Abstract
Objectives
To test the hypothesis that, without diagnostic changes in serum creatinine, increased NGAL levels identify patients with subclinical acute kidney injury (AKI) and, therefore, worse prognosis.
Background
Neutrophil gelatinase-associated lipocalin (NGAL) detects subclinical AKI hours to days before increases in serum creatinine indicate manifest loss of renal function.
Methods
We analyzed pooled data from 2,322 patients with cardiorenal syndrome type 1 from ten prospective observational studies of NGAL. We used the terms NGAL(−) or NGAL(+) according to study-specific NGAL cut-off for optimal AKI prediction and the terms sCREA(−) or sCREA(+) to consensus diagnostic increases in serum creatinine defining AKI. A-priori-defined outcomes included need for renal replacement therapy (primary endpoint), hospital mortality, their combination and duration of stay in intensive care and in-hospital.
Results
Of study patients, 1,296 (55.8%) were NGAL(−)/sCREA(−), 445 (19.2%) NGAL(+)/sCREA(−), 107 (4.6%) NGAL(−)/sCREA(+) and 474 (20.4%) NGAL(+)/sCREA(+). According to the four study groups, there was a stepwise increase in subsequent renal replacement therapy initiation, (NGAL(−)/sCREA(−): 0.0015% vs. NGAL(+)/sCREA(−): 2.5% [odds ratio 16.4, 95% CI 3.6–76.9, P<0.001], NGAL(−)/sCREA(+): 7.5% and NGAL(−)/sCREA(−): 8.0%, respectively), hospital mortality (4.8%, 12.4%, 8.4%, 14.7%, respectively) and their combination (four-group comparisons: all P<0.001). There was a similar and consistent progressive increase in median number of intensive care and in-hospital days with increasing biomarker positivity: NGAL(−)/sCREA(−): 4.2 and 8.8 days; NGAL(+)/sCREA(−): 7.1 and 17.0 days; NGAL(−)/sCREA(+): 6.5 and 17.8 days; NGAL(+)/sCREA(+): 9.0 and 21.9 days; four-group comparisons: P=0.003 and P=0.040, respectively. Urine and plasma NGAL indicated a similar outcome pattern.
Conclusions
In the absence of diagnostic increases in serum creatinine, NGAL detects patients with subclinical AKI who have an increased risk of adverse outcomes. The concept and definition of AKI may need re-assessment.
Keywords: Acute kidney injury (AKI), biomarker, creatinine, neutrophil gelatinase-associated lipocalin (NGAL), renal replacement therapy (RRT), mortality
INTRODUCTION
The current concept and diagnosis of acute kidney injury (AKI) are mainly based on diagnostic increases in serum creatinine indicating loss of excretory renal function. AKI is then classified according to either the RIFLE (1) or AKI Network consensus criteria (2). Although such diagnosis of AKI is of prognostic relevance (3,4), it is delayed by 24–72 hours compared to diagnosis by means of novel renal biomarkers of tubular injury like neutrophil gelatinase-associated lipocalin (NGAL) (5–7).
NGAL fulfils many characteristics for an ideal biomarker for AKI. It was discovered using unbiased transcriptomic approaches (8,9); it is rapidly induced and released from the injured distal nephron in experimental models and human disease (5,9,10); its urine and plasma concentrations increase proportionally to severity and duration of renal injury (9,11,12); its concentration rapidly decreases with attenuation of renal injury (13) and it is readily and easily measured in plasma (11) and urine (12). Finally, NGAL appears to play a key role in early AKI and local iron transport (10,14,15) providing biologic plausibility for its use as AKI biomarker.
Several studies have found NGAL useful compared to early measurements of serum creatinine in AKI (6,7,16,17). In response to renal injury, increases in NGAL levels predict AKI 24–72 hours before diagnostic creatinine increases (5–7,11,12) and are of prognostic value (18). Despite the above characteristics, NGAL’s ability to predict the development of AKI appears imperfect (19,20). However, the predictive value of NGAL improves with increasing RIFLE class of AKI (21), suggesting that limitations in its accuracy reflect the use of an imperfect test (serum creatinine) as study endpoint. Serum creatinine requires several hours to days to accumulate, it increases in serum only after 50% or more of renal function is lost and its concentration is affected by multiple confounding factors (22). Accordingly, we hypothesized that, without diagnostic increases in serum creatinine, NGAL(+) patients might have likely subclinical AKI and, therefore, carry a worse prognosis as indicated by the need for renal replacement therapy (RRT) and other patient-centered outcomes than NGAL(−) patients. We tested this hypothesis by analyzing pooled data from multiple centers and determined the clinical outcomes of subjects classified according to their NGAL and serum creatinine concentrations.
METHODS
We performed a summary patient-level analysis using summarized data from each study (see data collection form in the Appendix). For this purpose, we used data from a previously described multicenter data pool created to study the predictive value of NGAL for AKI defined by increases in serum creatinine (18). This pool was extended by newly identified clinical studies on NGAL as biomarker of AKI. Individual patient data were not available. Figure 1 displays the results of the search and data acquisition strategies.
Figure 1.
Flow of study selection.
NGAL, neutrophil gelatinase-associated lipocalin; RRT, renal replacement therapy.
Data pool creation
Two investigators (A.H.F. and P.R.M.) independently searched PUBMED, EMBASE, CENTRAL and abstracts submitted to the Congress of the American Society of Nephrology. They identified relevant articles or abstracts and independently screened studies for inclusion (Figure 1). Selection was restricted to published prospective cohort studies in humans investigating the diagnostic and prognostic ability of NGAL for AKI, need for renal replacement therapy and in-hospital mortality. Each independent biomarker study was approved by a local Institutional Review Board and all participants gave written informed consent. All studies originally were designed to examine the diagnostic accuracy of NGAL level, prospectively enrolled consecutive patients, had clearly defined enrolment and exclusion criteria and laboratory/research personnel and clinicians were blinded. All studies enrolled representative patient cohorts who might receive the test in clinical practice. Included studies had similar sample collection and processing and provided full datasets.
NGAL measurement and AKI definition
For the purpose of this study, we included urine and plasma NGAL values and reported those in a combined fashion and separately. We asked each author to use the measurement performed at least 24–48 hours before the diagnosis of AKI when NGAL was measured more than once or when no serum creatinine increase occurred to use NGAL measured at intensive care unit admission or 2–6 hours after any new renal insult. We defined AKI according to the RIFLE classification (1) as an increase in serum creatinine ≥50% from baseline to peak value within seven days of admission to the intensive care unit basing on daily serum creatinine measurement (RIFLE class R or worse). We choose RIFLE over the AKI Network classification (2) because of its greater sensitivity (23). Baseline serum creatinine was defined as the concentration obtained at outpatient departments or at hospital admission in cardiac surgery patients or at intensive care unit admission in critically ill patients when previous values were not available. Chronic kidney disease was defined as an estimated glomerular filtration rate <60 mL/min/1.73m2 calculated using the simplified Modification of Diet in Renal Disease (MDRD) study formula (24) in adults or the simplified Schwartz formula in children (25). Renal replacement therapy (RRT) was initiated based on center- and physician-specific practice and no patient received RRT for non-renal indications.
We applied the term “NGAL(−)” or “NGAL(+)” to indicate the absence or presence of tubular injury, as defined by each study-specific NGAL cut-off value for the optimal combination of sensitivity and specificity for AKI prediction (18). The early ‘research-based’ urine or plasma NGAL ELISA assays were internally valid but their cut-off values were not transferable to other ELISA assays or immunoblots used. We used the term serum creatinine “sCREA(−)” or “sCREA(+)” to indicate the absence or presence of manifest AKI as defined by RIFLE criteria.
Patients were classified as follows:
NGAL(−) / sCREA(−)
NGAL(+) / sCREA(−)
NGAL(−) / sCREA(+)
NGAL(+) / sCREA(+)
Patient outcomes
For the present study, we invited each investigator to analyze data from their individual patient cohort and return a specifically designed data collection form (see Appendix) recording demographic data, information on co-morbidities, and a-priori defined patient outcomes including renal replacement therapy initiation (primary endpoint), in-hospital mortality, the combination of both and the length of intensive care unit and hospital stay and according to patients’ NGAL and serum creatinine states as defined above.
Statistical Analysis
All analyses followed a preset statistical analysis plan, which included the above a-priori defined hypothesis. Data were tested on normality using histograms. When data were normally distributed, analysis-of-variance was used to compare numerical data of patients according to the defined groups. Otherwise, non-parametric testing was used (Kruskal-Wallis test for four-group comparison, Mann-Whitney test for two-group comparison). Fisher’s exact test or the chi-square test was applied for comparison of categorical values as appropriate. Analysis was repeated after weighing of the study endpoints according to the relative sample size of each study. We used SPSS Version 16.0 (SPSS Inc, Chicago, IL). A two-sided P-value of <0.05 was considered to be statistically significant.
RESULTS
Ten (6,7,11,12,19,20,26–29) out of 22 authors contacted returned complete data sets. The reasons for exclusion are reported in Figure 1.
We obtained data on 2,322 patients from America, Europe and Australia (Table 1) with the majority having cardiorenal syndrome type 1 (30). The incidence of AKI ranged from 15% to 49%. Patients’ characteristics and baseline peak NGAL levels are shown in Table 2. The majority of patients were NGAL(−)/sCREA(−), while 25% of patients developed AKI based on the RIFLE definition, 3% required renal replacement therapy and 8% died in-hospital. In patients with diabetes, NGAL(+)/sCREA(−) status was less common than NGAL(−)/sCREA(+). Chronic kidney disease was similarly common among NGAL(−)/sCREA(−) (11.0%) and NGAL(+)/sCREA(−) (10.6%) patients.
Table 1.
Characteristics of studies.
| Study | Population Type | AKI Etiology | AKI Incidence | No. of patients* | NGAL Measurement,† hrs | |
|---|---|---|---|---|---|---|
| Urine NGAL | Plasma NGAL | |||||
| Dent et al. (11) | Children | Cardiac surgery | 36% | - | 125 | 48 |
| Bennett et al. (12) | Children | Cardiac surgery | 49% | 194 | - | 48 |
| Krawczeski et al. (29) | Children | Cardiac surgery | 35% | 189 | 358 | 48 |
| Zappitelli et al. (27) | Adults | Critically ill | 30% | 33 | - | 36 |
| Cruz et al. (6) | Adults | Critically ill | 28% | - | 279 | 24 |
| Haase-Fielitz et al. (7) | Adults | Cardiac surgery | 23% | - | 100 | 48 |
| Koyner et al. (26) | Adults | Cardiac surgery | 16% | 52 | 51 | 36 |
| Wagener et al. (19) | Adults | Cardiac surgery | 15% | 383 | - | 36 |
| Martensson et al. (28) | Adults | Critically ill | 34% | 64 | 64 | 48 |
| Siew et al. (20) | Adults | Critically ill | 19% | 430 | - | 48 |
With NGAL measured at least 24 to 48 hours before the diagnosis of AKI or at ICU admission several hours after new renal insult and excluding patients with urinary tract infection.
Preceding the diagnosis of AKI based on RIFLE class R or worse (1).
AKI, acute kidney injury. NGAL, Neutrophil Gelatinase-associated Lipocalin.
Table 2.
Baseline characteristics of patients (N=2,322).
| NGAL(−) / sCREA(−) | NGAL(+) / sCREA(−) | NGAL(−) / sCREA(+) | NGAL(+) / sCREA(+) | P Value | |
|---|---|---|---|---|---|
| Number of patients, n | 1,296 (55.8%) | 445 (19.2%) | 107 (4.6%) | 474 (20.4%) | - |
| Age, y | 47.2 (4.5–61.6) | 53.5 (3.6–60.7) | 59.0 (4.4–67.3) | 50.4 (3.6–66.8) | 0.89 |
| Female, n | 475 (36.7%) | 181 (40.7%) | 42 (39.3%) | 210 (44.3%) | 0.028 |
| Chronic kidney disease*, n | 143 (11.0%) | 47 (10.6%) | 16 (15.0%) | 81 (17.1%) | 0.030 |
| Diabetes mellitus, n | 113 (8.7%) | 78 (17.5%) | 28 (26.2%) | 29 (6.1%) | <0.001 |
| Congestive heart failure, n | 67 (5.2%) | 51 (11.5%) | 10 (9.4%) | 14 (3.0%) | <0.001 |
| Peak NGAL, ng/mL | 59 (20–97) | 213 (117–1,124) | 69 (21–118) | 354 (208–1,888) | <0.001 |
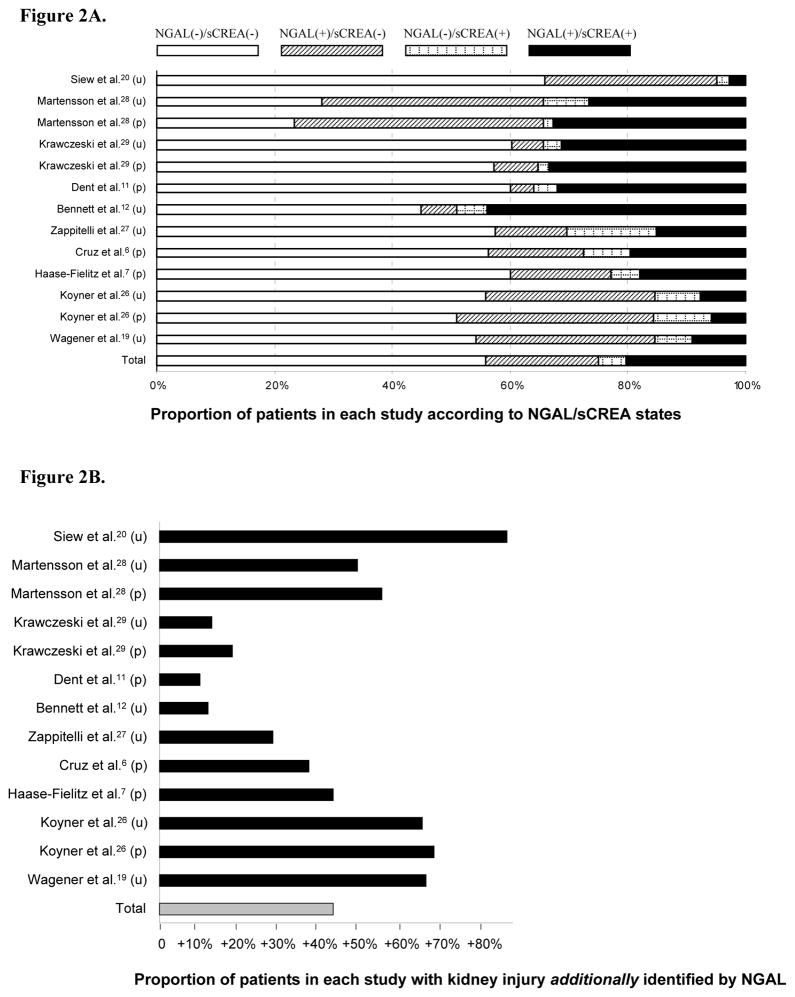
Figure 2A shows the proportion of patients in each study according to NGAL/sCREA status with about 20% of all patients being NGAL(+)/sCREA(−). Overall, 43% of patients diagnosed with AKI by means of NGAL would have been missed using creatinine criteria alone (Figure 2B).
Figure 2.
Figure 2A Proportion of patients according to biomarker states.
NGAL, neutrophil gelatinase-associated lipocalin; sCREA, serum creatinine; u, urine; p, plasma.
Figure 2B. Proportion of NGAL(+) / sCREA(−) patients in relation to the proportion of patients diagnosed to have AKI by conventional creatinine-based criteria (RIFLE classification [1]) and NGAL positivity.
Neutrophil Gelatinase-associated Lipocalin, NGAL; u, urine; p, plasma.
Patient outcomes
Treatment with RRT increased from 0.0015% in biomarker-negative patients to 2.5% in NGAL(+)/sCREA(−) patients, 7.5% in NGAL(−)/sCREA(+) patients and 8.0% in NGAL(+)/sCREA(+) patients (four-group comparison: P<0.001) (Figure 3). Specifically, more patients with NGAL+/sCREA- status needed RRT initiation than patients with NGAL-/sCREA- status; OR 16.4 [95% CI 3.6–76.9], P<0.001. Additional endpoints comparing these patient groups are shown in Table 3.
Figure 3.
Incidence of renal replacement therapy (RRT) initiation, in-hospital morality and a combination of both according to Neutrophil Gelatinase-associated Lipocalin (NGAL) and serum creatinine. There was a stepwise increase in all outcomes.
Table 3.
The value of NGAL complements prognosis in patients without diagnostic creatinine increase.
| Outcome | NGAL-/sCREA- vs. NGAL+/sCREA- |
|---|---|
| Need for RRT initiation, n | OR 16.4 (95% CI 3.6–76.9), P<0.001 |
| In-hospital mortality, n | OR 2.8 (95% CI 1.9–4.1), P<0.001 |
| Need for RRT initiation / In-hospital mortality, n | OR 3.6 (95% CI 2.5–5.2), P<0.001 |
| ICU stay* | 2.9 days; P=0.026 |
| Hospital stay* | 8.2 days; P=0.16 |
Median difference.
Hospital mortality doubled from biomarker negative patients to NGAL(+)/sCREA(−) status and more than tripled in patients positive for both biomarkers (four-group comparison: P<0.001) (Figure 3). More NGAL(+)/sCREA(−) patients (69/445; 15.5%) developed the composite endpoint of RRT initiation or in-hospital mortality when compared to biomarker negative patients (63/1,296; 4.9%), P<0.001. A greater proportion of NGAL(+)/sCREA(+) patients (84/474; 17.7%) reached the combined endpoint of RRT initiation and in-hospital mortality compared to patients negative for both biomarkers (odds ratio: 4.2 [95% CI 3.0–6.0]; P<0.001).
NGAL(+)/sCREA(−) patients had a >70% longer stay in the intensive care unit compared to patients negative for both biomarkers; (P=0.026) (Figure 4A). There was a gradual increase in median length of stay in the Intensive Care Unit with the shortest duration (days) for NGAL(−)/sCREA(−) patients (4.2 [2.2–6.4]), low intermediate duration for NGAL(−)/sCREA(+) patients (6.5 [3.0–11.7]), high intermediate duration for NGAL(+)/sCREA(−) patients (7.1 [5.4–10.3]) and longest duration for NGAL(+)/sCREA(+) patients (9.0 [8.0–14.0]); P=0.003 (four-group comparison).
Figure 4.
Length of stay (LOS) stratified by Neutrophil Gelatinase-associated Lipocalin (NGAL) and serum creatinine in the Intensive Care Unit (ICU) (A) and in-hospital (B). There was a stepwise increase in median LOS in ICU and in-hospital.
NGAL(+)/sCREA(−) patients spent twice as long in hospital compared to patients negative for both renal biomarkers; (P=0.16) (Figure 4B). The shortest hospital stay was for NGAL(−)/sCREA(−) patients (8.8 days [7.7–19.0]) with low intermediate values for NGAL(+)/sCREA(−) patients (17.0 [8.4–24.2]), high intermediate values for NGAL(−)/sCREA(+) patients (17.8 [5.1–26.4]) and longest stay for NGAL(+)/sCREA(+) patients (21.9 [15.8–29.9]); P=0.040 (four-group comparison).
Table 4 presents outcomes according to urine versus plasma NGAL confirming a similar outcome pattern independent of the biological material used to measure NGAL.
Table 4.
Outcome according to urine NGAL versus plasma NGAL.
| NGAL(−) / sCREA(−) | NGAL(+) / sCREA(−) | NGAL(−) / sCREA(+) | NGAL(+) / sCREA(+) | |
|---|---|---|---|---|
| Urine NGAL (N=1,345) | N=758 (56.4%) | N=307 (22.8%) | N=63 (4.7%) | N=217 (16.1%) |
| Need for RRT initiation, n | 2 (0.003%) | 9 (2.9%) | 2 (3.2%) | 16 (7.4%) |
| In-hospital mortality, n | 35 (4.6%) | 37 (12.1%) | 3 (4.8%) | 30 (13.8%) |
| ICU stay, days | 4.2 (2.1–8.5) | 7.6 (5.7–14.5) | 6.5 (3.0–11.6) | 10.4 (7.7–14.8) |
| Hospital stay, days | 8.8 (7.2–18.8) | 17.0 (8.2–24.6) | 18.3 (5.2–36.0) | 21.8 (11.3–24.1) |
|
| ||||
| Plasma NGAL (N=977) | N=538 (55.1%) | N=138 (14.1%) | N=44 (4.5%) | N=257 (26.3%) |
| Need for RRT initiation, n | 0 (0%) | 2 (1.5%) | 6 (13.6%) | 22 (8.6%) |
| In-hospital mortality, n | 27 (5.0%) | 18 (13.0%) | 6 (13.6%) | 40 (15.6%) |
| ICU stay, days | 4.0 (2.2–5.4) | 6.5 (4.3–9.0) | 5.1 (3.0–12.0) | 8.6 (8.0–11.9) |
| Hospital stay, days | 9.7 (7.3–20.3) | 14.6 (8.1–22.7) | 11.1 (4.5–21.1) | 25.5 (18.0–33.9) |
Linear values denote median (25th to 75th percentiles).
NGAL, Neutrophil Gelatinase-associated Lipocalin. RRT, renal replacement therapy; ICU, Intensive Care Unit.
DISCUSSION
Key study findings
We conducted a multicenter analysis of pooled data to explore the prognostic value of AKI detected by NGAL. We hypothesized that, without diagnostic increase in serum creatinine, NGAL(+) patients might have likely subclinical AKI and carry a worse prognosis than NGAL(−) patients. We found that a positive NGAL finding carried a similar risk of adverse outcome than a positive creatinine finding. We also found that NGAL(+)/sCREA(−) tests identified approximately 40% more AKI cases than sCREA(+) alone and that these patients were at greater risk of longer ICU and hospital stay, RRT and death compared to controls. As expected, NGAL(+)/sCREA(+) patients had the greatest risk of adverse outcomes. A smaller group of patients were NGAL(−)/sCREA(+), implying loss of renal function without evidence of acute tubular injury. These patients’ outcome was intermediate in severity. Finally, NGAL in the urine or plasma showed a similar pattern for the outcomes assessed.
Relation to previous studies
AKI may affect 20% to 30% of hospitalized patients; it carries significant costs and is independently associated with increased morbidity and mortality (31,32). Our study is consistent with other reports on the prognostic importance of serum creatinine increase (31,32). Similarly, NGAL has been repeatedly shown to predict the need for RRT and mortality in AKI (7,12,16). However, previous studies have also shown that NGAL failed to reliably predict changes in serum creatinine, even though its predictive value improved with increasing AKI severity (21).
Until now, the above findings have been interpreted as reflecting NGAL’s shortcomings as a biomarker (20). An alternative explanation, however, is that the limitation lies with serum creatinine as the diagnostic standard and the different nature of the signal provided. Thus, NGAL indicates tubular injury which precedes renal functional loss by several days and serum creatinine indicates subsequent loss of renal excretory function. Our findings suggest that a state of AKI likely exists when NGAL is increased, independent of serum creatinine increases.
Previous work supports the biological plausibility of the above notion and suggests that serum creatinine is a delayed, low-sensitivity and misleading biomarker of AKI (5,7), affected by many confounding factors (22). In this regard, several studies (33,34,35) suggest an analogy between the troponin/creatine kinase and the NGAL/creatinine relationship with a novel more sensitive biomarker identifying previously undetected organ injury. This increased diagnostic sensitivity of troponin is clinically relevant (33) and, in the field of cardiology, has altered the definition, diagnosis, and management of acute myocardial infarction. This concept may similarly apply to NGAL.
Significance of study findings
Potential explanations of study findings are given in Table 5. Our study suggests that acute tubular damage may occur without detectable loss of excretory function (and vice versa) and may predict worse clinical outcome and that NGAL and serum creatinine reflect distinct pathophysiological events. A changed view of AKI may change clinical practice and its treatment. Detection of elevated NGAL may enable more rapid conventional interventions or introduction of novel therapies to prevent or effectively treat such otherwise undetected cardiorenal syndrome type 1 (30). Novel renal biomarkers might facilitate standardization of early diagnosis and treatment. On the other hand, a normal NGAL result may inform clinical decision-making leading to improved use of hospital resources.
Table 5.
Possible combinations of NGAL and serum creatinine status.
| Diagnostic increase of NGAL (subclinical AKI, days before diagnostic serum creatinine increase) | Diagnostic increase of serum creatinine (manifest AKI) | Patient outcomes* | Potential explanations of study findings |
|---|---|---|---|
| Positive | Positive | Worse | Subclinical AKI, manifest AKI |
| Positive | Negative | Worse | Subclinical AKI |
| Negative | Positive | Worse | False negative NGAL / Glomerular impairment without tubular injury (prerenal azotemia) / NGAL negative because measurement was days before serum creatinine increase |
| Negative | Negative | Better | No subclinical AKI, no manifest AKI |
NGAL, Neutrophil Gelatinase-associated Lipocalin; AKI, acute kidney injury as defined by the RIFLE criteria (1) or AKI Network classification (2).
As there is no consent for such outcomes: e.g. need for renal replacement therapy (RRT) initiation; mortality, death during RRT, prolonged length of stay in in the Intensive Care Unit or in the hospital.
For the first time, we identified a substantial group of patients who do not fulfil current creatinine-based consensus criteria for AKI yet are likely to have acute tubular injury. We further demonstrated that these patients have a higher risk of adverse outcomes including death. These patients may benefit from medical attention. Such medical attention may carry a greater likelihood of success because changes in NGAL levels are rapid (hours) and changes in serum creatinine slow (days). By analogy with acute myocardial infarction (34), patients with AKI may now receive early intervention (13,36,37). In addition, our findings suggest that the definition of AKI should be refined by the application of criteria that include NGAL.
Since NGAL positive AKI, with or without the development of sCREA(+), is associated with poor patient outcomes, serum creatinine fails to identify likely AKI in some patients who are at increased risk of death. This observation does not imply that serum creatinine should be discarded as a marker of AKI. In fact, in most study patients, subclinical tubular injury preceded detectable decreases of renal function and when both occurred together clinical outcomes were worse. Thus, knowledge of NGAL levels modifies prognostic assessment not only in patients without an increase in serum creatinine but also in patients with creatinine-based consensus criteria for AKI. In these patients, however, AKI could only be diagnosed late, making any treatment less likely to succeed.
There may be alternative explanations for the NGAL(+)/sCREA(−) syndrome: Some of these patients might develop loss of renal function after the consensus period of seven days and NGAL identified these patients more than one week before such loss of function. A small number of patients might have died soon after NGAL testing, possibly too early to manifest sCREA(+) AKI. Finally, in a minority of patients, NGAL might have simply acted as a marker of inflammation, one of the most important risk factors for AKI.
We also identified a very small subgroup of patients with no biomarker evidence of tubular injury but loss of excretory function (NGAL(−)/sCREA(+), 4.6%). As NGAL, in contrast to serum creatinine, was not consistently serially measured, this observation might represent a false negative finding. However, ‘pre-renal azotemia’ (loss of function without tubular injury) may also explain such findings (38). Therefore, if NGAL was truly negative, these patients may represent a subgroup currently referred to as having pre-renal azotemia (39). In our study, NGAL(−)/sCREA(+) status was more common in elderly and diabetic patients and was still associated with a two-fold increase in risk of developing the composite outcome of death and renal replacement therapy compared to patients negative for both biomarkers (NGAL-/sCREA-). This is consistent with recent reports associating even transient azotemia with a greater risk of death (40). NGAL might now help identify some of these patients.
Although it is known that NGAL concentration is somewhat increased in patients with diabetic nephropathy (41–43), there is no information on acute NGAL responsiveness. On the other hand, it remains unknown whether patients in the NGAL(−)/sCREA(+) subgroup did not have active tubular damage as no biopsies were performed. However, there is evidence from animal experiments that prerenal azotemia does not translate into tubular damage or detection of NGAL in the urine or plasma (44,45).
Finally, NGAL detected approximately 40% of patients with probable AKI who were missed by consensus criteria. This proportion is similar to that identified by troponin in subjects with myocardial injury missed by conventional biomarkers (30).
This study cannot determine the source of NGAL, but the kidney should be the major source of NGAL. If plasma NGAL was produced outside the kidney and then filtered, the proximal renal tubules would completely reabsorb NGAL with no or minimal urine NGAL levels (10). Also, the decrease in glomerular filtration rate seen in AKI would decrease NGAL clearance resulting in decreased urine NGAL and increased plasma NGAL. Therefore, NGAL measured in the urine should either result largely from injured and not reabsorbing proximal tubules or arise from the injured distal nephron. Indeed, experimental evidence supports this view that NGAL in plasma might predominantly arise from the injured thick ascending tubules and the collecting ducts via back-leak from injured renal tissue - again reflecting renal damage. Basing on these results and our study findings with urine and plasma NGAL indicating a very similar patient outcome pattern, NGAL should be considered as a vigorous outcome marker in AKI.
Strengths and limitations of the study
This study has several strengths. It is multicentre and involved more than 2,000 patients at risk of AKI; it used data collected independently in different countries, assessed patients with diverse conditions and used different commercially available assays. However, it is retrospective in design with all the inherent imperfections of such studies and, because no individual patient data were obtained, meaningful meta-regression analysis was not possible. Our results might be affected by selection based on voluntary data contribution. The direction and the magnitude of this potential bias is unknown. Nonetheless, the findings show strength of association, temporality, consistency, biological plausibility and gradient, coherence with previous studies and are analogous to other fields of biomarker investigations. These features make it probable that the findings reflect a biological phenomenon (46). Future prospective studies should confirm histopathological agreement of NGAL with tubular injury, shown in animal experiments, and explore the prognostic value of other structural AKI biomarkers beyond NGAL (47), independent of serum creatinine. More importantly, they should test whether NGAL-based early diagnosis of AKI leads to the more successful and more timely deployment of therapies which, until now, could only be delivered late in the course of AKI (48,49) and improved outcomes.
Conclusions
The study findings show that NGAL complements serum creatinine in AKI diagnosis and prognosis. In a significant proportion of patients at renal risk, acute tubular damage may occur without loss of excretory function. Such NGAL(+) patients are at greater risk of adverse outcomes, including death and renal replacement therapy, both in the presence or absence of an increase in serum creatinine. These patients may be reasonably classified as having AKI, even though they do not fulfil current AKI consensus criteria. Their detection and the size of their cohort justify re-assessment of the concept and definition of AKI in patients with cardiorenal syndrome type 1.
Acknowledgments
The authors thank Dr Peter Schlattmann, Ph.D. (Dept of Biostatistics, University Hospital, Campus Mitte, Berlin, Germany) for the statistical insights he provided.
FINANCIAL SUPPORT MH is a fellow of the Alexander von Humboldt-Foundation, Bonn, Germany. AHF is a grant recipient of the Jackstädt Foundation, Essen, Germany; both non-profit foundations. JLK is supported via K23DK081616.
ABBREVIATIONS
- AKI
acute kidney injury
- NGAL
neutrophil gelatinase-associated lipocalin
- RIFLE
renal risk (R), injury (I), failure (F), loss of renal function (L), end stage renal disease (E) classification
- RRT
renal replacement therapy
- sCREA
serum creatinine
Appendix
| Setting of AKI | _________(1–4) |
| Cardiac surgery (1) | |
| Emergency department (2) | Citation of publication: |
| Contrast-induced nephropathy (3) | |
| General ICU/Critical Care patients (4) | |
|
| |
|
Sample size (Use NGAL concentration with best combination of sensitivity and specificity for prediction of RIFLE AKI: increase in serum creatinine >50% from baseline to peak value which was based on a rolling time window of seven days basing on daily serum creatinine measurement [=class R or worse]) |
NGAL-positive AND no AKI: n=_________ NGAL-negative AND no AKI: n=_________ NGAL-positive AND AKI: n=_________ NGAL-negative AND AKI: n=_________ |
|
| |
| Age (years, SD) | NGAL-positive AND no AKI: yr
NGAL-negative AND no AKI: yr NGAL-positive AND AKI: yr NGAL-negative AND AKI: yr |
|
| |
| Sex | NGAL-positive AND no AKI: Female n= NGAL-negative AND no AKI: Female n= NGAL-positive AND AKI: Female n= NGAL-negative AND AKI: Female n= |
|
| |
| Renal impairment (eGFR <60mL/min/1.73m2 by simplified MDRD formula or Schwartz formula) | NGAL-positive AND no AKI: n=_________ NGAL-negative AND no AKI: n=_________ NGAL-positive AND AKI: n=_________ NGAL-negative AND AKI: n=_________ |
|
| |
| Diabetes mellitus (on medication) | NGAL-positive AND no AKI: n=_________ NGAL-negative AND no AKI: n=_________ NGAL-positive AND AKI: n=_________ NGAL-negative AND AKI: n=_________ |
|
| |
| Chronic heart failure (NYHA III/IV OR LVEF < 40%) | NGAL-positive AND no AKI: n=_________ NGAL-negative AND no AKI: n=_________ NGAL-positive AND AKI: n=_________ NGAL-negative AND AKI: n=_________ |
|
| |
| Peak NGAL concentration (ng/mL, SD) | |
| NGAL-positive AND no AKI: | ng/mL |
| NGAL-negative AND no AKI: | ng/mL |
| NGAL-positive AND AKI: | ng/mL |
| NGAL-negative AND AKI: | ng/mL |
|
| |
| Length of stay in ICU | NGAL-positive AND no AKI: median______ (95% CI______ -______ ), days NGAL-negative AND no AKI: median______ (95% CI______ -______ ), days NGAL-positive AND AKI: median______ (95% CI______ -______ ), days NGAL-negative AND AKI: median______ (95% CI______ -______ ), days |
|
| |
| Length of stay in hospital | NGAL-positive AND no AKI: median (95% CI), days NGAL-negative AND no AKI: median (95% CI), days NGAL-positive AND AKI: median (95% CI), days NGAL-negative AND AKI: median (95% CI), days |
|
| |
| Number of patients with RRT initiation | |
| NGAL-positive AND no AKI: | n=________________ |
| NGAL-negative AND no AKI: | n=________________ |
| NGAL-positive AND AKI: | n=________________ |
| NGAL-negative AND AKI: | n=________________ |
|
| |
| In-hospital mortality | |
| NGAL-positive AND no AKI: | n=________________ |
| NGAL-negative AND no AKI: | n=________________ |
| NGAL-positive AND AKI: | n=________________ |
| NGAL-negative AND AKI: | n=________________ |
|
| |
| RRT initiation and in-hospital mortality | |
| NGAL-positive AND no AKI: | n=________________ |
| NGAL-negative AND no AKI: | n=________________ |
| NGAL-positive AND AKI: | n=________________ |
| NGAL-negative AND AKI: | n=________________ |
Footnotes
TRANSPARENCY DECLARATION
Drs Bellomo and Devarajan have acted as paid consultant to Abbott Diagnostics and Biosite Inc. Drs Ronco and Cruz have received an honorarium for speaking for Biosite Inc. Dr Haase has received an honorarium for speaking for Abbott and Diagnostics Biosite Inc. Both companies are involved in the development of NGAL assays to be applied in clinical practice.
References
- 1.Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: 2nd International Consensus Conference of the ADQI Group. Crit Care. 2004;8:R204–12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uchino S, Bellomo R, Goldsmith D, et al. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med. 2006;34:1913–7. doi: 10.1097/01.CCM.0000224227.70642.4F. [DOI] [PubMed] [Google Scholar]
- 4.Haase M, Bellomo R, Matalanis G, et al. A comparison of the RIFLE and Acute Kidney Injury Network classifications for cardiac surgery-associated acute kidney injury: a prospective cohort study. J Thorac Cardiovasc Surg. 2009;138:1370–6. doi: 10.1016/j.jtcvs.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–8. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 6.Cruz DN, de Cal M, Garzotto F, et al. Plasma neutrophil gelatinase-associated lipocalin is an early biomarker for acute kidney injury in an adult ICU population. Intensive Care Med. 2010;36:444–51. doi: 10.1007/s00134-009-1711-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haase-Fielitz A, Bellomo R, Devarajan P, et al. Novel and conventional serum biomarkers predicting acute kidney injury in adult cardiac surgery—a prospective cohort study. Crit Care Med. 2009;37:553–60. doi: 10.1097/CCM.0b013e318195846e. [DOI] [PubMed] [Google Scholar]
- 8.Supavekin S, Zhang W, Kucherlapati R, et al. Differential gene expression following early renal ischemia/reperfusion. Kidney Int. 2003;63:1714–24. doi: 10.1046/j.1523-1755.2003.00928.x. [DOI] [PubMed] [Google Scholar]
- 9.Mishra J, Ma Q, Prada A, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–43. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 10.Mori K, Lee HT, Rapoport D, et al. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest. 2005;115:610–21. doi: 10.1172/JCI23056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dent CL, Ma Q, Dastrala S, et al. Plasma neutrophil gelatinase-associated lipocalin predicts acute kidney injury, morbidity and mortality after pediatric cardiac surgery: a prospective uncontrolled cohort study. Crit Care. 2007;11:R127. doi: 10.1186/cc6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bennett M, Dent CL, Ma Q, et al. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol. 2008;3:665–73. doi: 10.2215/CJN.04010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haase M, Haase-Fielitz A, Bellomo R, et al. Sodium Bicarbonate to Prevent Acute Kidney Injury after Cardiopulmonary Bypass - A Multiple-blind, Randomized Controlled Trial. Crit Care Med. 2009;37:39–47. doi: 10.1097/CCM.0b013e318193216f. [DOI] [PubMed] [Google Scholar]
- 14.Mishra J, Mori K, Ma Q, et al. Amelioration of Ischemic Acute Renal Injury by Neutrophil Gelatinase–Associated Lipocalin. J Am Soc Nephrol. 2004;15:3073–82. doi: 10.1097/01.ASN.0000145013.44578.45. [DOI] [PubMed] [Google Scholar]
- 15.Haase M, Bellomo R, Haase-Fielitz A. Novel biomarkers, oxidative stress, and the role of labile iron toxicity in cardiopulmonary bypass-associated acute kidney injury. J Am Coll Cardiol. 2010;55:2024–33. doi: 10.1016/j.jacc.2009.12.046. [DOI] [PubMed] [Google Scholar]
- 16.Shapiro NI, Trzeciak S, Hollander JE, et al. The Diagnostic Accuracy of Plasma Neutrophil Gelatinase-Associated Lipocalin in the Prediction of Acute Kidney Injury in Emergency Department Patients With Suspected Sepsis. Ann Emerg Med. 2010 Apr 3; doi: 10.1016/j.annemergmed.2010.02.010. Epub. [DOI] [PubMed] [Google Scholar]
- 17.Hall IE, Yarlagadda SG, Coca SG, et al. IL-18 and urinary NGAL predict dialysis and graft recovery after kidney transplantation. J Am Soc Nephrol. 2010;21:189–97. doi: 10.1681/ASN.2009030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haase M, Bellomo R, Devarajan P, et al. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;54:1012–24. doi: 10.1053/j.ajkd.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 19.Wagener G, Gubitosa G, Wang S, Borregaard N, Kim M, Lee HT. Urinary neutrophil gelatinase-associated lipocalin and acute kidney injury after cardiac surgery [erratum in Am J Kidney Dis 2008;52:810] Am J Kidney Dis. 2008;52:425–33. doi: 10.1053/j.ajkd.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 20.Siew ED, Ware LB, Gebretsadik T, et al. Urine neutrophil gelatinase-associated lipocalin moderately predicts acute kidney injury in critically ill adults. J Am Soc Nephrol. 2009;20:1823–32. doi: 10.1681/ASN.2008070673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haase-Fielitz A, Bellomo R, Devarajan P, et al. The predictive performance of plasma neutrophil gelatinase-associated lipocalin (NGAL) increases with grade of acute kidney injury. Nephrol Dial Transplant. 2009;24:3349–54. doi: 10.1093/ndt/gfp234. [DOI] [PubMed] [Google Scholar]
- 22.Herget-Rosenthal S, Marggraf G, Huesing J, et al. Early detection of acute renal failure by serum cystatin C. Kidney Int. 2004;66:1115–22. doi: 10.1111/j.1523-1755.2004.00861.x. [DOI] [PubMed] [Google Scholar]
- 23.Joannidis M, Metnitz B, Bauer P, et al. Acute kidney injury in critically ill patients classified by AKIN versus RIFLE using the SAPS 3 database. Intensive Care Med. 2009;35:1692–702. doi: 10.1007/s00134-009-1530-4. [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Coresh J, Greene T, et al. Chronic Kidney Disease Epidemiology Collaboration. Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53:766–72. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz GJ, Muñoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–37. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koyner JL, Bennett MR, Worcester EM, et al. Urinary cystatin C as an early biomarker of acute kidney injury following adult cardiothoracic surgery. Kidney Int. 2008;74:1059–69. doi: 10.1038/ki.2008.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zappitelli M, Washburn KK, Arikan AA, et al. Urine neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in critically ill children: a prospective cohort study. Crit Care. 2007;11:R84. doi: 10.1186/cc6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mårtensson J, Bell M, Oldner A, Xu S, Venge P, Martling CR. Neutrophil gelatinase-associated lipocalin in adult septic patients with and without acute kidney injury. Intensive Care Med. 2010;36:1333–40. doi: 10.1007/s00134-010-1887-4. [DOI] [PubMed] [Google Scholar]
- 29.Krawczeski C, Woo J, Wang Y, et al. Neutrophil gelatinase-associated lipocalin (NGAL) in acute kidney injury after pediatric cardiac surgery. in revision 08/2010. [Google Scholar]
- 30.Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52:1527–39. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 31.Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–70. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 32.Lassnigg A, Schmid ER, Hiesmayr M, et al. Impact of minimal increases in serum creatinine on outcome in patients after cardiothoracic surgery: do we have to revise current definitions of acute renal failure? Crit Care Med. 2008;36:1129–37. doi: 10.1097/CCM.0b013e318169181a. [DOI] [PubMed] [Google Scholar]
- 33.White HD. Evolution of the definition of myocardial infarction: what are the implications of a new universal definition? Heart. 2008;94:679–84. doi: 10.1136/hrt.2007.130955. [DOI] [PubMed] [Google Scholar]
- 34.Roger VL, Killian JM, Weston SA, et al. Redefinition of myocardial infarction: prospective evaluation in the community. Circulation. 2006;114:790–7. doi: 10.1161/CIRCULATIONAHA.106.627505. [DOI] [PubMed] [Google Scholar]
- 35.Cantor WJ, Fitchett D, Borgundvaag B, et al. Routine early angioplasty after fibrinolysis for acute myocardial infarction. N Engl J Med. 2009;360:2705–18. doi: 10.1056/NEJMoa0808276. [DOI] [PubMed] [Google Scholar]
- 36.Sezai A, Hata M, Niino T, et al. Influence of continuous infusion of low-dose human atrial natriuretic peptide on renal function during cardiac surgery: a randomized controlled study. J Am Coll Cardiol. 2009;54:1058–64. doi: 10.1016/j.jacc.2009.05.047. [DOI] [PubMed] [Google Scholar]
- 37.Shiao CC, Wu VC, Li WY, et al. Late initiation of renal replacement therapy is associated with worse outcomes in acute kidney injury after major abdominal surgery. Crit Care. 2009;13:R171. doi: 10.1186/cc8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waikar SS, Betensky RA, Bonventre JV. Creatinine as the gold standard for kidney injury biomarker studies? Nephrol Dial Transplant. 2009;24:3263–5. doi: 10.1093/ndt/gfp428. [DOI] [PubMed] [Google Scholar]
- 39.Nickolas TL, O’Rourke MJ, Yang J, et al. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med. 2008;148:810–9. doi: 10.7326/0003-4819-148-11-200806030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uchino S, Bellomo R, Bagshaw SM, et al. Transient azotemia is associated with a high risk of death in hospitalised patients. Nephrol Dial Transplant. 2010;25:1833–9. doi: 10.1093/ndt/gfp624. [DOI] [PubMed] [Google Scholar]
- 41.Malyszko J, Bachorzewska-Gajewska H, Poniatowski B, Malyszko JS, Dobrzycki S. Urinary and serum biomarkers after cardiac catheterization in diabetic patients with stable angina and without severe chronic kidney disease. Ren Fail. 2009;31:910–9. doi: 10.3109/08860220903216113. [DOI] [PubMed] [Google Scholar]
- 42.Yang YH, He XJ, Chen SR, Wang L, Li EM, Xu LY. Changes of serum and urine neutrophil gelatinase-associated lipocalin in type-2 diabetic patients with nephropathy: one year observational follow-up study. Endocrine. 2009;36:45–51. doi: 10.1007/s12020-009-9187-x. [DOI] [PubMed] [Google Scholar]
- 43.Nielsen SE, Schjoedt KJ, Astrup AS, et al. Neutrophil Gelatinase-Associated Lipocalin (NGAL) and Kidney Injury Molecule 1 (KIM1) in patients with diabetic nephropathy: a cross-sectional study and the effects of lisinopril. Diabet Med. 2010;27:1144–50. doi: 10.1111/j.1464-5491.2010.03083.x. [DOI] [PubMed] [Google Scholar]
- 44.Kuwabara T, Mori K, Mukoyama M, et al. Urinary neutrophil gelatinase-associated lipocalin levels reflect damage to glomeruli, proximal tubules, and distal nephrons. Kidney Int. 2009;75:285–94. doi: 10.1038/ki.2008.499. [DOI] [PubMed] [Google Scholar]
- 45.Paragas N, Qiu A, Zhang Q, et al. Seeing Renal Stress in Real Time: The NGAL Reporter Mouse Detects the Response of Distal Tubular Segments In Vivo. Nat Med. 2010 in press. [Google Scholar]
- 46.Bradford-Hill AB. The environment and disease: association or causation. Proc R Soc Med. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walshe CM, Odeljay F, Ng S, Marsh B. Urinary glutathione-S-transferase as an early marker for rneal dysfunction in patients admitted to intensive care with sepsis. Crit Care Resusc. 2009;11:204–9. [PubMed] [Google Scholar]
- 48.Bagshaw SM, Delaney A, Haase M, Ghali WA, Bellomo R. Loop diuretics in the management of acute renal failure : a systematic review and meta-analysis. Crit Care Resusc. 2007;9:60–8. [PubMed] [Google Scholar]
- 49.Duke GJ. Renal protective agents: a review. Crit Care Resusc. 1999;1:265–75. [PubMed] [Google Scholar]