MassLynx software. A Waters Acquity BEH C18 column (2.1 × 50 mm) was used for metabolite separation. UPLC was performed with the following: A, water; B, acetonitrile; both A and B contained 0.1% formic acid. Gradient: initial gradient of 90% A for 0.5 minute, then a linear gradient to 20% A at 4 minutes, held until 5 minutes, then returning to initial conditions and held for an additional 2 minutes for column equilibration. The flow rate was 0.4 mL/minute, and the column temperature was maintained at 40°C. The Waters Xevo TQ-S was operated in MRM ESI+ mode. The following instrument conditions were used: 2.5kV capillary voltage, 150°C source temperature, desolvation gas flow rate of 950 L/hour at 600°C, source offset 50V, and cone gas flow of 150L/hour. The total run time was 7 minutes. The following MRM transitions were used to monitor each compound: clozapine (327.0 →192.0, 327.0→270.0), clozapine-N-oxide (343.0→192.0, 343.0→243.0), and chlorpropamide (as internal standard) (277.0→110.9). The optimal cone voltage and collision energy for each MRM transition was determined using IntelliStart software (Waters). All data were processed using TargetLynx software (Waters Corporation). Internal standard-normalized area under the peak (response) from serially diluted authentic standard solution was used to build calibration curve for each compound. The concentration of compounds was determined from the calibration curve multiplied by any dilution factor.
Supplementary Material
Acknowledgments
We thank A. Cummins, M. Malloy, E. Masseau, A. Horovitz, K. Lowe, J. Fredericks, B. Corgiat, S. Bhayana, and V. Der Minassian for their technical assistance. This study was supported by the Intramural Research Program of the National Institute of Mental Health (MAGE, WL, RCS, KK, FG, and BJR), PRESTO/JST and KAKENHI 15H05917 (TM).
Footnotes
Author contributions
MAGE, WL, RCS, & BJR designed the study. WL produced virus. RCS, WL & MAGE performed surgery. MAGE & BJR analyzed the behavioral data. RS, WL & MAGE analyzed the histological data. KK & FJG analyzed serum and CSF samples. HK, BJ, MH & TM performed and analyzed in vitro experiments. MAGE, WL, RCS, TM, & BJR wrote the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Note: Any Supplementary Information and Source Data files are available in the online version of the paper.
References
- 1.Clark AM, et al. J Neurosci. 2013;33:1833–1845. doi: 10.1523/JNEUROSCI.3605-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Minamimoto T, et al. J Neurophysiol. 2009;101:437–447. doi: 10.1152/jn.90959.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Essen DC, Maunsell JH. J Comp Neurol. 1980;191:255–281. doi: 10.1002/cne.901910208. [DOI] [PubMed] [Google Scholar]
- 4.Diester I, et al. Nat Neurosci. 2011;14:387–397. doi: 10.1038/nn.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks V. Reviews of Physiology, Biochemistry and Pharmacology. Vol. 95. Springer; Berlin Heidelberg: 1983. pp. 1–109. [Google Scholar]
- 6.Martin JH, Ghez C. Journal of Neuroscience Methods. 1999;86:145–159. doi: 10.1016/s0165-0270(98)00163-0. [DOI] [PubMed] [Google Scholar]
- 7.Haber SN, et al. J Neurosci. 2006;26:8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizzo MA, et al. Nat Biotechnol. 2004;22:445–449. doi: 10.1038/nbt945. [DOI] [PubMed] [Google Scholar]
- 9.Krashes MJ, et al. J Clin Invest. 2011;121:1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lerchner W, et al. Gene Ther. 2014;21:233–241. doi: 10.1038/gt.2013.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han X, et al. Neuron. 2009;62:191–198. doi: 10.1016/j.neuron.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Z, et al. Nat Neurosci. 2000;3:1307–1315. doi: 10.1038/81841. [DOI] [PubMed] [Google Scholar]
- 13.Meunier M, et al. J Neurosci. 1993;13:5418–5432. doi: 10.1523/JNEUROSCI.13-12-05418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fritz J, et al. Proc Natl Acad Sci U S A. 2005;102:9359–9364. doi: 10.1073/pnas.0503998102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turchi J, et al. Proc Natl Acad Sci U S A. 2005;102:2158–2161. doi: 10.1073/pnas.0409708102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang P, Nikolic D. Front Hum Neurosci. 2011;5:85. doi: 10.3389/fnhum.2011.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hays AV, et al. WESCON Conference Proceedings. 1982:1–10. [Google Scholar]
- 18.Bowman EM, et al. J Neurophysiol. 1996;75:1061–1073. doi: 10.1152/jn.1996.75.3.1061. [DOI] [PubMed] [Google Scholar]
- 19.Myers RH, et al. Wiley; 1995. [Google Scholar]
- 20.Simmons JM, et al. J Neurosci. 2010;30:15878–15887. doi: 10.1523/JNEUROSCI.1802-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker AE. The Journal of Comparative Neurology. 1940;73:59–86. [Google Scholar]
- 22.Barbas H, Pandya DN. J Comp Neurol. 1989;286:353–375. doi: 10.1002/cne.902860306. [DOI] [PubMed] [Google Scholar]
- 23.Pandya DN, Yeterian EH. Prog Brain Res. 1990;85:63–94. doi: 10.1016/s0079-6123(08)62676-x. [DOI] [PubMed] [Google Scholar]
- 24.Carmichael ST, Price JL. J Comp Neurol. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.