Abstract
Background
Vascular health factors frequently co-occur with Alzheimer’s disease (AD). A better understanding of how systemic vascular and cerebrovascular health intersects with clinical and pathological AD may inform prevention and treatment opportunities.
Objective
To establish the Vanderbilt Memory & Aging Project, a case-control longitudinal study investigating vascular health and brain aging, and describe baseline methodology and participant characteristics.
Methods
From September 2012 to November 2014, 335 participants age 60–92 were enrolled, including 168 individuals with mild cognitive impairment (MCI, 73 ± 8 years, 41% female) and 167 age-, sex-, and race-matched cognitively normal controls (NC, 72 ± 7 years, 41% female). At baseline, participants completed a physical and frailty examination, fasting blood draw, neuropsychological assessment, echocardiogram, cardiac MRI, and brain MRI. A subset underwent 24-hour ambulatory blood pressure monitoring and lumbar puncture for cerebrospinal fluid (CSF) collection.
Results
As designed, participant groups were comparable for age (p = 0.31), sex (p = 0.95), and race (p = 0.65). MCI participants had greater Framingham Stroke Risk Profile scores (p = 0.008), systolic blood pressure values (p = 0.008), and history of left ventricular hypertrophy (p = 0.04) than NC participants. As expected, MCI participants performed worse on all neuropsychological measures (p-values<0.001), were more likely to be APOE ε4 carriers (p = 0.02), and had enhanced CSF biomarkers, including lower Aβ42 (p = 0.02), higher total tau (p = 0.004), and higher p-tau (p = 0.02) compared to NC participants.
Conclusion
Diverse sources of baseline and longitudinal data will provide rich opportunities to investigate pathways linking vascular and cerebrovascular health, clinical and pathological AD, and neurodegeneration contributing to novel strategies to delay or prevent cognitive decline.
Keywords: Alzheimer’s disease, biomarkers, brain MRI, cardiac MRI, mild cognitive impairment, vascular risk factors
INTRODUCTION
As the population continues to age, cognitive decline and dementia are a rapidly growing public health issue. Alzheimer’s disease (AD) prevalence is expected to triple by 2050 [1]. While pharmacological targets to arrest or slow disease progression continue to be developed and evaluated, there is an urgent need to concurrently identify risk factors that contribute to the pathophysiology and clinical manifestation of AD for primary and secondary prevention purposes.
Vascular health is one major category of risk factors that is increasingly emphasized in the AD literature. Markers of systemic vascular health, such as blood pressure, vascular stiffness, and heart failure, are becoming more frequently linked to incident clinical AD [2] as well as cognitive [3], neuroimaging [4], cerebrospinal fluid (CSF) [5], and positron emission tomography [6] markers of pathological AD. Clinical and epidemiological data from members of our group suggest that, independent of shared vascular risk factors, even modest reductions in systemic hemodynamics (as assessed by cardiac output) are associated with clinically detectable neuropsychological impairment [7–9], reduced gray matter volume [9], increased white matter hyperintensities (WMH) [10], reduced normative cerebral perfusion values [11], and incident dementia, especially AD [12]. Collectively, research from our group and others suggest vascular health factors correlate with clinical AD. Whether this association is causal remains unknown.
In September 2012, we initiated a clinical research resource locally known as the Vanderbilt Memory & Aging Project. The overarching goal of this study is to test cross-sectional and longitudinal associations between both systemic vascular and cerebrovascular factors and brain health for the purpose of better understanding how vascular health relates to unhealthy brain changes with age. The novelty of our study lies in the role subclinical vascular disease plays in accelerating abnormal brain aging in older adults prior to the onset of clinical dementia, utilizing neuropsychological, neuroimaging, and CSF outcomes. Insights gained from this research may contribute to future novel strategies to delay or prevent cognitive decline. In the current paper, we describe the design, sampling, methodology (with an emphasis on brain aging phenotypes), and participant characteristics of the baseline Vanderbilt Memory & Aging Project cohort enrolled between September 2012 and November 2014.
MATERIAL AND METHODS
Study cohort eligibility and selection
The Vanderbilt Memory & Aging Project is a case-control longitudinal study investigating vascular health and brain aging launched in September 2012 at Vanderbilt University Medical Center in Nashville, TN. Participants were recruited through postal mailings, clinical referrals, radio advertisements, newsletters, research distribution emails, community events, websites, and word of mouth. Participants were required to speak English, have adequate auditory and visual acuity for testing, and have a reliable study partner (i.e., an informant the participant knew a minimum of 2 years at study enrollment with weekly contact and knowledge of the participant’s cognitive and functional abilities). Participants were excluded if they had MRI contraindication (i.e., ferrous metal in the body or history of claustrophobia), a history of neurological disease (e.g., dementia, multiple sclerosis), stroke, heart failure, major psychiatric illness (e.g., bipolar disorder, schizophrenia), head injury with loss of consciousness >5 min, chronic obstructive pulmonary disease, or a systemic or terminal illness (e.g., cancer) that could impact participation in follow-up examinations. The protocol was approved by the Vanderbilt University Medical Center Institutional Review Board, and written informed consent was obtained from all participants prior to data collection.
Participants meeting initial inclusion and exclusion criteria completed a 4-h screening visit, including a medical history and record review, a Clinical Dementia Rating (CDR) [13] interview with the participant and informant, an assessment of activities of daily living (Functional Activities Questionnaire) [14], and a comprehensive neuropsychological protocol (see Table 1). This information was used by a consensus team to determine study eligibility, including cognitive diagnosis (i.e., normal cognition (NC) and mild cognitive impairment (MCI)).
Table 1.
Neuropsychological Protocol – Screening Visit
| Domain | Test | Description | Range |
|---|---|---|---|
| Global Cognition | Montreal Cognitive Assessment [78] | Briefly measures global cognitive status | 0–30 |
| Learning and Memory | Selective Reminding Test Immediate Recall [79] | Measures immediate recall of verbal material using multiple list-learning trials with a selective reminding paradigm | 0–72 |
| Selective Reminding Test Delayed Recall [79] | Assesses delayed recall of a list of words | 0–12 | |
| Brief Visual Retention Test [80] | Measures recall for geometric designs | 0–10 | |
| Language | Vegetable Naming [81] | Assesses the ability to rapidly generate words from a specified category | n/a |
| Boston Naming Test-30 Item Odd Version [82] | Assesses confrontation naming and lexical retrieval abilities | 0–30 | |
| Attention | WAIS-III Digit Span, Forward [83] | Measures auditory attention span | 0–16 |
| Information Processing | Trail Making Test, Part A [84] | Measures speeded visual search and scanning | 0–150 |
| Executive Functioning | WAIS-III Digit Span, Backward [83] | Measures auditory working memory | 0–14 |
| Trail Making Test, Part B [84] | Measures sequencing and mental flexibility in a number and letter set-shifting task | 0–300 | |
| Stroop Color Word Inhibition Test [85] | Measures the ability to inhibit a rote response in favor of a novel response | 0–180 | |
| Controlled Oral Word Association [81] | Measures the ability to rapidly generate words from a specified letter category (CFL) | n/a | |
| Visuospatial Ability | WAIS-IV Block Design [86] | Measures visual perception and organizational abilities | 0–68 |
| Premorbid Function | Wide Range Achievement Test-3rd Edition Reading Subtest [16] | Measures reading ability for words with irregular sound to spelling correspondence | 0–57 |
| Mood | Geriatric Depression Scale [87] | Assesses features of depressed mood in older adults | 0–30 |
WAIS-III, Wechsler Adult Intelligence Scale 3rd Edition; WAIS-IV, Wechsler Adult Intelligence Scale 4th Edition.
Participants labeled as NC were required to have (a) CDR = 0 (no dementia), (b) no deficits in activities of daily living directly attributable to cognitive impairment, and (c) no evidence of neuropsychological impairment defined as standard scores falling 1.5 standard deviations within the age-adjusted normative mean.
MCI determinations are based upon National Institute on Aging/Alzheimer’s Association Workgroup core clinical criteria [15] defined as (a) CDR = 0 or 0.5 (reflecting mild severity of impairment), (b) relatively spared activities of daily living, (c) objective impairment for any neuropsychological test within a cognitive domain (i.e., performances falling greater than 1.5 standard deviations outside the age-adjusted normative mean or pre-morbid level of functioning based on educational attainment, occupational history, and Wide Range Achievement Test 3rd Edition Reading Subtest performance [16]), (d) concern of a cognitive change by the participant, informant or clinician based on information obtained during the clinical interview, and (e) absence of a dementing syndrome.
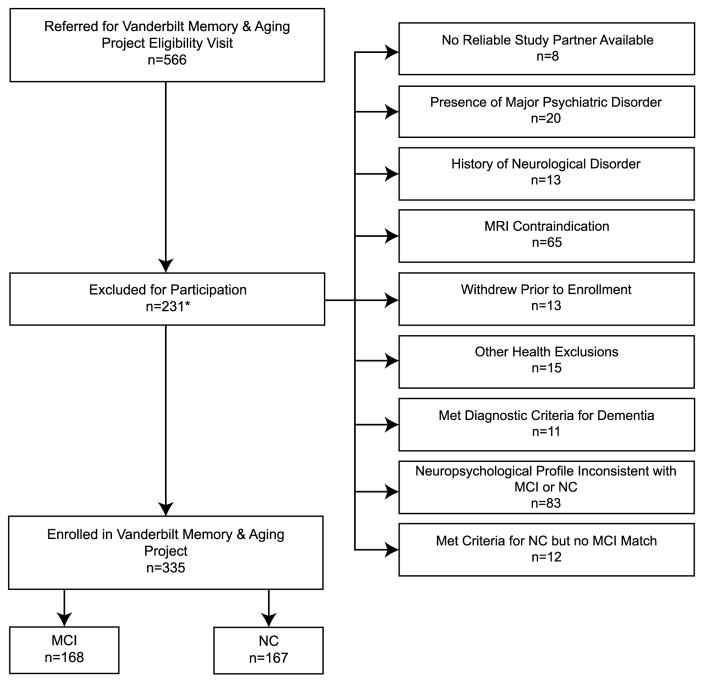
Given the case-control design, each MCI participant was enrolled first and then an age (defined as ±4 years), sex, and race (defined as White or non-White) NC ‘match’ was enrolled. See Fig. 1 for details on participant selection. Baseline procedures, conducted between September 2012 and November 2014, are described below. Longitudinal follow-up of the cohort is underway with procedures repeating at 18-month intervals for a minimum of 3 years (i.e., baseline, 18-month follow-up, and 36-month follow-up).
Fig. 1.
Participant Inclusion/Exclusion Details. *Exclusion categories delineated on the right side of the figure are not mutually exclusive. Specifically, of the 9 participants assigned to more than one category, 8 were included in Neuropsychological Profile Inconsistent with MCI or NC and in at least one other category (i.e., 2 in MRI Contraindication, 3 in Presence of Major Psychiatric Disorder, 2 in No Reliable Study Partner Available, and one in History of Neurological Disorder). Another participant was categorized both in Met Criteria for NC but no MCI Match and MRI Contraindication.
Fasting blood work, lumbar puncture, and biochemical analyses
A morning fasting venous blood draw was performed. A series of blood work was immediately performed, including complete blood count with differential and platelet count, comprehensive metabolic panel, lipid profile (with high density lipoprotein), high sensitivity C-reactive protein, anti-cardiolipin antibody, thyroid stimulating hormone, and hemoglobin A1C. A serum-based insulin assay was also processed. Remaining samples (i.e., 20 mLs of whole blood without anticoagulate reagents added for collecting serum, 10 mLs of EDTA anticoagulated whole blood for collecting plasma, and 2.5 mLs of whole blood in Paxgene tube) were prepared for storage at Vanderbilt. Plasma was separated from whole blood by centrifugation at 2000 g and 4°C for 15 min and stored in ten 0.5 mL aliquots. Remaining blood (including red and white cells) was stored separately in a single 5 mL tube for DNA analysis. Serum was separated by centrifuge at 2000 g and 4°C for 15 min and stored in two 0.5 mL aliquots and seven 1 to 1.5 mL aliquots. The remaining 4 to 5 mL blood clot was stored separately as a backup resource for DNA extraction. After processing and preparation, samples were stored at −80°C, including the Paxgene tube which was immediately frozen.
Plasma samples were analyzed in batch using the Milliplex Map Kit (EMD Millipore Corporation, Billerica, MA, Cat. No. HCCBP1MAG-58K) to measure leptin, interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) and vascular endothelial growth factor A (VEGF-A) according to manufacturer instructions. Seven working standards were generated by serial dilution (1 : 3) of the reconstituted standard provided in the kit. Two quality control (QC) samples were included in each plate run. Assay plate was read on Luminex 200 with XPONENT software. Milliplex Analyst 5.0 was used for data analysis. The mean intra-assay coefficients of variation (CVs) were as follows: leptin 5.6%, IL-6 5.9%, TNF-α 6.6%, and VEGF-A 5.9%.
For apolipoprotein E (APOE) genotyping, frozen whole blood was used for white blood cell extraction. Genotyping was performed using the commercially available TaqMan® single-nucleotide polymorphism genotyping assay from Applied Biosystems (Foster City, California, USA). Polymerase chain reaction (PCR) was carried out in 384-well plates in 5 μl reactions containing 5 ng of genomic DNA, Assays-on-Demand probe/primer mix, and TaqMan® Universal PCR Master Mix. Plates were scanned on a Life Technologies 7900HT real-time PCR machine after 50 cycles of standard PCR on a Life Technologies 9700 Thermal Cycler, and analyzed using Life Technologies SDS 2.4.1 software. Negative and positive controls were included on the plate for assay validation. Genotyping efficiency was >99%, and all duplicates were concordant as expected.
As an optional procedure, 233 participants consented to a morning fasting lumbar puncture. A total of 155 participants successfully completed the procedure to collect a maximum of 25 mLs of CSF with polypropylene syringes using a Sprotte 25-gauge spinal needle in an intervertebral lumbar space in the region between L2 and L5. Two mLs were drawn off for waste. Three mLs were processed for gross appearance, nucleated cells, total cell count, lymphocyte percentage, monocyte/macrophage percentage, glucose, and total protein. The remaining 20 mLs were immediately mixed and centrifuged at 2000 g and 4°C for 10 min. Supernatants were aliquoted in forty 0.5 mL polypropylene tubes and stored at −80°C. Samples were analyzed in batch using commercially available enzyme-linked immunosorbent assays (Fujirebio, Ghent, Belgium) to determine the levels of Aβ1-42 (INNOTEST® β-AMYLOID(1-42)), total tau (INNOTEST® hTAU), and tau phosphorylated at threonine 181 (p-tau; INNOTEST® PHOSPHO-TAU(181P)). The INNOTEST Aβ1-42 method employs antibodies that specifically bind the neo-epitopes of the first and last amino acid of the 42 amino acid-long Aβ sequence, thus measuring the full 42 amino acid-long Aβ42 form and not any N-terminally truncated species [17]. Additionally, Aβ forms ending at amino acid 38 and 40 were measured using the Aβ Triplex Assay (Meso Scale Discovery, Rockville, MD). This assay uses end-specific antibodies to capture Aβ peptides ending at amino acid 38, 40, or 42 and 6E10 (specific to amino acids 3 to 8) to detect them (the assay can thus measure both Aβ1-38/40/42 as well as N-terminally truncated Aβ forms). Meso Scale Discovery Aβ will hereafter be designated AβX-40 and AβX-42. Processing was completed by board certified laboratory technicians who were blinded to clinical information, as previously described [18]. Intra-assay CVs were <10%.
Physical examination and frailty assessment
Each participant completed a physical examination, including assessment of height, weight, hip circumference, and waist circumference. Frailty was assessed using standardized criteria from the Cardiovascular Health Study [19] as follows
Shrinkage/unintentional weight loss assessed by asking the participant if s/he has lost ≥10 lbs in weight over the previous 12 months or there has been a change >5% in body weight. If yes, the participant met this criterion.
Poor endurance/exhaustion assessed using two Center for Epidemiologic Studies Depression Scale [20] items: (a) I felt that everything I did was an effort, and (b) I could not get going. For each item, the participant is asked “How often in the last week did you feel this way?” with four response options: 0 = rarely or none of the time (<1 day), 1 = some or a little of the time (1–2 days), 2 = moderate amount of time (3–4 days), or 3 = most of the time. Participants endorsing either item with a 2 or 3 met this criterion.
Low physical activity assessed using a short version of the Minnesota Leisure Time Activity Questionnaire (MLTA), assessing weekly physical activity [21]. Sex-stratified cut-offs defined low physical activity as follows: men <383 kilo-calories per week and women <270 kilocalories per week.
Slowness assessed by asking participants to complete a 15-feet walk test. Sex- and height-stratified cut-offs defined slowness as follows:≥6 s for men with a height ≤173 cm or women with a height ≤159 cm;≥7 s for men with a height >173 cm or women with a height >159 cm.
Weakness defined by mean dominant hand grip strength measured across 3 trials using a hand held dynamometer. Sex- and body mass index (BMI)-quartile stratified cut-offs defined weakness as BMI ≤24 and grip strength ≤29 kg, BMI >24 but ≤28 and grip strength ≤30 kg, BMI >28 and grip strength ≤32 for men. For women, weakness was defined as BMI ≤23 and grip strength ≤17 kg, BMI >23 but ≤26 and grip strength ≤17.3 kg, BMI >26 but ≤29 and grip strength ≤18 kg or BMI >29 and grip strength ≤21 kg.
The presence or absence of each frailty criterion was summed for a possible range of 0–5. Participants were categorized as frail ≥3, pre-frail = 1 or 2, and not frail = 0. All frailty criterion were also recorded as continuous or ordinal measurements, including shrinkage (BMI), poor endurance (ordinal sum of exhaustion items ranging 0–6), low physical activity (kilocalories/week), slowness (gait speed in m/s), and weakness (mean dominant hand grip strength across 3 trials). Using these continuous or ordinal measurements, a composite frailty score was also constructed by converting each component raw score to a z-score using the cohort’s baseline mean stratified by sex and standard deviation and then computing an average z-score. Higher z-scores indicated poorer performance (higher frailty) [22].
Lifestyle questionnaires
Participants completed self-report questionnaires assessing physical activity, sleep, and nutrition habits as detailed below:
MLTA estimates leisure time physical activity beyond activities of daily living [21]. We applied the MLTA abbreviated version to assess caloric expenditure [19]. Participants estimated (in min/week) the frequency of physical activities performed in the past 2 weeks. Activity was expressed in expended kilocalories per week.
Community Healthy Activities Model Program for Seniors estimates physical activity by assessing caloric expenditure for activities typically undertaken by older adults [23]. Participants report frequency and duration of activities performed over a typical week during the past month, including sedentary activities (e.g., reading) to minimize social desirability. Caloric expenditure is calculated by multiplying the estimated duration of each activity by the metabolic equivalent value and then summing across activities.
National Cancer Institute Quick Food Scan estimates fat intake as a percentage of total energy [24]. Participants rate their frequency of consuming 15 food groups and reduced-fat butter or margarine over the last 12 months ranging 1 = never to 8 = more than once per day. Reduced-fat margarine usage as a replacement for butter is rated separately. Scoring is based on weighted least-squares estimates of regression coefficients for data from the U.S. Department of Agriculture’s 1994–1996 Continuing Survey of Food Intakes by Individuals [24]. The algorithm accounts for food frequency with age- and sex-specific portion sizes. The final score is expressed as total estimated percentage of dietary energy from fat.
Pittsburgh Sleep Quality Index assesses sleep quality over the previous month using 19 self-rated questions covering seven components of sleep health (i.e., sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medications, and daytime dysfunction [25]). Each component is scored from 0 = not during the past month to 3 = three or more times a week, then summed for a total ranging 0–21. Higher scores denote poorer sleep health. There are 5 additional questions for bed partners (i.e., completed by the informant if they had first-hand knowledge of the participant’s sleep behaviors), which are not included in the total score.
Subjective cognitive decline and functional activity questionnaires
Participants and their informants completed a series of questionnaires assessing perceived cognitive decline and functional activities. These questionnaires were not used for determination of diagnostic status or eligibility at screening.
Cognitive Changes Questionnaire is derived from frequently used cognitive decline questions [26–29]. Items assess memory and thinking changes, including comparing the participant’s cognition to same-aged peers, comparing the participant’s current cognition to past cognition, and rating the participant’s ability to complete daily tasks. Questions are answered on a 2-item (“yes” or “no”) or 3-item scale (“major problem, ” “minor problem, ” “no problem”). Total scores range 14–85 with higher scores indicating greater perceived change. Both participants and informants completed this questionnaire.
Everyday Cognition Questionnaire measures functional activities within memory, language, visuospatial, and executive function domains [26]. Each item rates the participant’s current ability compared to 10 years ago on a 4-item Likert scale where 1 = better or no change and 4 = consistently much worse. Total scores range 39–156 with higher scores indicating greater decline. Both participants and informants completed this questionnaire.
Cognitive Difficulties Scale is a 41-item measure of the frequency of everyday cognitive difficulties, such as trouble recalling frequently used phone numbers, trouble recalling the names of familiar people, and trouble thinking of the names of objects [28]. Questions are answered using a 5-item Likert scale where 0 = not at all and 4 = very often. Total scores range 0–164 with higher scores indicating greater cognitive difficulties. Only participants completed this questionnaire.
Memory Functioning Questionnaire measures participant ratings of current memory abilities [27]. Responses rely on a 7-item scale across six subcategories. First, global memory is rated from 1 = major problems to 7 = no problems. Second, memory failure frequency is rated on a scale of 1 = always to 7 = never. Third, individuals rate their short term memory (last month) and long term memory (6–10 years ago) on a scale of 1 = very badly to 7 = very well. Fourth, severity of actual memory failures are rated 1 = very serious to 7 = not serious. Fifth, mnemonic strategy use is rated as 1 = always to 7 = never. Finally, temporal comparisons of memory abilities are characterized as 1 = much worse to 7 = much better. Items were reverse scored so that a low score (minimum = 64) represented no memory complaints and a high score (maximum = 448) represented more memory complaints. Only participants completed this questionnaire.
Functional Capacities for Activities of Daily Living captures the participant’s changes in cognition and ability to complete everyday functional activities, such as watching television programs, using the stove, and telling time [30]. Questions are answered using a 5-item Likert scale ranging from 1 = not at all to 5 = very much. Total scores range 50–250 with higher scores indicating greater functional activity changes. Only informants complete this questionnaire.
Neuropsychological assessment
At baseline, all participants completed a common, comprehensive neuropsychological protocol assessing global cognition, language, information processing speed, executive functioning, visuospatial ability and episodic learning and memory. Measures were carefully selected to preclude floor or ceiling effects, enabling both detection of early cognitive decline and tracking of longitudinal changes. See Table 2 for details. A subset of participants (n = 231) also completed an addendum protocol assessing episodic learning and memory and visual and verbal working memory. These latter measures were selected to supplement the primary protocol to enhance detection of nuances in neuropsychological performance across the cognitive aging spectrum using an analysis of process and errors as previously suggested [31, 32]. See Table 3 for details. Note, baseline and addendum protocol performances were not used as part of the screening or selection of participants into the study.
Table 2.
Primary neuropsychological protocol: baseline and follow-up visits
| Domain | Test | Description | Range |
|---|---|---|---|
| Global Cognition | Montreal Cognitive Assessment [78] | Briefly measures global cognitive status | 0–30 |
| Learning and Memory | California Verbal Learning Test-II Trials 1–5 Total Learning [88] | Assesses total learning for a list of 16 words across 5 learning trials | 0–80 |
| California Verbal Learning Test-II Long Delay Free Recall [88] | Assesses delayed retrieval for a list of 16 words after a 20-minute delay | 0–16 | |
| Biber Figure Learning Test Trials 1–5 Total Learning [89] | Assesses immediate visuospatial learning for a set of 15 geometric designs presented across 5 learning trials | 0–225 | |
| Biber Figure Learning Test Delayed Recall [89] | Assesses delayed visuospatial learning for a set of 15 geometric designs after a 20-minute delay | 0–45 | |
| Language | Boston Naming Test-30 Item Even Version [82] | Assesses confrontation naming and lexical retrieval abilities | 0–30 |
| Animal Naming [81] | Measures the ability to rapidly generate words from a specified category | n/a | |
| Information Processing | WAIS-IV Coding [90] | Measures visual scanning and coding | 0–93 |
| DKEFS Color-Word Interference Test: Color Naming [91] | Measures speed for naming colors | 0–90 | |
| DKEFS Color-Word Interference Test: Word Reading [91] | Measures speed for word reading | 0–90 | |
| DKEFS Number Sequencing [91] | Measures visual scanning and attention in a number sequencing task | 0–150 | |
| Executive Functioning | DKEFS Tower Test [91] | Measures planning and problem solving abilities | 0–30 |
| DKEFS Number-Letter Switching [91] | Measures sequencing and mental flexibility in a number and letter set-shifting task | 0–240 | |
| DKEFS Color-Word Interference Test: Inhibition [91] | Measures ability to inhibit a conditioned response in favor of a novel response | 0–180 | |
| Letter Fluency [92] | Measures the ability to rapidly generate words from a specified letter category (FAS) | n/a | |
| Visuospatial Ability | Hooper Visual Organization Test [93] | Measures visual perceptual functioning and object recognition | 0–30 |
| Mood | Geriatric Depression Scale [87] | Assesses features of depressed mood in older adults | 0–30 |
DKEFS = Delis Kaplan Executive Function System; WAIS-IV = Wechsler Adult Intelligence Scale 4th Edition.
Table 3.
Addendum neuropsychological protocol: baseline and follow-up visits
| Domain | Test | Description | Range |
|---|---|---|---|
| Learning and Memory | Philadelphia Verbal Learning Test Trials 1–5 Total Recall [94] | Measures immediate recall of verbal learning using multiple-trial list-learning task | 0–60 |
| Philadelphia Verbal Learning Test Delayed Recall [94] | Assesses delayed recall of a list-learning task | 0–12 | |
| Working Memory | Backward Digit Task [95, 96]* | Measures auditory working memory | 0–21 |
| WMS-IV Symbol Span [97] | Measures visual working memory | 0–50 | |
| Mental Control Subtest [98]** | Measures ability to maintain mental set | 0–66 |
Brain magnetic resonance imaging
Participants were scanned on a 3T Phillips Achieva system (Best, The Netherlands) with an 8-channel SENSE receiver head coil [33] at the Vanderbilt University Institute of Imaging Science to gather the following sequences (see Table 4 for acquisition parameters and summary of imaging outcomes):
Table 4.
Brain MRI acquisition parameters
| Sequence | Spatial resolution | TR (ms) | TE (ms) | Slices | R | Flip Angle | Additional Details | Outcome Measure | |
|---|---|---|---|---|---|---|---|---|---|
| T1-weighted | fast field echo | 1×1×1 mm3 | 8.9 | 4.6 | 170 | 2 | 5° | – | Cortical thickness, gray matter ROIs |
| T2-weighted FLAIR | turbo spin echo inversion recovery | 0.45×0.45×4 mm3 | 11000 | 121 | 24 | 1.75 | 90° | – | WMH volume |
| SWI | fast field echo | 0.75×0.75×1.5 mm3 | 40 | 34 | 80 | 2 | 20° | – | Number and location of microhemorrhages |
| DTI | spin echo echo-planar imagine | 2×2×2 mm3 | 10000 | 60 | – | 2 | 90° | Δ = 28.9 ms, δ = 12.6 ms, 31 directions | FA, MD, RD, and AD |
| VE-pCASL | single-shot gradient echo-planar imaging | 3×3×7 mm3 | 3900 | 13 | 17 | 2 | – | labeling duration = 1650 ms, post-labeling delay = 1525 ms, Hanning-windowed labeling pulse train (pulse duration = 0.7 ms) | CBF, CVR |
| MRA | gradient echo | 0.35×0.35×0.50 mm3 | 6.8 | 3.8 | 150 | 2 | 12° | – | CoW variants |
| VWI | Noncontrasted 3D turbo-spin-echo anti-DRIVE | 0.6 × 0.6 × 1 mm3 | 1500 | 38.5 | 90 | 1.5 | 90° | – | ICA, MCA, ACA, and VB wall thickness |
FLAIR, fluid attenuated inversion recovery; SWI, susceptibility weighted imaging; DTI, diffusion tensor imaging; VE-pCASL, vessel-encoded pseudo-continuous arterial spin labeling; CBF, cerebral blood flow; CVR, cerebrovascular reactivity; MRA, magnetic resonance angiography; CoW, circle of Willis; VWI, vessel wall imaging; R, SENSE factor; ROIs, regions of interest; WMH, white matter hyperintensities; ICA, internal carotid artery; MCA, middle cerebral artery; ACA, anterior cerebral artery; VB, vertebrobasilar; FA, fractional anisotropy; MD, mean diffusivity; RD, radial diffusivity; AD, axial diffusivity.
T1-weighted Anatomical Imaging was acquired for tissue volume quantification and co-registration. T1-weighted images were post-processed using three methods:
FreeSurfer 5.1.0 (http://surfer.nmr.mgh.harvard.edu/): Standard FreeSurfer reconstruction steps were performed to calculate regions of interest (ROIs) and cortical thickness as previously described [34–37]. White and gray matter surfaces were manually inspected and corrected for registration, topological and segmentation defects. After manual intervention, images were re-processed through FreeSurfer to update the transformation template and segmentation information.
Voxel-based morphometry in Statistical Parametric Mapping (SPM8) (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/): As previously described T1 images were warped into Montreal Neuroimaging Institute (MNI) standard space using an affine and non-linear registration [38]. Using a smoothed average of gray matter in MNI as spatial prior probability maps and intensity information from the image, T1 images were segmented into gray matter, white matter, and CSF. Voxel intensities were multiplied by the local value in the deformation field from normalization and convolved with an isotropic Gaussian kernel.
Multi-Atlas Segmentation [39]: Images were post-processed with an established pipeline as previously described using NiftyReg affine registration of the T1 images to MNI space that geodesically selects appropriate atlases in pairwise registration framework using NiftyReg and ANTs [40]. Next, registered atlases were statistically fused using JIST with spatially varying performance estimation. Segmentation errors were corrected using the AdaBoost segmentation adaptor framework to calculate ROIs.
T2-weighted FLAIR was acquired for quantification of WMH. T1 and FLAIR images were post-processed using the Lesion Segmentation Tool toolbox for SPM8 as previously described [41]. Each T1-weighted image voxel was classified as gray matter, white matter, or CSF. FLAIR images were bias-corrected for field inhomogeneities and registered to the T1-weighted images. FLAIR intensity distribution of the three tissue classes were assigned, enabling detection of outliers. Neighboring voxels were classified iteratively and analyzed and assigned to lesion, white matter, or gray matter until no more voxels were assigned to a lesion.
Susceptibility Weighted Image with high resolution 3D T2* weighted gradient echo was used to capture microbleeds. Both magnitude and phase images were collected. For post-processing, a Hanning filter was first applied to remove slow-varying fluctuations on the unwrapped phase images. Then, a phase mask filter was created from the original phase image and multiplied 4 times with the magnitude image. For the phase mask, a triangular filter was used for vascular enhancement at all vessel orientations [42]. Finally, minimum intensity projection images were formed by combining 5 slices. The images were reviewed by a board-certified neuroradiologist (LTD, blinded to clinical information) to manually detect total number, hemisphere (right, left, both), predominant pattern (deep hemispheric, infratentorial, lobar), location (brainstem, lentiform nucleus, caudate nucleus, cerebellum, frontal, frontal periventricular, lobar, occipital, occipital periventricular, thalamus, parietal periventricular, temporal, temporal periventricular), and distribution (cortical, subcortical) of microbleeds.
Diffusion Tensor Imaging assessed white matter integrity. Images were post-processed with Vanderbilt University Institute for Imaging Science Center of Computational Imaging resources (Vanderbilt University, Nashville, TN) using an established pipeline previously described [43–46]. Standard tensor fitting methods were used to estimate the diffusion tensor and resulting tensor parameters fractional anisotropy (FA) and mean diffusivity (MD) [47]. FA maps were post-processed using two methods:
FSL Tract-Based Spatial Statistics (TBSS) was used for voxel wise analysis [48]. As previously described, TBSS projects participant FA data onto a mean FA tract skeleton [49–51]. Voxel wise cross-participant statistics were generated.
Eve White Matter Stamper was used to process FA white matter segmentation maps according to an established pipeline [52]. Mean FA values were calculated from ROIs defined by the Johns Hopkins Eve White Matter Atlas (http://cmrm.med.jhmi.edu/cmrm/atlas/human_data/file/AtlasExplanation2.htm).
Vessel-encoded Pseudo-Continuous Arterial Spin Labeling (VE-pCASL) assessed cerebral blood flow (CBF; rate of blood delivery to tissue, measured as mL blood/100 g tissue/min) in the microvasculature, separately in left and right internal carotid artery (ICA) and basilar flow territories [53]. Two general images were acquired, including a label image with signal from magnetically labeled blood water and a control image in the absence of a magnetic label. The label scenarios were acquired separately for (i) non-selective labeling of left and right ICAs and basilar artery, (ii) right ICA labeling only and (iii) basilar artery labeling only. This labeling paradigm allows for total CBF to be quantified but also for the CBF in separate flow territories to be quantified. Magnetic labeling was obtained by inverting the spins of incoming blood water using a Hanning-windowed pulse train in the major cervical arteries listed above. Data were corrected for motion applying rigid body transformations and baseline drift with FSL FLIRT. Data were post-processed using MATLAB. Whole brain CBF maps were generated by a sliding window approach subtracting a control image by an adjacent label image. CBF was calculated using a two-compartment perfusion model [54]. See Fig. 2 for illustration of reduced CBF. To allow for quantification of CBF in defined-cortical regions, a separately acquired equilibrium magnetization image (i.e., M0 image) with identical geometry and readout as the VE-pCASL scan but long repetition time (20 s) was registered to the structural T1 map. The T1 registered M0 map was registered to the standard MNI space [55]. Transformation matrices for these registrations were then applied to the CBF maps. ROIs were identified using the Multi-Atlas Segmentation, and region specific CBF values were calculated.
Fig. 2.
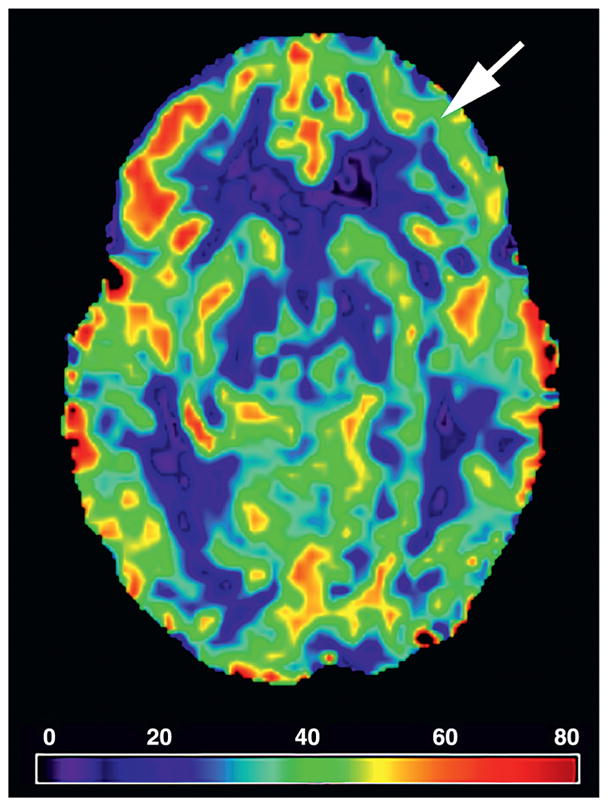
Vessel-Encoded pseudo-Continuous Arterial Spin Labeling. The white arrow illustrates a region of decreased cerebral blood flow.
Hypercapnic VE-pCASL with identical scan parameters as the VE-pCASL acquisition evaluated cerebrovascular reactivity (CVR). A non-rebreathing facemask delivered 5% CO2 and 95% normoxic normocapnic medical-grade air (approximately 79% N2, 21% O2) in a 2-block paradigm from compressed cylinders at a flow rate of 12 L/min. End-tidal CO2 and estimated arterial oxygen saturation from peripheral pulse oximetry were recorded throughout the protocol. Four scan volumes before the gas challenge were removed from the calculations to allow for blood gases to equilibrate. Percentage change in CBF (from baseline values due to the induced hypercapnia) was normalized by the end-tidal CO2 measures to calculate cerebral vascular reactivity [56].
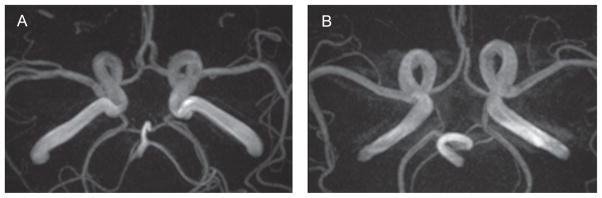
Magnetic Resonance Angiography (MRA) assessed the patency of the circle of Willis (CoW). Images were reviewed by a board-certified neuroradiologist (LTD) blinded to clinical information to manually code the patency of each of the seven segments of the CoW (i.e., anterior communicating artery, A1 segments of the left and right anterior cerebral arteries, left and right posterior communicating arteries, and P1 segments of the left and right posterior cerebral arteries). Each segment was coded as normal (≥0.8 mm), hypoplastic (<0.8 mm), or aplastic (invisible by MRA [57]). The entire CoW was further classified as one of 22 variants [58, 59]. See Fig. 3 for illustration of complete versus incomplete CoW.
Fig. 3.
Magnetic Resonance Angiography. Illustration of a complete (A) and incomplete (B) Circle of Willis.
Vessel Wall Imaging assessed the health of the macrovasculature. This novel 3D sequence uses an anti-DRIVE module with appropriately spaced repetition time to keep CSF magnetization near zero, while nulling the blood water signal using a long turbo-spin-echo pulse train, allowing visualization of the vessel walls. The field of view covers the large cerebral arteries of the CoW and segments of the middle cerebral arteries. Images were reviewed by a board-certified neuroradiologist (LTD) blinded to clinical information to manually measure the vessel wall thickness of the supraclinoid left and right ICA, proximal anterior cerebral artery, proximal middle cerebral artery, and basilar artery. See Fig. 4 for illustration of measurements.
Fig. 4.
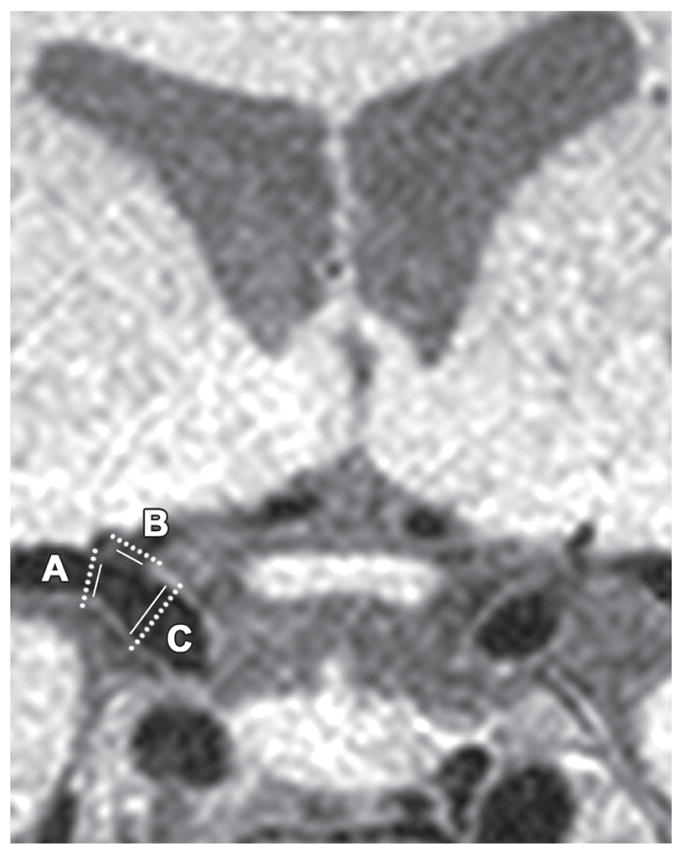
Vessel Wall Imaging. The dashed line illustrates measurement of the outer diameter of the vessel wall and the solid line illustrates measurement of the inner diameter of the vessel wall. The diameters are reported as inner/outer for each vessel as follows: (A) middle cerebral artery, 2.84/3.73 mm, (B) anterior cerebral artery 2.19/3.31 mm, and (C) internal carotid artery 3.67/4.90 mm.
Cardiac magnetic resonance imaging
Cardiac magnetic resonance (CMR) imaging was acquired using a 1.5T Siemens Avanto system (Erlangen, Germany) with a phased-array torso receiver coil. Left (LV) and right ventricular volumes and function were assessed using a breath-hold, electrocardiogram synchronized, cine steady-state free precession sequence with the following parameters: TR = 180 ms, TE = 1.1 ms, flip angle = 80°, field of view = 300–340 mm, and 156 × 192 matrix. Short and long axis imaging planes were acquired. Under the supervision of a board-certified cardiologist (FLR), a trained rater blinded to all clinical information coded the data. Ventricular mass and volumes were determined from the short axis image stack, 8–10 slices, 8 mm per slice, 0 mm inter-slice gap. Endocardial contours were traced in diastole and systole, and epicardial contours were traced in diastole using QMASS MR 7.6 Enterprise Solution (Medis, Leiden, Netherlands). Velocity encoded flow data was acquired from the proximal and descending thoracic aorta with slice angulation adjusted to maximize perpendicular flow. Flow data was analyzed by QFLOW 5.6 Enterprise Solution (Medis, Leiden, Netherlands), including total flow, net forward flow, and aortic area in systole and diastole. Aortic compliance was quantified from area change in systole and diastole as related to pulse pressure.
Longitudinal, circumferential, and radial strain were assessed for systole and diastole using a semiautomated algorithm in 2D Cardiac Performance Analysis MR (TomTec, Munich, Germany). Steady state free precession cine images from the 4-chamber, 2-chamber, and short axis orientations were postprocessed for strain determinations, including peak strain, strain rate, and time to peak strain. Strain results were determined per cardiac segment using the 17-segment model and averaged to yield a global value for each strain orientation. LV epicardial and endocardial contours were also traced in end diastole of standard cine short and long axis images.
Echocardiogram
Standard 2D, M-mode, and Doppler transthoracic echocardiography, including measurements of cardiac structure and function, was performed by a single research sonographer (JG) at the Vanderbilt University Medical Center Clinical Research Center on a Phillips IE33 cardiac ultrasound machine (Phillips Medical, Andover, MD). Digital images with measurements were sent to the Vanderbilt Heart Imaging Core Laboratory and confirmed by one of two board certified cardiologists (LAM, DKG) using commercially available software (HeartLab, AGFA Healthcare, Greenville, SC). All raters were blinded to clinical information.
Image acquisition and quantification was performed according to American Society of Echocardiography (ASE) guidelines [60–67]. Briefly, LV volumes and ejection fraction were calculated by the biplane Simpson’s method. LV mass was calculated from LV linear dimensions using the ASE recommended formula (0.8 × [1.04[ (LV internal diameter during diastole+posterior wall thickness during diastole+septum wall thickness during diastole)3 - (LV internal diameter during diastole)3]]+0.6 g) and indexed to body surface area. LV hypertrophy (LVH) was defined as LV mass index >115 g/m2 in men or >95 g/m2 in women. Left atrial (LA) maximum volume was measured by the modified Simpson’s method using apical 4- and 2-chamber views at the end-systolic frame preceding mitral valve opening and indexed to body surface area to derive LA volume index. Each valve was assessed for the presence and severity of stenosis and regurgitation with the over-reading cardiologist assigning one of the following grades: normal, trace, mild, mild to moderate, moderate, moderate to severe, or severe.
Early (E) and late (A) transmitral velocities and the E deceleration time were measured from pulsed wave spectral Doppler images acquired in the apical 4-chamber view with the sample volume positioned at the tip of the mitral leaflets. Peak lateral and septal mitral annular early relaxation and atrial contraction velocities were assessed using tissue Doppler imaging (TDI). LV filling pressures were estimated by E wave divided by average early relaxation velocities. Diastolic function was graded by the over-reading cardiologist as normal, grade I (impaired relaxation), grade II (pseudonormal), or grade III (restrictive) according to ASE recommendations. Right ventricular systolic function was assessed using TDI systolic velocity of the lateral tricuspid annulus and tricuspid annulus planar systolic excursion. Cardiac output was calculated as stroke volume × heart rate, where stroke volume was calculated from the LV outflow tract velocity time integral and diameter. The right ventricular systolic pressure was estimated from the peak tricuspid regurgitant velocity using the modified Bernoulli equation plus right atrial pressure. Right atrial pressure was estimated from the inferior vena cavamaximal diameter and change in size with inspiration.
Final values were taken from measurements of a single cardiac cycle for participants in normal sinus rhythm or the average of three cardiac cycles for those participants in atrial fibrillation. Atrial fibrillation at the time of echocardiography was determined by the absence of A waves on transmitral spectral Doppler flow and tissue Doppler mitral annular velocity profiles, as well as the lack of organized electrocardiographic P waves.
Ambulatory blood pressure monitoring
As an optional study procedure, a subset of participants (n = 301) wore a SpaceLabs 90207 monitor (SpaceLabs Inc., Redmond, WA) on the left arm for 24-consecutive hours of ambulatory blood pressure monitoring. Systolic blood pressure, diastolic blood pressure, pulse pressure, and heart rate readings were captured every 30 min for a maximum of 49 readings. Data were uploaded to a workstation using the SpaceLabs Healthcare ABP Report Management System (Version 3.0.5, 2010). For QC purposes, at least 39 readings in the 24-h monitoring period were required for inclusion, resulting in 34 exclusions. Datasets with >3 h gap between readings (n = 19) were excluded, 17 of whom were excluded for <39 readings, resulting in 265 datasets meeting QC.
Analytical plan
Prior to analysis, medical and vascular characteristics were calculated as follows. Systolic blood pressure was the mean of two measurements obtained prior to the echocardiogram. Diabetes mellitus included current fasting blood glucose ≥126 mg/dL, hemoglobin A1C ≥6.5%, or current oral hypoglycemic or insulin medication usage. Dyslipidemia included total cholesterol ≥200 mg/dL, high-density lipoprotein cholesterol <40 mg/dL, or lipid-lowering medication usage. Self-report was used to ascertain current cigarette smoking (i.e., yes/no within the year prior to baseline examination), prior cigarette smoking (i.e., yes/no ever prior to baseline examination), medication use, history of cancer, history of transient ischemic attack, and history of head injury with loss of consciousness <5 minutes. Cardiovascular disease (CVD) included coronary heart disease, angina, or myocardial infarction (note, heart failure was a study exclusion). History of sleep disorder included restless leg syndrome, sleep apnea, or REM sleep behavior disorder. Thyroid disease included self-report, thyroid stimulating hormone <0.45 or >4.5 mcunit/mL, prior thyroid surgery, or thyroid medication usage. Atrial fibrillation included self-report with supporting evidence from echocardiogram, CMR, or prior surgery or medication usage to treat atrial fibrillation. Framingham Stroke Risk Profile score included age, sex, systolic blood pressure, anti-hypertensive medication usage, diabetes mellitus, current cigarette smoking, LVH, CVD, and atrial fibrillation [68]. APOE ε4 carrier status was defined as positive (ε2/ε4, ε3/ε4, ε4/ε4) or negative (ε2/ε2, ε2/ε3, ε3/ε3).
Descriptive statistics for baseline demographic, genetic, biomarker, medical, and neuropsychological characteristics were calculated separately for NC and MCI participants. Between-group characteristics were statistically compared using Pearson chi-square test for categorical variables and Wilcoxon rank sum test for continuous variables. Significance was set a priori at p < 0.05. All analyses were conducted using R 3.2.0 (http://www.r-project.org).
RESULTS
Demographic characteristics
Of the 566 participants who completed eligibility visits, 335 were enrolled. At baseline, the cohort was age 73 ± 7 years with an education level of 16 ± 3 years, 41% of participants being female, and 13% self-identifying as a racial or ethnic minority. Sample characteristics for the entire cohort as well as between-group differences for NC and MCI participants are presented in Table 5.
Table 5.
Baseline participant characteristics
| Total n = 335 | NC n = 167 | MCI n = 168 | p-value | |
|---|---|---|---|---|
| Demographic Characteristics | ||||
| Age, years | 73 ± 7 | 72 ± 7 | 73 ± 8 | 0.31 |
| Sex, % female | 41 | 41 | 41 | 0.95 |
| Race, % white | 13 | 13 | 14 | 0.65 |
| Education, years | 15 ± 9 | 16 ± 2 | 15 ± 3 | <0.001 |
| AD Genetic, Family History, and Biomarker Characteristics | ||||
| APOE ε4 % | 34 | 28 | 40 | 0.02 |
| Family History of Dementia, % | 51 | 50 | 51 | 0.91 |
| Family History of AD, % | 25 | 26 | 24 | 0.61 |
| CSF AβX-40, pg/mL | 6137 ± 1640 | 6211 ± 1711 | 6062 ± 1572 | 0.85 |
| CSF AβX-42, pg/mL | 547 ± 266 | 593 ± 245 | 501 ± 279 | 0.009 |
| CSF Aβ1-42, pg/mL | 714 ± 245 | 761 ± 224 | 668 ± 258 | 0.02 |
| CSF total tau, pg/mL | 426 ± 226 | 374 ± 177 | 479 ± 258 | 0.004 |
| CSF p-tau, pg/mL | 61.0 ± 25.7 | 56.2 ± 22.2 | 66.0 ± 28.1 | 0.02 |
| Vascular Characteristics | ||||
| Dyslipidemia, % | 80 | 80 | 80 | 0.87 |
| Framingham Stroke Risk Profile, total | 12.5 ± 4.2 | 11.9 ± 4.1 | 13.1 ± 4.2 | 0.008 |
| Systolic blood pressure, mm Hg | 142 ± 18 | 140 ± 18 | 145 ± 18 | 0.008 |
| Anti-hypertensive medication usage, % | 56 | 54 | 59 | 0.35 |
| Diabetes, % | 19 | 15 | 22 | 0.10 |
| Cigarette Smoking Ever, % | 31 | 30 | 32 | 0.75 |
| Cigarette Smoking Current, % | 2 | 2 | 2 | 0.71 |
| Prevalent cardiovascular disease, % | 4 | 4 | 3 | 0.55 |
| Atrial fibrillation, % | 6 | 5 | 6 | 0.82 |
| Left ventricular hypertrophy, % | 5 | 2 | 7 | 0.04 |
| Health Characteristics | ||||
| History of Cancer, % | 26 | 26 | 26 | 0.97 |
| Prior Head Injury (LOC<5 minutes), % | 8 | 10 | 7 | 0.31 |
| History of Thyroid Disease, % | 20 | 17 | 24 | 0.13 |
| History of Transient Ischemic Attack, % | 6 | 7 | 5 | 0.64 |
| History of Sleep Disorder, % | 28 | 29 | 27 | 0.85 |
Values denoted as mean ± standard deviation or frequency; AD = Alzheimer’s disease; APOE = apolipoprotein E; CSF = cerebrospinal fluid; LOC = loss of consciousness.
All 335 participants completed required elements of the protocol, including fasting blood work, physical examination/frailty assessment, questionnaires, neuropsychological assessment, brain MRI, cardiac MRI, and echocardiogram. The exception is that 8 participants did not complete either the cardiac MRI (n = 3) or the brain MRI (n = 7). Of note, there were some differences in sample characteristics for those participants who consented to optional study components and those participants who did not consent. Specifically, when compared to participants who did not complete the optional lumbar puncture, participants completing the procedure were more likely to be male (p = 0.006) and self-identify as White non-Hispanic (p = 0.002) with lower FSRP scores (p = 0.005), anti-hypertensive medication usage (p = 0.001), history of cigarette smoking (p = 0.006), and history of atrial fibrillation (p = 0.02). As compared to participants who did not complete the optional ambulatory blood pressure monitoring protocol, participants completing the protocol were younger (p = 0.008) with lower education levels (p = 0.04, though means for both groups were at or above the college graduate level) and history of cigarette smoking (p = 0.04).
AD genetic, family history, and biomarker characteristics
There was a between-group difference for APOE ε4 (x2 = 5.7, p = 0.017), such that the MCI group had a higher prevalence of APOE ε4 carriers (40%) compared to the NC group (28%). However, the participant groups were comparable for self-reported family history of both dementia (x2 = 0.01, p = 0.91) and AD (x2 = 0.25, p = 0.61).
The groups differed on CSF biomarkers of AD in the expected direction, including AβX-42 (W(1,153) = 6.9, p = 0.009), Aβ1-42 (W(1,153) = 5.7, p = 0.018), total tau (W(1,153) = 8.4, p = 0.004), and p-tau levels (W(1,328) = 5.5, p = 0.02). The groups did not differ for CSF AβX-40 levels (W(1,153) = 0.04, p = 0.85).
Medical characteristics
The NC and MCI groups were similar for history of cancer (x2 = 0.001, p = 0.97), head injury with loss of consciousness <5 min (x2 = 1.04, p = 0.31), sleep disorder (x2 = 0.03, p = 0.85), transient ischemic attack (x2 = 0.23, p = 0.64), and thyroid disease (x2 = 2.34, p = 0.13).
The groups differed on Framingham Stroke Risk Profile (W(1,330) = 7.1, p = 0.008) with MCI participants having a higher mean risk profile score (13.0 ± 4.2) than NC participants (11.9 ± 4.1). The groups differed on LVH (x2 = 4.1, p = 0.04) with the MCI participants having a higher prevalence of LVH than the NC participants. The groups differed on systolic blood pressure (W(1,333)=7.1, p = 0.008) with MCI participants having higher mean values (144.9 ± 18.6) than NC participants (139.9 ± 17.6) despite comparable anti-hypertensive medication utilization (x2 = 0.86, p = 0.35). The groups did not differ on other vascular health characteristics, including dyslipidemia (x2 = 0.03, p = 0.87), diabetes (x2 = 2.76, p = 0.10), prevalent CVD (x2 = 0.36, p = 0.55), or atrial fibrillation (x2 = 0.05, p = 0.82). There were no group differences for prior smoking history (x2 = 0.10, p = 0.81) or current smoking prevalence (x2 = 0.14, p = 0.71). See Table 5 for details.
CDR, activities of daily living, and neuropsychological test performances
As designed, CDR global score differed between the two participant groups with 100% of NC participants having a CDR = 0 and 86% of MCI participants having a CDR = 0.5 (x2 = 251.0, p < 0.001). Activities of daily living, as assessed by the Functional Activities Questionnaire, were compromised in MCI participants as compared to NC participants (W(1,333) = 264.0, p < 0.001).
Between-group comparisons yielded baseline neuropsychological performance differences in all cognitive domains assessed such that MCI participants performed worse on all measures as compared to NC participants. Specifically, differences emerged for Montreal Cognitive Assessment (W(1,332) = 129.0, p < 0.001), Animal Naming (W(1,332) = 65.5, p < 0.001), Boston Naming Test-30 Item Even Version (W(1,332) = 46.7, p < 0.001), California Verbal Learning Test-II (CVLT-II) Trials 1–5 Total Learning (W(1,332) = 159.0, p < 0.001), CVLT-II Long Delay Free Recall (W(1,332) = 186.0, p < 0.001), Biber Figure Learning Test (BFLT) Trials 1–5 Total Learning (W(1,330) = 180.0, p < 0.001), BFLT Delayed Free Recall (W(1,330) = 148.0, p < 0.001), Coding (W(1,332) = 59.1, p < 0.001), Delis Kaplan Executive Function System (DKEFS) Color-Word Interference Test Word Reading (W(1,327) = 34.5, p < 0.001), DKEFS Color-Word Interference Test Color Naming (W(1,324) = 50.9, p < 0.001), DKEFS Number Sequencing (W(1,332)- = 47.8, p < 0.001), DKEFS Number Letter Sequencing (W(1,331) = 100.0, p < 0.001), DKEFS Color-Word Interference Test Inhibition (W(1,327) = 81.5, p < 0.001), DKEFS Tower Test (W(1,331) = 20.4, p < 0.001), Letter Fluency (W(1,333) = 63.3, p < 0.001), and Hooper Visual Organization Test (W(1,332) = 24.5, p < 0.001). Wide Range Achievement Test 3rd Edition Reading Subtest performance also differed between the two groups (W(1,333) = 20.3, p < 0.001). See Table 6 for details, including effect sizes.
Table 6.
Baseline neuropsychological and functional characteristics
| Total n = 335 | NC n = 167 | MCI n = 168 | p-value | d | |
|---|---|---|---|---|---|
| Clinical Dementia Rating*,† | |||||
| 0, % | 57 | 100 | 14 | <0.001 | – |
| 0.5, % | 43 | 0 | 86 | ||
| Functional Activities Questionnaire*,† | 1.3 ± 2.2 | 0.1 ± 0.3 | 2.5 ± 2.6 | <0.001 | −1.30 |
| Montreal Cognitive Assessment | 25.4 ± 3.3 | 27.1 ± 2.1 | 23.6 ± 3.4 | <0.001 | 1.24 |
| Animal Naming | 18.9 ± 5.4 | 21.1 ± 4.9 | 16.8 ± 5.1 | <0.001 | 0.86 |
| Boston Naming Test 30-item Even Version | 26.8 ± 3.1 | 27.9 ± 2.0 | 25.7 ± 3.6 | <0.001 | 0.76 |
| Coding | 52.6 ± 12.8 | 57.5 ± 11.4 | 47.6 ± 12.3 | <0.001 | 0.83 |
| DKEFS Number Sequencing† | 42.4 ± 19.4 | 35.8 ± 12.3 | 49.0 ± 22.7 | <0.001 | −0.72 |
| DKEFS Number-Letter Switching† | 107.5 ± 48.9 | 85.8 ± 33.6 | 129.5 ± 52.1 | <0.001 | −1.00 |
| DKEFS Tower Test | 14.9 ± 4.7 | 16.1 ± 4.4 | 13.7 ± 4.7 | <0.001 | 0.53 |
| DKEFS Color-Word Interference Test - Color Naming† | 32.2 ± 6.6 | 29.7 ± 4.8 | 34.7 ± 7.3 | <0.001 | −0.81 |
| DKEFS Color-Word Interference Test - Word Naming† | 24.4 ± 4.9 | 22.9 ± 4.2 | 25.8 ± 5.2 | <0.001 | −0.61 |
| DKEFS Color-Word Interference Test - Inhibition† | 69.4 ± 23.8 | 59.4 ± 13.5 | 79.2 ± 27.4 | <0.001 | −0.92 |
| Letter Fluency | 38.6 ± 11.9 | 43.3 ± 11.2 | 33.9 ± 10.6 | <0.001 | 0.86 |
| California Verbal Learning Test-II Trials 1–5 Total Learning | 40.4 ± 11.9 | 47.1 ± 9.4 | 33.7 ± 10.3 | <0.001 | 1.36 |
| California Verbal Learning Test-II Long Delay Free Recall | 8.0 ± 4.3 | 10.6 ± 3.3 | 5.5 ± 3.6 | <0.001 | 1.48 |
| Biber Figure Learning Test Trials 1–5 Total Learning | 112.3 ± 40.8 | 136.3 ± 30.1 | 88.2 ± 35.6 | <0.001 | 1.46 |
| Biber Figure Learning Test Delayed Recall | 26.8 ± 10.6 | 32.7 ± 7.5 | 21.0 ± 10.0 | <0.001 | 1.32 |
| Hooper Visual Organization Test | 24.4 ± 3.1 | 25.3 ± 2.5 | 23.5 ± 3.4 | <0.001 | 0.60 |
| Wide Range Achievement Test 3rd Edition, Reading Subtest* | 50.1 ± 5.1 | 51.4 ± 4.2 | 48.9 ± 5.5 | <0.001 | 0.51 |
| Geriatric Depression Scale† | 3.6 ± 3.6 | 2.4 ± 2.8 | 4.7 ± 4.0 | <0.001 | −0.67 |
Values denoted as mean ± standard deviation or frequency; DKEFS, Delis Kaplan Executive Function System;
variable collected at screening visit;
lower scores denote better performances; d, Cohen’s d effect size comparing NC and MCI groups.
DISCUSSION
The Vanderbilt Memory & Aging Project is a prospective case-control cohort study of 335 men and women diagnosed as NC or MCI who have consented to longitudinal follow-up every 18 months. Because the parent grant supporting cohort creation focuses on systemic vascular mechanisms underlying abnormal cognitive aging, study procedures are enriched for vascular assessments, including risk factor, proteomic, systemic, cardiac, and cerebrovascular data. Such detailed vascular phenotyping is coupled with a serum, plasma, and CSF biosample repository and neuropsychological assessment and multimodal brain MRI acquisition at repeat time points. Collectively, these data sources offer a unique opportunity to examine interrelations among vascular risk factors and disease, AD pathophysiology, and neurodegeneration on cognitive health among older adults. For example, applying a systems-based approach, we can examine complex pathways leading from vascular risk factors and disease to changes in cognition, CSF biomarkers of AD pathophysiology, and neuroimaging markers of microvasculature health, macrovasculature health, and neurodegeneration. Such studies will help elucidate whether the common co-occurrence of AD and cerebrovascular disease reflects convergence of two or more pathological processes or simply additive effects of independent pathological processes.
There are several existing older adult cohorts focused on cognitive aging with longitudinal follow-up. Examples include both single Center studies, such as the Oregon Brain Aging Study [69] and Religious Orders Study [70], and multi-site cooperatives like the National Alzheimer’s Coordinating Center [71] and Alzheimer’s Disease Neuroimaging Initiative [72]. The Vanderbilt Memory & Aging Project shares a few key similarities with these existing cohorts with respect to comparable demographic characteristics and AD risk profiles, such as age [71] and education levels [71, 73], AD genetic susceptibility based on APOE ε4 prevalence [74], and CSF biomarker status [75].
Despite these key similarities, there are several unique features to the Vanderbilt Memory & Aging Project. First, detailed baseline assessment of vascular risk factors, systemic vascular health, and cerebrovascular health present valuable opportunities for longitudinal investigation into the intersection of vascular mechanisms, AD pathology, and neurodegeneration on cognitive decline with age. Specifically, vascular mechanisms can be explored via systemic vascular variables from echocardiogram, CMR, and ambulatory blood pressure monitoring along with innovative cerebrovascular integrity assessments, including MRA of the CoW, vessel-encoded CBF and reactivity paradigms, vessel wall imaging in the brain, and microhemorrhages. These detailed vascular measurements can be related to extensive brain aging phenotypes, including comprehensive neuropsychological features, neuroimaging markers of gray and white matter integrity, and CSF biomarkers of amyloid deposition, tau aggregation, and neuronal injury. Furthermore, given the longitudinal nature of the study’s methodology, baseline storage of DNA, serum, plasma, and CSF samples will facilitate longitudinal biomarker analyses across the cognitive aging spectrum (i.e., normal cognition, MCI, and dementia). Thus, the Vanderbilt Memory & Aging Project complements the existing landscape of cohort studies by offering unique opportunities to investigate the emerging intersection of vascular disease, AD, and neurodegeneration on cognitive health.
Despite the strengths of our cohort, there are limitations. Individuals who participate in research studies, especially studies involving detailed medical assessments, are unlikely to be representative of the general population, limiting generalizability of results. For example, individuals consenting to undergo MRI are generally healthier (e.g., better systolic blood pressure, less likely to be obese, less coronary heart disease) than individuals who do not consent [76]. In the current study, we similarly observed that participants completing optional procedures in our protocol, such as lumbar puncture and ambulatory blood pressure monitoring, were healthier than participants who did not complete these optional study elements. It is likely that our cohort represents a healthier segment of the population, especially those participants completing optional procedures, which will limit generalizability of our findings. Another potential limitation is the criteria by which we enrolled individuals into the cognitively normal control group, such that all NC participants had a global CDR of 0 and no impairment on neuropsychological testing within 1.5 standard deviations of the normative data mean. We leveraged such criteria given our intention to enroll a cohort of individuals with normal cognition rather than a cognitive aging sample free of dementia or MCI. This strategy aligns with our case-control methodology, facilitating comparisons between individuals at high risk of decline and conversion (i.e., prodromal AD or dementia) and individuals at low risk of decline or conversion.
Detailed analyses from the rich resources available in this cohort will be valuable for advancing information about vascular mechanisms for cognitive decline in older adults. The longitudinal methods with repeat assessments for collecting serum, plasma, and CSF biospecimens, patient-reported outcomes, documentation of lifestyle factors, neuropsychological assessment, multi-modal brain MRI, echocardiogram, CMR, and ambulatory blood pressure monitoring will offer unique opportunities to enhance knowledge about the progression of AD and non-AD mechanisms for abnormal brain and cognitive aging. While our observational methods are unable to establish causal mechanisms independently, the data sources presented here provide a rich opportunity to examine the complex interplay among vascular risk factors and disease, AD pathophysiology, and neurodegeneration on brain health in older adults. Findings from our study can be used as converging evidence with other observational studies, animal research, and clinical trial findings to ultimately establish whether the link between vascular health and AD is correlative or causal [77].
Acknowledgments
This research was supported by Alzheimer’s Association IIRG-08-88733 (ALJ), R01-AG034962 (ALJ), R01-AG034962-S2 (ALJ), K24-AG046373 (ALJ), Paul B. Beeson Career Development Award in Aging K23-AG030962 (ALJ), R01-HL111516 (ALJ), UL1-TR000445 (Vanderbilt Clinical Translational Science Award from the National Center for Research Resources and National Institutes of Health), K12-HD043483 (KAG, SPB, TJH), K12-HL109019 (DKG), Paul B. Beeson Career Development Award in Aging K23-AG045966 (KAG), Paul B. Beeson Career Development Award in Aging K23-AG048347 (SPB), F32-AG046093 (EML), Vanderbilt Office of Clinical and Translational Scientist Development (SR), and the Vanderbilt Memory & Alzheimer’s Center. The authors would like to thank the dedicated Vanderbilt Memory & Aging Project participants and their loved ones, our devoted staff and trainees who contributed to recruitment, screening, and enrollment of the baseline cohort, and our biostatistics graduate research assistants, Lauren Samuels and Jacquelyn Neal.
Footnotes
Authors’ disclosures available online (http://www.j-alz.com/manuscript-disclosures/15-0914r2).
References
- 1.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: Prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 2.Qiu C, Winblad B, Marengoni A, Klarin I, Fastbom J, Fratiglioni L. Heart failure and risk of dementia and Alzheimer disease: A population-based cohort study. Arch Intern Med. 2006;166:1003–1008. doi: 10.1001/archinte.166.9.1003. [DOI] [PubMed] [Google Scholar]
- 3.Gifford KA, Badaracco M, Liu D, Tripodis Y, Gentile A, Lu Z, Palmisano J, Jefferson AL. Blood pressure and cognition among older adults: A meta-analysis. Arch Clin Neuropsychol. 2013;28:649–664. doi: 10.1093/arclin/act046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogels RL, Oosterman JM, van Harten B, Gouw AA, Schroeder-Tanka JM, Scheltens P, van der Flier WM, Weinstein HC. Neuroimaging and correlates of cognitive function among patients with heart failure. Dement Geriatr Cogn Disord. 2007;24:418–423. doi: 10.1159/000109811. [DOI] [PubMed] [Google Scholar]
- 5.Nation DA, Edland SD, Bondi MW, Salmon DP, Delano-Wood L, Peskind ER, Quinn JF, Galasko DR. Pulse pressure is associated with Alzheimer biomarkers in cognitively normal older adults. Neurology. 2013;81:2024–2027. doi: 10.1212/01.wnl.0000436935.47657.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes TM, Kuller LH, Barinas-Mitchell EJ, Mackey RH, McDade EM, Klunk WE, Aizenstein HJ, Cohen AD, Snitz BE, Mathis CA, Dekosky ST, Lopez OL. Pulse wave velocity is associated with beta-amyloid deposition in the brains of very elderly adults. Neurology. 2013;81:1711–1718. doi: 10.1212/01.wnl.0000435301.64776.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jefferson AL, Poppas A, Paul RH, Cohen RA. Systemic hypoperfusion is associated with executive dysfunction in geriatric cardiac patients. Neurobiol Aging. 2007;28:477–483. doi: 10.1016/j.neurobiolaging.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jefferson AL, Himali JJ, Au R, Seshadri S, DeCarli C, O’Donnell CJ, Wolf PA, Manning WJ, Beiser AS, Benjamin EJ. Relation of left ventricular ejection fraction to cognitive aging (from the Framingham Heart Study) Am J Cardiol. 2011;108:1346–1351. doi: 10.1016/j.amjcard.2011.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jefferson AL, Himali JJ, Beiser AS, Au R, Massaro JM, Seshadri S, Gona P, Salton CJ, DeCarli C, O’Donnell CJ, Benjamin EJ, Wolf PA, Manning WJ. Cardiac index is associated with brain aging: The Framingham Heart Study. Circulation. 2010;122:690–697. doi: 10.1161/CIRCULATIONAHA.109.905091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jefferson AL, Tate DF, Poppas A, Brickman AM, Paul RH, Gunstad J, Cohen RA. Lower cardiac output is associated with greater white matter hyperintensities in older adults with cardiovascular disease. J Am Geriatr Soc. 2007;55:1044–1048. doi: 10.1111/j.1532-5415.2007.01226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jefferson AL, Holland CM, Tate DF, Csapo I, Poppas A, Cohen RA, Guttmann CR. Atlas-derived perfusion correlates of white matter hyperintensities in patients with reduced cardiac output. Neurobiol Aging. 2011;32:133–139. doi: 10.1016/j.neurobiolaging.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jefferson AL, Beiser AS, Himali JJ, Seshadri S, O’Donnell CJ, Manning WJ, Wolf PA, Au R, Benjamin EJ. Low cardiac index is associated with incident dementia and Alzheimer disease: The Framingham Heart Study. Circulation. 2015;131:1333–1339. doi: 10.1161/CIRCULATIONAHA.114.012438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 14.Pfeffer RI, Kurosaki TT, Harrah CH, Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 15.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilkinson GS. Wide Range Achievement Test-3 (WRAT-3) Administration Manual. The Psychological Corporation; San Antonio, TX: 1993. [Google Scholar]
- 17.Brinkmalm G, Portelius E, Ohrfelt A, Mattsson N, Persson R, Gustavsson MK, Vite CH, Gobom J, Mansson JE, Nilsson J, Halim A, Larson G, Ruetschi U, Zetterberg H, Blennow K, Brinkmalm A. An online nano-LC-ESI-FTICR-MS method for comprehensive characterization of endogenous fragments from amyloid beta and amyloid precursor protein in human and cat cerebrospinal fluid. J Mass Spectrom. 2012;47:591–603. doi: 10.1002/jms.2987. [DOI] [PubMed] [Google Scholar]
- 18.Palmqvist S, Zetterberg H, Blennow K, Vestberg S, Andreasson U, Brooks DJ, Owenius R, Hagerstrom D, Wollmer P, Minthon L, Hansson O. Accuracy of brain amyloid detection in clinical practice using cerebrospinal fluid beta-amyloid 42: A cross-validation study against amyloid positron emission tomography. JAMA Neurol. 2014;71:1282–1289. doi: 10.1001/jamaneurol.2014.1358. [DOI] [PubMed] [Google Scholar]
- 19.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA Cardiovascular Health Study Collaborative Research G. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 20.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: Evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 21.Taylor HL, Jacobs DR, Jr, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31:741–755. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- 22.Buchman AS, Wilson RS, Bienias JL, Bennett DA. Change in frailty and risk of death in older persons. Exp Aging Res. 2009;35:61–82. doi: 10.1080/03610730802545051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: Outcomes for interventions. Med Sci Sports Exerc. 2001;33:1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Thompson FE, Midthune D, Subar AF, Kipnis V, Kahle LL, Schatzkin A. Development and evaluation of a short instrument to estimate usual dietary intake of percentage energy from fat. J Am Diet Assoc. 2007;107:760–767. doi: 10.1016/j.jada.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 26.Farias ST, Mungas D, Reed BR, Cahn-Weiner D, Jagust W, Baynes K, Decarli C. The measurement of everyday cognition (ECog): Scale development and psychometric properties. Neuropsychology. 2008;22:531–544. doi: 10.1037/0894-4105.22.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilewski MJ, Zelinski EM, Schaie KW. The Memory Functioning Questionnaire for assessment of memory complaints in adulthood and old age. Psychol Aging. 1990;5:482–490. doi: 10.1037//0882-7974.5.4.482. [DOI] [PubMed] [Google Scholar]
- 28.McNair D, Kahn R. Self-assessment of cognitive deficits. Mark Powley Associates; New Canaan, CT: 1983. [Google Scholar]
- 29.Reid LM, Maclullich AM. Subjective memory complaints and cognitive impairment in older people. Dement Geriatr Cogn Disord. 2006;22:471–485. doi: 10.1159/000096295. [DOI] [PubMed] [Google Scholar]
- 30.Glosser G, Gallo J, Duda N, de Vries JJ, Clark CM, Grossman M. Visual perceptual functions predict instrumental activities of daily living in patients with dementia. Neuropsychiatry Neuropsychol Behav Neurol. 2002;15:198–206. [PubMed] [Google Scholar]
- 31.Kaplan E. A process approach to neuropsychological assessment. In: Boll TE, Bryant BR, editors. Clinical neuropsychology and brain function: Research. Measurement, and practice: Master lectures. American Psychological Association; Washington, DC: 1988. [Google Scholar]
- 32.Kaplan E. The process approach to neuropsychological assessment of psychiatric patients. J Neuropsychiatry Clin Neurosci. 1990;2:72–87. doi: 10.1176/jnp.2.1.72. [DOI] [PubMed] [Google Scholar]
- 33.Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: Sensitivity encoding for fast MRI. Magn Reson Med. 1999;42:952–962. [PubMed] [Google Scholar]
- 34.Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, van der Kouwe A, Jenkins BG, Dale AM, Fischl B. Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology. 2002;58:695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- 35.Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale AM, Fischl B. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- 36.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, Dale AM. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23(Suppl 1):S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 38.Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 39.Asman AJ, Landman BA. Formulating spatially varying performance in the statistical fusion framework. IEEE Trans Med Imaging. 2012;31:1326–1336. doi: 10.1109/TMI.2012.2190992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Asman AJ, Landman BA. Non-local statistical label fusion for multi-atlas segmentation. Med Image Anal. 2013;17:194–208. doi: 10.1016/j.media.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmidt P, Gaser C, Arsic M, Buck D, Forschler A, Berthele A, Hoshi M, Ilg R, Schmid VJ, Zimmer C, Hemmer B, Muhlau M. An automated tool for detection of FLAIR-hyperintense white-matter lesions in Multiple Sclerosis. Neuroimage. 2012;59:3774–3783. doi: 10.1016/j.neuroimage.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 42.Martínez Santiesteban FM, Swanson SD, Noll DC, Anderson DJ. Object orientation independence of susceptibility weighted imaging by using a sigmoid-type phase window. Proc Intl Soc Mag Reson Med. 2006:Abstract No. 2399, 467. [Google Scholar]
- 43.Harrigan RL, Yvernault BC, Boyd BD, Damon SM, Gibney KD, Conrad BN, Phillips NS, Rogers BP, Gao Y, Landman BA. Vanderbilt University Institute of Imaging Science Center for Computational Imaging XNAT: A multimodal data archive and processing environment. Neuroimage. 2016;124(Pt B):1097–1101. doi: 10.1016/j.neuroimage.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lauzon CB, Asman AJ, Esparza ML, Burns SS, Fan Q, Gao Y, Anderson AW, Davis N, Cutting LE, Landman BA. Simultaneous analysis and quality assurance for diffusion tensor imaging. PLoS One. 2013;8:e61737. doi: 10.1371/journal.pone.0061737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cook PA, Bai Y, Nedjati-Gilani SKKS, Seunarine KK, Hall MG, Parker GJ, Alexander DC. Camino: Open-source diffusion-MRI reconstruction and processing. 14th Scientific Meeting of the International Society for Magnetic Resonance in Medicine; Seattle, WA, USA. 2006. [Google Scholar]
- 47.Chang LC, Jones DK, Pierpaoli C. RESTORE: Robust estimation of tensors by outlier rejection. Magn Reson Med. 2005;53:1088–1095. doi: 10.1002/mrm.20426. [DOI] [PubMed] [Google Scholar]
- 48.Andersson J, Jenkinson M, Smith S. Non-linear optimisation: FMRIB Technial Report TR07JA1. FMRIB Centre; Oxford, United Kingdom: 2007. [Google Scholar]
- 49.Andersson J, Jenkinson M, Smith S. Non-linear registration aka Spatial normalisation FMRIB Technial Report TR07JA2. FMRIB Centre; Oxford, United Kingdom: 2007. [Google Scholar]
- 50.Park Y, Diez-Silva M, Fu D, Popescu G, Choi W, Barman I, Suresh S, Feld MS. Static and dynamic light scattering of healthy and malaria-parasite invaded red blood cells. J Biomed Opt. 2010;15:020506. doi: 10.1117/1.3369966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Choi SW, Reise SP, Pilkonis PA, Hays RD, Cella D. Efficiency of static and computer adaptive short forms compared to full-length measures of depressive symptoms. Qual Life Res. 2010;19:125–136. doi: 10.1007/s11136-009-9560-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tian Q, Ferrucci L, Resnick SM, Simonsick EM, Shardell MD, Landman BA, Venkatraman VK, Gonzalez CE, Studenski SA. The effect of age and microstructural white matter integrity on lap time variation and fast-paced walking speed. Brain Imaging Behav. 2015 doi: 10.1007/s11682-015-9449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Donahue MJ, Hussey E, Rane S, Wilson T, van Osch M, Hartkamp N, Hendrikse J, Ally BA. Vessel-encoded arterial spin labeling (VE-ASL) reveals elevated flow territory asymmetry in older adults with substandard verbal memory performance. J Magn Reson Imaging. 2014;39:377–386. doi: 10.1002/jmri.24150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang J, Alsop DC, Li L, Listerud J, Gonzalez-At JB, Schnall MD, Detre JA. Comparison of quantitative perfusion imaging using arterial spin labeling at 1.5 and 4.0 Tesla. Magn Reson Med. 2002;48:242–254. doi: 10.1002/mrm.10211. [DOI] [PubMed] [Google Scholar]
- 55.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 56.Donahue MJ, Dethrage LM, Faraco CC, Jordan LC, Clemmons P, Singer R, Mocco J, Shyr Y, Desai A, O’Duffy A, Riebau D, Hermann L, Connors J, Kirshner H, Strother MK. Routine clinical evaluation of cerebrovascular reserve capacity using carbogen in patients with intracranial stenosis. Stroke. 2014;45:2335–2341. doi: 10.1161/STROKEAHA.114.005975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krabbe-Hartkamp MJ, van der Grond J, de Leeuw FE, de Groot JC, Algra A, Hillen B, Breteler MM, Mali WP. Circle of Willis: Morphologic variation on three-dimensional time-of-flight MR angiograms. Radiology. 1998;207:103–111. doi: 10.1148/radiology.207.1.9530305. [DOI] [PubMed] [Google Scholar]
- 58.Lazorthes G, Gouazé A, Santini J-J, Salamon G. Lécercle arteriel du cerveau (circulus arteriosus cerebri) Anat Clin. 1979;1:241–257. [Google Scholar]
- 59.Eftekhar B, Dadmehr M, Ansari S, Ghodsi M, Nazparvar B, Ketabchi E. Are the distributions of variations of circle of Willis different in different populations? - Results of an anatomical study and review of literature. BMC Neurology. 2006;6:22. doi: 10.1186/1471-2377-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 61.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. quiz 786–688. [DOI] [PubMed] [Google Scholar]
- 62.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 63.Quinones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA Doppler Quantification Task Force of the N, Standards Committee of the American Society of E. Recommendations for quantification of Doppler echocardiography: A report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15:167–184. doi: 10.1067/mje.2002.120202. [DOI] [PubMed] [Google Scholar]
- 64.Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quinones M American Society of E, European Association of E. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009;22:1–23. doi: 10.1016/j.echo.2008.11.029. quiz 101–102. [DOI] [PubMed] [Google Scholar]
- 65.Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weissman NJ American Society of E. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 66.Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, Galderisi M, Marwick T, Nagueh SF, Sengupta PP, Sicari R, Smiseth OA, Smulevitz B, Takeuchi M, Thomas JD, Vannan M, Voigt JU, Zamorano JL. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. J Am Soc Echocardiogr. 2011;24:277–313. doi: 10.1016/j.echo.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 67.Picard MH, Adams D, Bierig SM, Dent JM, Douglas PS, Gillam LD, Keller AM, Malenka DJ, Masoudi FA, McCulloch M, Pellikka PA, Peters PJ, Stainback RF, Strachan GM, Zoghbi WA American Society of E. American Society of Echocardiography recommendations for quality echocardiography laboratory operations. J Am Soc Echocardiogr. 2011;24:1–10. doi: 10.1016/j.echo.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 68.D’Agostino RB, Wolf PA, Belanger AJ, Kannel WB. Stroke risk profile: Adjustment for antihypertensive medication. The Framingham Study. Stroke. 1994;25:40–43. doi: 10.1161/01.str.25.1.40. [DOI] [PubMed] [Google Scholar]
- 69.Green MS, Kaye JA, Ball MJ. The Oregon brain aging study: Neuropathology accompanying healthy aging in the oldest old. Neurology. 2000;54:105–113. doi: 10.1212/wnl.54.1.105. [DOI] [PubMed] [Google Scholar]
- 70.Bennett DA, Schneider JA, Arvanitakis Z, Wilson RS. Overview and findings from the religious orders study. Curr Alzheimer Res. 2012;9:628–645. doi: 10.2174/156720512801322573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morris JC, Weintraub S, Chui HC, Cummings J, Decarli C, Ferris S, Foster NL, Galasko D, Graff-Radford N, Peskind ER, Beekly D, Ramos EM, Kukull WA. The Uniform Data Set (UDS): Clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20:210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 72.Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, Jack CR, Jr, Jagust WJ, Shaw LM, Toga AW, Trojanowski JQ, Weiner MW. Alzheimer’s Disease Neuroimaging Initiative (ADNI): Clinical characterization. Neurology. 2010;74:201–209. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Silbert LC, Dodge HH, Perkins LG, Sherbakov L, Lahna D, Erten-Lyons D, Woltjer R, Shinto L, Kaye JA. Trajectory of white matter hyperintensity burden preceding mild cognitive impairment. Neurology. 2012;79:741–747. doi: 10.1212/WNL.0b013e3182661f2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aisen PS, Petersen RC, Donohue MC, Gamst A, Raman R, Thomas RG, Walter S, Trojanowski JQ, Shaw LM, Beckett LA, Jack CR, Jr, Jagust W, Toga AW, Saykin AJ, Morris JC, Green RC, Weiner MW Alzheimer’s Disease Neuroimaging, Initiative. Clinical Core of the Alzheimer’s Disease Neuroimaging Initiative: Progress and plans. Alzheimers Dement. 2010;6:239–246. doi: 10.1016/j.jalz.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hohman TJ, Koran ME, Thornton-Wells TA Alzheimer’s Neuroimaging, Initiative. Genetic variation modifies risk for neurodegeneration based on biomarker status. Front Aging Neurosci. 2014;6:183. doi: 10.3389/fnagi.2014.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, Beiser A, D’Agostino R, Wolf PA. Measures of brain morphology and infarction in the framingham heart study: Establishing what is normal. Neurobiol Aging. 2005;26:491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 77.Page RM, Cole GE, Timmreck TC. Basic Epidemiological Methods and Biostatistics: A Practical Guidebook. Jones and Bartlett; 1995. [Google Scholar]
- 78.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 79.Klages JD, Fisk JD, Rockwood K. APOE genotype, memory test performance, and the risk of Alzheimer’s disease in the Canadian Study of Health and Aging. Dement Geriatr Cogn Disord. 2003;15:1–5. doi: 10.1159/000066670. [DOI] [PubMed] [Google Scholar]
- 80.Sivan AB. Benton visual retention test. Psychological Corporation; San Antonio, TX: 1992. [Google Scholar]
- 81.Goodglass H, Kaplan E. The assessment of aphasia and related disorders. Lea & Febiger; Philadelphia, PA: 1983. [Google Scholar]
- 82.Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Lea & Febiger; Philadelphia, PA: 1983. [Google Scholar]
- 83.Wechsler D. Wechsler Adult Intelligence Scale. 3. The Psychological Corporation; San Antonio, TX: 1997. (WAIS-III) Manual. [Google Scholar]
- 84.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 85.Golden C. Stroop colour and word test. Stoelting Co; Wood Dale IL: 1978. [Google Scholar]
- 86.Wechsler D. Wechsler Adult Intelligence Scale. 4. Pearson; San Antonio, TX: 2008. [Google Scholar]
- 87.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 88.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. 2. Psychological Corporation; San Antonio: 2000. [Google Scholar]
- 89.Glosser G, Gallo JL, Clark CM, Grossman M. Memory encoding and retrieval in frontotemporal dementia and Alzheimer’s disease. Neuropsychology. 2002;16:190–196. [PubMed] [Google Scholar]
- 90.Wechsler D. Wechsler Memory Scale-Revised. Psychological Corporation; San Antonio, Texas: 1987. [Google Scholar]
- 91.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (D-KEFS): Examiner’s Manual. The Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- 92.Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol. 1999;14:167–177. [PubMed] [Google Scholar]
- 93.Hooper H. Hooper Visual Organization Test (HVOT) Western Psychological Services; Los Angeles, CA: 1983. [Google Scholar]
- 94.Price CC, Garrett KD, Jefferson AL, Cosentino S, Tanner JJ, Penney DL, Swenson R, Giovannetti T, Bettcher BM, Libon DJ. Leukoaraiosis severity and list-learning in dementia. Clin Neuropsychol. 2009;23:944–961. doi: 10.1080/13854040802681664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lamar M, Price CC, Libon DJ, Penney DL, Kaplan E, Grossman M, Heilman KM. Alterations in working memory as a function of leukoaraiosis in dementia. Neuropsychologia. 2007;45:245–254. doi: 10.1016/j.neuropsychologia.2006.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kaplan E, Fein D, Morris R, Delis D. WAIS-R as a neuropsychological instrument. The Psychological Corporation; San Antonio TX: 1991. [Google Scholar]
- 97.Wechsler D. Wechsler Memory Scale. 4. Pearson; San Antonio, TX: 2009. [Google Scholar]
- 98.Lamar M, Price CC, Davis KL, Kaplan E, Libon DJ. Capacity to maintain mental set in dementia. Neuropsychologia. 2002;40:435–445. doi: 10.1016/s0028-3932(01)00125-7. [DOI] [PubMed] [Google Scholar]
- 99.Wechsler D. Wechsler Adult Intelligence Scale -Revised Manual. Psychological Corporation; New York, NY: 1981. [Google Scholar]