Abstract
While there is growing interest in understanding how marine life will respond to future ocean acidification, many coastal ecosystems currently experience intense acidification in response to upwelling, eutrophication, or riverine discharge. Such acidification can be inhibitory to calcifying animals, but less is known regarding how non-calcifying macroalgae may respond to elevated CO2. Here, we report on experiments performed during summer through fall with North Atlantic populations of Gracilaria and Ulva that were grown in situ within a mesotrophic estuary (Shinnecock Bay, NY, USA) or exposed to normal and elevated, but environmentally realistic, levels of pCO2 and/or nutrients (nitrogen and phosphorus). In nearly all experiments, the growth rates of Gracilaria were significantly increased by an average of 70% beyond in situ and control conditions when exposed to elevated levels of pCO2 (p<0.05), but were unaffected by nutrient enrichment. In contrast, the growth response of Ulva was more complex as this alga experienced significantly (p<0.05) increased growth rates in response to both elevated pCO2 and elevated nutrients and, in two cases, pCO2 and nutrients interacted to provide a synergistically enhanced growth rate for Ulva. Across all experiments, elevated pCO2 significantly increased Ulva growth rates by 30% (p<0.05), while the response to nutrients was smaller (p>0.05). The δ13C content of both Gracilaria and Ulva decreased two-to-three fold when grown under elevated pCO2 (p<0.001) and mixing models demonstrated these macroalgae experienced a physiological shift from near exclusive use of HCO3- to primarily CO2 use when exposed to elevated pCO2. This shift in carbon use coupled with significantly increased growth in response to elevated pCO2 suggests that photosynthesis of these algae was limited by their inorganic carbon supply. Given that eutrophication can yield elevated levels of pCO2, this study suggests that the overgrowth of macroalgae in eutrophic estuaries can be directly promoted by acidification, a process that will intensify in the coming decades.
Introduction
Ocean acidification is changing the chemistry of the ocean. Beyond reducing pH, the anthropogenic delivery of CO2 into surface oceans this century will differentially effect various pools of inorganic carbon, with CO2 and HCO3- expected to increase 260% and 20%, respectively, and CO32- levels expected to decrease 60% [1]. As the total dissolved inorganic carbon (DIC) pool shifts towards these predicted values, marine flora and fauna are expected to have a varied response with lower availability of CO32- inhibiting the growth of calcifying organisms [2–4] and higher CO2 levels potentially benefiting some, but not all, photosynthetic organisms [2, 5–6].
The extent to which uncalcified marine macroalgae benefit from anthropogenically-induced changes in carbonate chemistry is complex and not fully understood. While CO2 is an important carbon source for photosynthesis, the likelihood of elevated CO2 benefiting autotrophs is partly dependent on photosynthetic pathways utilized by algae. C3 plants that utilize RuBisCO as their initial carboxylating enzyme experience loss of fixed carbon due to photorespiration and may benefit from increased CO2 concentrations since RuBisCO is not substrate-saturated at current CO2 levels [1, 7]. In contrast, C4 plants that utilize phosphenolpyruvate carboxylase (PEPC) experience little photorespiratory loss due to use of carbon concentrating mechanisms (CCM) and thus may not benefit from increased CO2 since PEPC is substrate-saturated at current CO2 levels [1, 7]. Marine macroalgae acquire carbon through direct diffusive uptake of CO2 as well as active transport of CO2 and HCO3- [8]. Although the majority of macroalgae are C3 plants, they often make use of CCMs and extracellular carbonic anhydrase (CA) to convert HCO3- to CO2 for use by RuBisCO [1, 8–10]. However, there is significant variation in the photosynthetic strategies employed by different macroalgae regarding the use of extracellular CA as well as the degree to which HCO3- and/or CO2 can or cannot be utilized for photosynthesis [8]. Macroalgal growth in response to elevated pCO2 can also be manifested through non-photosynthetic means. Webber et al. [11] and Roger et al. [12] found that acclimation to elevated CO2 can result in decreased concentrations of RuBisCO, but results in an increase in soluble carbohydrate content that could enhance growth rates and alter the total carbon content of algal tissues.
Beyond the progressively increasing levels of CO2 in the world’s oceans due to the combustion of fossil fuels, there are strong sources of CO2 in coastal zones [13]. One of the most prominent CO2 sources in coastal zones appears to be eutrophication-enhanced microbial respiration [14–16]. The accumulation of respiratory CO2 from the degradation of excessive organic matter can lower seawater pH and commonly result in CO2 levels (>1,000 μatm) not predicted to occur in open ocean regions for more than a century [16]. The combination of excessive nutrients and elevated CO2 could have a variety of impacts on primary producers. It has been well-established that with excessive nutrient loading, dominance among benthic autotrophs can shift from seagrasses to fast-growing, ephemeral macroalgae such as Ulva and Gracilaria [17–18]. Furthermore, some species of Ulva including Ulva. rigida and U. lactuca have been shown to experience increased growth under elevated CO2 concentrations [19–20], while others have not [21]. Additionally, elevated CO2 levels could aid in the assimilation of nutrients by Ulva [22]. Ulva is well-known for the formation of green tides along eutrophied coastlines such as Brittany, France, and Qingdao, China [23–25]. Common rhodophytes such as Gracilaria have been shown to bloom in response to high nutrient concentrations [26] and, like some Ulva, may also benefit from elevated CO2 concentrations, although this has never been examined. In general, the dynamics of macroalgal communities in response to eutrophication and elevated CO2 are difficult to generalize, as both the slow- and fast-growing species have been hypothesized to benefit from elevated CO2 and nutrients and studies assessing the response of macrophytes to elevated CO2 have been limited [1].
The objective of this study was to assess how elevated concentrations of CO2 alone, and combined with elevated nutrient levels, affect the growth rates of two common species of temperate, bloom-forming macroalgae; the rhodophyte, Gracilaria, and the chlorophyte, Ulva. The overabundance of these macroalgae is commonly interpreted as a symptom of eutrophication and their overgrowth within estuaries can have a series of negative impacts on marine plants and animals [18, 27–28]. Macroalgae were exposed to ambient and elevated concentrations of CO2 with and without nutrient enrichment during experiments performed throughout their growing season and their growth responses, δ13C signatures, and elemental composition were evaluated.
Methods
Macroalgae Collection and Preparation
Macroalgae used for this study were collected from shallow regions of eastern Shinnecock Bay, NY, USA (40.85° N, 72.50° W; Fig 1) during low tide. Permission to access the water and collect the water and macroalgae was received from the Southampton Town Trustees, Southampton, NY, USA, who hold jurisdiction over Shinnecock Bay. Collections targeted large, well-pigmented, robust fronds of Ulva and Gracilaria that were transferred to dark, temperature-controlled containers filled with seawater and returned to the Stony Brook Southampton Marine Science Center within 15 minutes of collection. Individual thalli of Gracilaria approximately 5 cm in length were cut from the main plant and spun in a salad spinner to remove debris and epiphytes. Samples were then extensively rinsed with filtered (0.2 μm) seawater and placed into the salad spinner a second time to further remove debris, epiphytes, and excess seawater. Ulva samples were prepared by use of a small brass ring to cut circular sections approximately 3 cm in diameter from a singular, large sheet of Ulva with care taken to avoid the outer, potentially reproductive region of the plant [29]. Ulva circles were brought through the same cleaning procedures described for Gracilaria. Five additional circular samples of Ulva were created from the same vegetative plant and were placed between two transparency films and frozen for future analysis described below. All samples were weighed on an A&D EJ300 digital scale (± 0.01 g) to obtain initial wet weights in grams. To prevent desiccation, all samples were kept in individual, 100 mL filtered (0.2 μm) seawater-filled containers after spinning prior to use in experiments.
Fig 1. Shinnecock Bay, NY, USA.
Map of Shinnecock Bay, NY, USA. The star represents the shallow-water region where macroalgal collections occurred and in situ experiments were performed.
In situ growth experiments
In situ growth experiments with Gracilaria and Ulva were performed to assess the rate at which the macroalgae grew within the region of Shinnecock Bay from which they were collected. Experiments were performed monthly from June through November with two experiments performed September and October, for a total of eight experiments. Quadruplet, 0.25 m2 incubation cages constructed from ~1 cm2 wire mesh were attached to a four-armed (25 cm) umbrella fishing apparatus on a line with surface flotation and a bottom weight that kept cages suspended at 0.2 m [29]. Discrete and continuous measurements of light and temperature present during experiments were made using a LI-COR LI-1500 light sensor logger and HOBO pendant temperature and light loggers, respectively. Quadruplet thalli of each macroalgae species were placed in each cage for ~7 days after which the samples were recovered, brought to the lab, and rinsed, spun, re-rinsed, re-spun, and weighed as described above. Gracilaria samples were placed into small freezer bags for further analysis, whereas Ulva samples were placed between two transparency films and flattened with care to minimize folds. The surface areas of the experimental Ulva samples, in addition to the five initial Ulva samples were analyzed using SigmaScan Pro 5 [29]. Weight-based growth rates for both species were determined using the relative growth rate formula (growth d-1) = (ln Wfinal−ln Winitial) / (Δt) where Wfinal and Winitial are the final and initial weights in grams and Δt is the duration of the experiment in days.
Assessing the effects of elevated nutrients and pCO2
Parallel experiments were established to assess the effects of elevated nutrients and pCO2 on the growth of Gracilaria and Ulva. Thirty-six, 2.5 L polycarbonate bottles were acid-washed (10% HCl), liberally rinsed with deionized water before use, and rinsed and filled with 0.2 μm filtered seawater from eastern Shinnecock Bay. Experimental bottles were placed in an environmental control chamber set to approximate the temperature (16–21°C) and light intensity (~450–500 μmol s-1 m-2 on a 14 h: 10 h light dark cycle) present during in situ experiments and were randomly assigned, in triplicate (n = 3), to one of four treatments for each species: a control with ambient levels of pCO2 (~400 μatm) and no nutrients added, a treatment of enhanced nutrient levels (50μM nitrate, 3 μM phosphate), a treatment of elevated pCO2 (~2000 μatm), and a treatment of elevated pCO2 and nutrient levels (~2000 μatm, 50μM nitrate, 3 μM phosphate). These nutrient and pCO2 levels were higher than levels present at the collection site, but consistent with concentrations present in eutrophic US East Coast estuaries [16, 29]. Each bottle was aerated via a 1 mL, polystyrene serological pipette inserted to the bottom of each experimental bottle and via tygon tubing to an air source. Bottles were subjected to the control level of pCO2 (~400 μatm) and elevated (~2000 μatm) via use of a gas proportionator system (Cole Parmer® Flowmeter system, multitube frame) that mixed ambient air with 5% CO2 gas [3]. The δ13C of this tanked CO2 gas was determined to be -27.7‰ by syringe injection into a split/splitless inlet of a continuous flow gas chromatograph isotope ratio mass spectrometer (cf-GCIRMS, Finnegan MAT 253) using a 0.25μm x 30m poraplot column and a secondary standard referenced to V-PDB in the laboratory of Dr. John Mak (Stony Brook University). The mixtures of air and CO2 gas were delivered at a net flow rate of 500 ± 5 mL min-1 through an 18-way gang valve into the serological pipettes that fit through an opening drilled into the closed cap to the bottom of polycarbonate bottles. This delivery rate turned over the volume of experimental bottles >100 times daily, ensuring that desired pCO2 concentrations and pH levels were generally maintained [3]. Bubbling was established two days before the beginning of each experiment to ensure that pCO2 concentrations and pH levels had reached a state of equilibrium and experiments persisted for ~ one week. Measurements of pH within bottles were made throughout each experiment using an Orion Star A321 Plus electrode (± 0.001) calibrated prior to each use using National Institute of Standards and Technology (NIST) traceable standards. Measurements using this pH meter were highly similar to and never significantly different from scale-adjusted spectrophotometric pH measurements made using m-cresol purple as described by Dickson et al. [30]. DIC concentrations in bottles were measured using an EGM-4 Environmental Gas Analyzer (PP Systems) system that quantifies DIC levels after separating the gas phase from seawater via acidification using a Liqui-Cel Membrane (Membrana) [3]. This instrument provided a methodological precision better than ± 1% for replicated measurements of total dissolved inorganic carbon. The levels of DIC and pH within Dr. Andrew Dickson’s (University of California, San Diego, Scripps Institution of Oceanography) certified reference material (Batch 138, 141) were measured during every analytical run as a quality assurance measure; analysis of samples proceeded only after complete recovery of certified reference material was attained. pCO2 levels (mean of t = initial and t = final, Table 1) were calculated using measured levels of DIC, pH (NIST), temperature, and salinity, as well as the first and second dissociation constants of carbonic acid in seawater according to Roy et al. [31] using the program CO2SYS (http://cdiac.ornl.gov/ftp/co2sys/). The targeted levels of pCO2 resulted in actual pCO2 and pH values of 441 ± 72 μatm and 7.9 ± 0.1, respectively, for ambient conditions and 1941 ± 141 μatm and 7.3 ± 0.1, respectively, for the elevated CO2 conditions (Table 1).
Table 1. Mean pH, temperature, salinity, pCO2, DIC, and alkalinity present during experiments and starting dissolved inorganic nitrogen (DIN), and dissolved inorganic phosphorus (DIP) concentrations during experiments.
| Gracilaria | ||||||||||
| Treatment | pH | Temperature | Salinity | pCO2 | DIC | HCO3- | Alkalinity | DIN | DIP | |
| Control | 8.23±0.02 | 18.4±0.1 | 29.5±0.8 | 327±58 | 1520±73 | 1380±73 | 1790±76 | 5.42±0.87 | 0.72±0.11 | |
| Nutrients | 8.29±0.03 | 18.5±0.1 | 29.5±0.7 | 314±61 | 1400±68 | 1310±93 | 1720±71 | 55.42±8.86 | 3.72±0.58 | |
| CO2 | 7.37±0.01 | 18.6±0.1 | 29.8±0.6 | 2530±108 | 1760±60 | 1660±48 | 1710±59 | 5.42±0.87 | 0.72±0.11 | |
| CO2/Nutrients | 7.38±0.01 | 18.6±0.1 | 29.7±0.7 | 2380±114 | 1710±61 | 1630±49 | 1670±58 | 55.42±8.86 | 3.72±0.53 | |
| Ulva | ||||||||||
| Treatment | pH | Temperature | Salinity | pCO2 | DIC | HCO3- | Alkalinity | DIN | DIP | |
| Control | 8.27±0.02 | 18.5±0.1 | 29.3±0.8 | 329±55 | 1540±72 | 1380±70 | 1780±76 | 5.42±0.87 | 0.72±0.11 | |
| Nutrients | 8.35±0.03 | 18.5±0.1 | 29.6±0.7 | 328±56 | 1440±57 | 1330±63 | 1750±89 | 55.42±8.86 | 3.72±0.58 | |
| CO2 | 7.37±0.01 | 18.6±0.1 | 29.7±0.6 | 2510±102 | 1770±70 | 1650±56 | 1720±70 | 5.42±0.87 | 0.72±0.11 | |
| CO2/Nutrients | 7.40±0.01 | 18.6±0.1 | 29.8±0.6 | 2300±163 | 1740±55 | 1650±46 | 1700±58 | 55.42±8.86 | 3.72±0.53 | |
Mean values of pH (NBS scale), temperature (°C), salinity (g kg-1), pCO2 (μatm), DIC (μmol kgSW-1), HCO3- (μmol kgSW-1), alkalinity (μmol kgSW-1), DIN (μM), and DIP (μM) for Gracilaria and Ulva for June through November experiments.
Values represent means ± SE. Data from individual experiments appear within S1 Table.
Experiments began with the addition of nutrients and introduction of macroalgae into experimental bottles. Experiments were maintained for seven days, during which daily pH and temperature measurements of each individual bottle were made with the Orion Star A321. Continuous measurements of light and temperature present during experiments were made using a LI-COR LI-1500 light sensor logger and HOBO pendant temperature and light data loggers and continuous pH measurements were made within selected bottles using the Orion Star A321 pH meter. At the termination of experiments, final pH and temperature measurements were made and a final DIC sample from each bottle was analyzed as described above. After measuring DIC, each macroalgae sample was removed from their respective bottles and rinsed, spun, re-rinsed, re-spun, and weighed as described above. Gracilaria samples were placed into small freezer bags for further analysis, whereas Ulva samples were placed between two transparency films and flattened with care to minimize folds. The surface areas of the samples were analyzed using SigmaScan Pro 5. Weight-based growth rates for both species were determined as described above. Significant differences in growth rates during experiments were assessed using a three-way ANOVA within SigmaPlot 11.0 where the main treatment effects were pCO2 treatment (ambient or elevated), nutrients (none or enhanced), and date of experiment.
Tissue analyses
Identification of macroalgae was based on morphology, microscopy, known biogeography, and DNA sequencing. Gracilaria tikvahiae is one of the most common species of red algae along the North American east coast, is the only Gracilaria species native to the Northeast US [32–34], and displays a distinct, continuous, phylogenic lineage across the Canadian-Northeast-Mid-Atlantic US region [35]. The morphology and pigmentation of Gracilaria fronds used in this study were fully consistent with prior descriptions of Gracilaria tikvahiae in the region [32–33, 35] and this was considered to be the species of Gracilaria used during this study. In contrast to Gracilaria, identifying Ulva spp. across the Northeast US is more challenging due to the co-occurrence of multiple, morphologically similar species [36]. For this study, selected frozen Ulva samples were dried at 55°C and then homogenized into a fine powder using a mortar and pestle. DNA from selected samples were extracted using the CTAB method and the quality and quantity of nucleic acids were assessed by use of a Nanodrop 2000 spectrophotometer [29]. Next-generation DNA sequencing of ITS1 and ITS2 regions of the ribosome of samples [29, 36] was performed on extracted samples using an Illumina MiSeq at the Molecular Research Laboratory (Shallowater, TX, USA). Forward primer 18S1763 (5`-GGTGAACCTGCGGAGGGATCATT-3`) and reverse primer 5.8S142 (5`-TATTCCGACGCTGAGGCAG-3`) were used for amplification of ITS1 whereas for ITS2, forward primer 5.8S30 (5`-GCAACGATGAAGAACGCAGC-3`) and reverse primer ENT26S (5`-GCTTATTGATATGCTTAAGTTCAGCGGGT-3`) were used [29]. The sequences in samples (Genbank Accession #KU306346) had the greatest similarity with Ulva rigida which has been previously identified in NY estuaries [29] and the US Northeast [36] and are synonymous with other Ulva spp. (Ulva lactuca var. rigida). Due to the plastic nature of macroalgal taxonomic nomenclature as well as the high similarity in ITS sequences among Ulva species [36–37] for the purposes of this study, we refer to these algae simply as Ulva and for consistency, refer to Gracilaria tikvahiae as Gracilaria.
For carbon (C) and nitrogen (N) analyses, frozen samples were dried at 55°C, and then homogenized into a fine powder using a mortar and pestle. The total tissue nitrogen and carbon content of the homogenized samples were analyzed using a CE Instruments Flash EA 1112 elemental analyzer [38]. Samples were analyzed for δ13C signatures using an elemental analyzer interfaced to a Europa 20–20 isotope ratio mass spectrometer at the UC Davis Stable Isotope Facility [29]. Concentrations of nitrate, ammonium, and phosphate were measured using standard wet chemical methods [39].
Finally, isotope mixing models were developed to estimate the use of CO2 and HCO3- during experiments. The models considered the δ13C and biomass of macroalgal tissue before and after experiments, the δ13C of the tanked gas used for experiments (-27.7‰), the δ13C of the marine CO2 and HCO3- pool (-10‰ and 0‰, respectively; [40–42]), the fractionation of C during macroalgal uptake of CO2 and HCO3- (-20‰ and -10‰, respectively; [40–42]), the fractionation of C during conversion of tanked CO2 bubbled into experimental vessels to HCO3- (+10‰, respectively; [40–42]), and the concentration of DIC with and without the addition of tanked CO2 with the later providing an indication of the fraction of DIC contributed by the tanked gas compared to ambient air. We assumed that during the course of the experiment, the tanked CO2 gas came to equilibrium with the total DIC pool and thus that the HCO3- pool took on a lighter δ13C signature in a manner proportional to the fraction of the DIC pool comprised of tanked gas compared to ambient air. Next, since we dried and homogenized the entire experimental macroalgal fronds for subsequent analyses, we assumed that the δ13C signature of the algal tissue was proportionally representative of the fraction of original tissue (pre-experiment) with its original δ13C signature and that the tissue grown during the experiment would take on a δ13C value representative of the CO2 or HCO3- pool with a value made proportionally more negative by the tanked CO2. Finally, two sets of mixing models were run for each macroalgal species to estimate their δ13C signature based on using exclusively CO2 and exclusively HCO3- during experiments. One-way ANOVAs were used to assess the differences between the measured δ13C signature of the macroalgae and signatures calculated based on exclusive CO2 or HCO3- use and Tukey tests were used to assess differences between individual groups.
Results
Gracilaria
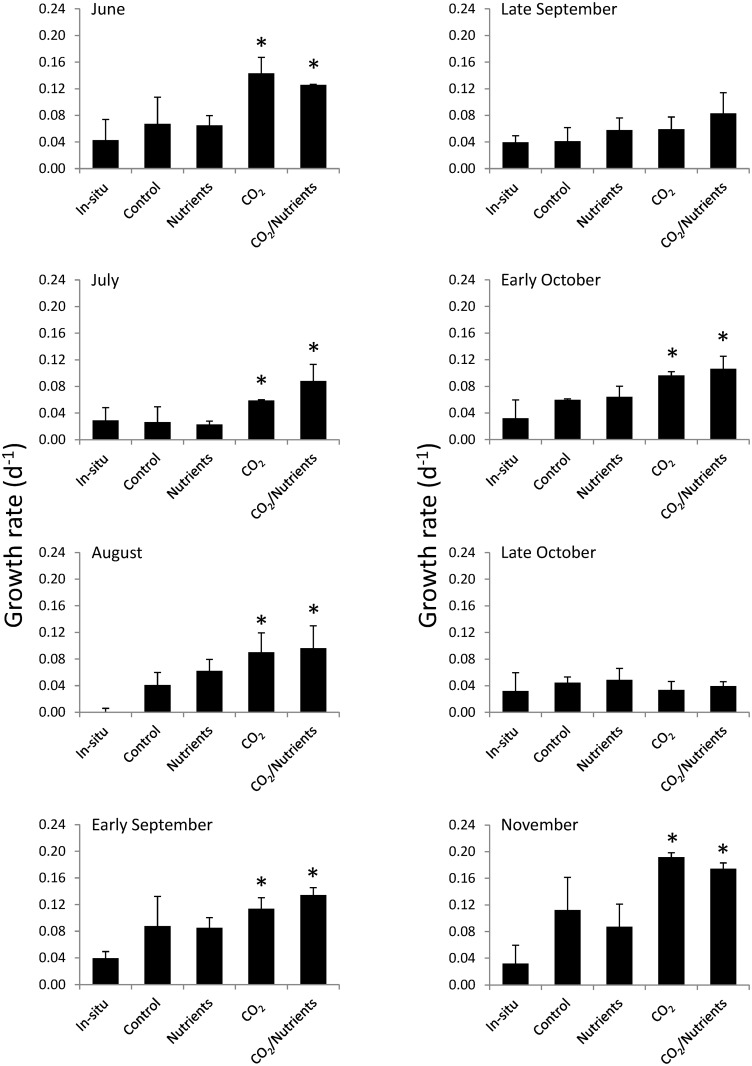
The in situ growth of Gracilaria in Shinnecock Bay was found to be highly similar to and not significantly different from growth rates within experimental control bottles with the exception of the August experiment, when experimental growth rates exceeded those in situ (Two-way ANOVA; p > 0.05; Fig 2; S2 Table). Gracilaria growth rates differed seasonally (Three-way ANOVA; p < 0.05; Fig 2; S2 Table). The experimental growth rates of Gracilaria were highly sensitive to changes in CO2 concentrations (Three-way ANOVA; p < 0.05; Fig 2; S2 Table). For six of the eight experiments, the growth of Gracilaria increased significantly when exposed to elevated CO2 concentrations (Tukey test; p < 0.05; Fig 2; S2 Table) with experiments during late September and late October being the exceptions to this trend. On average, growth rates under elevated CO2 were 70% higher than growth under ambient conditions (Fig 2). In contrast, the addition of nutrients did not significantly alter the growth rates of Gracilaria or yield statistically significant interactions with elevated pCO2 concentrations during any experiment (S2 Table).
Fig 2. Gracilaria growth rates.
Growth rates of Gracilaria exposed ambient and elevated CO2 conditions with and without nutrient additions for experiments performed August through November. Columns with an asterisk over them indicate significant results.
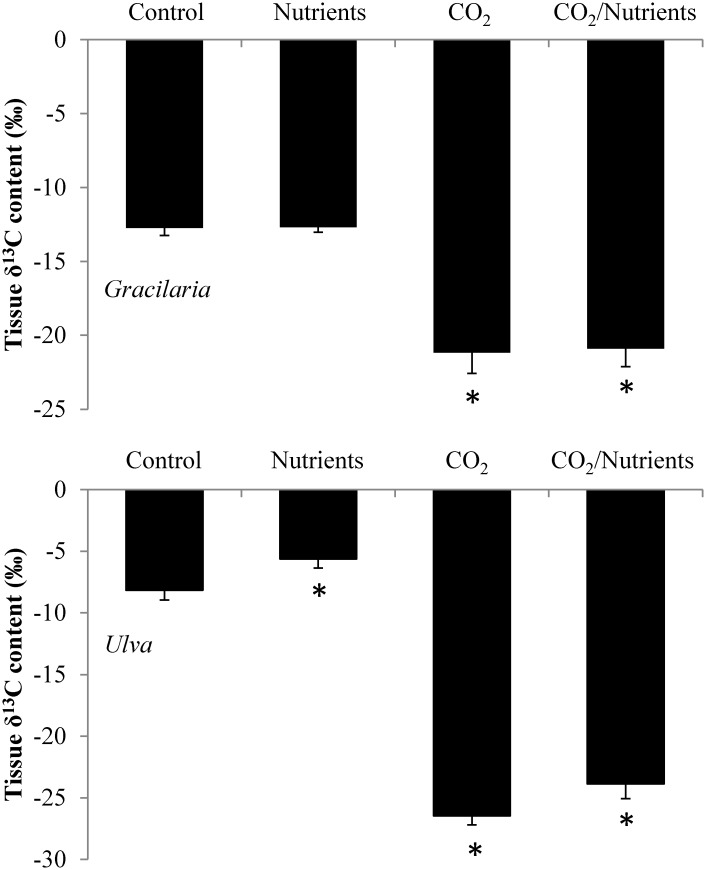
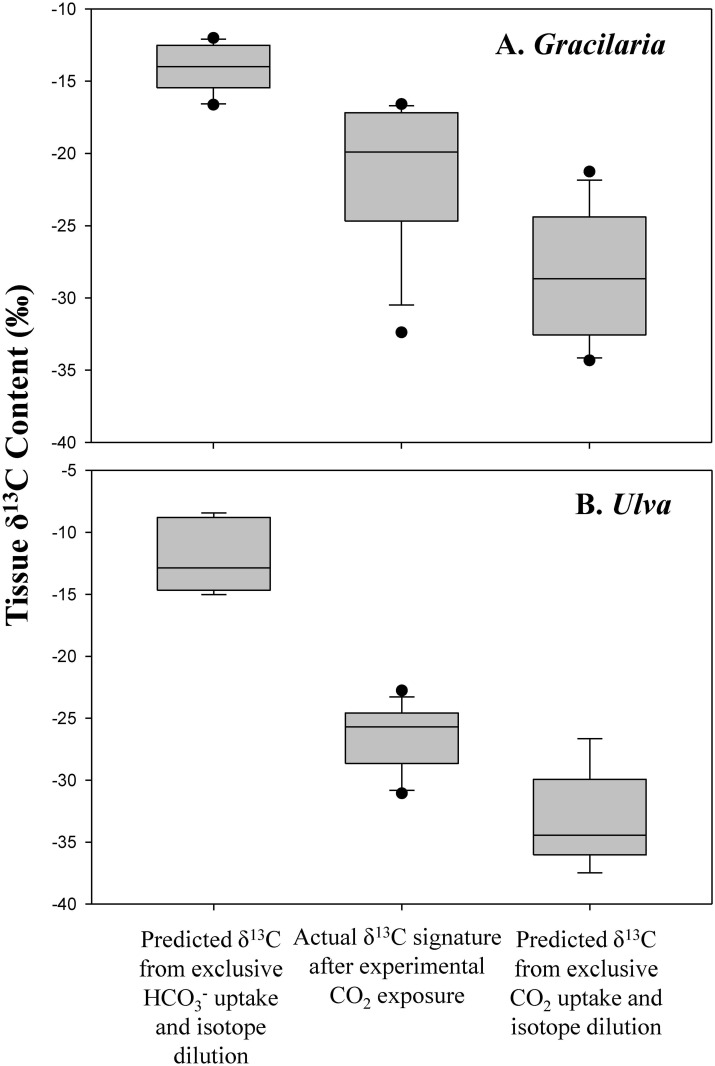
The stable carbon isotope (δ13C) content of Gracilaria was significantly reduced by exposure to elevated pCO2, with the average δ13C value of the ambient and elevated CO2 groups being, on average, -13‰ and -21‰, respectively (Three-way ANOVA; p<0.001; Fig 3; S2 and S3 Tables). The δ13C signatures of Gracilaria were not altered by nutrients but did differ by experiment (Three-way ANOVA; p<0.001; S2 and S3 Tables). Isotope mixing models demonstrated that when incubated with elevated pCO2 concentrations, Gracilaria δ13C signatures (-21‰) were significantly lower than values expected if their DIC was exclusively from use of HCO3- (-14‰) and significantly higher than expected from the use of exclusively CO2 (-28‰; Tukey test; p<0.001; Fig 4; S2 and S3 Tables). Quantitatively, the model suggested Gracilaria was using equal amounts of HCO3- and CO2 during experimental incubations with CO2 (Fig 4).
Fig 3. Macroalgal tissue δ13C.
δ13C content of Gracilaria and Ulva exposed to ambient and elevated CO2 conditions with and without nutrient additions for experiments performed August through November.
Fig 4. δ13C mixing model.
δ13C content of A) Gracilaria and B) Ulva exposed to elevated CO2 conditions compared with the δ13C signature expected from the exclusive use of CO2 or the exclusive use of HCO3-. Box plots depict the mean median (line within the boxes), 25th and 75th percentiles (lower and upper edges of the boxes), and 10th and 90th percentiles of the data (lower and upper error bars).
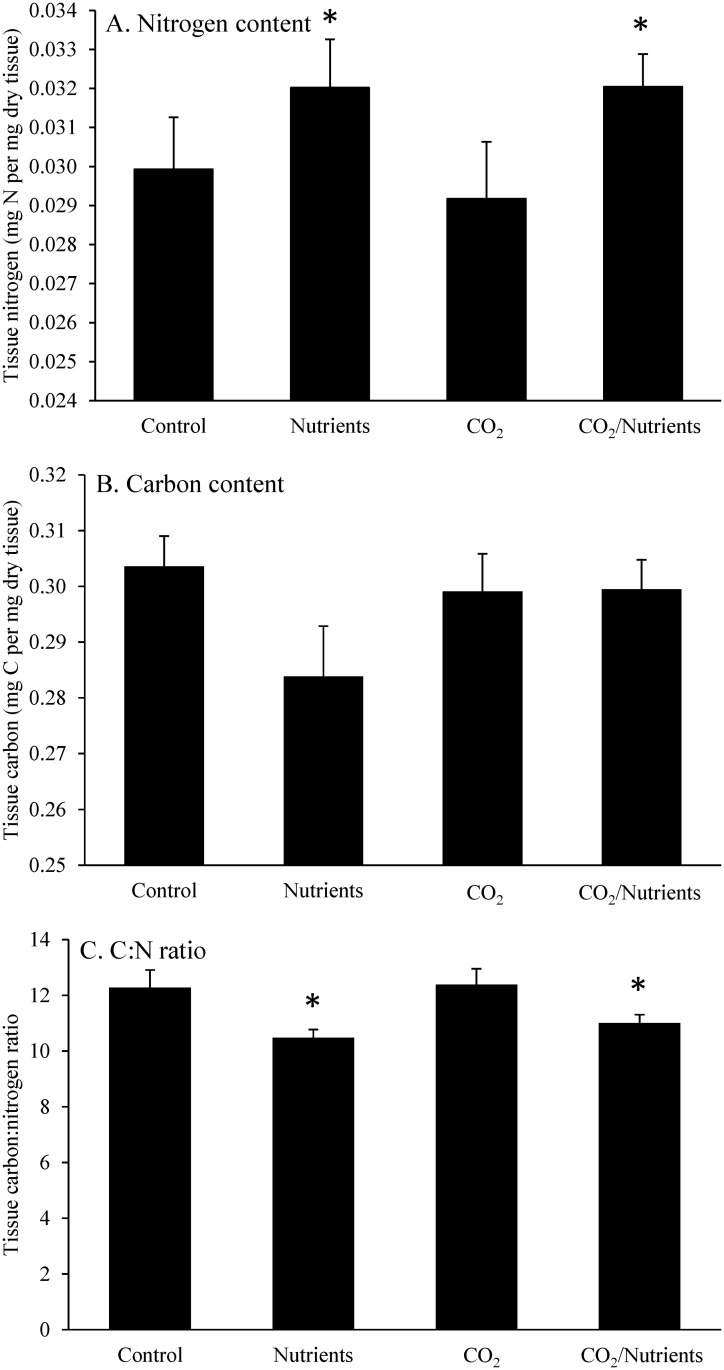
The nitrogen content of Gracilaria during experiments was found to be significantly higher in treatments that received nutrients and was found to differ seasonally (Three-way ANOVA; p < 0.05; S2 Table). On average, ambient and elevated nutrient treatments were found to have tissue nitrogen concentrations of 0.029 ± 0.005 and 0.032 ± 0.004 g N per g dry tissue, respectively (Fig 5; S4 Table). In contrast, the carbon content of Gracilaria was not significantly altered by pCO2 or nutrients, but did differ by seasonally (Three-way ANOVA; p < 0.05; Fig 5; S2 and S4 Tables). The tissue C:N ratio of Gracilaria was found to be significantly lower under elevated nutrient treatments (10.7 ± 0.2) compared to ambient nutrient treatments (12.3 ± 0.4) and differed seasonally (Three-way ANOVA; p < 0.05; Fig 5; S2 and S4 Tables). Tissue C:N ratio was not significantly changed in the CO2 treatments (S2 Table).
Fig 5. Gracilaria tissue nitrogen, carbon, and C:N.
Tissue nitrogen, carbon, and C:N content of Gracilaria exposed to ambient and elevated CO2 conditions with and without nutrient additions for experiments performed August through November.
Ulva
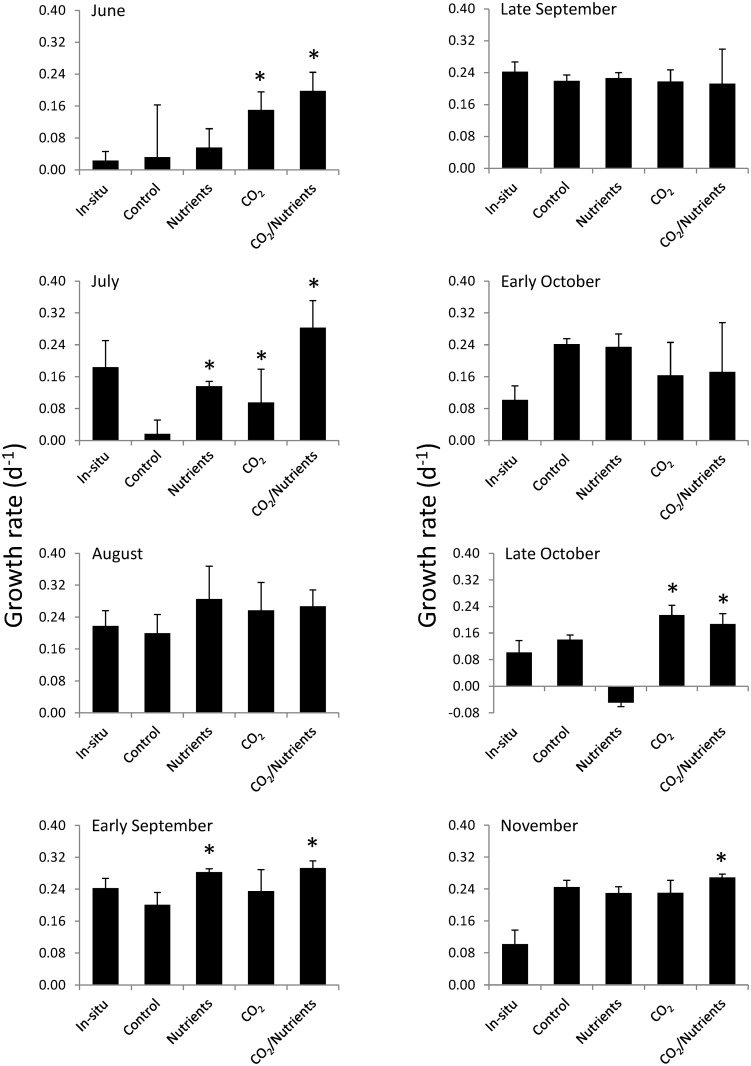
The growth rates of Ulva during in situ experiments did not differ statistically from those found within experimental control bottles except during experiments in early October and November when experimental control growth rates were greater than those observed in situ (Two-way ANOVA; p > 0.05; Fig 6; S2 Table). Ulva growth rates differed by experiment (Three-way ANOVA; p < 0.05; Fig 5; S2 Table). Ulva displayed more complex responses to nutrients and CO2 concentrations during experiments compared to Gracilaria. During experiments in June, July, and late October, Ulva growth rates significantly increased in response to elevated CO2 concentrations (Tukey test; p < 0.05; Fig 6; S2 Table). In addition, Ulva experienced significantly higher growth rates in response to higher nutrient levels during experiments performed during July and early September (Fig 6; Tukey test; p < 0.05; S2 Table). Finally, there was an interactive effect of CO2 and nutrients during the late October and November experiments during which these two factors synergistically increased the growth rates of Ulva (p < 0.05; S2 Table). On average, for all experiments, Ulva growth rates when exposed to elevated CO2 were 30% higher than ambient conditions (Fig 5; p<0.05; Three-way ANOVA; S2 Table) whereas the nutrients yielded a smaller, non-significant increase in growth rates (13%; Fig 6).
Fig 6. Ulva growth rates.
Growth rates of Ulva exposed to ambient and elevated CO2 conditions with and without nutrient additions for experiments performed August through November. Columns with an asterisk over them indicate significant results.
In a manner similar to Gracilaria, the δ13C content of Ulva was significantly reduced by exposure to elevated pCO2 (to -27‰) relative to control treatments value of -7‰ (Three-way ANOVA; p<0.001; Fig 3; S2 and S3 Tables). Unlike Gracilaria, however, the δ13C of Ulva was also effected by nutrients that yielded significantly higher values (-5‰) relative to control treatments (-7‰) and the δ13C differed by experiment (Three-way ANOVA; p<0.05; Fig 3; S2 and S3 Tables). Nutrients and CO2 did not interact to alter the δ13C of Ulva. Isotope mixing models indicated that when incubated with elevated pCO2 concentrations, Ulva δ13C signatures (-27‰) were significantly lower than values expected from the exclusively use of HCO3- (-12‰) and significantly higher than expected from the use of exclusively CO2 (-33‰; Tukey test; p<0.001; Fig 4; S2 Table). Quantitatively, the model suggested that for Ulva, during experimental incubations with elevated CO2, ~70% of their carbon came from CO2 and ~30% came from HCO3- (Fig 4).
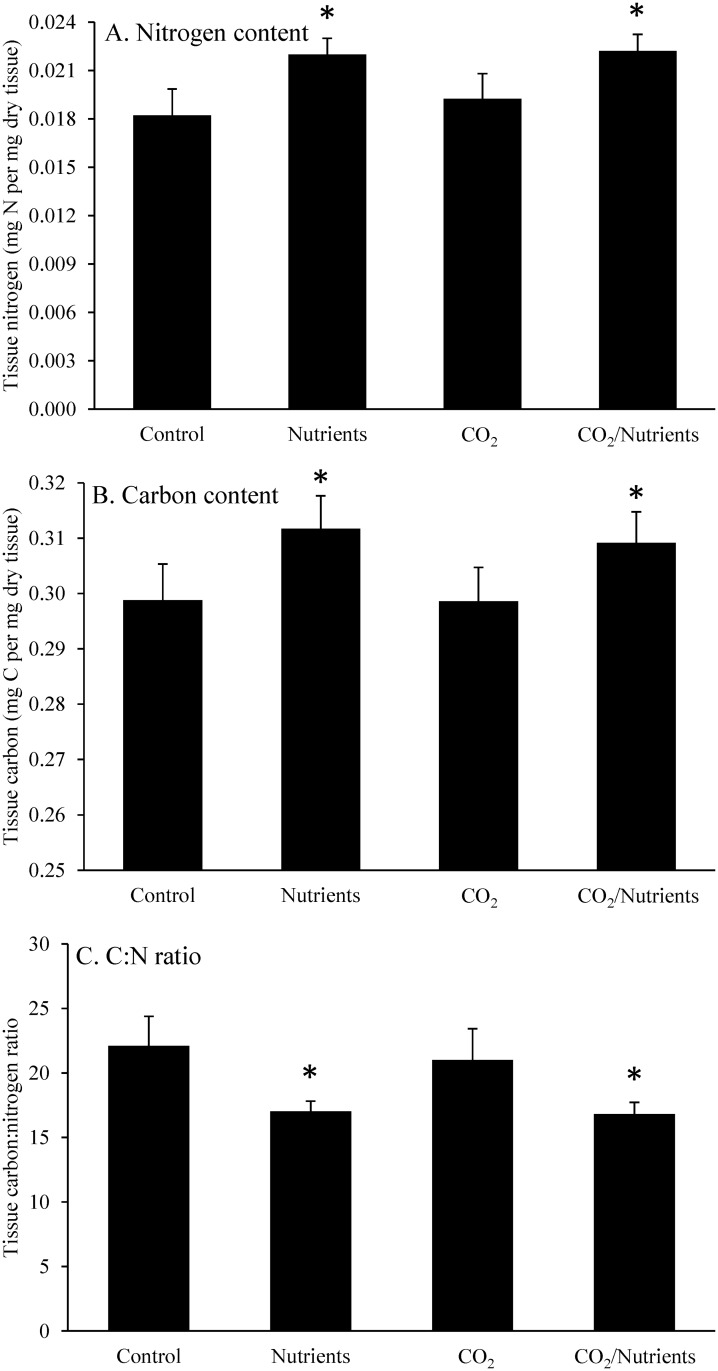
Also similar to Gracilaria, the nitrogen content of Ulva was significantly higher in elevated nutrient treatments (0.022 ± 0.004 g N per g dry tissue) compared to ambient nutrient treatments, regardless of pCO2 concentrations (0.019 ± 0.006 g N per g dry tissue; Three-way ANOVA; p < 0.05; Fig 7; S2 and S4 Tables). The carbon content of Ulva was not significantly altered by CO2 but was significantly increased by nutrients and differed by experiment (Three-way ANOVA; p < 0.05; Fig 7; S2 and S4 Tables). Tissue C:N was significantly lower in the elevated nutrient treatments (16.9 ± 0.6) than ambient nutrient treatments (21.5 ± 1.6) and differed by experiment (Three-way ANOVA; p < 0.05; Fig 7; S2 and S4 Tables).
Fig 7. Ulva tissue nitrogen, carbon, and C:N.
Tissue nitrogen, carbon, and C:N content of Ulva exposed to ambient and elevated CO2 conditions with and without nutrient additions for experiments performed August through November.
Discussion
During this study, elevated levels of pCO2 were found to significantly enhance the growth rates of two bloom-forming, estuarine macroalgae, Gracilaria and Ulva. These enhanced growth rates were accompanied by large and significant reductions in the δ13C content of the macroalgae. Concurrently, nutrients were found to enhance the growth of Ulva but not Gracilaria, and the combination of elevated nutrients and pCO2 were capable of synergistically promoting the growth of Ulva. Given that elevated pCO2 and acidification of coastal ecosystems are symptoms of eutrophication and that ocean acidification is enriching pCO2 concentrations in these systems, this study provides new insight regarding the present and future overgrowth of macroalgae in estuaries.
The effects of elevated CO2 concentrations on the growth of algae can depend on the precise carbon acquisition pathways utilized. C3 algae can benefit from high CO2 as their RuBisCO is not substrate-saturated at current CO2 levels (~400ppm) [7, 43]. Many macroalgae use HCO3- rather than dissolved CO2 under current seawater pCO2 concentrations and utilize CA to convert HCO3- to CO2 for use by RuBisCO [1, 8–10]. For example, Mercado et al. [44] found that the chlorophytes Ulva rigida and U. compressa (formerly Enteromorpha) do not receive enough CO2 through diffusive uptake alone at current CO2 levels and thus must use CCMs to acquire HCO3-. However, when exposed to elevated pCO2, macroalgae may down-regulate their CCMs, reduce the use of HCO3-, and begin to rely on CO2 as a primary C source [19, 45–47]. The energy made available from the down-regulation of the CCM may, in turn, be used for other purposes, such as increased vegetative growth [1] which we observed during this study.
Values of δ13C are often used to assess the types of carbon utilized by macroalgae. The δ13C of HCO3- is significantly higher (less negative) than that of CO2 in seawater and values of -10‰ or higher in macroalgae are reflective of the sole use HCO3- and CCMs whereas macroalgae relying wholly on diffusion of CO2 for carbon attain a value of -30‰ [40–41, 48]. When grown in ambient seawater, Ulva and Gracilaria had δ13C values of -8 and -13‰, values indicative of exclusive and near exclusive (85%) HCO3- use, respectively [40–41, 48]. The use of tanked CO2 gas with a known δ13C signature (-27.7‰) permitted that CO2 to be used as a tracer in mixing models and demonstrated that when incubated with elevated CO2, both macroalgal species switched their primary source of DIC. For Ulva, the change was the most dramatic as the three-fold decrease in δ13C signature was indicative of these algae going from exclusive use of HCO3- to, on average, 70% of their DIC originating from CO2 and only 30% from HCO3-. For Gracilaria, the change was less dramatic with but still notable as the alga went from ~85% HCO3- use under low pCO2 conditions to 50% CO2 use under high pCO2 conditions. Given the switch to increasing CO2 use by Ulva and Gracilaria and concurrent increase in growth experienced under elevated pCO2 concentrations, these algae may have down-regulated their CCMs permitting more energy to be dedicated to vegetative growth [1]. The significant increase in δ13C of Ulva when provided with nutrients further supports these hypotheses given that they experienced enhanced growth and presumably greater photosynthetic rates due to higher nutrient levels, causing a greater use of HCO3- via CCMs since additional CO2 was not available [21]. Finally, there are additional factors that could contribute to lowered δ13C values including preferential synthesis of lipids depleted in δ13C compared to proteins and carbohydrates [49] although the extent of fractionated associated with this process is small compared to changes observed during experiments presented here. Hence, the change in δ13C values during experiments suggest that when exposed to high concentrations of CO2, these bloom-forming macroalgae obtained a significantly larger fraction of their DIC from CO2 and often grew faster.
Elevated pCO2 concentrations did not alter the rate at which macroalgae took up and stored carbon (C) or nitrogen (N). The lack of change in tissue C content is consistent with the findings of Gordillo et al. [22] who reported no accumulation of soluble carbohydrates and no change in tissue C content for Ulva rigida fronds exposed to pCO2-enriched conditions. Despite the unchanged tissue C content, there were expected, significant increases in tissue N content within nutrient treatments. Both Ulva and Gracilaria have been shown to be able to rapidly assimilate and store nitrate [50–51] and have been shown to experience enhanced tissue N content when exposed to elevated levels of nitrate [28, 52]. While increases in the C:N ratio of macroalgae can reflect an increase in soluble carbohydrates during stimulation of growth rates in certain plants [53], during our study tissue C:N levels did not track growth rates. Given the observed changes in δ13C during exposure to high pCO2, we hypothesize that macroalgae responded to increased C availability by increasing, stoichiometrically-balanced growth rather than by storing more carbohydrates.
Eutrophication has been shown to promote coastal ocean acidification due to the accumulation of respiratory CO2 emanating from the microbial degradation of the excessive organic matter [16]. The present study has shown that Gracilaria and Ulva are capable of enhanced growth under elevated pCO2 levels and that Ulva can, on occasion, synergistically benefit from concurrently higher nutrient concentrations. Going forward, this finding may have broad implications as it demonstrates that, in some cases, the true impacts of elevated pCO2 on macroalgae may only be realized when excessive nutrients are present. Prior studies have demonstrated that elevated CO2 levels may have little effect on photosynthetic rates of some algae [10, 19, 47] but can result in increased biomass of Gracilaria sp., G. chilensis, and G. lemaneiformis [45–46] and Ulva rigida and U. lactuca [20, 22]. While Gracilaria can benefit from high nutrient concentrations [26], Ulva is capable of undergoing more rapid growth in eutrophic settings [29] due to a high maximum rate of uptake of ammonium and nitrate [17]. This was observed during the present study as Ulva growth rates were significantly higher than Gracilaria, and Ulva responded to nutrients more consistently than Gracilaria. Ulva is known to outcompete slower-growing algae in eutrophic estuaries, such as Saldanha Bay, South Africa [54], Britanny, France [25], and Qingdao, China [23]. The current study demonstrates that within eutrophied estuaries, seasonally elevated levels of pCO2 may be equally or more important than excessive nutrients in promoting algal growth. For example, Gracilaria grew faster in the presence of higher pCO2 levels but was unaffected by nutrients. Previously, it has been noted that more pristine estuaries are characterized by numerous, slower-growing macroalgal species while eutrophic estuaries are typically dominated by fewer, fast-growing, ephemeral macroalgal species [17–18, 24]. While nutrient loading and changes in light levels have been ascribed as the factors controlling these trends, the findings presented here suggest that elevated levels of pCO2 may be equally or more important for shaping estuarine macroalgal community composition.
The extent to which elevated levels of pCO2 affect the growth of macroalgae in estuaries will likely be influenced, in part, by physical mixing and circulation. In poorly flushed and/or mixed estuarine regions, diffusive boundary layers around seaweeds may limit DIC uptake [55–56] and thus higher ambient pCO2 may be more likely to be beneficial. In contrast, in high energy environments with fast-moving currents or wave-flow, boundary layers are less likely to develop [55–56] and additional pCO2 may be less likely to affect growth. During this study, macroalgae were vigorously bubbled at a rate that turned over the dissolved gas pool more than 700-times daily, a process that was unlikely to permit the development of boundary layers. This hypothesis is supported by the highly similar growth rates of thalli in a fairly high energy region of Shinnecock Bay during in situ experiments and in our control, experimental bottles for nearly all experiments. Hence, in our experiments, enhanced growth experienced during exposure to high levels of pCO2 were more likely a consequence of an intra-cellular, photosynthetic benefit for the algae rather than changes in external conditions.
The full implications of climate change for macroalgal communities are not fully understood, as studies of the effects of processes such as ocean acidification, rising temperatures, and changes in nutrient loading rates have been performed on a limited number of species. Porzio et al. [57] examined >100 species of macroalgae near volcanic CO2 vents in the Gulf of Naples, Italy, and found 20 species of calcium carbonate-containing macroalgae were no longer present under the acidification, whereas the ochryophyte Dictyota dichotoma and the rhodophyte Hildenbrandia rubra were most abundant within the high CO2 environment. Other studies have similarly found that tropical calcifying macroalgae indigenous to coral reefs are likely to be negatively impacted by ocean acidification [58–60]. Connell and Russell [61] found that elevated CO2 and temperature enhanced the growth of opportunistic turf-forming algae and that expansion of this algae inhibited the growth of kelp (Ecklonia radiata). As climate change processes promote increased pCO2, this and prior studies suggest that macroalgal communities may shift and favor rapid-growing and opportunistic species such as Ulva, Gracilaria, and turf algae, perhaps to the detriment of calcifying macroalgae and/or kelp.
The more rapid growth of some species of macroalgae will have important implications for other classes of marine autotrophs. The majority of seagrass species are C3 plants that are not currently substrate-saturated at current CO2 levels, with some, such as Zostera marina, showing enhanced photosynthesis and growth under elevated CO2 concentrations [1, 5]. Elevated nutrient loading, however, typically favors the dominance of macroalgae over seagrasses, as macroalgae are more competitive for high nutrient levels and can overgrow and shade seagrass [18]. Beyond CO2, climate change-induced warming may further favor macroalgae among submerged aquatic vegetation as many temperate species of seagrass exist at or near their upper level of thermal tolerance [62]. Finally, although highly excessive nutrient loading in estuaries with extended residence times are thought to ultimately favor the growth of phytoplankton blooms over macroalgae, the ability of both Ulva and Gracilaria to allelopathically inhibit the growth of phytoplankton [63–64] may allow macroalgae to remain dominant in high nutrient, high CO2 estuaries.
Macroalgal blooms can be harmful to marine life. Specifically, the overgrowth of macroalgae can cover critical benthic habitats and promote diel hypoxia/anoxia in estuaries [18, 28, 65] and Ulva has been shown to cause mortality in multiple calcifying animals including bivalves, barnacles, and larval crabs [27, 66–67]. Since these calcifying animals are also sensitive to high levels of CO2 [3, 68–70] the stimulation of harmful macroalgae such as Ulva under elevated pCO2 levels may represent a previously unrecognized, compounding environmental threat to some ocean animals. In contrast, other animals might benefit from predicted shifts in macroalgal communities. Some herbivorous fish of the families Blenniidae, Kyphosidae, and Siganidae selectively feed on filamentous and fleshy seaweeds such as Ulva [71] and Ulva lactuca can be an important nursery for juvenile blue crabs (Cahnectes sapidus) [72]. Furthermore, excessive nutrient loading generally enhances the nitrogen content and C:N ratio of macroalgal tissues, which could benefit herbivores feeding on such material [73]. Hence, while shifts in macroalgal communities caused by climate change and eutrophication may promote the prevalence of non-calcifying macroalgae over seagrasses and calcifying macroalgae and may be harmful to some marine mollusks, these shifts could benefit marine organisms that either graze on macroalgae or utilize it as a nursery.
Conclusion
This study demonstrated that two species of bloom-forming macroalgae experience fundamental changes in their photosynthetic physiology when exposed to high, but coastally realistic levels of pCO2 that led to significantly enhanced growth rates. Regarding Ulva, concurrently enhanced pCO2 and nutrient levels yielded synergistically increased growth. More studies are needed to understand the extent to which this phenomenon is applicable to other estuarine chlorophytes and rhodophytes as well as the ecosystem-wide implications of this phenomenon. Regardless, given that eutrophication can yield elevated levels of pCO2, this study suggests that the overgrowth of macroalgae in eutrophic estuaries may be promoted by acidification, a process that will intensify in coming decades.
Supporting Information
Values represent means ± SE.
(PDF)
(PDF)
Values represent means ± SE.
(PDF)
Values represent means ± SE.
(PDF)
Acknowledgments
We gratefully acknowledge the assistance of Dr. Dianna L. Berry during the molecular identification of Ulva during this study. We are appreciative of the logistical support provided by the Stony Brook Southampton Marine Science Center staff. We thank Dr. John Mak and Dr. Stuart Waugh for performing δ13C analysis CO2 gas. We thank Dr. Theresa Hattenrath-Lemann, Master Andrew Griffith, and Master Ryan Wallace for helpful feedback. This manuscript was greatly improved by the comments of three anonymous reviewers.
Data Availability
DNA sequences have been submitted to Genbank to accession and have been assigned Genbank Accession #KU306346.
Funding Statement
This work was supported by the Laurie Landeau Foundation to CJG, the Simons Foundation to CJG, and NOAA’s Ocean Acidification Program through award #NA12NOS4780148 from the National Centers for Coastal Ocean Science to CJG.
References
- 1.Koch M, Bowes G, Ross C, Zhang X-H. Climate change and ocean acidification effects on seagrasses and marine macroalgae. Global Change Biology. 2013; 19: 103–132. 10.1111/j.1365-2486.2012.02791.x [DOI] [PubMed] [Google Scholar]
- 2.Doney SC, Fabry VJ, Feely RA, Kleypas JA. Ocean Acidification: the Other CO2 Problem. Annual Review of Marine Science. 2009; 1: 169–192. [DOI] [PubMed] [Google Scholar]
- 3.Talmage SC, Gobler CJ. Effects of past, present, and future ocean carbon dioxide concentrations on the growth and survival of larval shellfish. Proceedings of the National Academy of Sciences of the United States of America. 2010; 107(40): 17246–17251. 10.1073/pnas.0913804107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gazeau F, Quiblier C, Jansen JM, Gattuso J-P, Middelburg JJ, Heip CHR. Impact of elevated CO2 on shellfish calcification. Geophysical Research Letters. 2007; 34: L07603. [Google Scholar]
- 5.Palacios SL, Zimmerman RC. Response of eelgrass Zostera marina to CO2 enrichment: possible impacts of climate change and potential for remediation of coastal habitats. Marine Ecology Progress Series. 2007; 344: 1–13. [Google Scholar]
- 6.Hattenrath-Lehmann TK, Smith JL, Wallace RB, Merlo LR, Koch F, Mittelsdorf H, et al. The effects of elevated CO2 on the growth and toxicity of field populations and cultures of the saxitoxin-producing dinoflagellate, Alexandrium fundyense. Limnology and Oceanography. 2015; 60: 198–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reiskind JB, Seamon PT, Bowes G. Alternative Methods of Photosynthetic Carbon Assimilation in Marine Macroalgae. Plant Physiology. 1988; 87: 686–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Badger M. The role of carbonic anhydrases in photosynthetic CO2 concentrating mechanisms. Photosynthesis Research. 2003; 77: 83–94. [DOI] [PubMed] [Google Scholar]
- 9.Gao K, McKinley KR. Use of macroalgae for marine biomass production and CO2 remediation: a review. Journal of Applied Phycology. 1994; 6: 45–60. [Google Scholar]
- 10.Israel A, Hophy M. Growth, photosynthetic properties and Rubisco activities and amounts of marine macroalgae grown under current and elevated seawater CO2 concentrations. Global Change Biology. 2002; 8: 831–840. [Google Scholar]
- 11.Webber AN, Nie GY, Long SP. Acclimation of photosynthetic proteins to rising atmospheric CO2. Photosynthesis Research. 1994; 39: 413–425. 10.1007/BF00014595 [DOI] [PubMed] [Google Scholar]
- 12.Rogers A, Fischer BU, Bryant J, Frehner M, Blum H, Raines CA, et al. Acclimation of Photosynthesis to Elevated CO2 under Low-Nitrogen Nutrition Is Affected by the Capacity for Assimilate Utilization. Perennial Ryegrass under Free-Air CO2 Enrichment. Plant Physiology. 1998; 118: 683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waldbusser GG, Salisbury JE. Ocean Acidification in the Coastal Zone: Multiple System Parameters, Frequency Domains, and Habitats. Annual Review of Marine Science. 2014; 6: 221–247. 10.1146/annurev-marine-121211-172238 [DOI] [PubMed] [Google Scholar]
- 14.Cai WJ, Hu X, Huang WJ, Murrell MC, Lehrter JC, Lohrenz SE, et al. Acidification of subsurface coastal waters enhanced by eutrophication. Nature Geoscience. 2011; 4: 766–770. [Google Scholar]
- 15.Melzner F, Thomsen J, Koeve W, Oschlies A, Gutowska MA, Bange HW, et al. Future ocean acidification will be amplified by hypoxia in coastal habitats. Marine Biology. 2013; 160: 1875–1888. [Google Scholar]
- 16.Wallace RB, Baumann H, Grear JS, Aller RC, Gobler CJ. Coastal ocean acidification: The other eutrophication problem. Estuarine, Coastal and Shelf Science. 2014; 148: 1–13. [Google Scholar]
- 17.Pedersen MF, Borum J. Nutrient control of estuarine macroalgae: growth strategy and the balance between nitrogen requirements and uptake. Marine Ecology Progress Series. 1997; 161: 155–163. [Google Scholar]
- 18.Valiela I, McClelland J, Hauxwell J, Behr PJ, Hersh D, Foreman K. Macroalgal blooms in shallow estuaries: Controls and ecophysiological and ecosystem consequences. Limnology and Oceanography. 1997; 42: 1105–1118. [Google Scholar]
- 19.Björk M, Ramazanov Z, Pedersén M. Inducible Mechanisms for HCO3- Utilization and Repression of Photorespiration in Protoplasts and Thalli of Three Species of Ulva (Chlorophyta). Journal of Phycology. 1993; 29: 166–173. [Google Scholar]
- 20.Olischläger M, Bartsch I, Gutow L, Wiencke C. Effects of ocean acidification on growth and physiology of Ulva lactuca (Chlorophyta) in a rockpool-scenario. Phycological Research. 2013; 61: 180–190. [Google Scholar]
- 21.Rautenberger R, Fernández PA, Strittmatter M, Heesch S, Cornwall CE, Hurd CL, et al. Saturating light and not increased carbon dioxide under ocean acidification drives photosynthesis and growth in Ulva rigida. Ecology and Evolution. 2015; 5(4): 874–888. 10.1002/ece3.1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordillo FJL, Niell FX, Figueroa FL. Non-photosynthetic enhancement of growth by high CO2 level in the nitrophilic seaweed Ulva rigida C. Agardh (Chlorophyta). Planta. 2001; 213: 64–70. [DOI] [PubMed] [Google Scholar]
- 23.Zhao J, Jiang P, Liu ZY, Wei W, Lin HZ, Li FC, et al. The Yellow Sea green tides were dominated by one species, Ulva (Enteromorpha) prolifera, from 2007 to 2011. Chinese Science Bulletin. 2013; 58(19): 2298–2302. [Google Scholar]
- 24.Smetacek V, Zingone A. Green and golden seaweed tides on the rise. Nature. 2013; 504: 84–88. 10.1038/nature12860 [DOI] [PubMed] [Google Scholar]
- 25.Perrot T, Rossi N, Ménesguen A, Dumas F. Modelling green macroalgal blooms on the coasts of Brittany, France to enhance water quality management. Journal of Marine Systems. 2014; 132: 38–53. [Google Scholar]
- 26.Ye CP, Zhang MC, Zhao JG, Yang YF, Zuo Y. Photosynthetic response of the macroalga, Gracilaria lemaneiformis (Rhodophyta), to various N and P levels at different temperatures. International Review of Hydrology. 2013; 98: 245–252. [Google Scholar]
- 27.Nelson TA, Lee DJ, Smith BC. Are “Green Tides” Harmful Algal Blooms? Toxic Properties of Water-Soluble Extracts from Two Bloom-Forming Macroalgae, Ulva fenestrate and Ulvaria obscura (Ulvophyceae). Journal of Phycology. 2003; 39: 874–879. [Google Scholar]
- 28.Liu D, Keesing JK, Xing Q, Shi P. World’s largest macroalgal bloom caused by expansion of seaweed aquaculture in China. Marine Pollution Bulletin. 2009; 58: 888–895. 10.1016/j.marpolbul.2009.01.013 [DOI] [PubMed] [Google Scholar]
- 29.Wallace RB, Gobler CJ. Factors Controlling Blooms of Microalgae and Macroalgae (Ulva rigida) in a Eutrophic, Urban Estuary: Jamaica Bay, NY, USA. Estuaries and Coasts. 2015; 38(2): 519–533. [Google Scholar]
- 30.Dickson AG, Sabine CL, Christian JR. Guide to best practices for ocean CO2 measurements. PICES Special Publication; 2007; 3: 191 pp. [Google Scholar]
- 31.Roy RN, Roy LN, Vogel KM, Porter-Moore C, Pearson T, Good CE, et al. The dissociation constants of carbonic acid in seawater at salinities 5 to 45 and temperatures 0 to 45°C. Marine Chemistry. 1993; 44: 249–267. [Google Scholar]
- 32.Schneider CW, Suyemoto MM, Yarish C. An annotated checklist of Connecticut Seaweeds Connecticut Geological and Natural History Survey. 1979; Bulletin; 108: 20 pp. [Google Scholar]
- 33.Sears JR. NEAS Keys to Benthic Marine Algae of the Northeastern Coast of North America from Long Island Sound to the Strait of Belle Isle. Northeast Algal Society; 1998; 1878301039: 161 pp. [Google Scholar]
- 34.Kim JK, Kraemer GP, Yarish C. Field scale evaluation of seaweed aquaculture as a nutrient bioextraction strategy in Long Island Sound and the Bronx River Estuary. Aquaculture. 2014; 433: 148–156. [Google Scholar]
- 35.Gurgel CFD, Fredericq S, Norris JN. Phylogeography of Gracilaria tikvahhiae (Gracilariaceae, Rhodophyta): a study of genetic discontinuity in a continuously distributed species based on molecular evidence. Journal of Phycology. 2004; 40(4): 748–758. [Google Scholar]
- 36.Hofmann L, Nettleton J, Neefus C, Mathieson A. Cryptic diversity of Ulva (Ulvales, Chlorophyta) in the Great Bay Estuarine System (Atlantic USA): introduced and indigenous distromatic species. European Journal of Phycology. 2010; 45: 230–239. [Google Scholar]
- 37.Kirkendale L, Saunders GW, Winberg P. A molecular survey of Ulva (Chlorophyta) in temperate Australia reveals enhanced levels of cosmopolitanism. Journal of Phycology. 2013; 49: 69–81. 10.1111/jpy.12016 [DOI] [PubMed] [Google Scholar]
- 38.Sharp JH. Improved analysis for particulate organic carbon and nitrogen from seawater. Limnology and Oceanography. 1974; 19: 984–989. [Google Scholar]
- 39.Parsons TR. A Manual of Chemical & Biological Methods for Seawater Analysis. Revised ed Philadelphia: Elsevier; 2013. [Google Scholar]
- 40.Maberly SC, Raven JA, Johnston AM. Discrimination between 12C and 13C by marine plants. Oecologia. 1992; 91: 481–492. [DOI] [PubMed] [Google Scholar]
- 41.Raven JA, Johnston AM, Kübler JE, Korb R, Mclnory SG, Handley LL, et al. Mechanistic interpretation of carbon isotope discrimination by marine macroalgae and seagrasses. Functional Plant Biology. 2002; 29: 355–378. [DOI] [PubMed] [Google Scholar]
- 42.Mook WG, Bommerson JC, Staverman WH. Carbon isotope fractionation between dissolved bicarbonate and gaseous carbon dioxide. Earth and Planetary Science Letters. 1974; 22: 169–176. [Google Scholar]
- 43.Gao K, Ji Y, Aruga Y. Relationship of CO2 concentrations to photosynthesis of intertidal macroalgae during emersion. Hydrobiologia. 1999; 398: 355–359. [Google Scholar]
- 44.Mercado JM, Gordillo FJL, Figueroa FL, Niell FX. External carbonic anhydrase and affinity for inorganic carbon in intertidal macroalgae. Journal of Experimental Marine Biology and Ecology. 1998; 221: 209–220. [Google Scholar]
- 45.Gao K, Aruga Y, Asada K, Kiyohara M. Influence of enhanced CO2 on growth and photosynthesis of the red algae Gracilaria sp. and G. chilensis. Journal of Applied Phycology. 1993; 5: 563–571. [Google Scholar]
- 46.Xu Z, Zou D, Gao K. Effects of elevated CO2 and phosphorus supply on growth, photosynthesis and nutrient uptake in the marine macroalga Gracilaria lemaneiformis (Rhodophyta). Botanica Marina. 2010; 53: 123–129. [Google Scholar]
- 47.Cornwall CE, Hepburn CD, Pritchard D, Currie KI, McGraw CM, Hunter KA, et al. Carbon-Use Strategies in Macroalgae: Differential Responses to Lowered pH and Implications for Ocean Acidification. Journal of Phycology. 2012; 48: 137–144. 10.1111/j.1529-8817.2011.01085.x [DOI] [PubMed] [Google Scholar]
- 48.Hepburn CD, Pritchard DW, Cornwall CE, McLeod RJ, Beardalls J, Raven JA, et al. Diversity of carbon use strategies in a kelp forest community: implications for a high CO2 ocean. Global Change Biology. 2011; 17: 2488–2497. [Google Scholar]
- 49.Hoefs J. Stable Isotope Geochemistry. 6th ed New York City: Springer; 2009. [Google Scholar]
- 50.Ryther JH, Corwin N, DeBusk TA, Williams LD. Nitrogen uptake and storage by the red alga Gracilaria tikvahiae (McLachlan, 1979). Aquaculture. 1981; 26: 107–115. [Google Scholar]
- 51.Fan X, Xu D, Wang Y, Zhang X, Cao S, Mou S, et al. The effect of nutrient concentrations, nutrient ratios and temperature on photosynthesis and nutrient uptake by Ulva prolifera: implications for the explosion in green tides. Journal of Applied Phycology. 2014; 26: 537–544. [Google Scholar]
- 52.Naldi M, Wheeler PA. Changes in nitrogen pools in Ulva fenestrata (Chlorophyta) and Gracilaria pacifica (Rhodophyta) under nitrate and ammonium enrichment. Journal of Phycology. 1999; 35: 70–77. [Google Scholar]
- 53.Fonseca F, Bowsher CG, Stulen I. Impact of elevated atmospheric CO2 on nitrate-reductase transcription and activity in leaves and roots of Plantago major. Physiologia Plantarum. 1997; 100: 55–61. [Google Scholar]
- 54.Anderson RJ, Monteiro PMS, Levitt GJ. The effect of localized eutrophication on competition between Ulva lactuca (Ulvaceae, Chlorophyta) and a commercial resource of Gracilaria verrucosa (Gracilariaceae, Rhodophyta). Hydrobiologia. 1996; 326/327: 291–296. [Google Scholar]
- 55.Wheeler WN. Effect of Boundary Layer Transport on the Fixation of Carbon by the Giant Kelp Macrocystis pyrifera. Marine Biology. 1980; 56(2): 103–110. [Google Scholar]
- 56.Koch EW. The effect of water flow on photosynthetic processes of the alga Ulva lactuca L. Hydrobiologia. 1993; 260(1): 457–462. [Google Scholar]
- 57.Porzio L, Buia MC, Hall-Spencer JM. Effects of ocean acidification on macroalgal communities. Journal of Experimental Marine Biology and Ecology. 2011; 400: 278–287. [Google Scholar]
- 58.Hofmann LC, Yildiz G, Hanelt D, Bischof K. Physiological responses of the calcifying rhodophyte, Corallina officinalis (L.), to future CO2 levels. Marine Biology. 2012; 159: 783–792. [Google Scholar]
- 59.Hofmann LC, Bischof K, Baggini C, Johnson A, Koop-Jakobsen K, Teichberg M. CO2 and inorganic nutrient enrichment affect the performance of a calcifying green alga and its noncalcifying epiphyte. Oecologia. 2015; 177: 1157–1169. 10.1007/s00442-015-3242-5 [DOI] [PubMed] [Google Scholar]
- 60.Harley CDG, Anderson KM, Demes KW, Jorve JP, Kordas RL, Coyle TA. Effects of Climate Change on Global Seaweed Communities. Journal of Phycology. 2012; 48: 1064–1078. 10.1111/j.1529-8817.2012.01224.x [DOI] [PubMed] [Google Scholar]
- 61.Connell SD, Russell BD. The direct effects of increasing CO2 and temperature on non-calcifying organisms: increasing the potential for phase shifts in kelp forests. Proceedings of the Royal Society B: Biological Sciences. 2010; 277: 1409–1415. 10.1098/rspb.2009.2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Short FT, Neckles HA. The effects of global climate change on seagrasses. Aquatic Botany. 1999; 63(3): 169–196. [Google Scholar]
- 63.Tang YZ, Gobler CJ. The green macroalga, Ulva lactuca, inhibits the growth of seven common harmful algal bloom species via allelopathy. Harmful Algae. 2011; 10:480–488. [Google Scholar]
- 64.Lu H, Xie H, Gong Y, Wang Q, Yang Y. Secondary metabolites from the seaweed Gracilaria lemaneiformis and their allelopathic effects on Skeletonema costatum. Biochemical Systematics and Ecology. 2011; 39(4): 397–400. [Google Scholar]
- 65.Valiela I, Cole ML. Comparative Evidence that Salt Marshes and Mangroves May Protect Seagrass Meadows from Land-derived Nitrogen Loads. Ecosystems. 2002; 5: 92–102. [Google Scholar]
- 66.Magre EJ. Ulva lactuca L. negatively affects Balanus balanoides (L.) (Cirripedia Thoracica) in tidepools. Crustaceana. 1974; 27(3): 231–234. [Google Scholar]
- 67.Johnson DA, Welsh BL. Detrimental effects of Ulva lactuca (L.) exudates and low oxygen on estuarine crab larvae. Journal of Experimental Marine Biology and Ecology. 1985; 86(1): 73–83. [Google Scholar]
- 68.Ries JB, Cohen AL, McCorkle DC. Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology. 2009; 37(12): 1131–1134. [Google Scholar]
- 69.Findlay HS, Burrows MT, Kendall MA, Spicer JI, Widdicombe S. Can ocean acidification affect population dynamics of the barnacle Semibalanus balanoides at its southern range edge? Ecology. 2010; 91(10): 2931–2940. [DOI] [PubMed] [Google Scholar]
- 70.Long WC, Swiney KM, Foy RJ. Effects of ocean acidification on the embryos and larvae of red king crab, Paralithodes camtschaticus. Marine Pollution Bulletin. 2013; 69(1): 38–47. [DOI] [PubMed] [Google Scholar]
- 71.Tolentino-Pablico G, Bailly N, Froese R, Elloran C. Seaweeds preferred by herbivorous fishes. Journal of Applied Phycology. 2008; 20: 933–938. [Google Scholar]
- 72.Wilson KA, Able KW, Heck KL Jr. Predation rates on juvenile blue crabs in estuarine nursery habitats: evidence for the importance of macroalgae (Ulva lactuca). Marine Ecology Progress Series. 1990; 58: 243–251. [Google Scholar]
- 73.Hemmi A, Jormalainen V. Nutrient enhancement increases performance of a marine herbivore via quality of its food alga. Ecology. 2002; 83(4): 1052–1064. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Values represent means ± SE.
(PDF)
(PDF)
Values represent means ± SE.
(PDF)
Values represent means ± SE.
(PDF)
Data Availability Statement
DNA sequences have been submitted to Genbank to accession and have been assigned Genbank Accession #KU306346.