Abstract
Chytridiomycosis, caused by the fungal pathogen Batrachochytrium dendrobatidis (Bd), is an emergent infectious disease partially responsible for worldwide amphibian population declines. The spread of Bd along highland habitats (> 500 meters above sea level, m a.s.l.) of Costa Rica and Panamá is well documented and has been linked to amphibian population collapses. In contrast, data are scarce on the prevalence and dispersal of Bd in lowland habitats where amphibians may be infected but asymptomatic. Here we describe the spread (2009 to 2014) of Bd across lowland habitats east of the Panamá Canal (< 500 m a.s.l.) with a focus on the Túngara frog (Physalaemus [Engystomops] pustulosus), one of the most common and abundant frog species in this region. Highland populations in western Panamá were already infected with Bd at the start of the study, which was consistent with previous studies indicating that Bd is enzootic in this region. In central Panamá, we collected the first positive samples in 2010, and by 2014, we detected Bd from remote sites in eastern Panamá (Darién National Park). We discuss the importance of studying Bd in lowland species, which may serve as potential reservoirs and agents of dispersal of Bd to highland species that are more susceptible to chytridiomycosis.
Introduction
Wildlife extinctions are not typically attributed to infectious diseases, yet there are a few examples showing pathogens as the direct cause of local extinctions [1–3]. Some of them have been linked to chytridiomycosis, an emerging infectious disease [4,5]. In amphibians, chytridiomycosis results from a skin infection caused by the chytrid fungus Batrachochytrium dendrobatidis (Bd). In the tropics, the most severe declines have been documented in comparatively cooler and humid areas above 500 m [6], most likely because lower temperatures (17–25°C) are optimal for Bd growth and water facilitates the propagation and dispersal of the aquatic, flagellated Bd zoospores [7]. Although the rapid spread of Bd into apparently Bd-free areas throughout the world is well-documented [8–12], the process by which Bd spreads is still not clearly understood [13].
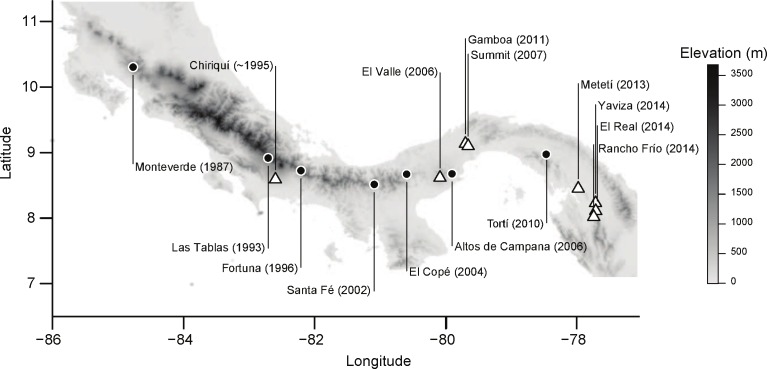
Bd has rapidly spread throughout the highlands of Central America. In the late 1980s, the disappearance of golden toads (Incilius periglenes) and declines of other anuran populations in the protected cloud forest of Monteverde, Costa Rica (Fig 1), were the first alerts to what later became a predictable pattern of declines spreading towards Panamá [14,15]. Shortly thereafter, between 1993 and 1997 additional cases of amphibian population declines or extinctions were reported in the highlands close to the border with Panamá at Las Tablas, Costa Rica, and Fortuna, Panamá (Fig 1)[16,17]. The sites experiencing population declines were located at altitudes above 500 m, and the fastest declining species were stream dwellers with aquatic tadpoles [6]. Bd was spreading west to east in a wave like pattern [8]. High elevation sites in western and central Panamá were then intensively studied prior to and during the arrival of Bd, these studies documented amphibian population declines after the arrival of Bd in this region (Fig 1)[16–19].
Fig 1. Sites where Bd has been detected in the past (filled circles), and sites where we sampled Túngara frog (Physalaemus [Engystomops] pustulosus) populations (open triangles).
Year in parenthesis corresponds to year of decline or the year that Bd was first detected.
Only two studies have investigated Bd infection spread in eastern Panamá. Rebollar et al. [20] sampled populations along the lowlands of east Panamá, and Woodhams et al. [19] sampled sites in central Panamá. These studies did not detect a clear pattern of wave-like spread from west to east as observed in the highlands of Panamá. It is also possible that another wave of Bd from South America crossed into Panamá [8]. Currently, the dynamics of Bd spread in the lowlands of Panamá, where environmental conditions are not ideal for Bd, are still unclear and require additional investigation.
Evaluating the spread of a pathogen in a single, abundant, and well-characterized host can help to predict spread dynamics while controlling for host phylogenetic diversity. The Túngara frog (Physalaemus [Engystomops] pustulosus) is a common species occupying lowland habitats ranging from Mexico to Colombia, Venezuela, and a small portion of the Guyana Shield. Its range parallels many highland regions where Bd and amphibian declines have been extensively documented [8–13]. The adults and tadpoles of this species use both permanent and ephemeral bodies of water in habitats ranging from urban areas to pristine forests. Thus, the Túngara frog is an ideal species in which to characterize the spread of Bd through populations of a lowland host. Here, we (i) report the Bd infection of túngara frogs at two sites west of the Panamá Canal where Bd is enzootic, and (ii) present data documenting the spread of Bd in the lowlands of central and east Panamá from 2009 to 2014.
Methods
We sampled Túngara frog populations in Panamá from 2009 to 2014, each year during the rainy season (June to November), which falls within their reproductive season. In most cases, populations were sampled during two reproductive seasons. Lowland sites in east and central Panamá ranged from 12 to 67 m in altitude (Fig 1). We also sampled highland populations at El Valle (elevation 600 m; Fig 1) in central Panamá, and at Chiriquí near the town of Cuesta de Piedra (elevation 460–967 m; Fig 1) in western Panamá. This altitude is the highest known Túngara population in Panamá. Chiriquí was also our western most site and was located 30 km east of sites where other amphibian species experienced declines in 1993 [17].
In central Panamá, we sampled two lowland sites east of the Panamá Canal, Gamboa and Summit, ranging from 46 to 98 m in elevation (Fig 1). These two lowland sites are located on the eastern side of the Panamá Canal but are separated by the Chagres River. Gamboa is a small town north of the river that is surrounded by rainforest. Here we included Pipeline Road, which intersects a protected rainforest in the Soberanía National Park. Summit is located south of the river and includes portions of the Soberanía National Park. We sampled along the main road, on trails and dirt roads within the National Park.
In east Panamá, we sampled four sites. Metetí and Yaviza are located along and at the very end of the Inter-American Highway, respectively (Fig 1). Here, we sampled disturbed, deforested habitats, in puddles on dirt roads and cattle ranches. Further east, along the Tuira River, we sampled at El Real (Fig 1), a small town surrounded by less disturbed habitat and in Darién National Park. In Darién National Park, we sampled around the Rancho Frío field station (Fig 1) at an average of 50 m of elevation. This site is predominantly primary rainforest with a tall canopy, thick lianas and an understory dominated by palms.
Time scale of surveys
We first sampled El Valle in 2009 and then Chiriquí in 2010, where Bd is thought to have arrived in the mid-1990s according to previous estimations [13]. In 2010, we also sampled Gamboa and Summit in central Panamá. Woodhams et al. [19] reported Bd in Summit in 2007. We then sampled Metetí and Yaviza in eastern Panamá during 2011, where we expected the front of the Bd wave to be just arriving. In 2013, we sampled all the populations previously surveyed. In 2014 we sampled in Gamboa, Yaviza and finally Darién National Park, which was the eastern most site and where we expected P. pustulosus populations to be Bd naive.
Bd sampling
We toe-clipped individuals to avoid recapture. To avoid cross contamination, we captured adults by hand using a new pair of nitrile gloves for each individual and kept them isolated in a plastic bag until processing. We swabbed the ventral area using a sterile cotton tip dry swab (Medical Wire & Equipment, model MW113 and MW110) following established procedures [21]. Swabs were stored in 90% ethanol or they were kept frozen until extraction, thus we expect no effects of sample storage on estimates of prevalence or infection intensity [22]. To avoid potential cross-contamination between sites, we bleached our rubber boots and vehicle tires and then rinsed them with tap water before leaving the collection site.
Real time quantitative PCR
To determine the prevalence of Bd in each population and the intensity of infection for individual frogs, we processed the swabs using quantitative PCR (qPCR) following the protocol developed by Boyle et al. [23] and modified by Kriger et al. [24]. For samples collected from 2009 to 2012, we used a Roche LightCycler 480 system with a high confidence setting to detect positive samples. For samples collected during 2009–2010, we did not quantify the number of zoospores due to lack of Bd standards. For samples collected during 2013 and 2014, we used TaqMan® Fast Advanced Master Mix (Applied Biosystems) and a StepOnePlus™ system. We used a dilution series of genomic DNA from strain JEL423 as our standard reference for the estimations of infection intensity in samples collected from 2011 to 2014. We calculated prevalence by using the ratio of positive samples to total samples per population and calculated 95% binomial confidence intervals. For each population, we calculated average infection intensity using the average number of zoospore equivalents (z.e.) inferred from qPCR among positive individuals.
To calculate the rate of Bd spread across sites east of the Canal, we used the distance between sites and the year we first detected infected frogs. We sampled frogs from June to November. If Bd arrived after sampling was completed for a given year, then we would have detected it the following year. We could not calculate the rate of spread in western Panamá because Bd was already present at the beginning of our sampling period. Thus, we approximated when Túngara frogs were first infected based on historical reports for sites near Chiriquí [8,13,19].
Ethical approval
All applicable institutional and/or national guidelines for the care and use of animals were followed. The protocol was approved by the Institutional Animal Care and Use Committee of the Smithsonian Tropical Research Institute (protocol number: 2011-0825-2014-02), and by the Autoridad Nacional del Ambiente (permit numbers: SE/A-81-09, SE/A-73-10, SE/A-48-10, SC/A-28-11, SE/A-83-11, SE/A-42-11, SE/A-30-12, SE/A-47-13, SC/A-9-14)
Results
In total, we sampled 1695 P. pustulosus adults across Panamá from 2009 to 2014 (Fig 1). Sample size, prevalence, and 95% binomial confidence intervals are shown in Table 1. We considered sites with sample sizes greater than 60 individuals and no positive samples as Bd-negative [25]. The site Chiriquí, in western Panamá, was positive for Bd in 2010 and 2013. In central Panamá, El Valle was positive for Bd in 2009. In 2010, Gamboa samples were negative for Bd, but Summit, which is only 8 km to the south, was positive. Bd reached Gamboa in 2011, and in following years we detected Bd positive samples in both Summit and Gamboa. By 2014, prevalence had reached 26% in Gamboa (Table 1). Populations in Metetí were Bd naive in 2011 but positive in 2013. Farther east, Yaviza was naive for Bd in 2013, but by 2014 prevalence reached approximately 6%. By 2014, Bd was also present in P. pustulosus populations from El Real and Rancho Frío, Darién National Park (Table 1).
Table 1. Bd prevalence and infection intensity per site and year in Túngara frog (Physalaemus [Engystomops] pustulosus) populations sampled in this study (n = number of individuals sampled; Positive = number of individuals detected positive for Bd; 95% CI = 95% binomial distribution confidence intervals; Average intensity = average of number of zoospore equivalents in infected frogs per population).
Sites are arranged west to east by longitude.
| Site | Year | N | Positive | Prevalence % (95% CI) | Average Intensity (± St.Dev) |
|---|---|---|---|---|---|
| Chiriquí | 2010 | 41 | 11 | 27 (14–43) | data not available |
| 2013 | 38 | 16 | 42 (26–60) | 3570 (± 12454) | |
| El Valle | 2009 | 5 | 3 | 60 (15–95) | data not available |
| Gamboa | 2010 | 321 | 0 | 0 (0–0.5) | 0 |
| 2011 | 111 | 7 | 6 (3–13) | 86 (± 117) | |
| 2012 | 205 | 26 | 13 (8–18) | 1617 (± 5485) | |
| 2013 | 166 | 35 | 21 (15–29) | 536 (± 2061) | |
| 2014 | 84 | 22 | 26 (17–37) | 209 (± 833) | |
| Summit | 2010 | 12 | 2 | 17 (2–48) | data not available |
| 2011 | 108 | 2 | 2 (0–6) | 10 (± 7) | |
| 2013 | 120 | 17 | 14 (9–22) | 89 (± 193) | |
| Metetí | 2011 | 91 | 0 | 0 (0–4) | 0 |
| 2013 | 94 | 2 | 2 (0–8) | 7 (± 3) | |
| Yaviza | 2013 | 68 | 0 | 0 (0–0.5) | 0 |
| 2014 | 41 | 3 | 7 (2–20) | 6 (± 1) | |
| El Real | 2014 | 40 | 2 | 5 (1–17) | 6 (± 1) |
| Rancho Frío | 2014 | 150 | 11 | 7 (4–13) | 48 (± 101) |
Rates of spread of Bd in Túngara frogs
If Bd spread eastward from Summit in central Panamá to Darién in eastern Panamá, then the front moved at an average rate of 54 km/year among these lowland Túngara frog populations. The rates of spread, however, vary substantially. Specifically, our data suggest that it took approximately one year for Bd to move the 8 km distance between Summit and Gamboa, which are separated by the Chagres River. In contrast, the rate of Bd spread from Summit to Metetí was 65 km/year, and 42 km/year from Metetí to Yaviza.
All data are available from the DRYAD Digital Repository (doi:10.5061/dryad.6bp92).
Discussion
Data for the spread of Bd in the lowlands of Middle America are scarce. Here we estimated the rate of spread of Bd in Túngara frogs based on the first detection of Bd, and we assumed that Bd spread in a wave-like fashion [8]. In the lowlands of Panamá, Bd spread at a similar rate among Túngara frogs (54 km/year) when compared to other amphibian species tested in this area (30–174 km/year) [19]. There was one exception. The rate of spread from Summit across the Chagres River to Gamboa was slower than the average (8 km/year).
In western Panamá, we sampled in Chiriquí, at elevations where amphibian population declines have been severe in the past [16]. Since Bd is now enzootic in this area [19] and at El Valle in central Panamá, it is not surprising that the Túngara frog populations from Chiriquí and El Valle were positive in 2010, and 2009, respectively. We also found the highest average loads of Bd in Chiriquí (3570 z.e., Table 1). We could not estimate the rate of Bd dispersal among Túngara frog populations between Chiriquí and El Valle as they were already infected at the time we sampled. In 2007, Woodhams et al. [19] reported Bd positive individuals (30% prevalence) among three species at Soberanía National Park, including the Summit area where we sampled in 2010, and they suggested that Bd was already enzootic during their study. In the same year, Gamboa, was still naive and we most likely sampled before Bd arrived. In 2011, we detected Bd at Gamboa for the first time, one year earlier than previously reported for this area [20].
We cannot discern whether the source of infection of the Gamboa populations was from Summit Túngara frogs or from other species of frogs in Gamboa. Regardless, there is a relatively large time lag between when Bd was detected in Túngara frogs from Gamboa compared to when Bd was first detected in Túngara frogs from Summit. The mechanism by which Bd spreads is unknown, but it has been suggested that Bd could survive and could be carried in mud, and thus easily dispersed by humans [26]. In Túngara frogs, Bd did not spread as fast as expected from Summit to Gamboa, which are only 8 km apart and connected by a well-traveled road and bridge over the Chagres River.
Certain geographic features, like rivers, could impede the spread of Bd. The Chagres River, which is about 100 m wide and has water all year long, separates Gamboa and Summit. Genetic studies demonstrate that the Chagres River is a geographical barrier for gene flow between Túngara frog populations [27], thus it is possible that limited migration between these populations could slow the spread of Bd whether Túngara frogs contracted Bd from conspecifics or heterospecifics. Bd does seem to have spread rapidly in the lowlands towards eastern Panamá. An alternative explanation for the spread of Bd from east to west throughout all of Panamá is that populations in Darién, and elsewhere in far eastern Panamá, were infected by a wave coming from the south [8]. These two scenarios should be tested through a phylogenetic analysis of Bd throughout the Túngara frog's range in Central and South America. As such data are currently unavailable, it is difficult to determine the recent origin of Bd in eastern Panamá; therefore, our estimates of rate of spread should be viewed with this potential caveat in mind.
Woodhams et al. [19] conservatively estimated that Bd spread east and would have reached Tortí in September of 2012, but [28] reported two positives out of 93 samples of other species of frogs at this site in 2010. There are no other published reports on the presence of Bd among amphibian species from this specific area. In 2011, we sampled in Metetí, approximately 70 km southeast of Tortí, and found this site to be Bd naive. Rebollar et al. [20] recorded Bd positive samples from Nuevo Vigía in 2012, just 26 km east of Metetí, where we detected low Bd prevalence in 2013, thus supporting a wave-like Bd spread from west to east in Túngara frogs.
Species that carry Bd asymptomatically and share the habitat with more vulnerable species can potentially function as Bd spreaders. Túngara frog population declines have not been reported and were not evident during our study. If the prevalence and dispersal of Bd is density dependent, then the spread of Bd along the lowlands might be enhanced by abundant and apparently resistant species serving as reservoirs. Túngara frogs are known to disperse between breeding sites at distances up to 200 m [29]. They also share the habitat with a wide variety of species; thus, they could contribute to the rapid dispersal of Bd if they are effective carriers. Moreover, if most species in the lowlands are less susceptible to Bd or Bd is less virulent [7], then the high diversity and abundance of hosts could further facilitate the dispersal of Bd.
Chytridiomycosis is linked to some of the most severe population declines and extinctions of wildlife yet recorded. While substantial efforts have been aimed at understanding the spread and pathogenicity in the more vulnerable highland frog species, we know relatively little about the dynamics of Bd in tropical lowland regions of the world. Lowland species, however, could be reservoirs and dispersal agents between areas where amphibian species are more vulnerable to Bd infection. As highlighted by our results, even though lowland regions are typically characterized by less favorable climatic conditions for Bd [7], by harboring asymptomatic Bd infections, lowland amphibian populations could potentially play an important role in the spread of Bd across tropical regions.
Acknowledgments
For help in the field we thank Emma and Lucy Ryan, Tony Alexander, Alex Jordan, Meghan Still, Ty Hoskin, Mahudy Díaz, Samuel Sucre, and for logistical support we thank SENAFRONT, ANAM, and STRI. We thank Teofil Nakov and Robert Puschendorf for comments on the manuscript. We also thank Michael R.J. Forstner at Texas State University for instrumentation support, and Mahudy Díaz at STRI for laboratory assistance.
Data Availability
All data are available from the DRYAD Digital Repository (doi:10.5061/dryad.6bp92).
Funding Statement
This work was funded by National Science Foundation (http://www.nsf.gov/), IBN 0517328 to MJR. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.MacPhee RDE, Greenwood AD. Infectious disease, endangerment, and extinction. Int J Evol Biol. 2013. January 16;2013:e571939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCallum H. Disease and the dynamics of extinction. Philos Trans R Soc B Biol Sci. 2012. October 19;367(1604):2828–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith KF, Sax DF, Lafferty KD. Evidence for the role of infectious disease in species extinction and endangerment. Conserv Biol. 2006. October 1;20(5):1349–57. [DOI] [PubMed] [Google Scholar]
- 4.Collins JP, Crump ML. Extinction in Our Times: Global Amphibian Decline. Oxford University Press; 2009. 477 p. [Google Scholar]
- 5.Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, McCraw SL, et al. Emerging fungal threats to animal, plant and ecosystem health. Nature. 2012. April 12;484(7393):186–94. 10.1038/nature10947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brem FMR, Lips KR. Batrachochytrium dendrobatidis infection patterns among Panamanian amphibian species, habitats and elevations during epizootic and enzootic stages. Dis Aquat Organ. 2008. September 24;81(3):189–202. 10.3354/dao01960 [DOI] [PubMed] [Google Scholar]
- 7.Piotrowski JS, Annis SL, Longcore JE. Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians. Mycologia. 2004. January 1;96(1):9–15. [PubMed] [Google Scholar]
- 8.Lips KR, Diffendorfer J, Mendelson JR, Sears MW. Riding the wave: reconciling the roles of disease and climate change in amphibian declines. PLoS Biol. 2008. March;6(3):e72 10.1371/journal.pbio.0060072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farrer RA, Weinert LA, Bielby J, Garner TWJ, Balloux F, Clare F, et al. Multiple emergences of genetically diverse amphibian-infecting chytrids include a globalized hypervirulent recombinant lineage. Proc Natl Acad Sci. 2011. November 15;108(46):18732–6. 10.1073/pnas.1111915108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher MC, Garner TWJ, Walker SF. Global emergence of Batrachochytrium dendrobatidis and amphibian chytridiomycosis in space, time, and host. Annu Rev Microbiol. 2009;63(1):291–310. [DOI] [PubMed] [Google Scholar]
- 11.James TY, Litvintseva AP, Vilgalys R, Morgan JAT, Taylor JW, Fisher MC, et al. Rapid global expansion of the fungal disease chytridiomycosis into declining and healthy amphibian populations. PLoS Pathog. 2009. May 29;5(5):e1000458 10.1371/journal.ppat.1000458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Velo-Antón G, Rodríguez D, Savage AE, Parra-Olea G, Lips KR, Zamudio KR. Amphibian-killing fungus loses genetic diversity as it spreads across the New World. Biol Conserv. 2012. February;146(1):213–8. [Google Scholar]
- 13.Phillips BL, Puschendorf R. Do pathogens become more virulent as they spread? Evidence from the amphibian declines in Central America. Proc R Soc B Biol Sci [Internet]. 2013. September 7 [cited 2013 Jul 29];280(1766). Available: http://rspb.royalsocietypublishing.org/content/280/1766/20131290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pounds JA, Crump ML. Amphibian declines and climate disturbance: the case of the Golden Toad and the Harlequin Frog. Conserv Biol. 1994. March 1;8(1):72–85. [Google Scholar]
- 15.Pounds JA, Fogden MPL, Savage JM, Gorman GC. Tests of null models for amphibian declines on a tropical mountain. Conserv Biol. 1997;11(6):1307–22. [Google Scholar]
- 16.Lips KR. Mass mortality and population declines of anurans at an upland site in western Panama. Conserv Biol. 1999;13(1):117–25. [Google Scholar]
- 17.Lips KR. Decline of a tropical montane amphibian fauna. Conserv Biol. 1998;12(1):106–17. [Google Scholar]
- 18.Lips KR, Brem F, Brenes R, Reeve JD, Alford RA, Voyles J, et al. Emerging infectious disease and the loss of biodiversity in a neotropical amphibian community. Proc Natl Acad Sci U S A. 2006. February 28;103(9):3165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woodhams DC, Kilburn VL, Reinert LK, Voyles J, Medina D, Ibáñez R, et al. Chytridiomycosis and amphibian population declines continue to spread eastward in Panama. EcoHealth. 2008. September 1;5(3):268–74. 10.1007/s10393-008-0190-0 [DOI] [PubMed] [Google Scholar]
- 20.Rebollar EA, Hughey MC, Harris RN, Domangue RJ, Medina D, Ibáñez R, et al. The lethal fungus Batrachochytrium dendrobatidis is present in lowland tropical forests of far eastern Panamá. PLoS ONE. 2014. April 16;9(4):e95484 10.1371/journal.pone.0095484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hyatt A, Boyle D, Olsen V, Boyle D, Berger L, Obendorf D, et al. Diagnostic assays and sampling protocols for the detection of Batrachochytrium dendrobatidis. Dis Aquat Organ. 2007;73:175–92. [DOI] [PubMed] [Google Scholar]
- 22.Sluys MV, Kriger KM, Phillott AD, Campbell R, Skerratt LF, Hero JM. Storage of samples at high temperatures reduces the amount of amphibian chytrid fungus Batrachochytrium dendrobatidis DNA detectable by PCR assay. Dis Aquat Organ. 2008. August 27;81(2):93–7. 10.3354/dao01953 [DOI] [PubMed] [Google Scholar]
- 23.Boyle D, Boyle D, Olsen V, Morgan J, Hyatt A. Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Dis Aquat Organ. 2004;60:141–8. [DOI] [PubMed] [Google Scholar]
- 24.Kriger KM, Hero J, Ashton KJ. Cost efficiency in the detection of chytridiomycosis using PCR assay. Dis Aquat Organ. 2006. July 25;71(2):149–54. [DOI] [PubMed] [Google Scholar]
- 25.Skerratt LF, Berger L, Hines HB, McDonald KR, Mendez D, Speare R. Survey protocol for detecting chytridiomycosis in all Australian frog populations. Dis Aquat Organ. 2008. July 7;80(2):85–94. 10.3354/dao01923 [DOI] [PubMed] [Google Scholar]
- 26.Johnson ML, Speare R. Possible modes of dissemination of the amphibian chytrid Batrachochytrium dendrobatidis in the environment. Dis Aquat Organ. 2005. July 18;65(3):181–6. [DOI] [PubMed] [Google Scholar]
- 27.Lampert KP, Rand AS, Mueller UG, Ryan MJ. Fine-scale genetic pattern and evidence for sex-biased dispersal in the túngara frog, Physalaemus pustulosus. Mol Ecol. 2003. December 1;12(12):3325–34. [DOI] [PubMed] [Google Scholar]
- 28.Küng D, Bigler L, Davis LR, Gratwicke B, Griffith E, Woodhams DC. Stability of microbiota facilitated by host immune regulation: informing probiotic strategies to manage amphibian disease. PLoS ONE. 2014. January 29;9(1):e87101 10.1371/journal.pone.0087101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marsh DM, Fegraus EH, Harrison S. Effects of breeding pond isolation on the spatial and temporal dynamics of pond use by the Tungara frog, Physalaemus pustulosus. J Anim Ecol. 1999. July 1;68(4):804–14. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available from the DRYAD Digital Repository (doi:10.5061/dryad.6bp92).