Abstract
Background
Ibrutinib is active in patients with relapsed/refractory (R/R) CLL. In patients treated with ibrutinib for R/R CLL, del(17p) identified by interphase fluorescence in situ hybridization (FISH) is associated with inferior progression-free survival, despite equivalent initial response rates. Del(17p) is frequently associated with complex metaphase karyotype (CKT); the prognostic significance of CKT in ibrutinib-treated patients has not been reported.
Methods
We reviewed 88 patients treated for R/R CLL at MD Anderson Cancer Center with investigational ibrutinib-based regimens from 2010–2013. Pre-treatment FISH and Lipopolysaccharide-stimulated metaphase cytogenetic analysis were performed on bone marrow.
Results
Adequate pre-treatment metaphase karyotype was available for 56/88 patients. Karyotype was complex in 21 of 56 cases; 17 of the 21 had del(17p) by FISH. Overall response rate, including partial remission with persistent lymphocytosis, was 94% with 17% complete responses. In multivariable analysis (MVA), only CKT was significantly associated with event-free survival (EFS) [HR 6.6 (1.7–25.6), p=0.006]. Fludarabine-refractory CLL [HR 6.9 (1.8–27.1), p=0.005] and CKT [HR 5.9 (1.6–22.2), p=0.008] were independently associated with inferior overall survival (OS) in MVA. Del(17p) by FISH was not significantly associated with EFS or OS in MVA.
Conclusions
CKT is a powerful predictor of outcome in ibrutinib-treated patients with R/R CLL and may be a stronger predictor of biological behavior than del(17p) by FISH. Given their relatively poor outcomes, patients with CKT are ideal candidates for studies of consolidative treatment strategies or novel treatment combinations.
Keywords: CLL, complex karyotype, del(17p), ibrutinib, relapsed and refractory
Introduction
Patients with CLL with del(17p) by FISH have poor outcomes when treated with chemotherapy or chemoimmunotherapy; median survival is approximately 2 years.1 Long-term survival can be achieved with allogeneic stem cell transplantation (alloSCT) in some patients; patients with del(17p) have outcomes similar to those without del(17p) post-alloSCT,2, 3 with approximately 50% 5-year survival.
The Bruton Tyrosine Kinase (BTK)-inhibitor ibrutinib4 is active in relapsed/refractory (R/R) CLL with a progression-free survival (PFS) of approximately 75% at 26 months.4 Patients with del(17p) have inferior PFS to those without,4 with a median PFS of approximately 28 months.5 Ibrutinib resistance has been associated in some cases with development of specific mutations in the B-cell receptor signaling pathway, most commonly the C481S mutation in BTK, which prevents irreversible binding of ibrutinib to BTK. Patients with del(17p) and/or complex metaphase karyotype (CKT) may be more likely to develop these mutations.6 The optimal treatment for patients refractory to ibrutinib is not established; response to subsequent salvage treatment is poor and survival short.7 The availability of BCR-inhibitors has made the decision regarding whether to proceed with alloSCT in high-risk patients more complex; there is no consensus regarding which patients with del(17p) should proceed to alloSCT and the optimal timing of alloSCT.
Del(17p) CLL is frequently associated with CKT, defined as ≥3 distinct chromosomal abnormalities present in more than one metaphase.8 CKT has been associated with inferior outcomes in patients with both treatment-naive (TN)9, 10 and R/R CLL undergoing therapy,11 including alloSCT;12 the prognostic significance of CKT in ibrutinib-treated patients is unknown. Obtaining metaphases for cytogenetic analysis has traditionally been challenging in CLL, due to the low ex vivo mitotic rate of CLL cells. Frequently, no abnormalities are detected due to only normal hematopoietic cells dividing ex vivo or only poor quality metaphases are obtained.10, 13 Array CGH or FISH do not require cell division14 and may detect abnormalities when no abnormal metaphases are obtained by conventional techniques. However, they provide limited information; array CGH detects genomic imbalances only and provides no information about balanced translocations or the mechanism of gain or loss; FISH only provides information about chromosomes specifically targeted by the probes used.8 Mitogens such as combined immunostimulatory CpG-oligonucleotides and IL-2 can be used to stimulate ex vivo growth of CLL cells; this substantially increases the yield of analyzeable metaphases and increases the proportion of cases in which cytogenetic abnormities are detected by conventional cytogenetic analysis to >80%.15 Over 20% of patients analyzed in this way will have a CKT, compared to <1% detected by FISH alone.15
We analyzed long-term follow-up data in 88 patients with R/R CLL treated with ibrutinib-based regimens to determine whether subgroups of patients with del(17p) with differing outcomes could be identified and to assess possible novel predictors of outcome, with a particular focus on the prognostic significance of CKT.
Patients and Methods
We reviewed 88 patients treated for R/R CLL at MDACC with investigational ibrutinib-based regimens from 2010–2013. We were interested in patients with long-term follow-up; therefore, only patients who had commenced treatment prior to June 2013 were included. Thirty-eight patients received ibrutinib monotherapy at either 420mg or 840mg/day; 36 received ibrutinib plus rituximab and 14 received ibrutinib plus bendamustine and rituximab (BR). Details of the combination regimens were presented previously.16, 17 All patients provided informed consent and all studies were IRB-approved and conducted according to the declaration of Helsinki. Pre-treatment FISH and lipopolysaccharide-stimulated metaphase cytogenetic analysis18 were performed on bone marrow, although the latter was not required per protocol. Material was considered adequate for karyotypic analysis if ≥10 metaphases were available in the presence of clonal abnormalities or ≥15 in the absence of clonal abnormalities.10 Clonal cytogenetic abnormalities were considered to be present when at least two or more metaphases showed identical chromosome gain or structural abnormalities or at least three metaphases showed identical chromosome loss.19
Statistical analyses were performed using SPSS version 22 (IBM Corp, Armonk, New York) and GraphPad Prism 6 (La Jolla, California). Descriptive statistics were used to summarize patient characteristics. Dichotomous variables were compared using Chi-square or Fisher’s exact test. Univariable survival analyses was performed using the Kaplan and Meier method20 and differences between groups assessed using the log-rank test.21 Multivariable analysis (MVA) for dichotomous variables was performed using logistic regression. Multivariable survival analysis was performed using the Cox proportional hazards model.22 Variables with p values <0.1 in univariable analyses (UVA) were included in the multivariable models and only cases with complete data for variables of interest were included in the multivariable models. Event-free survival (EFS) was defined as time from initiation of study drug to permanent cessation of study drug for any reason. Overall survival (OS) was defined as time from initiation of study drug to death. Patients were censored at their last follow up for EFS and survival outcomes. Final censoring date was 7/14/2014. EFS was chosen as an outcome measure rather than PFS in order to capture potentially significant events which may be associated with the presence of CKT, including the development of second cancers requiring systemic therapy and the decision to proceed to allogeneic stem cell transplantation. Seven patients who underwent allogeneic stem cell transplantation in PR or CR were censored for EFS analysis at the time of transplant, but continued to be followed for survival.
Results
Baseline patient characteristics are shown in Table 1.
Table 1.
Baseline patient characteristics
| Characteristic (N=88 unless stated) | |
|---|---|
| Age, median (range) | 66 (35–83) |
| # prior therapies, median (range) | 2 (1–12) |
| Male gender, n (%) | 67 (76) |
| Rai stage, n(%) | |
| 0–II | 36 (41) |
| III–IV | 52 (59) |
| Bulky adenopathy ≥5cm | 45 (51) |
| FISH hierarchy, n (%) (n=86) | |
| Del(13q) | 13 (15) |
| No abnormalities | 10 (12) |
| Trisomy 12 | 1 (1) |
| Del(11q) | 28 (33) |
| Del(17p) | 34 (40) |
| Complex metaphase karyotype, n (%) (n=56) | 21 (38) |
| IGHV mutation status, n (%) | |
| Unmutated | 72 (82) |
| Mutated | 7 (8) |
| No PCR product obtained | 9 (10) |
| Fludarabine-refractory, n (%) | 17 (19) |
| β2-microglobulin (mg/dL), n (%) (n=75) | |
| ≥4.0 | 43 (57) |
| <4.0 | 32 (43) |
| Treatment regimen, n (%) | |
| Ibrutinib monotherapy | 38 (43) |
| Ibrutinib + rituximab | 36 (41) |
| Ibrutinib + bendamustine and rituximab | 14 (16) |
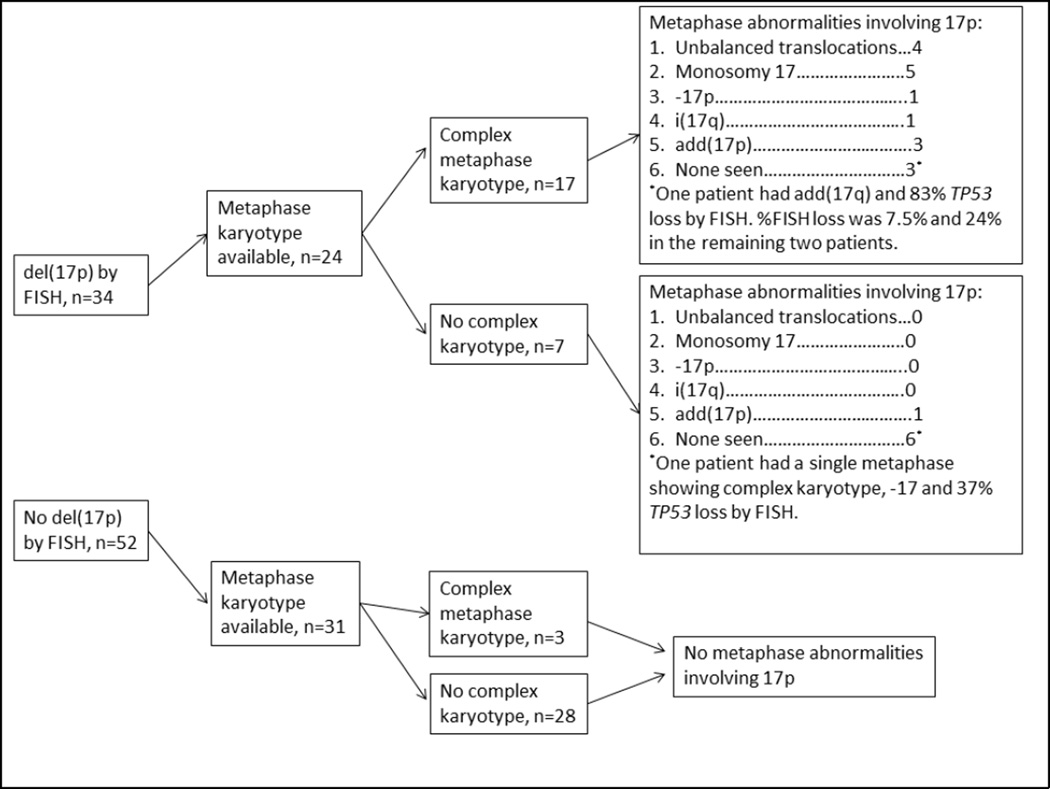
Relationship between metaphase karyotype analysis and del(17p) by FISH
Metaphase karyotype analysis was attempted in 63 patients and metaphases were adequate for analysis in 56. The 7 cases where metaphase analysis was attempted but inadequate data was available had poor quality or <10 metaphases generated. The relationship between FISH and metaphase karyotype results is shown in Figure 1: Among 21 patients with CKT, 17 had del(17p), [OR 22.7 (5.2–99.6) compared to non-del(17p) patients, p<0.001]; 3 had del(11q); 1 did not have FISH results available. There was no difference in percentage of cells with TP53 deletion by FISH, according to presence or absence of CKT [median 65 (7.5–86.0%) vs 37 (12.0–96.5%), p=0.32). The 7 patients with TP53 deletion by FISH but without CKT predominantly had a diploid karyotype (n=6), while one had add(17p).
Figure 1.
Associations between del(17p) by FISH and metaphase karyotype results.
There were 9 patients who had del(17p) detected by FISH but no abnormalities involving chromosome 17p noted by concurrent metaphase cytogenetic evaluation. Median %TP53 deletion by FISH in these patients was 30%, range (7.5–82). Six of these 9 patients had diploid cytogenetics or non-complex clonal abnormalities on metaphase cytogenetic analysis; two of these 6 cases had very low %TP53 deletion by FISH (7.5 and 12%) while the remaining 4 cases had ≥24% TP53 deletion by FISH; one of these patients had a single metaphase with complex cytogenetic abnormalities including monosomy 17, thus not meeting criteria for clonality, but had 37% TP53 deletion by FISH. Three patients had complex karyotype and del(17p) detected by FISH but no abnormalities involving 17p on conventional cytogenetics; two had low percentageTP53 deletion by FISH of 7.5% and 24%, while one had 83% TP53 deletion by FISH.
Response to treatment
The overall response rate (ORR) for the entire population was 94.3%; 17% achieved complete remission (CR). The ORR was not different according to baseline characteristics (Table 2). The CR rate was 50% among patients who received ibrutinib plus BR compared to 10.7% in patients who received ibrutinib monotherapy or ibrutinib plus rituximab [OR 40.1 (3.0–538.5), p=0.005].
Table 2.
Overall response and complete remission rates according to baseline characteristics.
| Characteristic | Overall response (%) |
p value | Complete remission (%) |
p value |
|---|---|---|---|---|
| Total cohort, N=88 | 83 (94.3) | 16 (18) | ||
| Age | ||||
| ≤65 yrs, n=46 | 45 (97.8) | 6 (13.0) | ||
| >65 yrs, n=42 | 38 (90.5) | 0.137 | 9 (21.4) | 0.296 |
| Gender | ||||
| Male, n=67 | 63 (94.0) | 15 (22.4) | ||
| Female, n=21 | 20 (95.2) | 0.835 | 0 (0) | 0.017 |
| Treatment regimen | ||||
| Ibrutinib monotherapy, n=38 | 35 (92.1) | 5 (13.2) | ||
| Ibrutinib + rituximab, n=36 | 34 (94.4) | 3 (8.3) | ||
| Ibrutinib +BR, n=14 | 14 (100) | 0.551 | 7 (50.0) | 0.001 |
| FISH Hierarchy | ||||
| Other, n=24 | 24 (100) | 8 (33.3) | ||
| Del(11q), n=28 | 27 (96.4) | 2 (7.1) | ||
| Del(17p), n=34 | 32 (94.1) | 0.904 | 5 (14.7) | 0.040 |
| IGHV Mutation Status | ||||
| Mutated, n=9 | 7 (100) | 4 (57.1) | ||
| Unmutated, n=72 | 67 (93.1) | 0.471 | 9 (12.5) | 0.012 |
| Complex karyotype | ||||
| Yes, n=21 | 19 (90.5) | 2 (9.5) | ||
| No, n=35 | 34 (97.1) | 0.283 | 6 (17.1) | 0.430 |
| Number of prior therapies | ||||
| <2, n=48 | 45 (93.8) | 10 (20.8) | ||
| ≥2, n=40 | 38 (95.0) | 0.801 | 5 (12.5) | 0.301 |
| Fludarabine-refractory | ||||
| Yes, n=17 | 15 (88.2) | 1 (5.9) | ||
| No, n=71 | 68 (95.8) | 0.228 | 14 (19.7) | 0.173 |
| B2M. | ||||
| ≥4.0mg/dL, n=43 | 43 (93.0) | 4 (9.3) | ||
| <4.0mg/dL, n=32 | 32 (100) | 0.127 | 8 (25.0) | 0.067 |
| Rai stage | ||||
| 0–II, n=36 | 35 (97.2) | 9 (25.0) | ||
| III–IV, n=52 | 48 (92.3) | 0.328 | 6 (11.5) | 0.099 |
| Bulky adenopathy (≥5cm) | ||||
| Yes, n=45 | 41 (91.1) | 5 (11.1) | ||
| No, n=43 | 42 (97.7) | 0.184 | 10 (23.3) | 0.130 |
In MVA, receiving a bendamustine-containing regimen was strongly associated with odds of achieving CR [OR 85.2 (3.2–2247.7), p=0.008]. Patients with unmutated IGHV gene [OR 0.001 (0.000–0.273), p=0.014] had lower odds of achieving CR, while patients with a baseline B2M ≥4.0 [OR 0.05 (0.002–1.13, p=0.051) showed a trend toward lower odds of achieving CR. There was no association between high-risk FISH, gender or advanced Rai stage and odds of achieving CR in MVA.
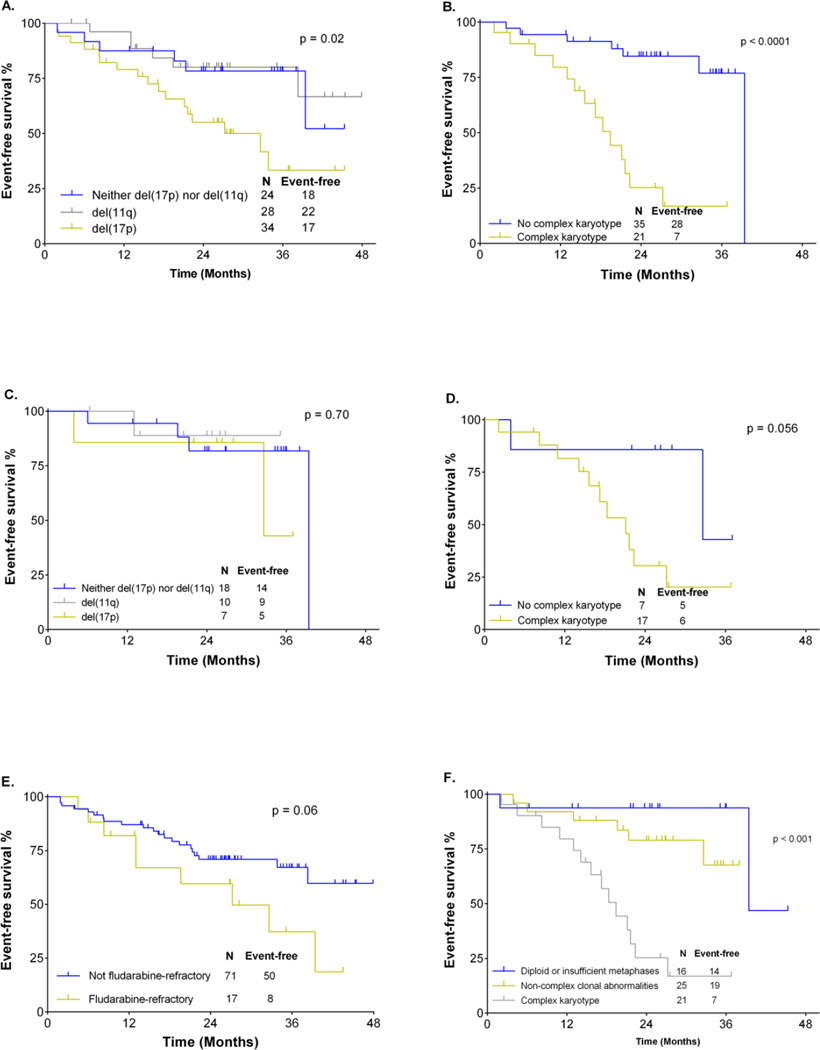
Event-free survival
Median follow-up for surviving patients was 28 months (range 14–48). In univariable analyses (UVA), the following were significantly associated with shorter event-free survival (EFS): female gender (median EFS 22mo vs NR, p=0.001), advanced Rai stage disease (median EFS for Rai III-IV 38.3mo vs NR for Rai 0-II, p=0.047), del(17p) (32mo vs. NR, p=0.021) and CKT (19 vs. 39mo, p<0.001), (Figure 2). There was a strong trend toward inferior EFS for patients with fludarabine-refractory disease (median EFS 27mo vs. not reached (NR), p=0.059). There was no association between EFS and percentage of cells with del(17p) ≥50% by FISH, age >65, mutation status, bulky lymphadenopathy ≥5cm, baseline β2-microglobulin ≥4.0mg/L or having received >2 prior therapies. In MVA, only CKT was significantly associated with EFS [HR 6.6 (1.7–25.6), p=0.006]. There was no association between del(17p) by FISH and EFS on MVA (p=0.995). When only patients with del(17p) were analyzed, there was a strong trend (p=0.056) toward an inferior EFS for those with complex karyotype, relative to those without (figure 2). We separately analyzed all patients where karyotyping was attempted and divided them into three groups: those with diploid karyotype or insufficient metaphases for analysis; those with complex karyotype; and those with non-complex clonal abnormalities; those with complex karyotype had an inferior EFS (p<0.0001). There was no significant difference in EFS in patients with diploid karyotype/insufficient metaphases compared to those with non-complex clonal abnormalities. (Figure 2).
Figure 2.
EFS analysis. A: EFS according to FISH hierarchy in all patients. B: EFS according to presence or absence of complex metaphase karyotype. C: EFS in patients without complex karyotype according to FISH. D: EFS in patients with del(17p) according to the co-existence or absence of complex karyotype. E: EFS in all patients according to the presence or absence of fludarabine-refractory disease. F: EFS according to karyotype group in all patients where karyotype was attempted.
Events during treatment and timing of events
Events and timing of events leading to permanent treatment cessation are shown in Table 3. Treatment was very well-tolerated, with permanent treatment cessation due to toxicity occurring in only 3 patients; all events occurred prior to 12 months of therapy (due to persistent diarrhea in one, gastrointestinal bleeding in the setting of acquired von Willebrand’s disease in one and recurrent infections in one). Nine patients developed progressive CLL and 5 developed Richter Transformation. Of note, all cases of progressive CLL developed beyond 6 months of therapy and 7 of 9 beyond 12 months of therapy. The 5 cases of Richter Transformation occurred at 2mo, 4.5mo 13mo, 21mo and 21.5mo post-initiation of therapy. The patient who transformed at 4.5 months had complex chromosomal abnormalities on metaphase analysis but <10 metaphases available for analysis and was therefore included in the unknown karyotype group. Of the 35 patients without CKT, only one developed disease progression and one developed Richter transformation, while none required therapy for other cancers; none of the 7 patients with del(17p) who did not also have CKT developed disease progression or other cancers, with the only two events in these 7 patients being a patient ceasing treatment after 4 months due to GI bleeding and a second patient dying in complete remission from her CLL at 32 months due to staphylococcal pneumonia. In contrast, four cases of CLL progression, three of Richter Transformation and three of other cancers requiring therapy were seen in the 21 patients with CKT. Seven patients underwent planned allogeneic stem cell transplantation while in remission and were censored for EFS analysis on the date ibrutinib was last taken.
Table 3.
Events and timing of events during study treatment.
| Event | Complex karyotype (n=21) |
No complex karyotype (n=35) |
Karyotype unknown (n=32) |
Total | <12mo of therapy |
≥12mo of therapy |
|---|---|---|---|---|---|---|
| Death in remission | 4 | 3 | 1 | 8 | 4 | 4 |
| Progressive disease | 4 | 1 | 4 | 9 | 2 | 7 |
| Richter transformation | 3 | 1 | 1 | 5 | 2 | 3 |
| Toxicity | 0 | 1 | 2 | 3 | 3 | 2 |
| Other cancer requiring therapy* | 3 | 0 | 0 | 3 | 0 | 3 |
| Allogeneic stem cell transplant† | 3 | 2 | 2 | 7 | 5 | 2 |
| Total events | 17 | 8 | 10 | 35 | 16 | 19 |
Ovarian cancer in 2 patients and acute myeloid leukemia in 1.
Patients undergoing planned allogeneic stem cell transplant were censored for EFS analysis at the last ibrutinib dose.
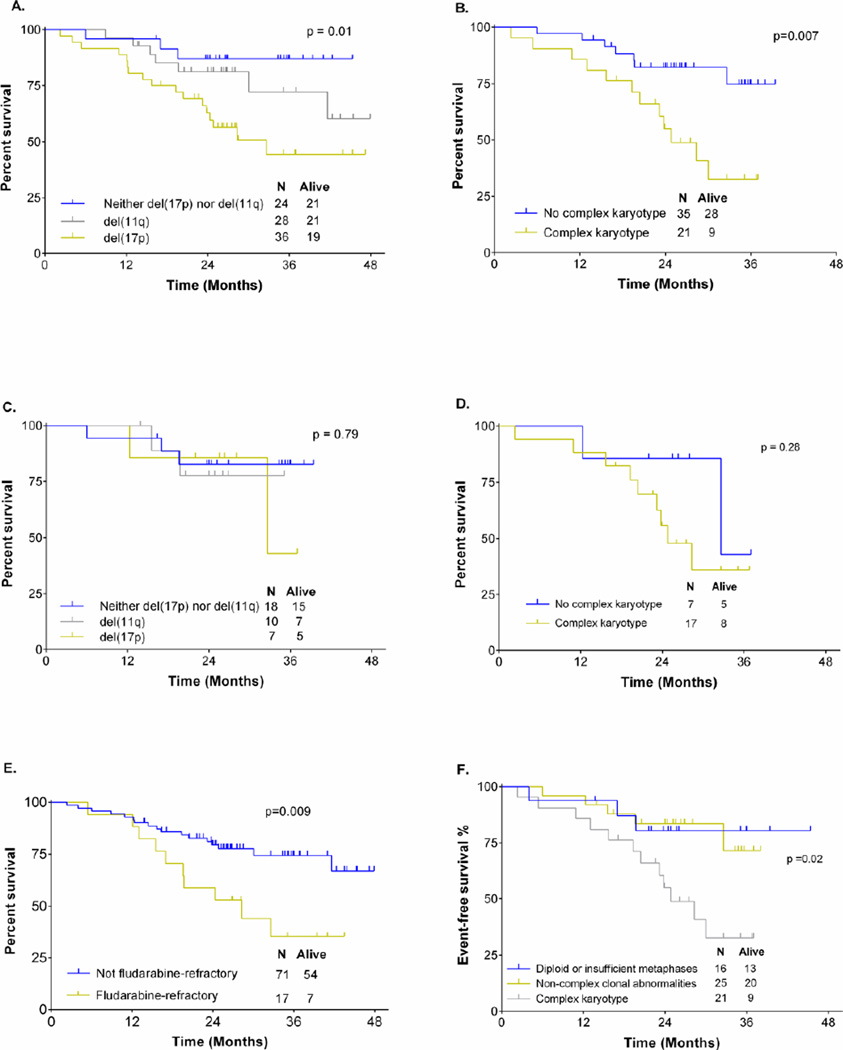
Overall survival
The following were significantly associated with shorter overall survival (OS) in UVA: fludarabine-refractory CLL (28mo vs. NR, p=0.009), del(17p) (33mo vs. NR, p=0.012), and CKT (25mo vs. NR, p=0.007).(Figure 3). There was a trend for shorter survival in patients with baseline B2M ≥4.0 (42mo vs. NR, p=0.078). There was no association between overall survival and percentage of cells with del(17p) by FISH, age >65 years, gender, advanced Rai stage, bulky lymphadenopathy ≥5cm, IGHV mutation status or >2 prior therapies. In MVA, fludarabine-refractory CLL [HR 6.9 (1.8–27.1), p=0.005] and CKT [HR 5.9 (1.6–22.2), p=0.008] were significantly and independently associated with shorter survival. There was no association between del(17p) and OS (p=0.885) in MVA. A separate analysis showed those with complex karyotype had an inferior survival (p=0.02) to those with non-complex clonal abnormalities or either diploid karyotype or insufficient metaphases for analysis. There was no significant difference in survival in patients with diploid karyotype or insufficient metaphases for analysis compared to those with non-complex clonal abnormalities. (Figure 3).
Figure 3.
Overall survival analysis. A: OS according to FISH in all patients. B: OS according to presence or absence of complex metaphase karyotype. C: OS according to FISH hierarchy, in patients without complex metaphase karyotype. D: OS in patients with del(17p) according to the co-existence or absence of complex karyotype. E: OS according to the presence or absence of fludarabine-refractory disease. F: OS according to karyotype group in all patients where karyotype was attempted.
Discussion
Ibrutinib represents a major advance in the treatment of patients with CLL, particularly those with high-risk R/R CLL. Patients with del(17p) have inferior PFS compared to patients without del(17p),4 but other baseline factors predictive for outcome have not been established. This retrospective analysis demonstrated that the presence of CKT at study entry was associated with inferior EFS and OS in R/R patients with CLL treated with ibrutinib-based regimens; the outcomes of patients with CKT are poor, with a median survival of only 25 months. In contrast, there was no association between del(17p) and EFS or OS on multivariable analysis. The association between del(17p) and inferior progression-free survival in patients treated with ibrutinib may therefore in large part be due to the co-existence of CKT, rather than due to the presence of del(17p) per se. Patients without CKT appeared to have excellent disease-specific outcomes with ibrutinib-based therapy, with a very low rate of CLL progression or Richter Transformation, including the subgroup of patients with del(17p); patients with del(17p) but without CKT had a relatively good outcome and appeared to have a low risk of disease progression, although small numbers limit conclusions in this sub-population. In addition, we identified fludarabine-refractory disease as associated with inferior overall survival in MVA.
Technical challenges associated with metaphase cytogenetic analysis have limited its widespread utilization in CLL. During the period of enrollment on the studies we report here, we used LPS-stimulation for culture of bone marrow specimens, which reportedly achieves rates of analyzable metaphases of approximately 50%.10, 23 The rate of successful metaphase generation was higher in our patient population (56/63 patients); this higher rate of metaphase generation may in part be explained by the relapsed/refractory population, who may have more proliferative CLL clones than in untreated populations. There were discrepancies between FISH and metaphase karyotyping in several cases. Notably, 9 of 24 patients with del(17p) detected by FISH had no clonal abnormalities involving 17p detected by metaphase karyotype. In 2 of 9 cases, the percentage of TP53 deletion by FISH was low, likely representing a sub-clonal population; metaphase analysis may, by chance, not have detected the 17p deletion due to its low frequency. In the remaining cases, which had ≥24% TP53 deletion by FISH, mitoses may have been obtained from non-neoplastic normal cells rather than the CLL population. These discrepant results illustrate the technical difficulties involved in conventional cytogenetic analysis in CLL and despite the important information provided by conventional karyotyping, FISH therefore remains an essential component of pre-treatment evaluation. It is possible that, due to the relative insensitivity of our karyotyping technique, successful metaphase generation is more likely in cases with aggressive CLL clones. To determine whether the ability to generate sufficient CLL metaphases for analysis was strongly associated with outcome, we divided all patients where karyotyping was attempted into three groups: those with CKT, those with non-complex clonal abnormalities and those with either diploid karyotype or insufficient clonal metaphases for analysis. Importantly, there was no difference in EFS or survival for patients with non-complex clonal abnormalities and those with either diploid karyotype or insufficient clonal abnormalties (a significant number of which likely represented technical failure to generate CLL metaphases for analysis). Recent technical advances, such as the use of CpG oligonucleotides and IL-2, pokeweed mitogen and phorbol myristate acetate (PMA), allow generation of sufficient metaphases for analysis in at least 80% of patients and may limit the number of patients misclassified as a result of technical failure.8, 23 Our laboratory recently started using this combination for conventional karyotyping in CLL. We plan to repeat this analysis using this newer karyotyping technique in a currently enrolling randomized study of 200 patients receiving ibrutinib +/− rituximab.
The precise mechanisms by which del(17p) and CKT contribute to the development of ibrutinib resistance are not clear. These patients may have a higher likelihood of developing specific resistance mutations such as the C481S mutation in BTK and activating mutations in PLCγ2, due to genomic instability;6 However, the presence of these mutations represent only a subset of cases with clinical drug resistance and additional gene mutations present at baseline or evolving during therapy may also be important; we did not have gene sequencing data available for patients treated on these studies. Elucidating other causes of ibrutinib resistance, to allow subsequent treatment to be rationally selected according to the specific resistance pattern in an individual, remains a major research priority.
Important questions remain unanswered regarding optimal management of high-risk patients, such as those with CKT, initially treated with ibrutinib-based regimens. In contrast to alloSCT, which achieves long-term remission in approximately 50% of patients with conventionally-defined high-risk CLL,2, 24, 25 long-term survival outcomes for high-risk patients treated with ibrutinib-based therapy are unknown; a median overall survival of only 25 months with ibrutinib-based treatment for patients with CKT, however, clearly indicates high-risk disease requiring development of more potent therapy. One potential strategy could be to perform alloSCT in high-risk patients after ibrutinib-based induction. However, while alloSCT has been shown to overcome the poor prognosis associated with del(17p),2, 3 only one study specifically addressed the outcomes of patients with CKT treated with alloSCT,12 and suggested that patients with CKT had poorer outcomes after alloSCT than those without CKT. This study was small and (N=51) the majority of patients received in vivo T cell depletion; In vivo T cell depletion with alemtuzumab as part of reduced intensity conditioning for alloSCT for CLL has been associated with higher relapse rate and poorer survival outcomes.2 Larger studies are required to address the important question of whether alloSCT ameliorates the poor outcome associated with CKT. The outcomes of patients treated with alloSCT after induction with BCR-inhibitors or BCL-2-inhibitors are also unknown, as is the optimal timing of alloSCT in patients responding to ibrutinib. The value of post-transplant maintenance treatment with novel agents will also be important to address in future studies. Finally, the potential for combinations of ibrutinib with other novel agents, particularly BCL-2 inhibitors, to induce deeper and more durable remissions, and whether this will in turn obviate the need for alloSCT in these high-risk patients needs to be assessed.
In summary, CKT may be associated with a particularly poor outcome in R/R CLL patients treated with ibrutinib-based regimens; in contrast, patients with del(17p) in the absence of CKT may have relatively favorable outcomes and require a less aggressive approach; these findings, however, require confirmation in larger groups of patients with more sensitive karyotyping techniques. Given their poor outcomes, patients with CKT are likely an ideal group for future studies of novel ibrutinib-based combinations or sequential therapies, including allogeneic stem cell transplant.
Key points.
A complex metaphase karyotype, determined by conventional cytogenetic analysis, is associated with very high-risk disease in patients with relapsed and/or refractory CLL treated with ibrutinib-based regimens.
Patients with del(17p) who do not have a complex karyotype may have a relatively favourable outcome.
Acknowledgments
Funding: PAT receives funding from CLL Global Research Foundation and a Haematology Society of Australia and New Zealand New Investigator Scholarship.
Funding info: P30 CA016672, Ronald DePinho.
Footnotes
Presented in part at the 56th annual American Society of Hematology meeting, San Francisco, 2014.
Conflict of interest: SOB and JAB received research funding from Pharmacyclics.
PAT provided clinical care to patients collected and analyzed data, performed statistical analysis and wrote the paper.
WGW, AF, SCS, ZE, NJ, HK and MJK were involved in the development of critical themes, provided clinical care to patients and co-wrote the paper.
FS performed statistical analysis and co-wrote the paper.
SOB and JB designed clinical studies, were involved in the development of critical themes, provided clinical care to patients and co-wrote the paper.
References
- 1.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–1174. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 2.Dreger P, Dohner H, Ritgen M, et al. Allogeneic stem cell transplantation provides durable disease control in poor-risk chronic lymphocytic leukemia: long-term clinical and MRD results of the German CLL Study Group CLL3X trial. Blood. 2010;116:2438–2447. doi: 10.1182/blood-2010-03-275420. [DOI] [PubMed] [Google Scholar]
- 3.Schetelig J, van Biezen A, Brand R, et al. Allogeneic hematopoietic stem-cell transplantation for chronic lymphocytic leukemia with 17p deletion: a retrospective European Group for Blood and Marrow Transplantation analysis. J Clin Oncol. 2008;26:5094–5100. doi: 10.1200/JCO.2008.16.2982. [DOI] [PubMed] [Google Scholar]
- 4.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Susan Mary O'Brien RRF, Coutre Steven E, Flinn Ian, Burger Jan Andreas, Blum Kristie A, Sharman Jeff Porter, Jones Jeffrey Alan, Wierda William G, Zhao Weiqiang, Heerema Nyla A, Johnson Amy J, Tran Anh, Zhou Cathy, Bilotti Elizabeth, James Danelle Frances, Byrd John C. Independent evaluation of ibrutinib efficacy 3 years post-initiation of monotherapy in patients with chronic lymphocytic leukemia/small lymphocytic leukemia including deletion 17p disease. J Clin Oncol. 2014;32:5s. (suppl; abstr 7014) [Google Scholar]
- 6.Woyach JA, Furman RR, Liu TM, et al. Resistance mechanisms for the Bruton's tyrosine kinase inhibitor ibrutinib. N Engl J Med. 2014;370:2286–2294. doi: 10.1056/NEJMoa1400029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jain P, Keating M, Wierda W, et al. Outcomes of patients with chronic lymphocytic leukemia (CLL) after discontinuing ibrutinib. 2015 doi: 10.1182/blood-2014-09-603670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haferlach C, Dicker F, Schnittger S, Kern W, Haferlach T. Comprehensive genetic characterization of CLL: a study on 506 cases analysed with chromosome banding analysis, interphase FISH, IgV(H) status and immunophenotyping. Leukemia. 2007;21:2442–2451. doi: 10.1038/sj.leu.2404935. [DOI] [PubMed] [Google Scholar]
- 9.Juliusson G, Robert KH, Ost A, et al. Prognostic information from cytogenetic analysis in chronic B-lymphocytic leukemia and leukemic immunocytoma. Blood. 1985;65:134–141. [PubMed] [Google Scholar]
- 10.Juliusson G, Oscier DG, Fitchett M, et al. Prognostic subgroups in B-cell chronic lymphocytic leukemia defined by specific chromosomal abnormalities. N Engl J Med. 1990;323:720–724. doi: 10.1056/NEJM199009133231105. [DOI] [PubMed] [Google Scholar]
- 11.Woyach JA, Lozanski G, Ruppert AS, et al. Outcome of patients with relapsed or refractory chronic lymphocytic leukemia treated with flavopiridol: impact of genetic features. Leukemia. 2012;26:1442–1444. doi: 10.1038/leu.2011.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaglowski SM, Ruppert AS, Heerema NA, et al. Complex karyotype predicts for inferior outcomes following reduced-intensity conditioning allogeneic transplant for chronic lymphocytic leukaemia. Br J Haematol. 2012;159:82–87. doi: 10.1111/j.1365-2141.2012.09239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oscier DG, Gardiner AC, Mould SJ, et al. Multivariate analysis of prognostic factors in CLL: clinical stage, IGVH gene mutational status, and loss or mutation of the p53 gene are independent prognostic factors. Blood. 2002;100:1177–1184. [PubMed] [Google Scholar]
- 14.Bentz M, Huck K, du Manoir S, et al. Comparative genomic hybridization in chronic B-cell leukemias shows a high incidence of chromosomal gains and losses. Blood. 1995;85:3610–3618. [PubMed] [Google Scholar]
- 15.Dicker F, Schnittger S, Haferlach T, Kern W, Schoch C. Immunostimulatory oligonucleotide-induced metaphase cytogenetics detect chromosomal aberrations in 80% of CLL patients: A study of 132 CLL cases with correlation to FISH, IgVH status, and CD38 expression. Blood. 2006;108:3152–3160. doi: 10.1182/blood-2006-02-005322. [DOI] [PubMed] [Google Scholar]
- 16.Burger JA, Keating MJ, Wierda WG, et al. Ibrutinib In Combination With Rituximab (iR) Is Well Tolerated and Induces a High Rate Of Durable Remissions In Patients With High-Risk Chronic Lymphocytic Leukemia (CLL): New, Updated Results Of a Phase II Trial In 40 Patients. 2013 [Google Scholar]
- 17.Brown JR, Barrientos JC, Barr PM, et al. Ibrutinib In Combination With Bendamustine and Rituximab Is Active and Tolerable In Patients With Relapsed/Refractory CLL/SLL: Final Results Of a Phase 1b Study. 2013 [Google Scholar]
- 18.Castoldi GL, Lanza F, Cuneo A. Cytogenetic aspects of B-cell chronic lymphocytic leukemia: their correlation with clinical stage and different polyclonal mitogens. Cancer Genet Cytogenet. 1987;26:75–84. doi: 10.1016/0165-4608(87)90135-x. [DOI] [PubMed] [Google Scholar]
- 19.Shaffer LGS, Campbell Marilyn L, Lynda J, editors. ISCN 2009: An International System for Human Cytogenetic Nomenclature. Basel: S. Karger; 2009. [Google Scholar]
- 20.Kaplan EL, Meier P. Nonparametric-Estimation from Incomplete Observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 21.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cox DRSE. Analysis of Binary Data. 2. London, United Kingdom: Chapman and Hall; 1989. [Google Scholar]
- 23.Muthusamy N, Breidenbach H, Andritsos L, et al. Enhanced detection of chromosomal abnormalities in chronic lymphocytic leukemia by conventional cytogenetics using CpG oligonucleotide in combination with pokeweed mitogen and phorbol myristate acetate. Cancer Genet. 2011;204:77–83. doi: 10.1016/j.cancergen.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khouri IF, Bassett R, Poindexter N, et al. Nonmyeloablative allogeneic stem cell transplantation in relapsed/refractory chronic lymphocytic leukemia: long-term follow-up, prognostic factors, and effect of human leukocyte histocompatibility antigen subtype on outcome. Cancer. 2011;117:4679–4688. doi: 10.1002/cncr.26091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sorror ML, Storer BE, Sandmaier BM, et al. Five-year follow-up of patients with advanced chronic lymphocytic leukemia treated with allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. J Clin Oncol. 2008;26:4912–4920. doi: 10.1200/JCO.2007.15.4757. [DOI] [PMC free article] [PubMed] [Google Scholar]