Abstract
BACKGROUND
Evidence suggests that moderate alcohol consumption may protect against cognitive decline and dementia. However, uncertainty remains over the patterns of drinking that are most beneficial.
OBJECTIVE
To examine associations between amount and frequency of alcohol consumption with multiple domains of cognitive function in a well-characterized cohort of older community-dwelling adults in southern California.
DESIGN
Observational, cross-sectional cohort study.
SETTING
A research visit between 1988–1992 in Rancho Bernardo, California.
PARTICIPANTS
1624 participants of the Rancho Bernardo Study (mean age ± SD = 73.2 ± 9.3 years).
Measurements
Participants completed a neuropsychological test battery, self-administered questionnaires on alcohol consumption and lifestyle, and a clinical health evaluation. We classified participants according to average amount of alcohol intake into never, former, moderate, heavy and excessive drinkers, and according to frequency of alcohol intake, into non-drinkers, rare, infrequent, frequent and daily drinkers. We examined the association between alcohol intake and cognitive function, controlling for age, sex, education, exercise, smoking, waist-hip ratio, hypertension and self-assessed health.
RESULTS
Amount and frequency of alcohol intake were significantly associated with cognitive function, even after controlling for potentially related health and lifestyle variables. Global and executive function showed positive linear associations with amount and frequency of alcohol intake, whereas visual memory showed an inverted U-shaped association with alcohol intake, with better performance for moderate and infrequent drinkers than for non-drinkers, excessive drinkers or daily drinkers.
CONCLUSIONS
In several cognitive domains, moderate, regular alcohol intake was associated with better cognitive function relative to not drinking or drinking less frequently. This suggests that beneficial cognitive effects of alcohol intake may be achieved with low levels of drinking that are unlikely to be associated with adverse effects in an aging population.
Keywords: Cognitive aging, drinking frequency, drinking quantity, executive function, visual memory
Introduction
Cognitive decline and dementia are growing public health concerns for our aging population. Identifying modifiable lifestyle factors that promote successful cognitive aging is of mounting importance. A large body of evidence indicates that moderate consumption of alcohol is cardioprotective (see 1 for review). It remains unclear, however, whether moderate alcohol intake also has neuroprotective effects, conferring resistance to age-related cognitive decline and dementia. The existing literature on the association between alcohol and cognitive function is inconclusive: some studies report a beneficial effect of alcohol on brain health and cognitive function while others report no clear association (2–6). These inconsistencies may stem from multiple important sources of variability across studies, including inadequate control for co-varying health and lifestyle factors, failure to distinguish true abstainers from former drinkers, lack of full characterization of drinking patterns, heterogeneity of study populations in terms of physiological, genetic or demographic factors, and differences in cognitive domains tested.
One of the most significant challenges to unraveling the alcohol-cognition association is the need to account for potential health and lifestyle factors that covary with alcohol consumption; individuals who drink moderately have lower rates of disability, diabetes, bone fractures, coronary heart disease and overall mortality (see 7 for review), which may reflect the healthier lifestyle that frequently accompanies social drinking in older age. Thus, to isolate the effects of alcohol on cognitive function, it is critical to account for overall health status as well as other lifestyle factors that may mediate better cognition through improved health.
Another potential confounder of the alcohol-cognition association is the inclusion of former drinkers with life-time abstainers in non-drinking reference groups. As individuals may choose to abstain or quit drinking alcohol for various reasons (8, 9) including personal preference, religious beliefs, environmental influences or health issues, combining these heterogeneous groups for comparison against alcohol consumers could either enhance or obscure the effects of alcohol (10). Life-time abstainers may be less likely than former drinkers to use other substances, such as tobacco, that may affect health and cognitive function. Former drinkers may quit drinking due to poor health, which may be related to, or independent of, complications of prior alcohol intake. In support of this “sick quitter” hypothesis (11), former drinkers have been found to report poorer subjective health and have elevated mortality rates compared to lifetime abstainers and moderate drinkers (12, 13). Thus, it is important to separate abstainers from former drinkers when examining associations of alcohol use with cognitive function.
The pattern of alcohol consumption, in terms of both the average amount and frequency of intake, is also an important consideration. Among studies reporting a positive association between alcohol and cognitive function, there is little consensus over which quantities or frequencies of alcohol intake are most beneficial. The dose-response association between alcohol and cognitive function is poorly characterized; many studies report U-shaped (14, 15) or J-shaped (16, 17) associations, with negative effects associated with non-drinking and drinking to excess. However, other studies have observed a linear association between alcohol and cognition (15, 18, 19), suggesting that the upper bound to a protective dose of alcohol may be quite high or poorly represented in the populations examined. Although less attention has been devoted to understanding how drinking frequency impacts cognition, this is an equally critical consideration. For instance, the effects of seven weekly alcoholic beverages are likely to differ depending on whether those drinks are spread throughout the week or consumed in one day. Thus far, evidence pertaining to this issue has been limited and inconclusive; high frequency drinking has been associated with both positive (18, 19) and negative (20, 21) cognitive outcomes. Furthermore, drinking quantity and frequency are likely correlated, and it remains to be seen whether these factors independently influence cognitive function or if one is the predominant modulator.
Physiological, genetic and lifestyle factors may further influence an individual’s response to alcohol. For instance, cognitive benefits of alcohol may differ between men and women (5, 15, 22), between carriers and non-carriers of the apolipoprotein E (ApoE) ε4 allele (23–25) or between those with lower and higher levels of education or baseline intelligence (26, 27), and physiological responses may differ between those of different races or ethnicities (28). It is therefore important to control for such sources of heterogeneity when examining response patterns to alcohol across study participants.
Finally, alcohol may not affect all cognitive functions to the same degree. For instance, past studies have suggested that alcohol may be particularly beneficial for global cognition, executive function or learning, and less protective against speed of processing or verbal memory impairments [10,15,17,19,29]. Some of the inconsistent findings across past studies may stem from the evaluation of different, or limited, cognitive outcome measures.
To develop guidelines for healthy alcohol consumption that might be cognitively protective with advancing age, it is essential to identify the habits that are most beneficial, and to dissociate the direct effects of alcohol from confounding demographic or lifestyle factors. To address these outstanding questions, we examined how drinking habits, characterized by volume and frequency of intake, relate to cognitive function in older age. We further examined drinking history to assess whether past drinking differentially impacts cognition, relative to lifetime abstinence or drinking into later life. Cognitive outcomes included multiple neuropsychological test measures to comprehensively evaluate the association between alcohol and a range of cognitive domains.
Subjects and Methods
Study Population
The study population included community-dwelling participants of the Rancho Bernardo Study (RBS), a population-based study of healthy aging in a Southern California community. Between 1972 and 1974, 6629 individuals (82% of all adult residents) enrolled in the study; 2040 (80% of surviving members) returned for a follow-up research visit between 1988 and 1992, the focus of this study.
Participants at the 1988–92 visit were excluded if they were less than age 50 years (N = 8), had insufficient data about alcohol consumption for classification of drinking habits (N = 3), incomplete neuropsychological test data (N = 383), or missing education information (N = 22). After excluding these 416 participants, 1624 individuals remained (977 women, 647 men; mean age ± SD = 73.2 ± 9.3 years; range 51–99 years).
Study procedures were approved by the University of California, San Diego Human Research Protections Program Board, and all participants provided informed written consent prior to participation.
Cognitive Assessment
A neuropsychological test battery comprising five tests was administered by a trained interviewer to evaluate cognitive function in multiple domains (30). The Mini-Mental State Examination (MMSE) (31) assesses orientation, attention, language and memory, providing a measure of global cognition. Scores range from 0 to 30, with higher scores reflecting better performance. The Trails Making Test, Part B (Trails B) (32) is a measure of executive function and attention. Participants draw lines to connect, in order, a sequence of alternating letters and numbers. Performance is measured as seconds required for task completion, with a maximum time of 300 seconds; higher scores reflect poorer performance. The category fluency test assesses semantic fluency by asking participants to name as many animals as possible within 60 seconds (33). The Heaton Visual Reproduction Test evaluates short and long-term visual memory (34). A series of complex images are briefly presented and subjects are asked to reproduce the stimuli from memory immediately and again after a 20 minute delay. The Buschke-Fuld Selective Reminding Test evaluates verbal learning and memory (35). A set of ten words are read and participants are immediately asked to recall the words. Participants are reminded of any omitted words and asked to recall all ten words again; the process is repeated six times. The long-term memory retrieval measure, which assesses the ability to retrieve words from long-term storage, was used in the present study.
Classification of Alcohol Consumption
Information about alcohol consumption was acquired from a self-administered questionnaire completed at home and reviewed at the clinic visit. Participants were asked whether they had consumed an alcoholic beverage in the preceding 12 months, and if not, if there was ever a time when they consumed at least one drink per year. Participants who reported drinking in the preceding 12 months were asked how frequently they drank alcohol over the previous 30 days (not at all, once, 2–3 times, 1–2 days per week, 3–4 days per week, 5–6 days per week, every day) and how many drinks, on average, they consumed on days they drank. A single drink was defined as a 12-ounce glass of beer, a 5-ounce glass of wine, or 1.25 ounces of hard liquor. Data on alcohol use patterns from a prior visit (between 1984 and 1987) were used to corroborate self-reports from participants who indicated that they did not previously consume alcohol.
Based on their responses, participants were classified as never-drinkers (N = 63), former drinkers (drank previously, but not during the past year; N = 147) and current drinkers (N = 1414). Current drinkers were further classified according to the quantity and frequency of alcohol intake over the prior 30 days. To group individuals by quantity of intake, frequency of intake over the past 30 days was multiplied by amount consumed on days that they drank. For ease of interpretation, this total was divided by 30 to yield an estimate of the average number of drinks consumed per day. Participants were then classified using sex-specific guidelines [36] into light to moderate drinkers (average of less than one drink per day for women and less than two drinks per day for men; N = 952, referred to hereafter as moderate drinkers), heavy drinkers (average of one to less than three drinks per day for women, two to less than four drinks per day for men; N = 397), and excessive drinkers (average of three or more drinks per day for women, four or more drinks per day for men; N = 65). For grouping according to frequency of alcohol intake, current drinkers were classified as those who drank rarely (one or fewer days per month, N = 278), infrequently (two days per month to two days per week; N = 400), frequently (three to four days per week; N = 151), or daily or near-daily (five or more days per week; N = 585, referred to here as “daily drinkers”).
Covariate Assessment
Height, waist and hip girth were measured in the clinic with participants wearing light clothing. Waist-hip ratio was calculated as an estimate of central adiposity. Blood pressure readings were measured twice on seated, resting participants by a nurse trained on the Hypertension Detection and Follow-up Program (Hypertension Detection and Follow-Up Program Cooperative Group, 1976). The mean of the two measures, taken five minutes apart, was used for analysis. Participants were considered hypertensive if they had an average systolic blood pressure reading above 140, diastolic blood pressure reading above 90, were taking antihypertensive medication, or reported a physician diagnosis of hypertension.
Standardized questionnaires, completed at home but reviewed during the clinic visit, were used to obtain information about medical history, medication use, self-assessed health (the extent to which the participant felt limited by poor health), smoking history (never, former, current), and exercise using the Godin Leisure-Time Exercise questionnaire (37). The Godin Leisure-Time Exercise score is computed as a composite score of the frequency of light, moderate and strenuous exercise during an average week. Medication use was verified by a trained nurse who examined pill containers and prescriptions brought to the clinic. ApoE genotype was available for a subset of 1376 participants. DNA was extracted by Sequana Therapeutics (La Jolla, CA) using standard techniques (Puregene; Gentra, Minneapolis, Minn) as previously described (38).
Statistical analysis
Univariate general linear modeling (GLM) was used to examine whether cognitive performance, as assessed by the MMSE, Trails B, category fluency, Heaton Visual Reproduction and Buschke Selective Reminding tests, differed according to amount and frequency of alcohol intake. GLM or chi-squared tests were conducted to test whether demographic or lifestyle variables differed according to the amount or frequency of alcohol consumption. Initial analyses examined potential interaction effects of sex with amount and frequency of alcohol intake on cognitive function. Because there were no significant interaction effects, models were adjusted for sex rather than stratified by sex. In addition to sex, base models included age and education. Fully adjusted models included covariates that were significantly associated (p < 0.05) with at least one cognitive function test (exercise, smoking, waist-hip ratio, systolic and diastolic blood pressure, antihypertensive medication, hypertension and health). For variables with strong collinearity (e.g., blood pressure, antihypertensive medication and hypertension), the variable with the strongest association (hypertension) was included in the fully adjusted model. Significant main effects were followed by pairwise comparisons (least significant difference) between groups.
In analyses on the subset of participants with ApoE genotype data, GLM was performed to examine whether associations of alcohol with cognitive function differed between carriers (N = 291) and non-carriers (N = 1050) of the ApoE-ε4 allele. Because of small numbers and opposing directions of dementia risk associated with an ε2ε4 genotype (N = 35) (39), these individuals were excluded from analysis.
Because amount and frequency of alcohol intake were correlated, we performed exploratory analyses on amount of intake holding frequency of intake constant, and on frequency of intake holding amount of intake constant.
Analyses were conducted using SPSS version 22 (SPSS Inc., Chicago, IL, USA); a two-sided p < 0.05 was considered significant. Analyses were not adjusted for multiple comparisons.
Results
Amount of alcohol consumption
Comparisons of demographic and lifestyle characteristics according to amount of alcohol consumption are presented in Table 1. There were significant differences in sex, age, smoking status, waist-hip ratio, diastolic blood pressure and overall health by amount of alcohol consumed. A greater proportion of men than women were moderate drinkers (p < 0.001). Excessive drinkers were younger than all other groups (p’s < 0.05). Never-drinkers were less likely to smoke than former or current drinkers, and the proportion of former and current smokers increased with increasing amounts of alcohol intake (p < 0.001). Excessive drinkers had higher diastolic blood pressure and, after adjustment for age and sex, larger waist-hip ratios than all other groups (p’s < 0.05). Former drinkers reported worse health than all current drinkers (p’s < 0.01), and heavy drinkers reported better health than never- (p = 0.01) and moderate drinkers (p < 0.001). Education, exercise, systolic blood pressure, antihypertensive medication, hypertension, and the proportion of ApoE-ε4 carriers did not differ by quantity of alcohol intake.
Table 1.
Demographic and lifestyle characteristics by amount of alcohol consumption
| Never N = 63 | Former N = 147 | Moderate N = 952 | Heavy N = 397 | Excessive N = 65 | p-value | |
|---|---|---|---|---|---|---|
| Sex (% within sex across drinking categories) | ||||||
| Men (N = 647) | 1 | 8 | 65 | 21 | 4 | < 0.001 |
| Women (N = 977) | 6 | 10 | 54 | 26 | 4 | |
| Demographic Characteristics | ||||||
| Age (years) | 73.4 (10.3) | 74.2 (9.3) | 73.7 (9.5) | 72.3 (8.6) | 69.6 (8.3) | 0.001 |
| Education (years) | 14.6 (2.8) | 14.0 (3.0) | 14.6 (2.9) | 14.6 (2.5) | 14.6 (2.2) | 0.17 |
| Exercise* | 17.1 (19.8) | 18.4 (20.7) | 19.6 (18.1) | 19.5 (18.2) | 19.3 (19.9) | 0.82 |
| Smoking | < 0.001 | |||||
| (% former) | 8 | 37 | 48 | 55 | 60 | |
| (% current) | 2 | 9 | 6 | 14 | 28 | |
| Waist-hip ratio† | 0.84 (0.06) | 0.84 (0.06) | 0.84 (0.06) | 0.84 (0.06) | 0.87 (0.06) | 0.003 |
| Systolic blood pressure (mm HG) | 138 (20) | 139 (25) | 135 (20) | 136 (19) | 136 (18) | 0.26 |
| Diastolic blood pressure (mm HG) | 75 (9) | 76 (10) | 76 (9) | 76 (9) | 79 (9) | 0.04 |
| Antihypertensive medication (% medicated) | 37 | 51 | 44 | 39 | 35 | 0.05 |
| Hypertension (%) | 59 | 68 | 60 | 56 | 62 | 0.17 |
| Health‡ | 1.87 (0.87) | 2.05 (1.05) | 1.75 (0.89) | 1.57 (0.78) | 1.65 (0.72) | < 0.001 |
| ApoE§ (% ε4 carriers) | 24 | 23 | 23 | 19 | 16 | 0.55 |
Values shown are mean (standard deviation) unless otherwise noted;
Exercise is expressed as Godin Leisure-Time Exercise scores, with higher scores representing higher levels of exercise;
Waist-hip ratio is adjusted for age and sex;
Self-assessed health is on a scale from 1 to 5, with higher scores representing worse health;
ApoE genotype data were available for a subset of participants (83%).
Differences in cognitive function test performance by quantity of alcohol consumption are shown in Figure 1. In both the base and fully adjusted models, a main effect of alcohol was found for MMSE (base: F(4,1616) = 2.77, p = 0.03; full: F(4,1592) = 2.92, p = 0.02), Trails B (base: F(4,1616) = 3.20, p = 0.01; full: F(4,1592) = 2.44, p = 0.05) and immediate visual recall scores (base: F(4,1616) = 3.27, p = 0.01; full: F(4,1592) = 2.95, p = 0.02). Pairwise comparisons in the fully adjusted models revealed lower MMSE scores for never- and former drinkers than moderate and heavy drinkers (p’s < 0.05). On the Trails B, never-drinkers scored worse than all current drinkers regardless of quantity, and former drinkers scored worse than heavy drinkers (p’s < 0.05). Immediate visual recall scores were lower for never- than moderate drinkers, and for excessive drinkers compared to former, moderate or heavy drinkers (p’s < 0.05). Category fluency and delayed visual recall scores differed significantly by drinking quantity in base models, but not in fully adjusted models (category fluency base: F(4,1616) = 2.56, p = 0.04; full: F(4,1592) = 2.04, p = 0.09; delayed visual recall (base: F(4,1616) = 2.41, p = 0.05; full: F(4,1592) = 2.30, p = 0.06). The effect of alcohol was not significant in either base or fully adjusted models for the Selective Reminding Test (base: F(4,1616) = 2.08, p = 0.08; full: F(4,1592) = 1.71, p = 0.14). There were no significant interactions involving ApoE genotype for any cognitive function measure (p’s > 0.10).
Figure 1.
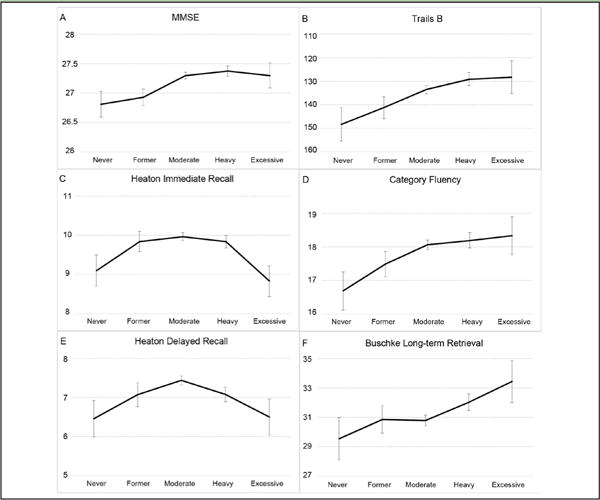
Multivariable adjusted means on each neuropsychological test are shown for each alcohol consumption group. Trails B scores are plotted with an inverted scale because lower scores indicate better performance. Scores differed significantly by amount of alcohol intake on the MMSE (A), Trails B (B) and immediate visual recall (C) tests. There were no significant effects of alcohol on category fluency (D), delayed visual recall (E) or Selective Reminding (F) scores. (p’s < 0.05, adjusted for age, sex, education, exercise, smoking, waist-hip ratio, hypertension and health.) Error bars represent the standard error of the mean.
Frequency of alcohol consumption
Because there were no significant differences between former drinkers and life-time abstainers in terms of quantity of drinking, these groups were collapsed into a single non-drinking group for analyses of drinking frequency. Demographic and lifestyle characteristics by drinking frequency are presented in Table 2. There were significant differences in sex, age, education, smoking status, systolic blood pressure, antihypertensive medication, hypertension and health according to drinking frequency. A relatively greater proportion of women than men were non-drinkers and infrequent drinkers, whereas a greater proportion of men were daily drinkers (p < 0.001). Frequent drinkers were younger than all other groups (p’s < 0.001). Daily drinkers were more highly educated than non- and rare drinkers (p’s < 0.05). The proportion of former and current smokers increased with more frequent drinking (p < 0.001). Systolic blood pressure was lower for frequent drinkers than non-, rare and daily drinkers, and for infrequent drinkers than non-drinkers (p’s < 0.05). A greater percentage of non- and rare drinkers were taking antihypertensive medications (p = 0.001). Non- and rare drinkers were most likely, whereas frequent drinkers were least likely, to have hypertension (p < 0.001). Non-and rare drinkers reported poorer health than infrequent, frequent and daily drinkers (p’s < 0.01). Exercise, waist-hip ratio, diastolic blood pressure and proportion of ApoE-ε4 carriers did not differ by drinking frequency.
Table 2.
Demographic and lifestyle characteristics by frequency of alcohol consumption
| Non-drinkers N = 210 | Rare N = 278 | Infrequent N = 400 | Frequent N = 151 | Daily N = 585 | p-value | |
|---|---|---|---|---|---|---|
| Sex (% within sex across drinking categories) | ||||||
| Men (N = 647) | 9 | 15 | 21 | 10 | 45 | < 0.001 |
| Women (N = 977) | 15 | 18 | 27 | 9 | 30 | |
| Demographic Characteristics | ||||||
| Age (years) | 73.9 (9.5) | 74.9 (9.8) | 73.5 (9.5) | 69.4 (9.1) | 73.0 (8.5) | < 0.001 |
| Education (years) | 14.2 (2.9) | 14.3 (3.0) | 14.6 (2.8) | 14.6 (2.6) | 14.8 (2.6) | 0.03 |
| Exercise* | 18.0 (20.4) | 18.5 (18.4) | 19.3 (17.3) | 20.3 (20.4) | 20.1 (18.1) | 0.54 |
| Smoking | < 0.001 | |||||
| (% former) | 28 | 42 | 45 | 48 | 59 | |
| (% current) | 7 | 4 | 6 | 10 | 14 | |
| Waist-hip ratio† | 0.84 (0.06) | 0.84 (0.06) | 0.83 (0.06) | 0.84 (0.06) | 0.84 (0.06) | 0.11 |
| Systolic blood pressure (mm HG) | 139 (23) | 137 (21) | 134 (20) | 131 (19) | 136 (19) | 0.008 |
| Diastolic blood pressure (mm HG) | 75 (10) | 75 (10) | 75 (9) | 77 (9) | 76 (9) | 0.08 |
| Antihypertensive medication (% medicated) | 47 | 53 | 42 | 36 | 39 | 0.001 |
| Hypertension (%) | 65 | 68 | 57 | 48 | 59 | < 0.001 |
| Health‡ | 2.00 (1.00) | 1.93 (1.00) | 1.71 (0.84) | 1.60 (0.87) | 1.60 (0.77) | < 0.001 |
| ApoE§ (% ε4 carriers) | 23 | 22 | 20 | 24 | 21 | 0.93 |
Values shown are mean (standard deviation) unless otherwise note;
Exercise is expressed as Godin Leisure-Time Exercise scores, with higher scores representing higher levels of exercise;
Waist-hip ratio is adjusted for age and sex;
Self-assessed health is on a scale from 1 to 5, with higher scores representing worse health;
ApoE genotype data were available for a subset of participants (83%).
Differences in cognitive function by drinking frequency are presented in Figure 2. In the base and full models, scores on the MMSE (base: F(4,1616) = 2.64, p = 0.03; full: F(4,1592) = 2.85, p = 0.02), Trails B (base: F(4,1616) = 3.51, p = 0.007; full: F(4,1592) = 2.54, p = 0.04) and immediate (base: F(4,1616) = 3.64, p = 0.006; full: F(4,1592) = 3.26, p = 0.01) and delayed (base: F(4,1616) = 2.84, p = 0.02; full: F(4,1592) = 2.56, p = 0.04) visual recall tests differed significantly by drinking frequency. Non-drinkers had significantly lower MMSE scores than drinkers, regardless of drinking frequency (p’s < 0.05). Trails B performance was significantly poorer for nondrinkers than infrequent, frequent and daily drinkers (p’s < 0.05). Infrequent drinkers had significantly higher immediate visual recall scores than non-, rare and daily drinkers (p’s < 0.01) and higher delayed visual recall scores than non- and daily drinkers (p’s < 0.05). Performance on the category fluency (base: F(4,1616) = 1.85, p = 0.12; full: F(4,1592) = 1.82, p = 0.12) and Selective Reminding (base: F(4,1616) = 0.67, p = 0.61; full: F(4,1592) = 0.64, p = 0.64) tests did not significantly differ as a function of frequency of alcohol intake. There were no significant interactions involving ApoE genotype for any cognitive function measure (p’s > 0.05).
Figure 2.
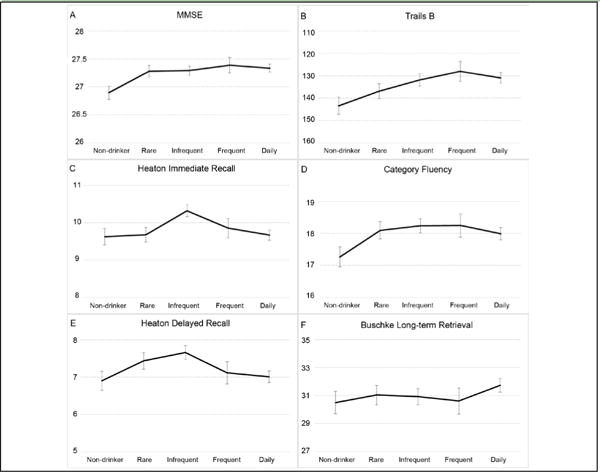
Multivariable adjusted means on each neuropsychological test are plotted according to average frequency of alcohol consumption. Trails B scores are plotted with an inverted scale because lower scores indicate better performance. MMSE (A), Trails B (B), immediate (C) and delayed visual recall (E) scores significantly differed by frequency of alcohol consumption. There were no significant differences by drinking frequency on category fluency (D) or Selective Reminding (F) scores. (p’s < 0.05, adjusted for age, sex, education, exercise, smoking, waist-hip ratio, hypertension and health.) Error bars represent the standard error of the mean.
Independent effects of amount and frequency of alcohol intake
To examine how quantity of drinking related to frequency of drinking, we examined the distribution of alcohol quantity groups across the alcohol frequency groups. Of the non-drinking group, 70% were former drinkers and 30% were life-time abstainers. All individuals in the rare drinking group consumed moderate amounts of alcohol. Infrequent and frequent drinkers were mainly moderate drinkers (infrequent drinkers: >99% moderate, <1%; heavy; frequent drinkers: 74% moderate, 26% heavy), and most daily drinkers were heavy drinkers (28% moderate, 61% heavy, 11% excessive).
To test whether quantity of alcohol intake affected cognitive function independently of frequency of intake, we restricted analyses to the subgroup of individuals who drank daily because this group contained individuals who consumed moderate (N = 163), heavy (N = 357) and excessive (N = 65) amounts of alcohol. To examine whether frequency of intake influenced cognitive function independently of amount of intake, we restricted analyses to the moderate drinking group because this group included individuals who drank rarely (N = 278), infrequently (N = 399), frequently (N = 112) and daily (N = 163).
The patterns of results for both sets of exploratory analyses were very similar to results on the full cohort (compare Figure 1 with Supplemental Figure 1, and Figure 2 with Supplemental Figure 2). When including only those who drank daily in the current drinker group, there was a similar linear positive association of alcohol quantity with MMSE and Trails B scores, and an inverted U-shaped association with visual memory, as observed in the full analysis. The effect of alcohol quantity was significant in the base model for Trails B scores (base: F(4,787) = 3.48, p = 0.008; full: (F(4,773) = 2.20, p = 0.07). When including only individuals who drank moderately in the current drinker group, similar associations of the frequency of alcohol intake with MMSE, Trails B and visual memory performance were again observed. The effect of drinking frequency was significant for Trails B scores in the base model (base: F(4,1154) = 3.03, p = 0.02; full: F(4,1135) = 2.28, p = 0.06) and for immediate visual memory in the base and full models (base: F(4,1154) = 3.18, p = 0.01; full: F(4,1135) = 2.78, p = 0.03).
Discussion
This study reports a positive association between alcohol intake and cognitive function across multiple domains in older adults, consistent with prior reports that alcohol intake may reduce risk of cognitive decline with age (2, 3). Individuals who drank moderate to heavy quantities of alcohol (up to three drinks per day for women, or four for men) scored higher on tests of global cognition, executive function and immediate visual memory than did lifetime abstainers and former drinkers. Excessive drinkers, those who consumed three or more drinks per day for women, or four or more drinks per day for men, performed worse on tests of visual memory than did those who drank less.
Frequency of alcohol intake was also related to cognitive function. Regular alcohol consumption (two days per month to four days per week) was associated with better cognitive performance compared to less or more frequent drinking. Specifically, those who drank infrequently to daily showed significantly better global and executive function than non-drinkers, while infrequent drinkers showed better visual memory performance than non-drinkers and daily drinkers. Partitioning non-drinkers into never- and former drinkers for the frequency analyses did not materially change the results. Notably, significant differences by drinking frequency were found even when holding amount of alcohol intake constant.
Stratifications according to quantity and frequency of alcohol intake showed distinct dose-response curves for different neuropsychological tests, suggesting that alcohol may be differentially associated with distinct cognitive processes. In line with prior reports (10, 17, 19, 9), we found linear positive associations of amount and frequency of alcohol intake with global cognition and executive function. In contrast, visual memory scores showed inverted U-shaped associations with alcohol, being low in non-drinkers and in the heaviest and most frequent drinkers. This suggests that visual memory may be more vulnerable to adverse effects of higher doses of alcohol than global and executive functions. Alcohol intake was unrelated to verbal memory in our study. Although some studies have reported positive effects (19, 29, 40), others suggest that alcohol is only weakly protective against verbal memory impairment (10, 18).
Although we observed linear associations of the amount of alcohol intake with global and executive function, our population comprised predominantly light to moderate alcohol consumers. The heaviest drinkers consumed fewer than nine drinks daily, and only 16 individuals (less than 1%) demonstrated patterns consistent with alcohol abuse, namely, consuming five or more drinks daily (41). Notably, our study demonstrated beneficial cognitive patterns even with moderate, infrequent alcohol consumption. This is important when considering lifestyle recommendations for successful cognitive aging, as excessive alcohol can also have negative health consequences. Although excessive chronic alcohol intake and binge drinking are associated with neuronal damage and cognitive impairment regardless of age, older adults may be particularly at risk of adverse effects from even lower amounts of intake. They have greater biological susceptibility to alcohol than younger adults, are more likely to suffer from medical conditions that may be exacerbated by alcohol intake, take more prescription medications that may interact with alcohol, and may suffer more severe consequences from alcohol-related accidents, such as falls (7, 42).
This study observed no difference in cognitive function between abstainers and former drinkers. Prior studies that have attempted to dissociate these groups have yielded conflicting results, reporting both poorer psychomotor attention for former drinkers than lifetime abstainers (10), and no difference in verbal memory or global cognition between these groups (40, 43). In our study, former drinkers reported the poorest health of all groups, consistent with prior reports (12, 13). It is possible that their poorer health may have overridden any benefit from prior alcohol consumption or that continued alcohol intake, rather than prior intake, may be essential for any alcohol-related cognitive protection. Here, the benefits of current alcohol intake persisted after accounting for self-assessed health and other health-related variables, suggesting that the associations between alcohol and cognitive function are not attributable to health-related group differences.
Although some studies indicate that alcohol influences cognitive function similarly in men and women (19), others suggest that women experience greater benefit from moderate drinking (18, 44, 45) and greater detriment from heavy drinking (5). While we found that sex did not interact with the alcohol-cognition association, we cannot conclude that cognitive responses to alcohol do not, in fact, differ between sexes. As our sample included fewer men than women, it is possible that sex differences would become apparent in a larger, more sex-balanced, population.
We also did not find significant interactions between ApoE genotype and the alcohol-cognition association. While some prior studies report that alcohol is more protective for non-carriers of the ε4 allele (23, 24), others report greater benefit for carriers (25) or no interaction (19, 29) with ApoE genotype. Here, the lack of an interaction with ApoE status suggests that ApoE genotype is either not a strong moderator of alcohol’s protective effects, or that its influence depends on other factors not assessed here.
Although the mechanisms by which moderate alcohol appears to be protective remain unclear, both cardiovascular and neuroprotective processes have been implicated (46). There is considerable evidence that moderate drinking evokes positive cardiovascular effects, possibly via higher HDL, lower blood pressure or anti-inflammatory properties (1, 47). Such changes may confer improved cerebrovascular health and, consequently, better cognitive function. It is also possible that the alcohol-cognition association may be directly mediated by protective neural effects, such as attenuation of neuroinflammation, synaptic damage, apoptosis, or oxidative stress (48, 49). Controlling for hypertension in this study did not alter our results. Further research is necessary to determine whether other cardioprotective properties of alcohol mediate its association with cognitive function.
The present study has several limitations. The population was a relatively homogeneous group of predominantly white, middle-class Americans with at least a high school education. Although this limits generalizability, it reduces confounding due to differences in race or ethnicity, socioeconomic status, education, and access to healthcare. We used self-reported alcohol use, which can be unreliable, particularly among individuals with cognitive impairment, with participants under-estimating alcohol intake (50) or providing conflicting responses over time (51). However, we corroborated reports of non-drinking with self-report four years earlier. Further, self-reported alcohol use in the RBS cohort was shown to correlate highly with alcohol use as ascertained separately by a trained dietician during a dietary recall interview and has been indirectly validated through its correlation with plasma aspartate aminotransferase and HDL cholesterol in this study (52, 53). Excessive drinkers are poorly represented in RBS, limiting our power to assess the effects of excessive drinking. We were also unable to examine associations as a function of type of alcohol consumed, since information on type of alcohol consumed was not collected in this study visit; however, there is some evidence that alcohol type may differentially affect cognitive function, with greater benefit for wine than beer or liquor (54, 55). The exploratory analyses examining the independent effects of drinking quantity and frequency were limited by small sample sizes; further investigation is required to better characterize the independent or interactive influences of the amount and frequency of alcohol consumption. Finally, given the cross-sectional, observational nature of this study, it is not possible to claim causation. Other factors that were not accounted for here may underlie observed associations between alcohol and cognitive function.
This study also includes several notable strengths. The population was large and relatively homogeneous, thus increasing statistical power and reducing confounding related to variability across participants. This allowed us to better clarify inconclusive findings from a previous analysis on a subset of the same dataset (5). Furthermore, individuals were well characterized in terms of demographics, lifestyle and health, allowing us to examine the impact of many potentially related variables on the alcohol-cognition association.
In conclusion, moderate, regular alcohol intake was associated with better global, executive and visual memory functions among older adults, even with control for a number of potentially related health and lifestyle variables. This suggests that continued moderate alcohol consumption in older age may be beneficial for cognitive health. Further research is necessary to clarify biological mechanisms by which alcohol might exert benefits, and to better understand whether the association of alcohol with cognitive function varies across socioeconomic, racial or ethnic groups.
Acknowledgments
Funding: This study is supported by NIH grant NIAAA R01 AA021187. The Rancho Bernardo Study was funded by research grants AG028507 and AG018339 from the NIA and grant DK31801 from the NIDDK.
Footnotes
Conflict of interests No authors declare a conflict of interest.
Ethical standards: The authors declare that the study procedures comply with the current ethical standards for investigation involving human subjects in the United States.
References
- 1.Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. Bmj. 2011;342:d671. doi: 10.1136/bmj.d671. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anstey KJ, Mack HA, Cherbuin N. Alcohol consumption as a risk factor for dementia and cognitive decline: meta-analysis of prospective studies. The American journal of geriatric psychiatry: official journal of the American Association for Geriatric Psychiatry. 2009;17:542–555. doi: 10.1097/JGP.0b013e3181a2fd07. 2009. [DOI] [PubMed] [Google Scholar]
- 3.Beydoun MA, Beydoun HA, Gamaldo AA, Teel A, Zonderman AB, Wang Y. Epidemiologic studies of modifiable factors associated with cognition and dementia: systematic review and meta-analysis. BMC Public Health. 2014;14:643. doi: 10.1186/1471-2458-14-643. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peters R, Peters J, Warner J, Beckett N, Bulpitt C. Alcohol, dementia and cognitive decline in the elderly: a systematic review. Age and ageing. 2008;37:505–512. doi: 10.1093/ageing/afn095. 2008. [DOI] [PubMed] [Google Scholar]
- 5.Edelstein SL, Kritz-Silverstein D, Barrett-Connor E. Prospective association of smoking and alcohol use with cognitive function in an elderly cohort. Journal of women’s health/the official publication of the Society for the Advancement of Women’s Health Research. 1998;7:1271–1281. doi: 10.1089/jwh.1998.7.1271. 1998. [DOI] [PubMed] [Google Scholar]
- 6.Pilleron S, Desport JC, Jesus P, Mbelesso P, Ndamba-Bandzouzi B, Dartigues JF, Clement JP, Preux PM, Guerchet M. Diet, Alcohol Consumption and Cognitive Disorders in Central Africa: A Study from the EPIDEMCA Program. The journal of nutrition, health & aging. 2015;19:657–667. doi: 10.1007/s12603-015-0487-y. 2015. [DOI] [PubMed] [Google Scholar]
- 7.Ferreira MP, Weems MK. Alcohol consumption by aging adults in the United States: health benefits and detriments. Journal of the American Dietetic Association. 2008;108:1668–1676. doi: 10.1016/j.jada.2008.07.011. 2008. [DOI] [PubMed] [Google Scholar]
- 8.Bernards S, Graham K, Kuendig H, Hettige S, Obot I. ‘I have no interest in drinking’: a cross-national comparison of reasons why men and women abstain from alcohol use. Addiction. 2009;104:1658–1668. doi: 10.1111/j.1360-0443.2009.02667.x. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaillant GE. The Natural History of Alcoholism Revisited. Cambridge, MA: 1995. 1995. [Google Scholar]
- 10.Ganguli M, Vander Bilt J, Saxton JA, Shen C, Dodge HH. Alcohol consumption and cognitive function in late life: a longitudinal community study. Neurology. 2005;65:1210–1217. doi: 10.1212/01.wnl.0000180520.35181.24. 2005. [DOI] [PubMed] [Google Scholar]
- 11.Shaper AG, Wannamethee G, Walker M. Alcohol and Mortality in British Men - Explaining the U-Shaped Curve. Lancet. 1988;2:1267–1273. doi: 10.1016/s0140-6736(88)92890-5. 1988. [DOI] [PubMed] [Google Scholar]
- 12.Gmel G, Gutjahr E, Rehm J. How stable is the risk curve between alcohol and all-cause mortality and what factors influence the shape? A precision-weighted hierarchical meta-analysis. European journal of epidemiology. 2003;18:631–642. doi: 10.1023/a:1024805021504. 2003. [DOI] [PubMed] [Google Scholar]
- 13.Friesema IH, Zwietering PJ, Veenstra MY, Knottnerus JA, Garretsen HF, Lemmens PH. Alcohol intake and cardiovascular disease and mortality: the role of pre-existing disease. Journal of epidemiology and community health. 2007;61:441–446. doi: 10.1136/jech.2006.050419. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elias PK, Elias MF, D’Agostino RB, Silbershatz H, Wolf PA. Alcohol consumption and cognitive performance in the Framingham Heart Study. American journal of epidemiology. 1999;150:580–589. doi: 10.1093/oxfordjournals.aje.a010056. 1999. [DOI] [PubMed] [Google Scholar]
- 15.Bond GE, Burr R, McCurry SM, Graves AB, Larson EB. Alcohol, aging, and cognitive performance in a cohort of Japanese Americans aged 65 and older: the Kame project. Int Psychogeriatr. 2001;13:207–223. doi: 10.1017/s1041610201007591. 2001. [DOI] [PubMed] [Google Scholar]
- 16.Hendrie HC, Gao S, Hall KS, Hui SL, Unverzagt FW. The relationship between alcohol consumption, cognitive performance, and daily functioning in an urban sample of older black Americans. Journal of the American Geriatrics Society. 1996;44:1158–1165. doi: 10.1111/j.1532-5415.1996.tb01364.x. 1996. [DOI] [PubMed] [Google Scholar]
- 17.Reid MC, Van Ness PH, Hawkins KA, Towle V, Concato J, Guo Z. Light to moderate alcohol consumption is associated with better cognitive function among older male veterans receiving primary care. Journal of geriatric psychiatry and neurology. 2006;19:98–105. doi: 10.1177/0891988706286513. 2006. [DOI] [PubMed] [Google Scholar]
- 18.Britton A, Singh-Manoux A, Marmot M. Alcohol consumption and cognitive function in the Whitehall II Study. American journal of epidemiology. 2004;160:240–247. doi: 10.1093/aje/kwh206. 2004. [DOI] [PubMed] [Google Scholar]
- 19.Ngandu T, Helkala EL, Soininen H, Winblad B, Tuomilehto J, Nissinen A, Kivipelto M. Alcohol drinking and cognitive functions: findings from the Cardiovascular Risk Factors Aging and Dementia (CAIDE) Study. Dement Geriatr Cogn Disord. 2007;23:140–149. doi: 10.1159/000097995. 2007. [DOI] [PubMed] [Google Scholar]
- 20.Gross AL, Rebok GW, Ford DE, Chu AY, Gallo JJ, Liang KY, Meoni LA, Shihab HM, Wang NY, Klag MJ. Alcohol consumption and domain-specific cognitive function in older adults: longitudinal data from the Johns Hopkins Precursors Study. J Gerontol B Psychol Sci Soc Sci. 2011;66:39–47. doi: 10.1093/geronb/gbq062. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou HZ, Karliner JS, Gray MO. Moderate alcohol consumption induces sustained cardiac protection by activating PKC-epsilon and Akt. American journal of physiology Heart and circulatory physiology. 2002;283:H165–174. doi: 10.1152/ajpheart.00408.2001. [DOI] [PubMed] [Google Scholar]
- 22.Richards M, Hardy R, Wadsworth ME. Alcohol consumption and midlife cognitive change in the British 1946 birth cohort study. Alcohol and alcoholism. 2005;40:112–117. doi: 10.1093/alcalc/agh126. [DOI] [PubMed] [Google Scholar]
- 23.Dufouil C, Tzourio C, Brayne C, Berr C, Amouyel P, Alperovitch A. Influence of apolipoprotein E genotype on the risk of cognitive deterioration in moderate drinkers and smokers. Epidemiology. 2000;11:280–284. doi: 10.1097/00001648-200005000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Virtaa JJ, Jarvenpaa T, Heikkila K, Perola M, Koskenvuo M, Raiha I, Rinne JO, Kaprio J. Midlife alcohol consumption and later risk of cognitive impairment: a twin follow-up study. J Alzheimers Dis. 2010;22:939–948. doi: 10.3233/JAD-2010-100870. [DOI] [PubMed] [Google Scholar]
- 25.Carmelli D, Swan GE, Reed T, Schellenberg GD, Christian JC. The effect of apolipoprotein E epsilon4 in the relationships of smoking and drinking to cognitive function. Neuroepidemiology. 1999;18:125–133. doi: 10.1159/000026204. 26204. [DOI] [PubMed] [Google Scholar]
- 26.Corley J, Jia X, Brett CE, Gow AJ, Starr JM, Kyle JA, McNeill G, Deary IJ. Alcohol intake and cognitive abilities in old age: the Lothian Birth Cohort 1936 study. Neuropsychology. 2011;25:166–175. doi: 10.1037/a0021571. [DOI] [PubMed] [Google Scholar]
- 27.Krahn D, Freese J, Hauser R, Barry K, Goodman B. Alcohol use and cognition at mid-life: the importance of adjusting for baseline cognitive ability and educational attainment. Alcoholism, clinical and experimental research. 2003;27:1162–1166. doi: 10.1097/01.ALC.0000078060.18662.C1. [DOI] [PubMed] [Google Scholar]
- 28.Edenberg HJ. The genetics of alcohol metabolism: role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol research & health: the journal of the National Institute on Alcohol Abuse and Alcoholism. 2007;30:5–13. [PMC free article] [PubMed] [Google Scholar]
- 29.Stampfer MJ, Kang JH, Chen J, Cherry R, Grodstein F. Effects of moderate alcohol consumption on cognitive function in women. N Engl J Med. 2005;352:245–253. doi: 10.1056/NEJMoa041152. [DOI] [PubMed] [Google Scholar]
- 30.Wiederholt WC, Cahn D, Butters NM, Salmon DP, Kritz-Silverstein D, Barrett-Connor E. Effects of age, gender and education on selected neuropsychological tests in an elderly community cohort. Journal of the American Geriatrics Society. 1993;41:639–647. doi: 10.1111/j.1532-5415.1993.tb06738.x. [DOI] [PubMed] [Google Scholar]
- 31.Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. Journal of the American Geriatrics Society. 1992;40:922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 32.Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Perceptual and motor skills. 1958;8:271–276. [Google Scholar]
- 33.Borkowsk JG, Benton AL, Spreen O. Word Fluency and Brain Damage. Neuropsychologia. 1967;5:135–&. doi: 10.1016/0028-3932(67)90015-2. [DOI] [Google Scholar]
- 34.Russell EW. Multiple Scoring Method for Assessment of Complex Memory Functions. J Consult Clin Psych. 1975;43:800–809. doi: 10.1037/0022-006x.43.6.800. [DOI] [Google Scholar]
- 35.Buschke H, Fuld PA. Evaluating Storage, Retention, and Retrieval in Disordered Memory and Learning. Neurology. 1974;24:1019–1025. doi: 10.1212/wnl.24.11.1019. [DOI] [PubMed] [Google Scholar]
- 36.USDA. Dietary Guidelines for Americans. 7. Washington, DC: US Government Printing Office; 2010. Foods and Food Components to Reduce; pp. 30–32. [Google Scholar]
- 37.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Canadian journal of applied sport sciences Journal canadien des sciences appliquees au sport. 1985;10:141–146. [PubMed] [Google Scholar]
- 38.Siest G, Pillot T, Regis-Bailly A, Leininger-Muller B, Steinmetz J, Galteau MM, Visvikis S. Apolipoprotein E: an important gene and protein to follow in laboratory medicine. Clinical chemistry. 1995;41:1068–1086. [PubMed] [Google Scholar]
- 39.Corder EH, Saunders AM, Risch NJ, Strittmatter WJ, Schmechel DE, Gaskell PC, Jr, Rimmler JB, Locke PA, Conneally PM, Schmader KE, et al. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet. 1994;7:180–184. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- 40.Au Yeung SL, Jiang C, Zhang W, Lam TH, Cheng KK, Leung GM, Schooling CM. Moderate alcohol use and cognitive function in the Guangzhou Biobank Cohort study. Ann Epidemiol. 2010;20:873–882. doi: 10.1016/j.annepidem.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 41.Knight JR, Wechsler H, Kuo M, Seibring M, Weitzman ER, Schuckit MA. Alcohol abuse and dependence among U.S. college students. Journal of studies on alcohol. 2002;63:263–270. doi: 10.15288/jsa.2002.63.263. [DOI] [PubMed] [Google Scholar]
- 42.Reid MC, Boutros NN, O’Connor PG, Cadariu A, Concato J. The health-related effects of alcohol use in older persons: a systematic review. Substance abuse: official publication of the Association for Medical Education and Research in Substance Abuse. 2002;23:149–164. doi: 10.1080/08897070209511485. [DOI] [PubMed] [Google Scholar]
- 43.Lobo E, Dufouil C, Marcos G, Quetglas B, Saz P, Guallar E, Lobo A, Workgroup Z. Is there an association between low-to-moderate alcohol consumption and risk of cognitive decline? American journal of epidemiology. 2010;172:708–716. doi: 10.1093/aje/kwq187. [DOI] [PubMed] [Google Scholar]
- 44.Kalmijn S, van Boxtel MP, Verschuren MW, Jolles J, Launer LJ. Cigarette smoking and alcohol consumption in relation to cognitive performance in middle age. American journal of epidemiology. 2002;156:936–944. doi: 10.1093/aje/kwf135. [DOI] [PubMed] [Google Scholar]
- 45.Dufouil C, Ducimetiere P, Alperovitch A. Sex differences in the association between alcohol consumption and cognitive performance. American journal of epidemiology. 1997;146:405–412. doi: 10.1093/oxfordjournals.aje.a009293. [DOI] [PubMed] [Google Scholar]
- 46.Collins MA, Neafsey EJ, Mukamal KJ, Gray MO, Parks DA, Das DK, Korthuis RJ. Alcohol in moderation, cardioprotection, and neuroprotection: epidemiological considerations and mechanistic studies. Alcoholism, clinical and experimental research. 2009;33:206–219. doi: 10.1111/j.1530-0277.2008.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hines LM, Rimm EB. Moderate alcohol consumption and coronary heart disease: a review. Postgraduate medical journal. 2001;77:747–752. doi: 10.1136/pmj.77.914.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bate C, Williams A. Ethanol protects cultured neurons against amyloid-beta and alpha-synuclein-induced synapse damage. Neuropharmacology. 2011;61:1406–1412. doi: 10.1016/j.neuropharm.2011.08.030. [DOI] [PubMed] [Google Scholar]
- 49.Liao SL, Chen WY, Raung SL, Chen CJ. Ethanol attenuates ischemic and hypoxic injury in rat brain and cultured neurons. Neuroreport. 2003;14:2089–2094. doi: 10.1097/01.wnr.0000093754.20088.bc. [DOI] [PubMed] [Google Scholar]
- 50.Devos-Comby L, Lange JE. «My drink is larger than yours»? A literature review of self-defined drink sizes and standard drinks. Current drug abuse reviews. 2008;1:162–176. doi: 10.2174/1874473710801020162. [DOI] [PubMed] [Google Scholar]
- 51.Caldwell TM, Rodgers B, Power C, Clark C, Stansfeld SA. Drinking histories of self-identified lifetime abstainers and occasional drinkers: findings from the 1958 British Birth Cohort Study. Alcohol and alcoholism. 2006;41:650–654. doi: 10.1093/alcalc/agl088. [DOI] [PubMed] [Google Scholar]
- 52.Jones BR, Barrett-Connor E, Criqui MH, Holdbrook MJ. A community study of calorie and nutrient intake in drinkers and nondrinkers of alcohol. Am J Clin Nutr. 1982;35:135–139. doi: 10.1093/ajcn/35.1.135. [DOI] [PubMed] [Google Scholar]
- 53.Barrett-Connor E, Suarez L. A community study of alcohol and other factors associated with the distribution of high density lipoprotein cholesterol in older vs. younger men. American journal of epidemiology. 1982;115:888–893. doi: 10.1093/oxfordjournals.aje.a113376. [DOI] [PubMed] [Google Scholar]
- 54.Arntzen KA, Schirmer H, Wilsgaard T, Mathiesen EB. Moderate wine consumption is associated with better cognitive test results: a 7 year follow up of 5033 subjects in the Tromso Study. Acta neurologica Scandinavica Supplementum. 2010:23–29. doi: 10.1111/j.1600-0404.2010.01371.x. [DOI] [PubMed] [Google Scholar]
- 55.Truelsen T, Thudium D, Gronbaek M, Copenhagen City Heart S Amount and type of alcohol and risk of dementia: the Copenhagen City Heart Study. Neurology. 2002;59:1313–1319. doi: 10.1212/01.wnl.0000031421.50369.e7. [DOI] [PubMed] [Google Scholar]


