Abstract
Objective
To test a predictive model of the associations among prepregnancy body mass index (BMI), third-trimester biological and behavioral variables, and symptoms of depression at 4 weeks postpartum.
Design
Secondary data analysis from a longitudinal, biobehavioral repeated measures study of women during the third trimester of pregnancy through 6 months postpartum.
Setting
Communities surrounding a midwestern and a western U.S. city.
Participants
Participants were 111 women enrolled in their third trimesters of pregnancy who were studied through 4 weeks postpartum.
Methods
Whole blood and saliva were used for biological measures, and validated questionnaires were used for behavioral measures. Principal component analysis and path analysis with principal component variables were used to iteratively test the model.
Results
There were three statistically significant direct effects in the model: the path from prepregnancy BMI to inflammation, the path from prepregnancy BMI to stress, and the path from stress to symptoms of depression at 4 weeks postpartum. Indirect effects of prepregnancy BMI on postpartum depression through intervening variables were not statistically significant, nor was the model-based total effect of prepregnancy BMI on postpartum depression.
Conclusion
Stress was significantly linked to prepregnancy BMI and postpartum depression. This finding highlights continuing possibilities for improving outcomes for mothers, infants, and families through stress-mitigating preventative strategies.
Keywords: biobehavioral predictive model, postpartum depression, prepregnancy obesity, inflammation, stress
Approximately 13% of new mothers will suffer from postpartum depression (PPD), making it the most frequent complication of childbirth (Beck, 2008; Centers for Disease Control and Prevention, 2009; Gaynes et al., 2005). Although there is an abundance of evidence regarding the incidence, prevalence, and psychosocial predictive factors of PPD, a persistent gap remains in the identification of women at high risk for developing PPD. A gap also exists regarding the possible biological determinants of PPD. The purpose of this study was to address this gap in knowledge by investigating the relationship between prepregnancy body mass index (BMI) and PPD, in particular, to test a predictive model of the associations among prepregnancy BMI, third trimester biological (pro-inflammatory cytokines interleukin-6 [IL-6] and tumor necrosis factor-alpha [TNF-α], leptin, and cortisol levels) and behavioral (symptoms of depression and perceived stress) variables, and symptoms of depression at 4 weeks postpartum.
Background
Major depressive disorders, including PPD, are recognized worldwide as leading causes of disability (Horowitz, Murphy, Gregory, & Wojcik, 2010). PPD has far-reaching consequences that extend beyond the maternal–infant dyad to the family unit, the community, and society at large. This devastating disorder is frequently undetected, and the mother experiences prolonged periods of emotional disturbance and disability; in severe cases, the disorder may lead to suicide or infanticide (Beck, 2002; Milgrom, Ericksen, & Gemmill, 2006). The quality of early maternal–infant relationships is a crucial component of early brain development, and the lack of a secure relationship may lead to impaired cognitive and emotional development in the infant and subsequent behavioral problems later in life (Knitzer, Theberge, & Johnson, 2008; Koutra et al., 2013; Letourneau, Tramonte, & Willms, 2013). Consequently, PPD is a public health problem.
Similarly, obesity is a worldwide public health problem that has reached epidemic proportions. It is estimated that 35.3% of all women over the age of 20 and 31.9% of women aged 20 to 39 years are obese (Flegal, Carroll, Kit, & Ogden, 2012). Additionally, gestational weight gain is on the rise (Chu, Callaghan, Bish, & D'Angelo, 2009). Prepregnancy obesity and excessive gestational weight gain are associated with potential short-term and long-term consequences for the mother and the infant, including the risk of depression (Corwin, 2011; Molyneaux, Poston, Ashurst-Williams, & Howard, 2014).
Emerging evidence suggests that prepregnancy obesity may be a biological determinant that increases susceptibility to PPD (Atlantis & Baker, 2008; LaCoursiere, Barrett-Connor, O'Hara, Hutton, & Varner, 2010; Luppino et al., 2010). In a recent study, investigators examined the association between maternal obesity and symptoms of PPD and anxiety in a group of severely obese women compared with women who were lean. After controlling for multiple confounding factors, PPD scores were significantly higher in severely obese women than lean women (p = 0.01; Mina et al., 2015). Likewise, in a recent systematic review and meta-analysis, investigators reported that women who were obese before pregnancy had significantly higher odds of developing PPD (OR 1.30, 95% CI [1.20-1.42]) compared with normal weight women (Molyneaux et al., 2014).
Emerging evidence suggests that prepregnancy body mass index may be a biological determinant that increases susceptibility to symptoms of postpartum depression.
The inflammatory state that accompanies excess adipose tissue may serve as a mediator between obesity and symptoms of depression. Compared with lean adipose tissue, obese adipose tissue secretes increased pro-inflammatory molecules, such as Interleukin 6 IL-6, TNF-α, and leptin (Dandona et al., 1998; Hotamisligil, Arner, Caro, Atkinson, & Spiegelman, 1995; Ziccardi et al., 2002). In studies of the non-pregnant population, investigators consistently link these pro-inflammatory molecules to symptoms of depression (Abassi, Hosseini, Modabbernia, Ashrafi, & Akhondzadeh, 2012; Krishnan et al., 2007; Tyring et al., 2006; Zautra et al., 2004).
Additionally, obesity is associated with chronic stress (DeVriendt, Moreno, & DeHenauw, 2009; Friedman et al., 2005). Chronic stress is linked to an individual’s inability to maintain homeostasis, which leads to physical and behavioral maladaptive responses. Physically, chronic stress produces changes in the hypothalamic–pituitary–adrenal (HPA) axis, specifically, hypercortisolism (Charmandari, Tsigos, & Chrousos, 2005). Behaviorally, chronic life stress is associated with symptoms of depression, and of particular interest is the association between perceived stress and symptoms of PPD (Corwin, Brownstead, Barton, Heckard, & Morin, 2005; Corwin et al., 2015; Gao, Chan, & Mao, 2009; Razurel, Kaiser, Sellenet, & Epiney, 2013).
Although the exact mechanisms by which these variables may alter human emotional behavior remain unclear, exposure to chronic inflammation and chronic stress may lead to altered signaling in the brain that results in cognitive and behavioral changes in vulnerable individuals. Investigations to explore these phenomena are crucial to expand knowledge and develop potential interventions aimed at improving health during this critical time for mother and baby. In our current study, we hypothesized that prepregnancy BMI would be positively associated with symptoms of depression at 4 weeks postpartum, as mediated by third-trimester pro-inflammatory markers (IL-6, TNF-α, and leptin) and biobehavioral markers of stress (symptoms of depression, perceived stress, and cortisol).
Methods
Design and Study Population
Our study was a secondary analysis of data from a grant awarded to the second author from the National Institutes of Health, National Institute of Nursing Research. The purpose of the primary study was to examine the interaction between the inflammatory immune response and the HPA axis and the possible association of this interaction with PPD. This study was a longitudinal, biobehavioral, repeated measures investigation of women during the third trimester of pregnancy through 4 weeks postpartum. Prenatal inclusion criteria included age between 18 and 40 years, pregnancy less than 36 weeks, anticipated vaginal birth of a singleton infant, and being free of chronic illness or pregnancy complications. Continued criteria for inclusion post-birth included an uncomplicated vaginal birth and discharge of mother and newborn together within 72 hours of birth. Women also needed to live within a 30-mile radius of the laboratory and be free of infection at the time of the postpartum home visit.
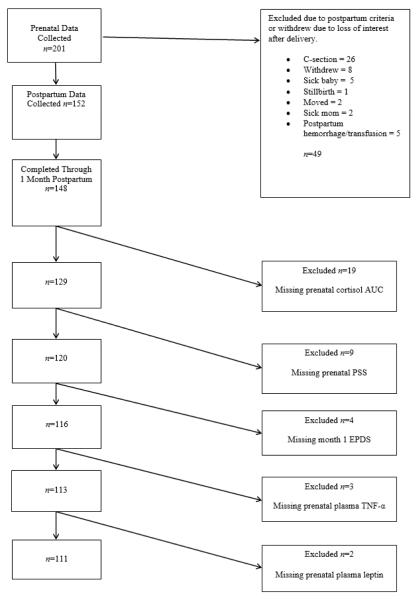
Women were recruited during the second or third trimester of pregnancy through flyers placed at support groups, prenatal clinics, and community billboards. Participants who met inclusion criteria during a screening phone call were scheduled for an initial home visit between 32 and 36 weeks of pregnancy. Prenatal data were collected from 201 women. Of these women, 49 (24%) were excluded due to postpartum criteria, whereas 4 others left the study post-birth prior to week 4 postpartum; a total of 148 women completed the study through 4 weeks postpartum. Missing data were approached using listwise deletion, and the progression to the final sample size of 111 women for the secondary analysis is shown in Figure 1. Based on this sample of 111 participants, an alpha of .05, and power of .80, this study had sufficient power to detect relatively small effect size correlations of r = 0.26.
Figure 1.
Participant loss flowchart. AUC = area under the curve; PSS = Perceived Stress Scale; EPDS = Edinburgh Postnatal Depression Scale; TNF-α = tumor necrosis factor alpha.
Procedures
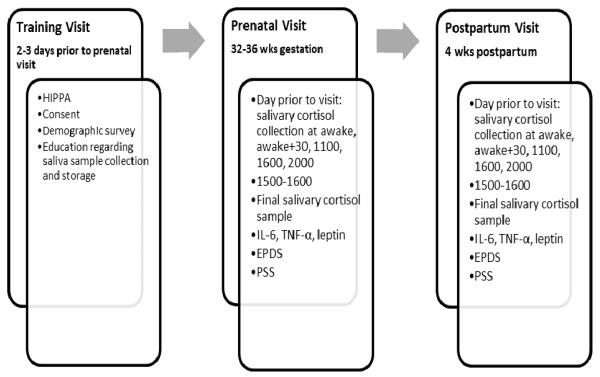
The study protocol was divided into three stages (see Figure 2). In stage 1, women were prepared for participation in the study. At the first home visit, the study protocol was reviewed and informed consent obtained. Participants completed a demographic survey that included age, race, ethnicity, household composition, marital status, educational status, height, and prepregnancy weight. Participants also were trained to collect saliva samples using saliva swabs to determine cortisol levels, and they practiced the technique under the supervision of the research nurse. The visit concluded with the scheduling of the prenatal visit within 7 days for sample collection and completion of questionnaires. Sample collection visits occurred between 3:30 and 4:30 pm to control for variability in cortisol and cytokine levels.
Figure 2.
Study timeline. HIPAA = Health Insurance Portability and Accountability Act; IL-6 = interleukin-6; TNF-α = tumor necrosis factor-alpha; EPDS = Edinburgh Postnatal Depression Scale; PSS = Perceived Stress Scale.
The protocols for the prenatal and postpartum visits were essentially the same. One day prior to the visit, participants collected saliva samples on awakening, 30 minutes later, and at 11:00 am, 4:00 pm, and 8:00 pm. During the scheduled home visit, women collected a fresh saliva sample and then completed the Edinburgh Postnatal Depression Scale (EPDS), the Perceived Stress Scale (PSS), and a current health survey. Blood was drawn from an antecubital vein into tubes that contained ethylenediaminetetraacetic acid for later measurement of the inflammatory molecules IL-6, TNF-α, and leptin. All samples were kept on ice for transport to the laboratory, where they were processed. At the end of the prenatal visit, subjects were compensated and received a reminder card to call within 2 days after the births of their infants.
Due to the crucial nature of the timing of cortisol sample collection to the study of HPA axis functioning, a detailed plan was developed to assist women with collecting samples on time. A reminder call was made to each woman the night before the scheduled collections, as well as approximately 5 minutes before each scheduled saliva collection time. Participants were compensated for each call answered. In addition, the saliva-sampling swabs were stored in a medication electronic monitoring system. Each time a sample was collected, the participant removed the swab’s bottle cap, which triggered an electronic recording of the event. These recordings were reviewed later, and participants were compensated if samples were collected at the appropriate times.
Measures
Body mass index
BMI is a commonly used measure of adiposity secondary to ease of measurement. It has been found to correspond fairly well with overall percentage of body fat (Flegal et al., 2009). BMI was calculated from self-reported prenatal weight and height. Although it is common to divide BMI into categories, for the purposes of this secondary analysis, BMI was treated as a continuous variable.
Questionnaires
The 10-item EPDS was used to screen for symptoms of depression (Cox, Holden, & Sagovsky, 1987). The EPDS is the most widely used PPD screening tool and has been validated in antepartum and postpartum populations (Gibson, McKenzie-McHarg, Shakespeare, Price, & Gray, 2009; Horowitz et al., 2010). Answers to statements such as I have looked forward with enjoyment to things are scored from 0 (As much as I ever did) to 3 (Hardly at all). A woman’s score is summed, with a total possible score of 0 to 30; higher scores correspond to higher levels of symptoms of PPD (Cox et al., 1987). In the original validation study, the EPDS had a specificity of 78%, a sensitivity of 86%, and a positive predictive value of 73% for symptoms of PPD, compared with a diagnostic clinical interview (Cox et al., 1987).
The PSS is a 14-item self-report measure of the degree to which an individual perceives life events as stressful over the past month (Cohen, Kamarck, & Memelstein, 1983). The responses are based on a Likert-scale from 0 (never) to 4 (very often). Responses are summed (range, 0 to 56), and higher scores are associated with higher levels of perceived stress. In the original study, the PSS demonstrated consistent reliability, with Cronbach alpha coefficients ranging from .84 to .86 in a young adult population (Cohen et al., 1983).
Biological measures
On reaching the laboratory, blood samples were centrifuged for 8 minutes at 2,000 rpm. Plasma aliquots were placed in 1.5-ml polypropylene microtubes and stored at –70°C until assayed using a Proinflammatory Ultra-Sensitive assay and quantitative multiplex array technology (Mesco Scale Discovery, Gaithersburg, Maryland). Intra-assay coefficient of variation was < 5%, and inter-assay coefficient of variation was < 10%. Leptin was assayed using quantitative multiplex array ELISA (enzyme-linked immunosorbent assay).
Saliva samples were centrifuged for 3 minutes at 3,000 rpm. Aliquots were placed in 1.5-ml polypropylene microtubes and stored at –70°C until assayed. Salivary cortisol was assayed using an expanded-range high-sensitivity enzyme immunoassay kit. The cortisol detection limit of the kit is .018 mg/dl and shows minimal cross-reactivity (4% or less) with other steroids. The intra-assay coefficient of variation was 4.3%, and the inter-assay coefficient of variation was 5.2%. The cortisol area under the curve (AUC) was calculated with respect to ground and used as the measurement to represent cortisol (Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, 2003).
Statistical Analysis
In addition to the primary independent and dependent variables of prepregnancy BMI and PPD symptoms at 4 weeks postpartum, two groups of intervening variables were considered. The first group was theoretically linked to inflammation and consisted of IL-6, TNF-α, and leptin levels. The second group was theoretically linked to stress and consisted of the lab-measured variable of third-trimester cortisol AUC levels and the questionnaire-measured variables of third-trimester EPDS score and third-trimester PSS score. Data analysis was conducted using IBM Statistical Package for the Social Sciences, Version 21, and maximum likelihood estimators were calculated in in IBM Statistical Package for the Social Sciences Analysis of Moment Structures, Version 21.
Descriptive statistics of means and standard deviations were calculated for all interval-level variables, and frequencies and percentages were calculated for categorical variables. The lab-measured variables of IL-6, TNF-α, leptin, and cortisol AUC were all log-transformed due to strong positive skewness, whereas the questionnaire-measured variables of prenatal EPDS score and EPDS score at 4 weeks postpartum were left untransformed to preserve their more widely understood scales.
Due to sample size considerations, component-based path analysis was used instead of structural equation modeling to test the proposed model via a multi-step process. First, principal component analysis was performed on the group of stress-linked variables and the group of inflammation-linked variables. Screening tests for principal component analysis included calculation of the Kaiser–Meyer–Olkin measure for sample adequacy and the use of Bartlett’s test of sphericity to test for overall deviation from the hypothesis of no intra-correlation. Components were extracted using the eigenvalue greater than one criterion and examined to confirm component loadings greater than .7, where applicable. Extracted components were then used in a path analysis model, which allowed for the calculation of direct, indirect, and total effects. Fit for the path model was examined using the Hu–Bentler criteria (Hu & Bentler, 1999).
Stress-linked variables in the third trimester of pregnancy were the strongest predictor of symptoms of depression at 4 weeks postpartum.
Results
Sample Characteristics
Demographic characteristics of the participants and descriptive statistics for each study variable are summarized in Tables 1 and 2. More than 80% of participants were White and married, and approximately 75% reported no history of depression. The mean (SD) age of study participants was 29.28 (5.08) years, with a range of 18 to 40 years. The mean (SD) BMI was 24.49 (3.99) kg/m2, with a range of 17.75 to 36.35 kg/m2.
Table 1.
Participant Characteristics (N = 111)
| Characteristic | Frequency | % |
|---|---|---|
| Body Mass Index Category | ||
| < 18.5 | 4 | 3.6 |
| 18.5-24.9 | 78 | 70.3 |
| 25-29.9 | 18 | 16.2 |
| ≥ 30 | 11 | 9.9 |
| Race | ||
| White | 92 | 82.9 |
| Black | 6 | 5.4 |
| Asian or Pacific Islander | 4 | 3.6 |
| American Indian or Alaska Native | 1 | 0.9 |
| Other | 8 | 7.2 |
| Marital Status | ||
| Single | 9 | 8.1 |
| Married | 94 | 84.7 |
| Partnered | 6 | 5.4 |
| Other | 2 | 1.8 |
| History of Depression | ||
| No | 82 | 73.9 |
| Yes | 29 | 26.1 |
Table 2.
Descriptive Statistics for Raw Data (N = 111)
| Variable | Mean | SD | Range | Skew | Kurtosis |
|---|---|---|---|---|---|
| BMI (kg/m2) | 23.49 | 3.99 | 17.75-36.35 | 1.128 | 1.174 |
| IL-6 (pg/ml) | 1.98 | 3.37 | 0.33-35.15 | 8.819 | 86.47 |
| TNF-α (pg/ml) | 6.37 | 4.41 | 0.00-22.31 | 1.737 | 2.851 |
| Leptin (ng/ml) | 45407.89 | 37981.64 | 2864-227080 | 1.984 | 5.281 |
| Cortisol AUC | 3.31 | 1.02 | 1.88-7.43 | 1.583 | 3.340 |
| Prenatal EPDS | 4.54 | 3.71 | 0.00-19.0 | 1.194 | 1.162 |
| Prenatal PSS | 20.10 | 6.37 | 7.0-43.0 | 0.943 | 1.442 |
| Month 1 EPDS | 3.31 | 3.32 | 0.00-19 | 1.654 | 4.267 |
Note. BMI = body mass index; IL-6 = interleukin-6; TNF-α = tumor necrosis factor-alpha; AUC = area under the curve; EPDS = Edinburgh Postnatal Depression Scale; PSS = Perceived Stress Scale; SD = standard deviation.
Model Characteristics
The initial theoretical model consisted of the independent and dependent variables of prepregnancy BMI and PPD symptoms at 4 weeks postpartum with two groups of intervening variables. Principal component analysis of the inflammation-linked variables of IL-6, TNF-α, and leptin showed a single inflammation component with an eigenvalue of one and a minimum component loading of greater than .6 (IL-6 = .852, TNF-α = .633, leptin = .685). This component explained 53.12% of the variance. In contrast, principal component analysis of the stress-linked variables of third-trimester EPDS score, third-trimester PSS score, and third-trimester cortisol AUC suggested a two-component structure in which third-trimester EPDS score and third-trimester PSS score loaded strongly (EPDS = .909, PSS = .904) onto the primary component and explained 84.36% of the variance. However, cortisol AUC had a loading of only 0.279 onto this component. Accordingly, cortisol AUC was considered separately from a stress component related to third-trimester EPDS score and third-trimester PSS score in the subsequent path analysis.
Based on the results of principal component analysis, the initial theoretical model was modified to include three intervening variables (inflammation, psychological stress, and cortisol). As shown in Table 3, model fit indicators were acceptable for a majority of the measures considered, although there were troubling indicators of large residuals. Modification indices were calculated in an attempt to develop a better fitting model. The modification indices threshold was set at 4. Using this threshold, the analysis resulted in no suggested modifications.
Table 3.
Model Fit Indices
| Fit Indices | χ2/df | CFI | NNFI | SRMR | RMSEA |
|---|---|---|---|---|---|
| Coefficient Value | 2.825/3 | 1.000 | 1.013 | 0.450 | 0.000 |
Note. CFI = comparative fit index; NNFI = non-normed fit index; SRMR = standardized root mean square residual; RMSEA = root mean square error of approximation. Good fit is indicated by CFI > 0.95, NNFI > 0.95, SRMR < 0.05, and RMSEA < 0.08.
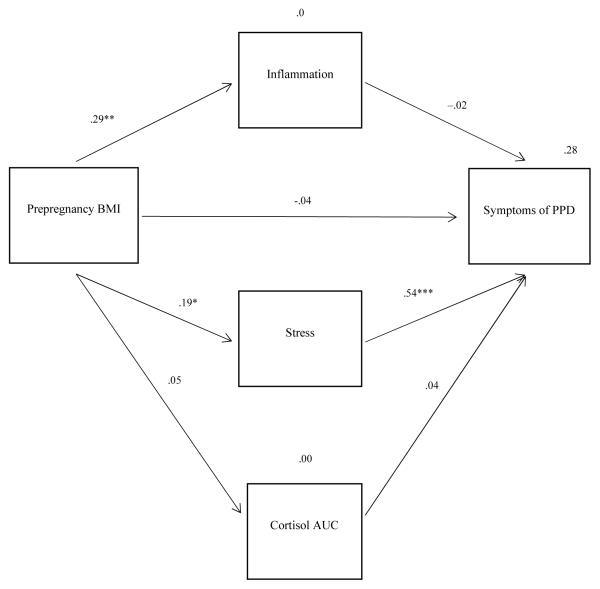
Figure 3 shows the path analysis model along with standardized path coefficients. Three statistically significant direct effects existed. The path from prepregnancy BMI to inflammation had a significant, moderate effect (standardized coefficient = .290, p = .001); the path from prepregnancy BMI to stress had a significant, small effect (standardized coefficient = .190, p = .043); and the path from stress to symptoms of depression at 4 weeks postpartum had a strong, significant effect (standardized coefficient = .538, p < .001). Due to weak and statistically nonsignificant direct effects in other portions of the model, indirect effects of prepregnancy BMI on PPD through intervening variables were all nonsignificant, as was the model-based total effect of prepregnancy BMI on PPD.
Figure 3.
Final model with standardized path coefficients. BMI = body mass index; PPD = postpartum depression; AUC = area under the curve. *p < .05. **p = .01. ***p < .001.
To better understand the separate and cumulative effects of the prenatal EPDS and PSS scores on postpartum EPDS score, we examined individual bivariate correlations and a multiple regression model. Prenatal EPDS scores (r = .484, p < .001) and prenatal PSS scores (r = .493, p < .001) were significantly correlated with EPDS scores at 4 weeks postpartum. Additionally, multiple regression analysis with both variables as predictors of postpartum EPDS score showed that prenatal EPDS scores (b = .241, t = 2.38, p = .019) and prenatal PSS scores (b = .158, t = 2.69, p = .008) were significant predictors of postpartum EPDS scores. Moreover, the prenatal PSS scores alone explained 24.3% of the variance in the postpartum EPDS scores, whereas for the overall model with the prenatal PSS scores and the prenatal EPDS scores, 28.3% of the variance in postpartum EPDS scores was explained.
Discussion
Our findings did not support a model in which prepregnancy BMI was associated with symptoms of PPD. However, analysis of the data revealed several findings of interest, including the relationship between stress in the third trimester of pregnancy and symptoms of depression at 4 weeks postpartum. This principal component of stress-linked variables represented symptoms of depression and perceived stress. It is generally accepted that perceived stress, prenatally and postpartum, is associated with symptoms of PPD (Corwin et al., 2005; Corwin et al., 2015; Gao et al., 2009). However, typical prenatal screening consists only of a self-report measure of symptoms of depression, such as the EPDS. Stress and symptoms of depression are two very different constructs, and neglecting to screen for stress in addition to symptoms of depression may lead to the lack of identification of a large group of women who are at high risk for development of prenatal or PPD.
During pregnancy, stress-screening protocols may be used to identify a large group of women at risk for symptoms of depression during the early postpartum period.
Furthermore, researchers are now using a biological susceptibility model aimed to identify women who are genetically susceptible to environmental influence. In a study of 1,206 mothers, evidence was found of a significant gene × environment interaction. However, more importantly, evidence was found that by altering the stressful environment, the genetic effect could be altered (Mitchel et al., 2011). This emerging knowledge, combined with the knowledge that prenatal stress is one of the most significant predictors of PPD, brings to light the need for significant reform to current screening practices for at-risk women. This may include the use of biomarkers, a comprehensive set of screening tools, and an adjustment in the timing of screening.
A second important finding in our study was the association between prepregnancy BMI and third-trimester inflammatory markers. These findings add to the knowledge surrounding obesity in pregnancy. Results from our study as well as other published studies support the fact that obesity in pregnancy is an inflammatory state. Although not specifically examined in this study, it is thought that many of the short-term and long-term consequences associated with obesity in pregnancy stem from this inflammatory state. For example, Coussons-Read et al. (2012) found a positive association between increased levels of pro-inflammatory cytokines and an increased risk of preterm birth. Additionally, authors of a recent systematic review of the literature reported that IL-6 levels in women with preeclampsia were significantly higher than in pregnant women without preeclampsia (mean difference = 7.96 pg/ml, 95% CI [4.72, 11.10]; Lau et al., 2013).
Although the mechanisms by which this inflammatory state may alter human mental health remain unclear, published research involving non-pregnant individuals consistently supports an association between an activated immune system and symptoms of depression (Abassi et al., 2012; Capuron et al., 2009; Krishnan et al., 2007). However, investigations involving pregnant and postpartum women offer conflicting results, and recent findings from our group highlight the complexity of the relationship between inflammation, stress, and PPD, reinforcing the need to consider the interaction between inflammatory cytokines and the stress response (Blackmore et al., 2011; Cassidy-Bushrow, Peters, Johnson, & Templin, 2012; Corwin & Pajer, 2008; Corwin et al., 2015). Additional reasons for these inconsistencies in the above studies may arise from the lack of use of a standard measure to screen for symptoms of depression in pregnant and postpartum women. The use of a measure that is not specifically designed to measure symptoms of PPD may significantly alter the validity of the results (Beck, 2001). Likewise, lack of knowledge of the complex maternal–fetal immune system, coupled with inconsistent sampling of biomarkers, may contribute to these discrepancies.
Increased stress is a common predictor of deprssion symptoms, and a common biological finding associated with depression in non-pregnant and non-postpartum individuals is elevated levels of cortisol (Charmandari et al., 2005). As mentioned earlier, cortisol AUC did not load on to either primary component and was considered separately in our model. Current theories suggest that a potential reason for this is that hypercortisolism is an indicator of chronic HPA axis dysfunction as opposed to a correlate of a measure of perceived stress in the past month (Tafet & Nemeroff, 2015). Additionally, during pregnancy and the postpartum period, the normal physiological functioning of the HPA axis is significantly altered. Levels of corticotropin-releasing hormone (CRH), adrenocorticotropic hormone, and cortisol all increase significantly during pregnancy secondary to increased production of CRH from the placenta and fetal membranes (Kalantaridou et al., 2010). Cortisol levels peak during the third trimester of pregnancy, and following delivery, these hormones return to prepregnancy levels. Due to the association of elevated levels of cortisol with symptoms of depression in the general population, many researchers have focused on this alteration as a potential determinant in the etiology of PPD. However, research in this population does not support an independent relationship between prenatal cortisol levels and symptoms of depression (Corwin et al., 2005; Groer & Morgan, 2007). The findings of our study are consistent with published research reporting no relationship between third-trimester cortisol AUC and symptoms of depression at 4 weeks postpartum. This further supports the conclusion that cortisol levels in pregnancy may not be a reliable predictor of PPD in this population.
Finally, we hypothesized that prepregnancy BMI would be positively associated with symptoms of depression at 4 weeks postpartum as mediated by third-trimester pro-inflammatory markers and biobehavioral markers of stress. Results of the path analysis failed to support this hypothesis. Available published research provides support for an association between prepregnancy BMI and symptoms of PPD. In a large study, women (N = 3,439) who were obese prepregnancy (BMI ≥ 29 kg/m2) had a 1.5 times greater likelihood of reporting increased depression symptoms (OR = 1.53, 95% CI [1.15, 2.02]) at 2 to 6 months postpartum than women who were normal weight (BMI, 19.9-25.9 kg/m2; LaCoursiere, Baksh, Bloebaum, & Varner, 2006). Molyneaux et al. (2014) found similar results in a recent systematic review and meta-analysis. A total of 4,687 studies were reviewed, of which 62 met the criteria for inclusion. These 62 studies included 540,373 women. Studies were included if researchers assessed the symptoms of depression at any time during pregnancy or within the first year postpartum. Women who were obese prepregnancy had 1.43 times the odds of suffering prenatal symptoms of depression compared with women who were not obese (OR = 1.43, 95% CI [1.27, 1.61], p < .001) and had 1.3 times the odds of developing symptoms of PPD than did normal-weight women (OR = 1.3, 95% CI [1.20, 1.42], p < .001; Molyneaux et al., 2014).
Importantly, the time frame used for assessment of symptoms of PPD is not consistent across the literature. The American Psychological Association (2013) now uses the term peripartum depression, which is inclusive of the onset of depression symptoms during pregnancy or in the first 4 weeks after birth. In our secondary analysis, symptoms of depression were measured at 4 weeks postpartum. The time frames used in the previously cited studies ranged from 6 weeks to 1 year postpartum. Lack of consensus regarding the time frame for the diagnosis of PPD is common in research (O'Hara & McCabe, 2013). Authors of a more recent study (N = 1,507) reported that one in five women experienced symptoms of depression during pregnancy or in the first 12 months postpartum (Woolhouse, Gartland, Mensah, & Brown, 2014). Therefore, extending the time frame to 12 months postpartum may greatly increase the identification of women suffering from symptoms of PPD.
Limitations
As with all studies, several limitations were present in our study. In a secondary analysis of data, several factors are predetermined in the parent study, such as sample size. A larger sample size would have increased the power of this study to detect an effect and may have increased the representativeness of the sample. For example, in our sample of 111 women, the BMI range was 17.75 kg/m2 to 36.35 kg/m2, but the mean was 23.49 kg/m2. Additionally, more than 80% of the women were White, pointing to limited inclusion of minority women. Continued enrollment criteria chosen by the principal investigator also may have affected the representativeness of the sample. For example, in the original study, women who experienced cesarean births were excluded from the study because of the potential for surgical birth to alter the inflammatory profile of the participant. Although this may have been relevant to the original study, inflammatory markers used in this study were measured in the third trimester of pregnancy, well before the surgical birth.
Current published research shows that obesity is associated with an increased rate of a cesarean birth (Hollowell et al., 2014). Consequently, exclusion of this subset of women from the study may have biased the results. Relatedly, the large drop in sample size (see Figure 1) from enrollment to analysis secondary to missing data may have reduced statistical power, and any data elements that were not missing at random could have biased analyses. Further, in this secondary analysis, the principal investigator identified variables pertinent to the original study a priori based on theoretical underpinnings most relevant to the study’s purpose. Self-reported height and weight, for example, might have been replaced, instead, with clinical measures had they been elements of primary data collection. Finally, due to the data included in the dataset available for analysis, it was not possible to compute estimates of reliability for the EPDS and PSS.
Clinical Implications
It is well documented that PPD has devastating consequences for all involved. The findings of our study point to several opportunities to improve the outcomes of women, infants, and their families. Most important is the finding of a strong, significant association between the complex construct of stress and symptoms of PPD, as well as the finding that the PSS score alone accounted for a significant amount of the variation in postpartum EPDS scores. These findings point to the need for a paradigm shift in prenatal care, with the emphasis being on risk evaluation and preventative strategies as opposed to diagnosis and treatment of depressive symptoms. In a recent Cochrane Review, authors reported that early identification of mothers at high risk for PPD assisted in preventing approximately one third of the cases of PPD (RR = 0.66, [95% CI 0.50-0.88]; Dennis & Dowswell, 2013). The American College of Obstetricians and Gynecologists (2015) currently recommends that providers screen for symptoms of depression and anxiety at least once during the perinatal period. However, investigators have identified significant psychosocial risk factors for PPD, including prenatal depression and anxiety, as well as major life events, lack of social support, substance abuse, a history of trauma, and a previous history of depression (Austin, 2014; Beck, 2001; Milgrom et al., 2008). This information suggests that current strategies for screening, health promotion, and prevention of illness, specifically mental health in pregnancy and postpartum, may need to change to include screening for psychosocial stressors. Nurses are ideally suited to play a key role in this process through the development of early comprehensive screening programs and interventions that promote healthy behaviors and a positive psychological status (Dennis & Dowswell, 2013).
A second important finding in our study was the association between prepregnancy BMI and increased inflammatory markers. This information adds to the existing scientific knowledge that obesity in pregnancy is associated with a pro-inflammatory state. There is a growing body of evidence that increased levels of pro-inflammatory molecules are associated with adverse pregnancy outcomes. Armed with this knowledge, nurses can assist women with setting realistic weight management goals, bearing in mind that even small reductions in BMI can dramatically improve the woman’s inflammatory profile and may subsequently prevent adverse outcomes (Dandona et al., 1998; Ziccardi et al., 2002). Additionally, nurses can develop evidence-based lifestyle modification programs to promote healthy prepregnancy weight, maintain healthy gestational weight gain, and minimize postpartum weight retention to optimize maternal and newborn outcomes.
Recommendations for Future Research
Building on the findings of our study, future research is needed to develop a comprehensive, biobehavioral screening program to identify women at high risk for developing depression, prenatally and postpartum. Evidence from our study suggests that a single tool designed to measure symptoms of depression alone may not be adequate to capture at-risk women. Future research is needed to explore a comprehensive set of tools with which to best identify this group of vulnerable women before the onset of depression symptoms and allow for the initiation of preventative interventions and prevention of adverse outcomes.
Furthermore, evidence from our study did not support inflammatory markers in pregnancy as a biological predictor of symptoms of PPD. Continued research is needed to fill the gap in knowledge regarding potential biological determinants of PPD. For example, researchers are beginning to explore potential gene × environment interactions in the development of depressive symptoms in pregnancy and postpartum. Results from our study support that stress-linked variables are the strongest predictor of symptoms of PPD. Therefore, examination of genetic biomarkers involved in the complex physiologic stress response may lead to important new knowledge. Specific genotypes may alter a woman’s resilience against chronic environmental stressors. Of particular relevance for nurse researchers is the interaction of these specific genotypes with the environment. Although the specific genotype is not changeable, exploration of these gen × environment interactions and the association with PPD symptoms may identify paths among variables that are modifiable through nursing intervention.
Conclusion
In general, researchers have focused on psychosocial risk factors that contribute to the development of PPD, neglecting potential biological determinants. Although our findings did not support a relationship between prepregnancy BMI and symptoms of depression at 4 weeks postpartum, the association between stress-linked variables and symptoms of depression at 4 weeks postpartum has important implications for nursing practice and future research. Our findings highlight the need for continued research to expand the knowledge regarding biological determinants of PPD. Identification of biobehavioral pathways in the etiology of depression in pregnancy and the postpartum period may elucidate opportunities for preventative strategies that significantly improve outcomes for mothers, infants, and families.
Acknowledgement
Funded in part by an award from the National Institutes of Health (R01NR011278). The authors thank Anne Mattarella for her assistance with this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure The authors report no conflict of interest or relevant financial relationships. Nancy K. Lowe is the editor of JOGNN. She was excluded from the peer review and editorial decision for this article.
Contributor Information
Sharon L. Ruyak, University of New Mexico, College of Nursing, Albuquerque, NM..
Elizabeth J. Corwin, Emory University, Nell Hodgson Woodruff School of Nursing, Atlanta, GA..
Nancy K. Lowe, University of Colorado–Denver, College of Nursing, Aurora, CO..
Madalynn Neu, University of Colorado–Denver, College of Nursing, Aurora, CO..
Blake Boursaw, University of New Mexico, College of Nursing, Albuquerque, NM..
References
- Abassi S, Hosseini F, Modabbernia A, Ashrafi M, Akhondzadeh S. Effect of celecoxib add-on treatment on symptoms and serum IL-6 concentrations on patients with major depressive disorders: A randomized double-blind placebo-controlled study. Journal of Affective Disorders. 2012;141:308–314. doi: 10.1016/j.jad.2012.03.033. [DOI] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists Committee opinion no. 630: Screening for perinatal depression. Obstetrics and Gynecology. 2015;125(5):1268–1271. doi: 10.1097/01.AOG.0000465192.34779.dc. [DOI] [PubMed] [Google Scholar]
- Atlantis E, Baker M. Obesity effects on depression: Systematic review of epidemiological studies. International Journal of Obesity. 2008;32:881–891. doi: 10.1038/ijo.2008.54. [DOI] [PubMed] [Google Scholar]
- Austin M-P. Marcé International Society position statement on psychosocial assessment and depression screening in perinatal women. Best Practice & Research Clinical Obstetrics & Gynaecology. 2014;28(1):179–187. doi: 10.1016/j.bpobgyn.2013.08.016. http://doi.org/10.1016/j.bpobgyn.2013.08.016. [DOI] [PubMed] [Google Scholar]
- Beck CT. Predictors of postpartum depression: An update. Nursing Research. 2001;50:275–285. doi: 10.1097/00006199-200109000-00004. [DOI] [PubMed] [Google Scholar]
- Beck CT. Postpartum depression: A metasynthesis. Qualitative Health Research. 2002;12:453–472. doi: 10.1177/104973202129120016. [DOI] [PubMed] [Google Scholar]
- Beck CT. State of the science on postpartum depression: What nurse researchers have contributed—Part 1. The American Journal of Maternal/Child Nursing. 2008;33:121–126. doi: 10.1097/01.NMC.0000313421.97236.cf. [DOI] [PubMed] [Google Scholar]
- Blackmore ER, Moynihan JA, Rubinow DR, Pressman EK, Gilchrist M, O'Connor TG. Psychiatric symptoms and proinflammatory cytokines in pregnancy. Pschosomatic Medicine. 2011;73:656–673. doi: 10.1097/PSY.0b013e31822fc277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Fornwalt FB, Knight BT, Harvey PD, Ninan PT, Miller AH. Does cytokine-induced depression differ from idiopathic major depression in medically healthy individuals? Journal of Affective Disorders. 2009;119:181–185. doi: 10.1016/j.jad.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy-Bushrow AE, Peters RM, Johnson DA, Templin TN. Assoication of depressive symptoms with inflammatory biomarkers among pregnant African American women. Journal of Reproductive Immunology. 2012;94:202–209. doi: 10.1016/j.jri.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Pregnancy risk assessment monitoring system (PRAMS): PRAMS and postpartum depression. 2009 Retrieved from http://www.cdc.gov/PRAMS/PPD.htm.
- Charmandari E, Tsigos C, Chrousos GP. Endocrinology of the stress response. Annual Review of Physiology. 2005;67:259–284. doi: 10.1146/annurev.physiol.67.040403.120816. [DOI] [PubMed] [Google Scholar]
- Chu SY, Callaghan WM, Bish CL, D'Angelo D. Gestational weight gain by body mass index among US women delivering live births, 2004-2005: Fueling future obesity. American Journal of Obstetrics and Gynecology. 2009;200:271–273. doi: 10.1016/j.ajog.2008.09.879. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Memelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- Corwin E. Biological link between maternal obesity and postpartum depression; Paper presented at the Western Institute of Nursing Research Conference; Las Vegas, NV. Apr, 2011. [Google Scholar]
- Corwin E, Brownstead J, Barton N, Heckard S, Morin K. The impact of fatigue on the development of postpartum depression. Journal of Obstetric, Gynecologic, and Neonatal Nursing. 2005;34:577–586. doi: 10.1177/0884217505279997. [DOI] [PubMed] [Google Scholar]
- Corwin E, Pajer K. The psychoneuroimmunology of postpartum depression. Journal of Women's Health. 2008;17:1529–1534. doi: 10.1089/jwh.2007.0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin EJ, Pajer K, Paul S, Lowe N, Weber M, McCarthy DO. Bidirectional psychoneuroimmune interactions in the early postpartum period influence risk of postpartum depression. Brain, Behavior, and Immunity. 2015;49:86–93. doi: 10.1016/j.bbi.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussons-Read ME, Lobel M, Carey JC, Kreither MO, D’Anna K, Argys L, Cole S. The occurrence of preterm delivery is linked to pregnancy-specific distress and elevated inflammatory markers across gestation. Brain, Behavior, and Immunity. 2012;26:650–659. doi: 10.1016/j.bbi.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: Development of the 10-item Edinburgh Postnatal Depression Scale. The British Journal of Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- Dandona P, Weinstock R, Thusu K, Abdel-Rhaman E, Aljad A, Wadden T. Tumor necorosis factor-alpha in sera of obese patients: fall with weight loss. Journal of Clinical Endocrinology and Metabolism. 1998;83:2907–2910. doi: 10.1210/jcem.83.8.5026. [DOI] [PubMed] [Google Scholar]
- Dennis CL, Dowswell T. Psychosocial and psychological interventions for preventing postpartum depression. Cochrane Database of Systematic Reviews. 2013;2013(2):1–211. doi: 10.1002/14651858.CD001134.pub3. [DOI] [PubMed] [Google Scholar]
- DeVriendt T, Moreno LA, DeHenauw S. Chronic stress and obesity in adolescents: Scientific evidence and methodological issues for epidemiological research. Nutrition, Metabolism, & Cardiovascular Diseases. 2009;19:511–519. doi: 10.1016/j.numecd.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Shepard JA, Looker AC, Graubard BI, Borrud LG, Ogden CL, Schenker N. Comparisons of percentage of body fat, body mass index, waist circumfrence, and waist-stature ratio in adults. American Journal of Clinical Nutrition. 2009;89:500–508. doi: 10.3945/ajcn.2008.26847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman KE, Reichmann SK, Costanzo PR, Zelli A, Ashmore JA, Musante GJ. Weight stigmatization and ideological beliefs: Relation to psychological functioing in obese adults. Obesity Research. 2005;13:907–916. doi: 10.1038/oby.2005.105. [DOI] [PubMed] [Google Scholar]
- Gao L, Chan SW, Mao Q. Depression, perceived stress, and social support among first time Chinese mothers and fathers in the postpartum period. Research in Nursing and Health. 2009;32:50–58. doi: 10.1002/nur.20306. [DOI] [PubMed] [Google Scholar]
- Gaynes BN, Gavin N, Meltzer-Brody S, Lohr KN, Swinson T, Gartlehner G, Miller WC. Perinatal depression: Prevalence, screening accuracy, and screening outcomes. Agency for Healthcare Research and Quality; Rockville, MD: 2005. AHRQ Publication No. 05-E006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson J, McKenzie-McHarg K, Shakespeare J, Price J, Gray R. A systematic review of studies validating the Edinburgh Postnatal Depression Scale in antepartum and postpartum women. Acta Psychiatrica Scandinavica. 2009;119:350–364. doi: 10.1111/j:1600-0447.2009.01363.x. [DOI] [PubMed] [Google Scholar]
- Groer MW, Morgan K. Immune, health, and endocrine characteristics of depressed postpartum mothers. Psychoneuroendocrinology. 2007;32:133–139. doi: 10.1016/j.psyneuen.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Hollowell J, Pillas D, Rowe R, Linsell L, Knight M, Brockelhurst P. The impact of maternal obesity on intrapartum outcomes in otherwise low risk women: Secondary analysis of the birthplace national prospective cohort study. BJOG. 2014:343–355. doi: 10.1111/1471-0528.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz JA, Murphy CA, Gregory KE, Wojcik J. A community-based initiative to identify mothers at risk for postpartum depression. Journal of Obstetric, Gynecologic, and Neonatal Nursing. 2010;40:52–61. doi: 10.1111/j.1552-6909.2010.01199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. Jounal of Clinical Investigation. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Bentler P. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- Kalantaridou SN, Zoumakis E, Makrigiannakis A, Lavasidis LG, Vrekoussis T, Chrousos GP. Corticotropin-releasing hormone, stress and human reproduction: An update. Journal of Reproductive Immunology. 2010;85:33–39. doi: 10.1016/j.jri.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Knitzer J, Theberge S, Johnson K. Reducing maternal depression and its impact on young children: Toward a responsive early childhood framework. National Center for Children in Poverty; New York, NY: 2008. [Google Scholar]
- Koutra K, Chatzi L, Bagkeris M, Vassilaki M, Bitsios P, Kogevinas M. Antenatal and postnatal materanl health as determinants of infant neurodevelopment at 18 months of age in a maternal-child cohort (Rhea Study) in Crete, Greece. Social Psychiatry and Psychiatric Epidemiology. 2013;48:1335–1345. doi: 10.1007/s00127-012-0636-0. [DOI] [PubMed] [Google Scholar]
- Krishnan R, Cella D, Leonardi C, Papp K, Gottlieb AB, Dunn M, Jahreis A. Effects of etanercept therapy on fatigue and symptoms of depression in subjects treated for moderate to severe plaque psoriasis for up to 96 weeks. British Journal of Dermatology. 2007;157:1275–1277. doi: 10.1111/j.1365-2133.2007.08205.x. [DOI] [PubMed] [Google Scholar]
- LaCoursiere DY, Baksh L, Bloebaum L, Varner MW. Maternal body mass index and self-reported postpartum depressive symptoms. Maternal and Child Health Journal. 2006;10:385–390. doi: 10.1007/s10995-006-0075-1. [DOI] [PubMed] [Google Scholar]
- LaCoursiere DY, Barrett-Connor E, O'Hara MW, Hutton A, Varner MW. The association between prepregnancy obesity and screening positive for psotpartum depression. International Journal of Obstetrics and Gynecology. 2010;117:1011–1018. doi: 10.1111/j.1471-0528.2010.02569.x. [DOI] [PubMed] [Google Scholar]
- Lau SY, Guild SJ, Barrett CJ, Chen Q, McCowan L, Jordan V, Chamley LW. Tumor necrosis factor-alpha, interleukin-6, and interleukin-10 levels are altered in preeclampsia: A systematic review and meta-analysis. American Journal of Reproductive Immunology. 2013;70:412–427. doi: 10.1111/aji.12138. [DOI] [PubMed] [Google Scholar]
- Letourneau NL, Tramonte L, Willms D. Maternal depression, family functioning, and children's longitudinal development. Journal of Pediatric Nursing. 2013;28:223–234. doi: 10.1016/j.pedn.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Luppino F, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx B, Zitman FG. Overweight, obesity, and depression: A systematic review and meta-analysis of longitudinal studies. Archives of General Psychiatry. 2010;67:220–229. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- Milgrom J, Ericksen J, Gemmill AW. Stressful impact of depression on early maternal-infant relations. Stress and Health. 2006;22:229–238. doi: 10.1002/smi.1101. [DOI] [Google Scholar]
- Milgrom J, Gemmill AW, Bilszta JL, Hayes B, Barnett B, Brooks J, Buist A. Antenatal risk factors for postnatal depression: A large prospective study. Journal of Affective Disorders. 2008;108:147–157. doi: 10.1016/j.jad.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Mina TH, Denison FC, Forbes S, Stirrat LI, Norman JE, Reynolds RM. Associations of mood symptoms with ante- and postnatal weight change in obese pregnancy are not mediated by cortisol. Psychological Medicine. 2015;45:3133–3146. doi: 10.1017/S0033291715001087. http://doi.org/10.1017/S0033291715001087. [DOI] [PubMed] [Google Scholar]
- Mitchel C, Notterman D, Brooks-Gunn J, Hobcraft J, Garfinkel I, Jaeger K, McLanahan S. Role of mother's genes in environment in postpartum depression. PNAS. 2011;108:8189–8193. doi: 10.1073/pnas.1014129108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneaux E, Poston L, Ashurst-Williams S, Howard LM. Obesity and mental disorders during pregnancy and postpartum a systematic review and meta-analysis. Obstetrics and Gynecology. 2014;123:857–867. doi: 10.1097/AOG.000000000000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara MW, McCabe JE. Postpartum depression: current status and future directions. Annual Review of Clinical Psychology. 2013;9:379–407. doi: 10.1146/annurev-clinpsy-050212-185612. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of area under the curve represent measures of total hormone concentration versus time-dependent chnage. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/S0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Razurel C, Kaiser B, Sellenet C, Epiney M. Relation between perceived stress, social support, and coping strategies and maternal well-being: a review of the literature. Women and Health. 2013;53:74–99. doi: 10.1080/03630242.2012.732681. [DOI] [PubMed] [Google Scholar]
- Tafet GE, Nemeroff CB. The Links Between Stress and Depression: Psychoneuroendocrinological, Genetic, and Environmental Interactions. The Journal of Neuropsychiatry and Clinical Neurosciences. 2015 doi: 10.1176/appi.neuropsych.15030053. appineuropsych15030053. http://doi.org/10.1176/appi.neuropsych.15030053. [DOI] [PubMed] [Google Scholar]
- Tyring S, Gottlieb AB, Papp K, Gordon K, Leonardi C, Wang A, Krishnan R. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: Double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367(9504):29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- Woolhouse H, Gartland D, Mensah F, Brown SJ. Maternal depression from early pregnancy to 4 years psotpartum in a prospective pregnancy cohort study: Implications for primary care. BJOG. 2014:1–11. doi: 10.1111/1471-0528.12839. [DOI] [PubMed] [Google Scholar]
- Zautra AJ, Yocum DC, Villanueva I, Smith B, Davis MC, Attrep J, Irwin M. Immune activation and depression in women with rheumatoid arthritis. The Journal of Rheumatology. 2004;31:457–463. [PubMed] [Google Scholar]
- Ziccardi P, Nappo F, Giugliano G, Esposito K, Marfella R, Cioffi M, Giugliano D. Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circulation. 2002;105:804–809. doi: 10.1161/hc0702.104279. [DOI] [PubMed] [Google Scholar]