Twenty-five years after its initial development, coronary artery calcium (CAC) scanning has become a relatively inexpensive test that has been extensively validated as a potent noninvasive means for assessing the burden of coronary atherosclerosis in asymptomatic individuals. A proportional relationship between the magnitude of CAC abnormality and the frequency of subsequent cardiac events over long term follow-up has been consistently demonstrated, including observations from large patient and population-based cohorts [1–3]. Incremental prognostic value over standard clinical assessments including the Framingham Risk Score and other scores of global risk has also been consistently reported [3–4]. Consequently, the application of CAC scanning for assessing asymptomatic patients with intermediate clinical risk has now become part of clinical guidelines[5–6].
Information from CAC scanning may be used to favorably alter patient management in clinical practice. As an example, in the Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research (EISNER) trial, subjects were randomized to routine risk management with and without a concomitant CAC scan [7]. In the scan group, incurred costs and intensity of treatment increased with high CAC scores, but decreased in the zero CAC subgroup. This counterbalance resulted in no net increase in downstream medical costs, which had been an initial concern with CAC scanning. While there have been no subsequent large randomized clinical trials involving the effect of CAC scanning on patients management, its ability to aid in clinical management of selected asymptomatic patients is now endorsed in recent preventive guidelines [8].
Beyond its use for risk-stratifying asymptomatic individuals, there is now strong reason to consider applications of CAC scanning in symptomatic or asymptomatic patients being evaluated for obstructive CAD. Several of these potential uses of CAC scanning arise from its combination with the application of stress myocardial perfusion imaging (MPI) (Table). CAC scanning can be used to improve the selection of patients for stress MPI procedures, aid in the overall risk stratification and guidance of management in patients undergoing MPI, and improve the actual interpretation of MPI results. In the present issue of Circulation: Cardiovascular Imaging, Engbers et al report findings relating to two of the potential applications as they relate to MPI: the use of CAC scanning to select patients for stress MPI and the combined use of CAC scanning and MPI for predicting overall patient risk [9].
Table.
Clinical applications of combining coronary artery calcium scanning and myocardial perfusion imaging
|
Selection of patients for MPI
The potential exists to incorporate the results of CAC scanning into the Bayesian analysis of the likelihood in hemodynamically significant CAD. Given the dramatic reduction in the frequency of abnormal stress MPI procedures over the last two decades [10], a need for better selection of patients for cardiac imaging procedures has become imperative within our increasingly value-based environment. Since the MPI study is designed to evaluate ischemia—whether for diagnostic or prognostic reasons—the pre-test likelihood of ischemia is of paramount importance in determining the need for ischemia testing. The Diamond-Forrester classification of pre-test likelihood of obstructive CAD (11), while having proven of immense clinical importance over three decades, is currently inaccurate and overestimates CAD likelihood [12–13]. The potential use of CAC scanning for better selection of patients for stress imaging is based on an underappreciated proportional relationship between the magnitude of CAC abnormality and the likelihood of obstructive CAD (14–15). Of interest, Diamond and Forrester initially suggested the incorporation of coronary fluoroscopy in their landmark publication regarding the Bayesian assessment of CAD likelihood [11]. Data confirming this relationship with CT based CAC scanning was later reported by Budoff et al (16). Given the need to better predict the likelihood of ischemia, one might have expected that there would have by now been extensive investigation into the clinical use of CAC scanning for guiding patient selection for MPI testing. In this regard, a recent meta-analysis assessed all studies that have reported the relationship between CAC scan results and the frequency of myocardial ischemia on MPI over a 15 year period (2000 to 2015) [17]. During this time, there were 20 publications that examined the relationship between CAC results and myocardial ischemia. However, most of these were very small studies, and only five of these studies involved patient populations with >500 patients. This paucity reflects the relative lack of interest in exploring and developing this potential clinical application. Most notably, the meta-analysis revealed a literature that is quite deficient in reporting and analyzing clinical parameters that might influence the relationship between CAC abnormality and inducible imyocardial schemia, such as the presence and quality of patients’ chest pain.
In the present study, Engbers et al examined the relationship between CAC score and inducible myocardial ischemia in a large cohort of 4,897 patients, dwarfing the size of all prior publications in this regard [9]. All patients were referred for testing because of a clinical suspicion of CAD, and the vast majority of patients had an intermediate likelihood of CAD. As in prior studies, a proportional relationship was observed between the magnitude of CAC abnormality and the frequency of inducible myocardial ischemia. Yet while useful, this larger analysis still does not sufficiently establish how to best use CAC scanning for selecting patients for cardiac stress testing.
This is because in the aforementioned meta-analysis, there was a marked variation in the frequency of ischemia in each CAC subgroup [17]. For instance, among CAC scores of 0, the frequency of ischemia varied from 6.4% 28.6%, and among those with CAC scores >400, the frequency of ischemia ranged from 11.7% to 65.7%. Many factors could account for these differences, including differences in the acuity of the patients, the frequency of co-morbid medical conditions, the concentration of CAD risk factors, the intensity of medical therapies, and a variety of technical factors, including a propensity for readers at some centers to read myocardial perfusion studies with a greater or lesser threshold for interpreting studies as abnormal. To-date, there has only been limited study as to how these individual factors might govern the relationship between the magnitude of CAC abnormality and the likelihood of ischemia. The present study by Engbers et al serves to emphasize this important limitation in the literature. In the present study, the presence of ischemia was 12% among the patients with a normal CAC scan. Such a frequency would preclude the use of a zero CAC scan for excluding the likelihood of inducible myocardial ischemia in symptomatic patients. At the same time, the presence of ischemia among the patients with zero CAC scores did not serve to increase patients’ clinical risk.
This observation begs for further analysis. One of the possible explanations may be that the investigators employed too lenient a criterion for interpreting studies as abnormal. In the present study, a summed difference score ≥2 was employed to define ischemia. By contrast, in many institutions, a score ≥4 is employed to define abnormality. This difference is magnified by the finding that most ischemic defects among those without CAC were small defects. This finding may be the dominant reason for the increased frequency of abnormal MPI in this study compared to prior reports. Thus, how many studies that were characterized as mild ischemia in the present study might be characterized as normal at other institutions? One approach to addressing this clinical question is to report results according to standardized and unbiased quantitative analysis.
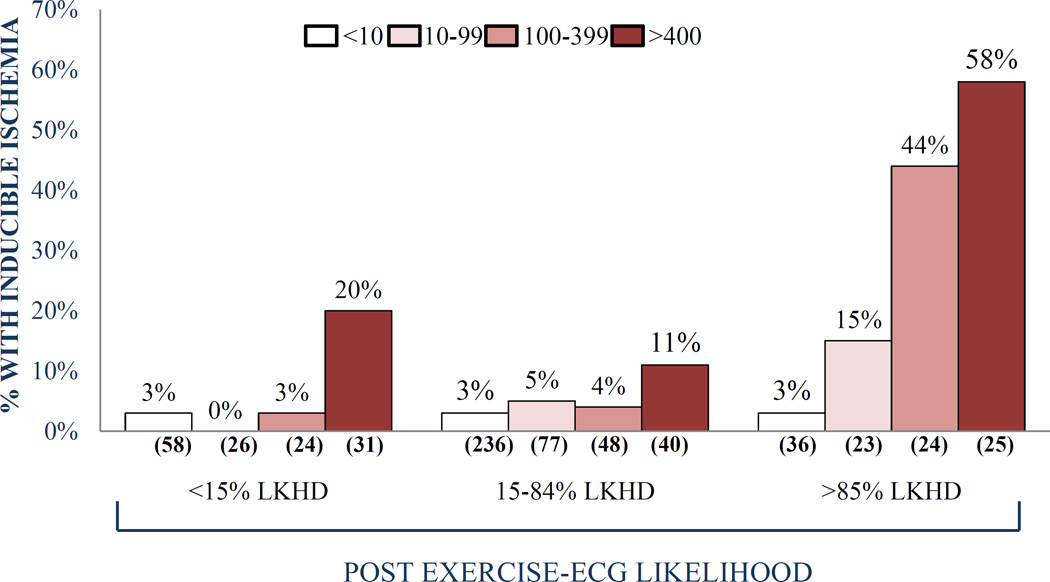
Alternatively, clinical factors may be important drivers of the higher rate of ischemia observed in the present study. Here, various clinical factors could be particularly relevant, such as gender, CAD risk factors, chest pain and exercise capacity. For instance, studies involving the comparison of diabetes to non-diabetics suggest the former have a higher frequency of inducible myocardial ischemia among subjects with an “intermediate” CAC score (100–400 range) [18]. In another study, the “threshold” CAC score for ischemia was substantially lower among patients with typical angina than in patients with atypical chest pain [19]. In a third study, exercise capacity modified the frequency of ischemia associated with intermediate CAC scores (20). Further, combining the results of chest pain and exercise ECG testing resulted in a markedly lower threshold of ischemia among patients with a high likelihood of CAD compared to patients with an intermediate likelihood of CAD (Figure) [19]. As in most prior studies, these potential modifiers of the relationship between CAC score and myocardial ischemia were not assessed in the present study. For instance, the study did not report chest pain symptoms and because pharmacologic testing was performed on a routine basis, no exercise data were available. Because the reported frequencies of ischemia according to CAC score range so widely, there is an important need to evaluate how clinical and technical factors modify this relationship.
Figure.
The frequency of ischemic stress/rest myocardial perfusion imaging (MPI) studies according to baseline coronary calcium scores among patients divided according to post-test likelihood (LKHD) of obstructive coronary artery disease (CAD), incorporating the results of age, gender, CAD risk factors, chest pain, and exercise ECG results. Both among patients with low and intermediate CAD likelihood, the frequency of ischemia was low for patients with CAC scores <400. By contrast, the CAC “threshold” for ischemia was substantially lower among patients with a high likelihood of CAD. Reproduced from Rozanski et al19, with permission of the publisher.©Springer 2007.
Combined use of MPI and CAC scanning for risk assessment
CAC scanning can serve as an adjunct to predicting clinical outcomes based on the results of stress imaging [21]. For instance, Chang et al followed 1,126 generally asymptomatic patients for a median of 6.9 years for the cardiac death, nonfatal myocardial infarction and the need for coronary revascularization [22]. In patients both with and without inducible myocardial ischemia, the event rate increased with increasing CAC score. The highest event rates occurred among both patients with high CAC scores and inducible myocardial ischemia. The present study of Engbers et al extends these findings to a more symptomatic population undergoing MPI with a hybrid SPECT/CT system. The added prognostic value of the CAC score to MPI in a symptomatic population was also previously assessed with hybrid PET/CT as reported by Schenker et al [23]. Such hybrid systems—whether SPECT/CT or PET/CT—provide the opportunity to routinely add CAC scanning in patients undergoing MPI.
In the present study, CAC abnormality and the presence of myocardial ischemia were found to provide synergistic information in predicting major adverse cardiac events (MACE), defined as death, non-fatal myocardial infarction and late revascularization. When the CAC score was zero, the frequency of MACE was very low, regardless of the presence of ischemia. For each subsequent CAC score grouping, MACE was more frequent if myocardial ischemia was also present. This study thus provides strong confirmatory evidence for the complementary use of CAC score and stress test results for predicting subsequent cardiac events.
Aid in interpretation of nuclear MPI studies
The use of CAC scanning might also aid the interpretation of MPI studies, particularly in the setting of borderline MPI abnormalities or when there is discordance between the MPI results and clinical or ECG responses to stress. In the presence of a borderline perfusion defect, the finding of a zero or low CAC score can lead to a test being interpreted as normal. In contrast, the finding of a borderline MPI study is found in a patient with extensive CAC or a high CAC score can lead to a study being interpreted as abnormal. The added value of combined CAC scanning with MPI with quantitative MPI analysis was recently demonstrated by Brodov et al, who found that this led to greater accuracy in predicting obstructive CAD [24]. This was accomplished by development of a novel combined CAC-MPI score, by logistic regression methods, which allowed assignment of the quantitive post-test probability of the obstructive disease on a per-vessel or per-patient basis in an objective quantitative manner. Such combined score does not need exact registration, but rather the per-vessel CAC score. It could be readily obtained in patients who have a prior CAC scan from a separate CT scanner or from a single session on a hybrid SPECT/CT or PET/CT scanner.
For future applications, modified protocols for attenuation correction have been proposed in which a separately acquired CAC scan could also be used with image registration to provide attenuation MPI maps that could eliminate soft-tissue artifacts by attenuation correction of the stand-alone SPECT or PET systems (25). The possibility that CAC scanning might improve assessments of MPI even with stand-alone SPECT systems has also been suggested. Schepis et al described that a separately acquired CAC scan could provide attenuation maps that could be used for attenuation correction of SPECT-MPI, thus potentially providing an aid to reducing image artifacts [26]. Further, software methods have been proposed for MPI to CT registration, which can register a single CAC or CT attenuation correction scan to both stress and rest MPI, replacing the two separate low-dose CT acquisitions currently required for attenuation correction (27). A simulation study also suggests that the combined use of CAC scanning with a stress only SPECT MPI study might potentially reduce the number of patients who require a subsequent rest MPI study (28).
Other future directions
In the coming era of value-based imaging, tests must improve outcomes or reduce costs. In order for an imaging test to improve outcomes, it must lead to a change in therapy. A drawback of stress imaging without anatomic assessment in patients with suspected CAD is that the methods detect only patients with hemodynamically significant lesions and fail to identify patients with subclinical atherosclerosis in whom aggressive medical and lifestyle modification might prevent subsequent cardiac events. Thus, there is little impact of normal MPI test results, found in the vast majority of patients, upon subsequent patient medical management. Since preventive therapies have been established to reduce cardiac events, identifying patients with normal MPI who have coronary atherosclerosis might lead to a change in preventive therapy which in turn may improve clinical outcomes. The ability of coronary CTA to assess the presence of both non-obstructive as well as obstructive CAD provides an advantage over stress imaging alone in guiding patient management [29]; however, CTA, when assessed for anatomic disease alone may has the potential to lead to an increased use of coronary angiography [13, 30]. The combination of stress MPI with CAC might result in a lower frequency of invasive coronary angiography than associated with coronary CTA, while at the same time resulting in effective initiation or cessation of preventive therapies. Thus, the combined functional and anatomic information of MPI with CAC scanning could increase the frequency of changes in management based on SPECT MPI alone.
The evidence to date suggests that the use of CAC scanning will aid in better selection of patients for MPI, improved diagnostic interpretation, improved prognostic assessment, and greater change in therapy after MPI. It is likely that this combination will be a strong factor in improving the “value” of MPI procedures. Is the routine combination of these two tests ready for prime time? It may not be proven, but it merits further study and even at this time should be strongly considered.
Supplementary Material
Footnotes
Disclosures
None.
REFERENCES
- 1.Budoff MJ, Shaw LJ, Liu ST, Weinstein SR, Mosler TP, Tseng PH, Flores FR, Callister TQ, Raggi P, Berman DS. Long-term prognosis associated with coronary calcification: observation from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49:1860–1870. doi: 10.1016/j.jacc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 2.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O'Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary Calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336-5. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 3.Erbel R, Mohlenkamp S, Moebus S, Schmermund A, Lehmann N, Stang A, Dragano N, Grönemeyer D, Seibel R, Kälsch H, Bröcker-Preuss M, Mann K, Siegrist J, Jöckel KH Heinz Nixdorf Recall Study Investigative Group. Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis: the Heinz Nixdorf Recall study. J Am Coll Cardiol. 2010;56:1397–1406. doi: 10.1016/j.jacc.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 4.Yeboah J, McCleland RL, Polonsky TS, Burke GL, Sibley CT, O'Leary D, Carr JJ, Goff DC, Greenland P, Herrington DM. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308:788–795. doi: 10.1001/jama.2012.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, Foster E, Hlatky MA, Hodgson JM, Kushner FG, Lauer MS, Shaw LJ, Smith SC, Jr, Taylor AJ, Weintraub WS, Wenger NK, Jacobs AK, Smith SC, Jr, Anderson JL, Albert N, Buller CE, Creager MA, Ettinger SM, Guyton RA, Halperin JL, Hochman JS, Kushner FG, Nishimura R, Ohman EM, Page RL, Stevenson WG, Tarkington LG, Yancy CW American College of Cardiology Foundation; American Heart Association. 2010 ACCF/AHA Guideline for Assessment of Cardiovascular Risk in Asymptomatic Adults: Executive Summary A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the American Society of Echocardiography, American Society of Nuclear Cardiology, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. J Am Coll of Cardiol. 2010;56:2182–2199. [Google Scholar]
- 6.Stone NJ, Robinson J, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr, Watson K, Wilson PW American College of Cardiology/American Heart Association Task Force on Practice Guidelines. ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults. A Report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;63:2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Rozanski A, Gransar H, Shaw LJ, Kim J, Miranda-Peats L, Wong N, Rana JS, Orakzai R, Hayes SW, Friedman JD, Thomson LE, Polk D, Min J, Budoff MJ, Berman DS. Impact of coronary artery calcium scanning on coronary risk factors and downstream testing: The EISNER (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research) prospective randomized trial. J Am Coll Cardiol. 2011;57:1622–1632. doi: 10.1016/j.jacc.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goff DC, Lloyd-Jones MD, Bennett G, Coady S, D'Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Jr, Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 9.Engbers EM, Timmer JR, Ottervanger JP, Mouden M, Knollema S, Jager PL. Prognostic value of coronary artery calcium scoring in addition to SPECT myocardial perfusion imaging in symptomatic patients. Circ Cardiovasc Imaging. 2016;9:e003966. doi: 10.1161/CIRCIMAGING.115.003966. [DOI] [PubMed] [Google Scholar]
- 10.Rozanski A, Gransar H, Hayes SW, Min J, Friedman JD, Thomson LE, Berman DS. Temporal trends in the frequency of inducible myocardial ischemia during cardiac stress testing: 1991–2009. J Am Coll Cardiol. 2013;61:1054–1065. doi: 10.1016/j.jacc.2012.11.056. [DOI] [PubMed] [Google Scholar]
- 11.Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med. 1979;300:1350–1358. doi: 10.1056/NEJM197906143002402. [DOI] [PubMed] [Google Scholar]
- 12.Cheng VY, Berman DS, Rozanski A, Dunning AM, Achenbach S, Al-Mallah M, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Chinnaiyan K, Chow BJ, Delago A, Gomez M, Hadamitzky M, Hausleiter J, Karlsberg RP, Kaufmann P, Lin FY, Maffei E, Raff GL, Villines TC, Shaw LJ, Min JK. Performance of the traditional age, sex, and angina typically-based approach for estimating pretest probability of angiographically significant coronary artery disease in patients undergoing coronary computed tomographic angiography: results from the multinational coronary CT angiography evaluation for clinical outcomes: an international multicenter registry (CONFIRM) Circulation. 2011;124:2423–2432. 1–8. doi: 10.1161/CIRCULATIONAHA.111.039255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Douglas PS, Hoffmann U. Anatomical versus Functional Testing for Coronary Artery Disease. N Engl J Med. 2015;373:91. doi: 10.1056/NEJMc1505594. [DOI] [PubMed] [Google Scholar]
- 14.He ZX, Hedrick TD, Pratt CM, Verani MS, Aquino V, Roberts R, Mahmarian JJ. Severity of coronary artery calcification by electron beam computed tomography predicts silent myocardial ischemia. Circulation. 2000;101:244–251. doi: 10.1161/01.cir.101.3.244. [DOI] [PubMed] [Google Scholar]
- 15.Berman DS, Wong ND, Gransar H, Miranda-Peats R, Dahlbeck J, Hayes SW, Friedman JD, Kang X, Polk D, Hachamovitch R, Shaw L, Rozanski A. Relationship between stress-induced myocardial ischemia and atherosclerosis measured by coronary calcium tomography. J Am Coll Cardiol. 2004;44:923–930. doi: 10.1016/j.jacc.2004.06.042. [DOI] [PubMed] [Google Scholar]
- 16.Budoff MJ, Diamond GA, Raggi P, Arad Y, Guerci AD, Callister TQ, Berman D. Continuous probabilistic prediction of angiographically significant coronary artery disease using electron beam tomography. Circulation. 2002;105:1791–1796. doi: 10.1161/01.cir.0000014483.43921.8c. [DOI] [PubMed] [Google Scholar]
- 17.Bavishi C, Argulian E, Chaterjee S, Rozanski A. CACS and the Frequency of Stress-Induced Myocardial Ischemia During MPI: A Meta-Analysis. JACC Cardiovasc Imaging. 2016 Apr 6; doi: 10.1016/j.jcmg.2015.11.023. pii: S1936-878X(16)30024-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Wong ND, Rozanski A, Gransar H, Miranda-Peats R, Kang X, Hayes S, Shaw L, Friedman J, Polk D, Berman DS. Metabolic syndrome and diabetes are associated with an increased likelihood of inducible myocardial ischemia among patients with subclinical atherosclerosis. Diabetes Care. 2005;28:1445–1450. doi: 10.2337/diacare.28.6.1445. [DOI] [PubMed] [Google Scholar]
- 19.Rozanski A, Gransar H, Wong ND, Shaw LJ, Miranda-Peats R, Hayes SW, Friedman JD, Berman DS. Use of coronary calcium scanning for predicting inducible myocardial ischemia: Influence of patients' clinical presentation. J Nucl Cardiol. 2007;14:669–679. doi: 10.1016/j.nuclcard.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 20.LaMonte MJ, FitzGerald SJ, Levine BD, Church TS, Kampert JB, Nichaman MZ, Gibbons LW, Blair SN. Coronary artery calcium, exercise tolerance, and CHD events in asymptomatic men. Atherosclerosis. 2006;189:157–162. doi: 10.1016/j.atherosclerosis.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 21.Rana JS, Rozanski A, Berman DS. Combination of myocardial perfusion imaging and coronary artery calcium scanning: potential synergies for improving risk assessment in subjects with suspected coronary artery disease. Curr Atheroscler Rep. 2011;13:381–389. doi: 10.1007/s11883-011-0192-1. [DOI] [PubMed] [Google Scholar]
- 22.Chang SM, Nabi F, Xu J, Peterson LE, Achari A, Pratt CM, Mahmarian JJ. The coronary artery calcium score and stress myocardial perfusion imaging provide independent and complementary prediction of cardiac risk. J Am Coll Cardiol. 2009;54:1872–1882. doi: 10.1016/j.jacc.2009.05.071. [DOI] [PubMed] [Google Scholar]
- 23.Schenker MP, Dorbola S, Hong EC, Rybicki FJ, Hachamovitch R, Kwong RY, Di Carli MF. Interrelation of coronary calcification, myocardial ischemia, and outcomes in patients with intermediate likelihood of coronary artery disease: a combined positron emission tomography/computed tomography study. Circulation. 2008;117:1693–1700. doi: 10.1161/CIRCULATIONAHA.107.717512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brodov Y, Gransar H, Dey D, Shalev A, Germano G, Friedman JD, Hayes SW, Thomson LE, Rogatko A, Berman DS, Slomka PJ. Combined Quantitative Assessment of Myocardial Perfusion and Coronary Artery Calcium Score by Hybrid 82Rb PET/CT Improves Detection of Coronary Artery Disease. J Nucl Med. 2015;56:1345–1350. doi: 10.2967/jnumed.114.153429. [DOI] [PubMed] [Google Scholar]
- 25.Kaster TS, Dwivedi G, Susser L, Renaud JM, Beanlands RS, Chow BJ, deKemp RA. Single low-dose CT scan optimized for rest-stress PET attenuation correction and quantification of coronary artery calcium. J Nucl Cardiol. 2015;22:419–428. doi: 10.1007/s12350-014-0026-y. [DOI] [PubMed] [Google Scholar]
- 26.Schepis T, Gaemperli O, Koepfli P, Rüegg C, Burger C, Leschka S, Desbiolles L, Husmann L, Alkadhi H, Kaufmann PA. Use of coronary calcium score scans from stand-alone multislice computed tomography for attenuation correction of myocardial perfusion SPECT. Eur J Nucl Med Mol Imaging. 2007;34:11–19. doi: 10.1007/s00259-006-0173-8. [DOI] [PubMed] [Google Scholar]
- 27.Zaidi H, Nkoulou R, Bond S, Baskin A, Schindler T, Ratib O, Declerck J. Computed tomography calcium score scan for attenuation correction of N-13 ammonia cardiac positron emission tomography: effect of respiratory phase and registration method. Int J Cardiovasc Imaging. 2013;29:1351–1360. doi: 10.1007/s10554-013-0207-9. [DOI] [PubMed] [Google Scholar]
- 28.Uretsky S, Cohen R, Argulian E, Balasundaram K, Supariwala A, Subero M, Sinha S, Paladugu K, DePuey EG, Rozanski A. Combining stress-only myocardial perfusion imaging with coronary calcium scanning as a new paradigm for initial patient work-up: An exploratory analysis. J Nucl Cardiol. 2015;22:89–97. doi: 10.1007/s12350-014-9958-5. [DOI] [PubMed] [Google Scholar]
- 29.SCOT-HEART investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicenter trial. Lancet. 2015;385:2383–2391. doi: 10.1016/S0140-6736(15)60291-4. [DOI] [PubMed] [Google Scholar]
- 30.Uretsky S, Argulian E, Supariwala A, Agarwal SK, El-Hayek G, Chavez P, Awan H, Jagarlamudi A, Puppala SP, Cohen R, Rozanski A. Comparative effectiveness of coronary CT angiography vs stress cardiac imaging in patients following hospital admission for chest pain work-up: The Prospective First Evaluation in Chest Pain (PERFECT) Trial. J Nucl Cardiol. 2016 doi: 10.1007/s12350-015-0354-6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.