Abstract
Human adenoviruses can cause serious disseminated infections including death in immunosuppressed patients, especially pediatric allogeneic hematopoietic stem cell transplant (allo-HSCT) patients. There are no drugs approved to treat such infections. Cidofovir is used intravenously in many transplant clinics, probably with some effect, but controlled trials have not been completed. Cidofovir is an acyclic nucleoside phosphonate analog of cytidine monophosphate. Following conversion to its diphosphate form within cells, cidofovir is a preferred substrate for the adenovirus DNA polymerase, leading to viral DNA chain termination. Problems with cidofovir include poor cellular uptake and nephrotoxicity. Brincidofovir, a lipid-linked derivative of cidofovir which is active against five families of double-stranded DNA viruses, represents a major advance in anti-adenovirus therapy. It is administered orally, taken up readily by cells followed by release of cidofovir within cells, and is not nephrotoxic. Brincidofovir, under development by Chimerix, Inc., is being evaluated against adenovirus infections in transplant patients including allo-HSCT patients in a phase III clinical trial (AdVise Study). Preliminary results indicate that brincidofovir is safe and very effective at decreasing adenovirus viremia and adenovirus-induced pathogenicity and mortality. Anti-adenovirus adoptive T cell therapy is another very promising approach to treating allo-HSCT patients as demonstrated in clinical studies.
Keywords: adenovirus, Brincidofovir, cidofovir, Syrian hamster animal model
Human adenoviruses (AdV) have a duplex DNA genome that encodes about 35 genes. There are more than 60 serotypes (types) that form 7 species, A-G. AdV in general cause asymptomatic or symptomatic respiratory, ocular, and gastrointestinal infections that, except for epidemic keratoconjunctivitis, are self-limiting in otherwise healthy individuals (1, 2). However, AdV can be a major problem in severely immunosuppressed patients, in particular pediatric patients undergoing allogeneic hematopoietic stem cell transplantation (allo-HSCT). The incidence of AdV infection in pediatric allo-HSCT ranges from 5-6% to 42-47% (1, 2). Mortality rates are up to 26% with symptomatic infection and up to 80% for disseminated disease (1, 2). In solid organ transplants, incidence ranges from 4% to 10% in pediatric liver transplants and up to 57% in small bowel recipients. Mortality ranges from 18% in kidney transplants to 53% in liver recipients. With disseminated AdV disease there is multi-organ involvement and two or more AdV-positive PCR assays for peripheral blood and other fluids (e.g. urine, bronchoalveolar fluid, cerebrospinal fluid). Death due to AdV is associated with multi-organ failure and persisting or increasing AdV levels in peripheral blood (1). Risk factors for AdV disease and death include young age, receipt of mismatched graft, receipt of T cell-depleted graft, acute graft versus host disease, isolation of AdV early after transplantation, persistent isolation of AdV from more than one site, initial high AdV level in the blood, and intensification of immunosuppression (2). Another important risk factor is the rising of AdV levels in serial stool samples above the threshold of 106 copies per gram (3).
Unfortunately, despite the seriousness of AdV disease following transplantation, there are no drugs approved to treat AdV infections. Intravenous cidofovir is used in many transplant clinics (4), probably with some efficacy (although controlled studies have not been completed). Cidofovir, an acyclic nucleoside phosphonate, is a nucleotide analog of cytosine. Upon conversion by cells to the diphosphate form, the compound is a preferred substrate for the AdV DNA polymerase, leading to viral DNA chain termination. A problem with cidofovir is poor cellular uptake (because of the phosphate group). Also, it is associated with leukopenia and neutropenia, and is a substrate for organic anion transporter 1, leading to accumulation of cidofovir in renal tubules and associated nephrotoxicity.
A major advance in anti-AdV therapy has been made in recent years with the development by Chimerix, Inc., of brincidofovir (BCV; previously named CMX001), a broad spectrum anti-viral active against 5 families of double-stranded DNA viruses (reviewed in (5)). BCV (3-hexadecyloxy-1-propanol-cidofovir) is a lipid-linked derivative of cidofovir. The lipid moiety allows BCV to be administered orally and to enter cells readily. Within cells, the lipid moiety is cleaved away by phospholipases, leaving cidofovir which cannot exit cells readily. BCV is not a substrate for organic anion transporter 1, so it does not accumulate in renal tubules and cause nephrotoxicity. The SUPPRESS trial, which investigates the ability of BCV to prevent clinically significant CMV infections in HSCT recipients, recently completed enrollment (https://clinicaltrials.gov/ct2/show/NCT01769170). Importantly, the study will also seek to determine if BCV can prevent other dsDNA viral infections in this high-risk population, including BK virus, other human herpesviruses including HHV-6 and EBV, and adenovirus.
Studies in cell culture indicate that BCV inhibits the replication of multiple AdV serotypes, with EC50s in the ∼0.02 μM range (6). In an immunosuppressed Syrian hamster model that is permissive for replication of adenovirus serotype 5 (Ad5), BCV was very effective at inhibiting Ad5 replication and pathology (7, 8) (Fig. 1). In a dramatic case report, BCV eradicated a disseminated AdV serotype 2 infection in a pediatric allo-HSCT patient (9) (Fig. 2).
Figure 1. Brincidofovir (BCV) decreases adenovirus type 5 (Ad5)-induced lesions in the liver.
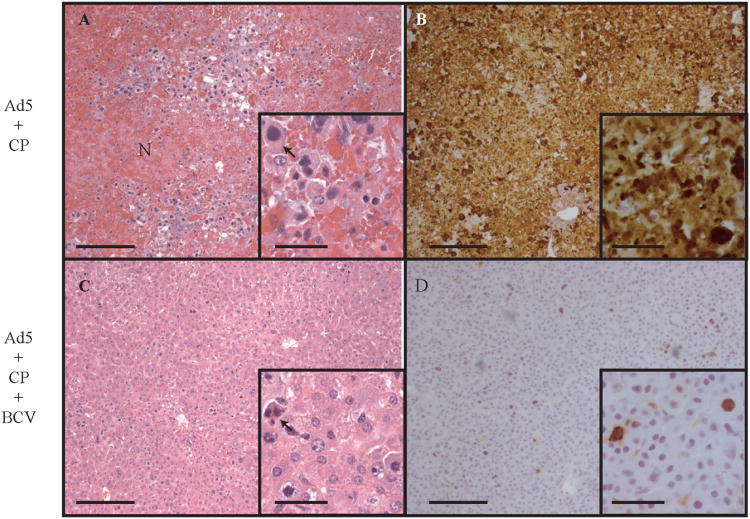
Syrian hamsters were immunosuppressed using cyclophosphamide, then infected intravenously with 1.9 × 1012 virus particles/kg of Ad5. Livers of hamsters sacrificed at day 6 were subjected to histopathological and immunohistochemistry (IHC) evaluation. Animals infected with Ad5 and not treated with BCV exhibited extensive coagulation necrosis throughout the liver (A) and widespread replication of Ad5, demonstrated by staining for the AdV fiber protein (B). Treatment of Ad5-infected hamsters with BCV resulted in a significant reduction in hepatocellular injury (C) and greatly reduced IHC staining for fiber (D). The arrows indicate intranuclear inclusion bodies. The scale bars represent 200 mm for the larger pictures and 50 mm for the insets. N, necrosis. (Reproduced from [12] Copyright 2007 National Academy of Sciences U.S.A.)
Figure 2. Plasma adenovirus (AdV) levels prior to (day +132 posttransplant) and during (day +133) treatment with brincidofovir (BCV).
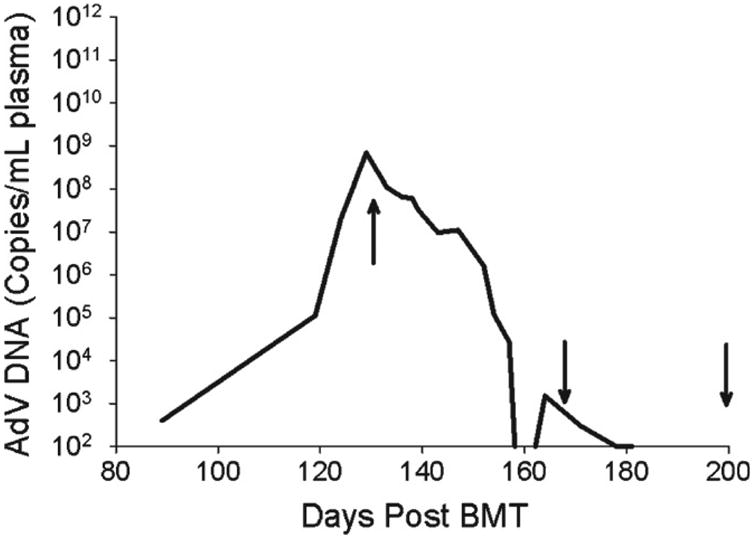
AdV was first detected day +89 posttransplant, with a continued rise in AdV load despite administration of intravenous cidofovir days +92 to +132 posttransplant and intravenous immunoglobulin (IVIG) (day +129 posttransplant). Treatment with BCV was initiated on day +133 posttransplant at 2 mg/kg administered twice weekly increasing to 3 mg/kg after the sixth dose. After the virus became undetectable (less than 1012 copies/mL) (day +159 posttransplant), administration of BCV continued at 3 mg/kg but the schedule was reduced to once weekly for maintenance. Arrows indicate timing of 1st, 10th, and 20th BCV doses. (Reproduced with permission from [14])
BCV is being evaluated for safety and efficacy against AdV in clinical trials (10). In March, 2014, a phase III trial was initiated (AdVise Study, NCT02087306) entitled “Phase III, Open-labeled, Multicenter Study of the Safety and Efficacy of Brincidofovir (CMX001) in the Treatment of Early Versus Late AdV Infection (Chimerix Study CMX001-0304)”. The primary objective is “to compare the overall mortality at week 24 of subjects treated with BCV during asymptomatic adenovirus viremia or early AdV infection to the mortality at week 24 for subjects treated with BCV during symptomatic or late AdV disease”.
Preliminary results have been presented in abstract form (11, 12) (see the web site for Chimerix, Inc.). Subjects receive 100 mg BCV biweekly (or 2 mg/kg biweekly if subjects are <50 kg) for 12 weeks, with 12 week follow-up with BCV available for possible AdV relapse. Three subject cohorts were defined. Cohort A consists of allo-HSCT patients at “high risk of AdV disease progression” (asymptomatic patients with plasma AdV at ≥1000 genome copies/ml or patients symptomatic in one organ system and plasma AdV <1000 copies/ml). Cohort B has allo-HSCT patients with “disseminated AdV disease” (symptomatic in one organ system with plasma AdV ≥ 1000 copies/ml or symptomatic in two or more organ systems). Cohort C consists of all other patients (e.g. solid organ transplants). As of January, 2015, data were available from 85 patients, 18 in Cohort A, 54 in Cohort B, and 13 in Cohort C. In Cohort B, the median age was 12 years (range 8 months to 69 years), the median date of treatment after HSCT was 77 days, and the median absolute lymphocyte count was 350 cells per μl. One third of patients had graft versus host disease at enrollment, and 41% of patients had prior intravenous cidofovir therapy. The median AdV copy level in plasma was 4.5 log10/ml and the median treatment was 12 doses over 39 days. Of the 54 patients in Cohort B with detected plasma AdV at baseline, 66% had a decrease of >3 log10 copies/ml or to undetectable levels in the plasma. The mean decrease in plasma AdV was about 10-fold after one week of treatment. AdV was cleared from plasma, urine, stool, and respiratory secretions in a majority of cases. The mortality was 37% after a median follow-up of 75 days, compared to 50% to 80% mortality as reported in the literature (1, 2). About two-thirds of Cohort B patients were co-infected with other DNA viruses at enrollment. No deaths or serious adverse events were attributed to BCV. These preliminary clinical data appear to be encouraging and support continued clinical testing (11, 12).
Anti-AdV adoptive T cell therapy is another promising approach for treating AdV. The loss of virus-specific CD4 and CD8 T cells is a critical contributor to sensitivity to opportunistic viral infections, among them AdV infections (1). As the reconstitution of this compartment of immune cells is usually slow, researchers attempted to adoptively transfer virus specific T cells into T cell-depleted patients to fight the infection until their immune system sufficiently recovered. Although AdV-specific T cells are present in the peripheral blood of healthy adults only at low frequencies, transfer of donor leukocytes was reported to have a beneficial effect with a patient with life-threatening AdV infection. However, the transfer of alloreactive T cells could possibly result in graft versus host disease. Thus, a process to select and enrich virus-specific T cells is required to develop an applicable therapy. As the target population for this type of therapy is recipients of some form of hematopoietic transplant, one obvious source is lymphocytes derived from the same donor as the transplant. In early experiments, the lymphocytes were harvested, stimulated overnight with viral antigens, and then the activated cells were selected using magnetic beads linked to antibodies recognizing IFNγ (13). While this treatment was efficacious in 5 of 6 evaluable patients, the small number of AdV-specific T cells present in the donor's blood meant that it took 7 to 14 weeks for the cells to expand in the recipient (13). This may prove to be too late for patients with high virus load. Although the same research group recently reported increased survival after repetitive transfer of ex vivo stimulated – predominantly Th1 –T cells (14), other researchers took a different approach and devised various methods for expanding AdV-specific T cells in vitro. With these protocols, donor leukocytes are harvested, and then stimulated and expanded in vitro using peptide-MHC I tetramers (15). This procedure yields a batch of AdV-specific T cells in 2 weeks. The peptides can be representatives of immunodominant T cell epitopes from one virus, or even multiple viruses (16). Fortunately, for AdVs, hexon epitopes cross reactive among most types are used (1), thus decreasing the number of epitopes that need to be included. To further decrease the time until a batch of T cells can be injected into a patient, researchers used an “off the shelf” approach: they prepared cryopreserved preparations of AdV-specific T cells from donors representing frequent HLA haplotypes, and used these cells to treat immunocompromised patients with AdV infections (16). This approach is also useful in cases when AdV-specific T cells from the donor are not available (e.g. for cord blood transplant, cadaver donor, AdV naïve donor, etc.). Clearly, the results from these cell therapy trials are encouraging. Further, as adoptive T-cell therapies utilize a different mechanism to curb AdV infection than antiviral drugs, one can anticipate that the combination of the two modalities would yield a synergistic increase in efficacy.
Ganciclovir has been reported to have anti-AdV activity in some clinical reports and it and valganciclovir were effective against Ad5 replication and pathogenesis in the immunosuppressed Syrian hamster model (17, 18), so perhaps these compounds have potential for anti-AdV therapy.
In summary, intravenous cidofovir is probably efficacious against AdV infections in some settings, but it is nephrotoxic. BCV appears to be a much better anti-AdV drug with apparent efficacy and low toxicity in clinical trials (10-12). Employment of AdV-specific T cells for adoptive T cell therapy appears to be promising as well (14)
Footnotes
Financial and competing interests disclosure: Chimeric Inc. provided a research contract to Saint Louis University to evaluate the efficacy of CMX001 against adenovirus replication in the immunosuppressed Syrian hamster model from January 1st 2007 to December 31st 2010. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed
Contributor Information
William S.M. Wold, Email: woldws@slu.edu, Saint Louis University School of Medicine, Department of Molecular Microbiology and Immunology, 1100 S. Grand Boulevard, St. Louis, MO 63104, Ph: 314-977-8857, Fax: 314-977-8717.
Karoly Toth, Email: toth@slu.edu, Saint Louis University School of Medicine, Department of Molecular Microbiology and Immunology, 1100 S. Grand Boulevard, St. Louis, MO 63104, Ph: 314-977-8338, Fax: 314-977-8717.
References
- 1**.Lion T. Adenovirus infections in immunocompetent and immunocompromised patients. Clin Microbiol Rev. 2014;27(3):441–62. doi: 10.1128/CMR.00116-13. Recent review highlighting the seriousness of AdV infections in immunocompromised humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sandkovsky U, Vargas L, Florescu DF. Adenovirus: current epidemiology and emerging approaches to prevention and treatment. Current infectious disease reports. 2014;16(8):416. doi: 10.1007/s11908-014-0416-y. [DOI] [PubMed] [Google Scholar]
- 3.Lion T, Kosulin K, Landlinger C, et al. Monitoring of adenovirus load in stool by real-time PCR permits early detection of impending invasive infection in patients after allogeneic stem cell transplantation. Leukemia. 2010;24(4):706–14. doi: 10.1038/leu.2010.4. [DOI] [PubMed] [Google Scholar]
- 4.Lugthart G, Oomen MA, Jol-van der Zijde CM, et al. The effect of cidofovir on adenovirus plasma DNA levels in stem cell transplantation recipients without T cell reconstitution. Biol Blood Marrow Transplant. 2015;21(2):293–9. doi: 10.1016/j.bbmt.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Florescu DF, Keck MA. Development of CMX001 (Brincidofovir) for the treatment of serious diseases or conditions caused by dsDNA viruses. Expert review of anti-infective therapy. 2014;12(10):1171–8. doi: 10.1586/14787210.2014.948847. [DOI] [PubMed] [Google Scholar]
- 6.Hartline CB, Gustin KM, Wan WB, et al. Ether lipid-ester prodrugs of acyclic nucleoside phosphonates: activity against adenovirus replication in vitro. J Infect Dis. 2005;191(3):396–9. doi: 10.1086/426831. First study to demontrate the in vitro activity of BCV against AdV. [DOI] [PubMed] [Google Scholar]
- 7*.Toth K, Spencer JF, Dhar D, et al. Hexadecyloxypropyl-cidofovir, CMX001, prevents adenovirus-induced mortality in a permissive, immunosuppressed animal model. Proc Natl Acad Sci U S A. 2008;105(20):7293–7. doi: 10.1073/pnas.0800200105. First study to demonstrate the efficacy of BCV against AdV in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tollefson AE, Spencer JF, Ying B, et al. Cidofovir and brincidofovir reduce the pathology caused by systemic infection with human type 5 adenovirus in immunosuppressed Syrian hamsters, while ribavirin is largely ineffective in this model. Antiviral Res. 2014;112:38–46. doi: 10.1016/j.antiviral.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Paolino K, Sande J, Perez E, et al. Eradication of disseminated adenovirus infection in a pediatric hematopoietic stem cell transplantation recipient using the novel antiviral agent CMX001. J Clin Virol. 2011;50(2):167–70. doi: 10.1016/j.jcv.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 10*.Florescu DF, Pergam SA, Neely MN, et al. Safety and efficacy of CMX001 as salvage therapy for severe adenovirus infections in immunocompromised patients. Biol Blood Marrow Transplant. 2012;18(5):731–8. doi: 10.1016/j.bbmt.2011.09.007. Retrospective study demonstrating the clinical efficacy of BCV against AdV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11**.Grimley M, Papanicolaou G, Maron G, et al. 41st Annual Meeting of the European Society for Blood and Marrow Transplantation. Istanbul, Turkey: 2015. Improved Outcomes in Allogeneic Hematopoietic Cell Transplant (allo HCT) Patients Treated with Brincidofovir for Disseminated Adenovirus AdV) Disease Compared to Literature: Updated Preliminary Results from the AdVise (CMX001-304) Study. Describes preliminary result of an ongoing clinical trial. [Google Scholar]
- 12**.Grimley M, Maron G, Prasad V, et al. Bone Marrow Transplant Tandem Meetings. San Diego, CA: 2015. Preliminary Results from the AdVise Study Evaluating Brincidofovir (BCV, CMX001) for the Treatment of Disseminated and High-Risk Adenovirus (AdV) Infection. Describes preliminary result of an ongoing clinical trial. [Google Scholar]
- 13.Khanna R, Smith C. Cellular immune therapy for viral infections in transplant patients. Indian J Med Res. 2013;138(5):796–807. [PMC free article] [PubMed] [Google Scholar]
- 14.Feucht J, Opherk K, Lang P, et al. Adoptive T-cell therapy with hexon-specific Th1 cells as a treatment of refractory adenovirus infection after HSCT. Blood. 2015;125(12):1986–94. doi: 10.1182/blood-2014-06-573725. [DOI] [PubMed] [Google Scholar]
- 15*.Geyeregger R, Freimuller C, Stevanovic S, et al. Short-term in-vitro expansion improves monitoring and allows affordable generation of virus-specific T-cells against several viruses for a broad clinical application. PloS one. 2013;8(4):e59592. doi: 10.1371/journal.pone.0059592. Adaptive transfer of in vitro-expanded self-derived T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16**.Leen AM, Heslop HE, Brenner MK. Antiviral T-cell therapy. Immunol Rev. 2014;258(1):12–29. doi: 10.1111/imr.12138. Recent review on the status of antiviral T-cell therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toth K, Ying B, Tollefson AE, et al. Valganciclovir inhibits human adenovirus replication and pathology in permissive immunosuppressed female and male Syrian hamsters. Viruses. 2015;7(3):1409–28. doi: 10.3390/v7031409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ying B, Tollefson AE, Spencer JF, et al. Ganciclovir inhibits human adenovirus replication and pathogenicity in permissive immunosuppressed Syrian hamsters. Antimicrob Agents Chemother. 2014;58(12):7171–81. doi: 10.1128/AAC.03860-14. [DOI] [PMC free article] [PubMed] [Google Scholar]


