Summary
Background
Atrial fibrillation (AF) is a common supraventricular arrhythmia. ECG-gated MDCT seems to be currently a method of choice for pre-ablation anatomical mapping due to an excellent resolution and truly isotropic three-dimensional nature. The aim of this study was to establish the between-subject variability and inter-observer reproducibility of anatomical evaluation of the pulmonary veins (PV) and the left atrium (LA) using computed tomography.
Material/Methods
A retrospective analysis included 42 patients with AF, who were scheduled for a cardiac CT for ablation planning. Images were assessed by two independent radiologists using a semi-automatic software tool. The left atrium anatomy (volume, AP diameter), anatomy of the pulmonary veins (number, ostia diameters and surface area) were evaluated. The relative between-subject variability and the inter-observer variability of measurements were calculated.
Results
The heart rate during scanning ranged from 50 to 133/min. (mean 79.1/min.) and all examinations were of adequate image quality. Accessory pulmonary veins were found in 24% of patients. Between-subject variability of the PV ostial cross-sectional area ranged from 33% to 48%. The variability of the left atrium size was 21% for the diameter and 35% for the volume. The inter-observer agreement for the detection of accessory pulmonary veins was good (κ=0.73; 95% CI, 0.54–0.93).
Conclusions
Between-subject variability of the pulmonary vein ostial cross-sectional area and the left artial volume is substantial. The anatomical assessment of the pulmonary vein ostia and the left atrium size in computed tomography presents a good inter-observer reproducibility.
MeSH Keywords: Atrial Fibrillation, Heart Atria, Multidetector Computed Tomography
Background
Atrial fibrillation (AF) is a common supraventricular arrhythmia, which affects 2–3% of the European and North American population [1]. According to the HRS/EHRA/ECAS consensus, catheter ablation is one of the methods that are recommended for AF treatment both in refractory fibrillation or in patients intolerant to antiarrhythmic medication. Moreover, the ablation is being increasingly used as a primary method of choice in selected patients [2].
Since the anatomy of the left atrium (LA) and pulmonary veins (PV) is variable, detailed knowledge of diameters and anatomical relationships is essential for ablation planning [3]. Both fluoroscopy and echocardiography present limited accuracy in the adequate depiction of the LA/PV topography [4]. Gadolinium-enhanced MRI gives an excellent access to anatomy of the left atrium and pulmonary veins [5]. However, this modality application is limited in patients with implanted pacemakers or defibrillators. Moreover, the quality of PV mapping based on MRI strongly depends on the experience of the technician. Therefore, ECG-gated MDCT seems to be currently the method of choice for pre-ablation anatomical mapping due to an excellent resolution and truly isotropic three-dimensional nature [3].
Measurement of the diameters of the pulmonary veins determines the treatment. Therefore, quantification of the variability in these measurements should be known. Clinical experience and data from previous reports suggest that there is a substantial variability in the morphology of normal pulmonary veins [6]. Consequently, this variability may influence inter-reader reproducibility of measurements. Therefore, the purpose of this study was to establish the between-subject variability and inter-observer reproducibility of measurements of PV and LA diameters.
Material and Methods
A retrospective analysis included 42 patients with AF, who were scheduled for a cardiac CT for ablation planning. The subjects were from 23 to 73 years of age (mean age 54.3 years). Examinations were performed on a 128-slice scanner (Somatom DEFINITION AS+, Siemens Healthcare, Forchheim, Germany) with retrospective ECG gating, using 0.6 mm collimation and 3-phase contrast bolus (Iomeron 400, Bracco, Milan, Italy) at a rate of 6.0 ml/sec. No pharmacological premedication was used.
All images were assessed by two independent radiologists using Philips Brilliance Workspace v. 4.5 and EP Planning, a semi-automatic software tool. The left atrium anatomy (volume, AP diameter), anatomy of the pulmonary veins (number, ostia diameters and surface area) and relation between the pulmonary vein ostia and the esophagus were evaluated.
The size of the pulmonary vein ostia was expressed as their two diameters (a, b), and the cross-sectional area. The area was calculated by the software based on a sum of pixels on the cross-section. Eccentricity of the ostia was calculated using a standard equation for ellipse:
The relative between-subject variability of results was calculated as a ratio between the mean value and the standard deviation of the mean. Parametric data were expressed as mean values and their 95% confidence intervals (95% CI). Normality of data was tested using a Kolmogorov-Smirnov test. Significance of differences between measures was tested using ANOVA and t-test. Agreement between observers in detecting accessory pulmonary veins was tested using inter-rater weighted kappa (κ). Inter-observer variability of parametric measures (x1, x2) was calculated as a relative variability:
and was assessed on Bland-Altman plots [7]. A P-value of <0.05 was considered significant. Statistical analyses were performed using Statistica 10 (StatSoft Inc., Tulsa, OK) and MedCalc Statistical Software version 13.3 (MedCalc Software bvba, Ostend, Belgium)
Results
The heart rate during scanning ranged from 50 to 133/min. (mean 79.1/min.) and all examinations were of adequate image quality. Results of the measurement of pulmonary vein ostia and atria are presented in Table 1.
Table 1.
Results of the measurement of the pulmonary vein ostia and atria by the reference observer. The size of the ostia is presented using two diameters and the area of cross-section. The left atrium size is defined using the antero-posterior diameter and total volume.
| Mean | 95% CI of the mean | Min. value | Max. value | |
|---|---|---|---|---|
| RSPV diam. a | 17.4 | 16.0–18.7 | 10 | 32 |
| RSPV diam. b | 23.5 | 21.9–25.1 | 15 | 43 |
| RSPV area | 333 | 283–382 | 129 | 1090 |
| RIPV diam. a | 15.1 | 14.2–16.0 | 7 | 21 |
| RIPV diam. b | 19.0 | 17.9–20.0 | 8 | 27 |
| RIPV area | 230 | 205–255 | 30 | 460 |
| LSPV diam. a | 14.9 | 13.9–15.9 | 9 | 23 |
| LSPV diam. b | 22.3 | 21.3–23.4 | 16 | 30 |
| LSPV area | 271 | 242–299 | 120 | 540 |
| LIPV diam. a | 12.4 | 11.5–13.3 | 7 | 20 |
| LIPV diam. b | 18.5 | 17.5–19.4 | 13 | 26 |
| LIPV area | 184 | 162–205.3 | 90 | 417 |
| LA diam. | 42.8 | 39.9–45.6 | 15 | 63 |
| LA volume | 105 | 93–116 | 63 | 207 |
Eccentricity of the ostial shape differed significantly between veins (P<0.001) and was the lowest in RIPV (0.58; 95% CI, 0.53 to 0.62) and the highest for LSPV (0.72; 95% CI, 0.68 to 0.76) – Figure 1. The ostial area of all veins significantly positively correlated with the left atrium volume (P<0.05). Moreover, the area of both superior and inferior veins was correlated between both sides. Accessory pulmonary veins were found in 10 cases (24%): one vein in 5 patients (12%) and two veins in 5 subjects.
Figure 1.
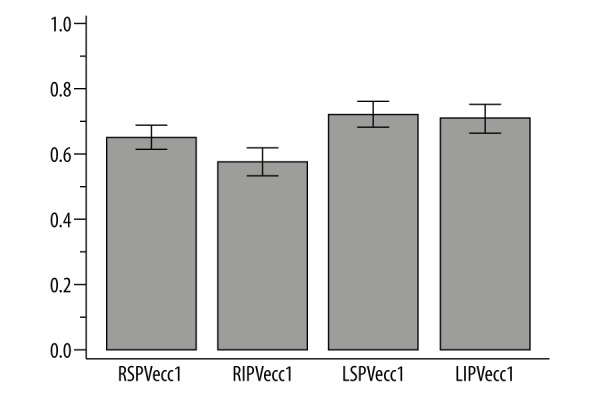
Mean eccentricity of pulmonary veins with its 95% CI.
Between-subject variability of the ostial cross-sectional area ranged from 33% for LSPV to 48% for RSPV – Figure 2. The between-subject variability of diameters was lower, and ranged from 14% to 24%. The variability of the left atrium size was 21% for the diameter and 35% for the volume.
Figure 2.
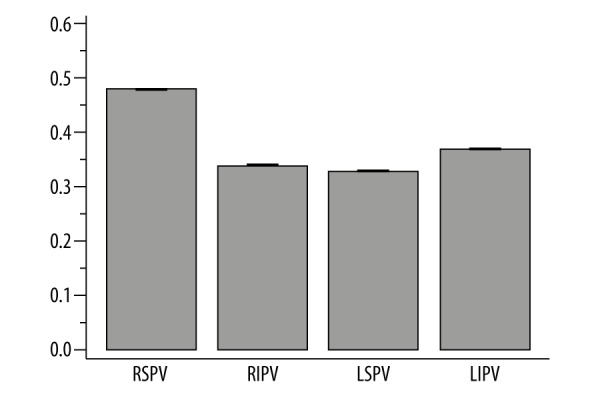
Between-subject variability of the pulmonary vein ostial cross-sectional area.
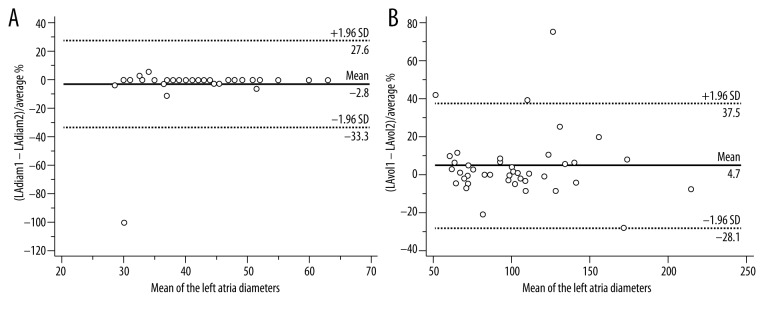
The inter-rater agreement for the detection of accessory pulmonary veins was good (κ=0.73; 95% CI, 0.54–0.93). The inter-observer variability values are presented in Table 2. The mean inter-observer variability was the lowest for the left atrium diameter (3%) and was equally highest for the pulmonary vein ostia cross-sectional areas (12%). The pulmonary veins did not differ regarding the variability of measurements. The inter-observer variability of the left atrial diameter and volume were not significantly different either – Figure 3.
Table 2.
The mean inter-observer variability of the assessed morphological parameters.
| Mean | Min. value | Max. value | |
|---|---|---|---|
| RSPV diam. a | 6% | 0% | 32% |
| RSPV diam. b | 7% | 0% | 33% |
| RSPV area | 12% | 0% | 64% |
| RIPV diam. a | 7% | 0% | 44% |
| RIPV diam. b | 7% | 0% | 27% |
| RIPV area | 12% | 0% | 68% |
| LSPV diam. a | 6% | 0% | 20% |
| LSPV diam. b | 8% | 0% | 57% |
| LSPV area | 12% | 0% | 91% |
| LIPV diam. a | 7% | 0% | 44% |
| LIPV diam. b | 8% | 0% | 53% |
| LIPV area | 12% | 0% | 51% |
| LA diam. | 3% | 0% | 100% |
| LA volume | 10% | 0% | 76% |
Figure 3.
Bland-Altman plots for the inter-observer variability of the left atrial diameter (A) and volume (B).
Discussion
It has been shown that generation of AF is related mainly to the morphological bands of ectopic atrial myocardium that extend to the PV ostia and present their own electrical activity [8]. Therefore, this ectopic myocardium became a target of ablation to treat AF [2]. Ablation points are established based on electrical mapping. However, a precise pre-procedural depiction of LA and PV anatomy may reduce the time of ablation and increase the success rate [3].
Apart from imaging of the coronary arteries in various clinical scenarios [9,10], current applications of cardiac computed tomography can be extended onto coronary calcium scoring, planning of electrophysiological procedures, and, recently, to functional analysis of the myocardium [11–13]. CTA of the left atrium and pulmonary veins is easily tolerated by patients as the scanning time is usually less than 10 s when using the latest generation of scanners. The main disadvantage of CTA is ionizing radiation. According to Eurpopean guidelines, pre-operative CT is considered a significant source of radiation to the patient with a dose as high as 32 mSv [14]. However, the scientific background of such guidelines remains a matter of debate since contemporary dose reduction techniques allow for substantial dose reduction [15–17].
Anatomical measurements of LA and PV may provide useful predictive information in AF patients scheduled for ablation. In a study including 232 subjects the LA maximum volume was significantly associated with chronicity and presence of AF (OR=1.06; 95% CI, 1.03–1.10; P=.0003) even after adjustment for traditional risk factors [18]. Left atrial tissue characteristics may also play an important role in induction and perpetuation. In a study by Dewland et al. AF patients presented 15.5% thinner LA walls. In linear mixed models adjusting for demographics, clinical variables, and other CT measurements, the average LA wall thickness, interatrial septum, LA appendage, and anterior walls remained significantly thinner in AF patients than in healthy controls. After adjusting for the same potential confounders, the history of AF was associated in their material with reduced density in the LA anterior wall and increased density below the right inferior pulmonary vein and in the LA appendage [19].
A typical pattern of PV anatomy may be found in 70–81% of healthy subjects [17]. A large study, which included CTA examinations of 783 patients without AF allowed for classification of 18 anatomical variations of the right pulmonary veins and 8 variations of the left pulmonary veins [17]. The size of PV ostia has been also recognized as a predictor of AF recurrence after ablation. In the study by Kiuchi et al. a multivariate analysis showed that the cross-sectional area of the right upper PV was associated with AF recurrence (OR: 0.41, CI: 0.21–0.77, p=0.006) [20]. Tsyganov et al. evaluated the correlation between PV anatomy and immediate and long-term success of PV isolation with two balloon-based ablation catheter techniques. They observed a variant PV anatomy in 32–40% of their patients. Depending on the technique used, the follow-up at one year after treatment showed AF recurrence in 16–24% of subjects. There were no significant predictors of an early technical success. However, a larger left superior PV and a larger left inferior PV were associated with a worse long-term outcome (p<0.004). Still, there was no absolute cut-off between PV anatomy and clinical success [21].
The most comprehensive assessment of PV measurement reproducibility was published by Yuan et al. [22]. They found that PV ostial measurements were less variable when made by a single observer than by multiple observers, and mean diameter measurements are more precise than a single, maximum diameter measurement. An average standard error of the PV area measurement (SEM) was 16 for one reader and 33 for multiple readers. SEM of PV diameter was 0.7 and 1.8, respectively. In our study, the inter-observer variability was smaller for PV diamaters than for cross-sectional areas as well. Wolf et al. measured the left atria using the same postprocessing software but in a semi-automated and manual mode. In their study, the interobserver agreement of VA volume was excellent for both methods with mean absolute differences of 0.4±2.12 ml and 1.10±2.93 ml, respectively. The interobserver variability was almost equal for both methods [23]. In a study by Mahabadi et al. 3-dimensional threshold-based LA volume measurements had a variability of 8–16% [24], which was close to our results.
Conclusions
Between-subject variability of the pulmonary vein ostial cross-sectional area and the left artial volume are substantial. The anatomical assessment of the pulmonary vein ostia and the left atrium size in computed tomography presents a good inter-observer reproducibility.
References
- 1.Zoni-Berisso M, Lercari F, Carazza T, Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol. 2014;6:213–20. doi: 10.2147/CLEP.S47385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS Expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. J Interv Card Electrophysiol. 2012;33(2):171–257. doi: 10.1007/s10840-012-9672-7. [DOI] [PubMed] [Google Scholar]
- 3.Kiuchi K, Yoshida A, Takei A, et al. Topographic variability of the left atrium and pulmonary veins assessed by 3D-CT predicts the recurrence of atrial fibrillation after catheter ablation. J Arrhythm. 2015;31(5):286–92. doi: 10.1016/j.joa.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lacomis JM, Wigginton W, Fuhrman C, et al. Multi-detector row CT of the left atrium and pulmonary veins before radio-frequency catheter ablation for atrial fibrillation. Radiographics. 2003;23:S35–50. doi: 10.1148/rg.23si035508. [DOI] [PubMed] [Google Scholar]
- 5.Tsao HM, Yu WC, Cheng HC, et al. Pulmonary vein dilation in patients with atrial fibrillation: detection by magnetic resonance imaging. J Cardiovasc Electrophysiol. 2001;12:809–13. doi: 10.1046/j.1540-8167.2001.00809.x. [DOI] [PubMed] [Google Scholar]
- 6.Cronin P, Kelly AM, Gross BH, et al. Reliability of MDCT in characterizing pulmonary venous drainage, diameter and distance to first bifurcation: An interobserver study. Acad Radiol. 2007;14(4):437–44. doi: 10.1016/j.acra.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 7.Serafin Z, Lasek W, Laskowska K. Phantom-calibrated versus automatic coronary artery mass quantification with multidetector-row computed tomography: In vitro and in vivo study. Acta Radiol. 2008;49(9):1007–15. doi: 10.1080/02841850802314770. [DOI] [PubMed] [Google Scholar]
- 8.Douglas YL, Jongbloed MR, Deruiter MC, Gittenberger-de Groot AC. Normal and abnormal development of pulmonary veins: State of the art and correlation with clinical entities. Int J Cardiol. 2011;147(1):13–24. doi: 10.1016/j.ijcard.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Koplay M, Celik M, Avcı A, et al. Comparison between prospectively electrocardiogram-gated high-pitch mode and retrospectively electrocardiogram-gated mode for dual-source CT coronary angiography. Pol J Radiol. 2015;80:561–68. doi: 10.12659/PJR.895232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rychter M, Radomski M, Sukiennik A, et al. Coronary artery aneurysm after implantation of an endothelial progenitor cell capturing stent. Kardiol Pol. 2012;70(6):641–44. [PubMed] [Google Scholar]
- 11.Esposito A, Palmisano A, Antunes S, et al. Cardiac CT with delayed enhancement in the characterization of ventricular tachycardia structural substrate: Relationship between CT-segmented scar and electro-anatomic mapping. JACC Cardiovasc Imaging. 2016 doi: 10.1016/j.jcmg.2015.10.024. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Kiuchi K, Yoshida A, Takei A, et al. Topographic variability of the left atrium and pulmonary veins assessed by 3D-CT predicts the recurrence of atrial fibrillation after catheter ablation. J Arrhythm. 2015;31(5):286–92. doi: 10.1016/j.joa.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serafin Z, Nawrocka E, Thabit SA, et al. Coronary artery calcifications in renal graft recipients at the time of transplantation. Med Sci Monit. 2007;13(Suppl 1):83–89. [PubMed] [Google Scholar]
- 14.Heidbuchel H, Wittkampf FH, Vano E, et al. Practical ways to reduce radiation dose for patients and staff during device implantations and electrophysiological procedures. Europace. 2014;16(7):946–64. doi: 10.1093/europace/eut409. [DOI] [PubMed] [Google Scholar]
- 15.Sardanelli F, Bashir H, Berzaczy D, et al. The role of imaging specialists as authors of systematic reviews on diagnostic and interventional imaging and its impact on scientific quality: Report from the EuroAIM Evidence-based Radiology Working Group. Radiology. 2014;272(2):533–40. doi: 10.1148/radiol.14131730. [DOI] [PubMed] [Google Scholar]
- 16.Jongbloed MR, Dirksen MS, Bax JJ, et al. Atrial fi brillation: Multi-detector row CT of pulmonary vein anatomy prior to radiofrequency catheter ablation – initial experience. Radiology. 2005;234(3):702–9. doi: 10.1148/radiol.2343031047. [DOI] [PubMed] [Google Scholar]
- 17.Tekbas G, Gumus H, Onder H, et al. Evaluation of pulmonary vein variations and anomalies with 64 slice multi detector computed tomography. Wien Klin Wochenschr. 2012;124(1–2):3–10. doi: 10.1007/s00508-011-0086-9. [DOI] [PubMed] [Google Scholar]
- 18.Stojanovska J, Cronin P, Gross BH, et al. Left atrial function and maximum volume as determined by MDCT are independently associated with atrial fibrillation. Acad Radiol. 2014;21(9):1162–71. doi: 10.1016/j.acra.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 19.Dewland TA, Wintermark M, Vaysman A, et al. Use of computed tomography to identify atrial fibrillation associated differences in left atrial wall thickness and density. Pacing Clin Electrophysiol. 2013;36(1):55–62. doi: 10.1111/pace.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiuchi K, Yoshida A, Takei A, et al. Topographic variability of the left atrium and pulmonary veins assessed by 3D-CT predicts the recurrence of atrial fibrillation after catheter ablation. J Arrhythm. 2015;31(5):286–92. doi: 10.1016/j.joa.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsyganov A, Petru J, Skoda J, et al. Anatomical predictors for successful pulmonary vein isolation using balloon-based technologies in atrial fibrillation. J Interv Card Electrophysiol. 2015;44(3):265–71. doi: 10.1007/s10840-015-0068-3. [DOI] [PubMed] [Google Scholar]
- 22.Yuan XP, Bach D, Skanes A, Drangova M. Assessment of intra- and interobserver variability of pulmonary vein measurements from CT angiography. Acad Radiol. 2004;11(11):1211–18. doi: 10.1016/j.acra.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 23.Wolf F, Ourednicek P, Loewe C, et al. Evaluation of left atrial function by multidetector computed tomography before left atrial radiofrequency-catheter ablation: Comparison of a manual and automated 3D volume segmentation method. Eur J Radiol. 2010;75(2):e141–46. doi: 10.1016/j.ejrad.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 24.Mahabadi AA, Samy B, Seneviratne SK, et al. Quantitative assessment of left atrial volume by electrocardiographic-gated contrast-enhanced multidetector computed tomography. J Cardiovasc Comput Tomogr. 2009;3(2):80–87. doi: 10.1016/j.jcct.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]