Abstract
Half of the patients who present with unstable angina (UA) develop recurrent symptoms over the subsequent year. Identification of patients destined to develop such adverse events would be clinically valuable, but current tools do not allow for this discrimination. Fibrocytes are bone marrow-derived progenitor cells that co-express markers of leukocytes and fibroblasts and are released into the circulation in the context of tissue injury. We hypothesized that, in patients with UA, the number of circulating fibrocytes predicts subsequent adverse events. We enrolled 55 subjects with UA, 18 with chronic stable angina (CSA), and 22 controls and correlated their concentration of circulating fibrocytes to clinical events (recurrent angina, myocardial infarction, revascularization, or death) over the subsequent year. Subjects with UA had a >2-fold higher median concentration of both total and activated fibrocytes compared to subjects with CSA and controls. In UA subjects, the concentration of total fibrocytes identified those who developed recurrent angina requiring revascularization (time dependent AUC 0.85) and was superior to risk stratification using Thrombolysis in Myocardial Infarction (TIMI) risk score and N-terminal pro B-type natriuretic peptide levels (AUC 0.53 and 0.56, respectively, p<0.001). After multivariable adjustment for TIMI predicted death, MI, or recurrent ischemia, total fibrocyte level was associated with recurrent angina (HR 1.016 per 10,000 cells/mL increase, 95% CI 1.007 to 1.024; p<0.001). Circulating fibrocytes are elevated in patients with UA and successfully risk stratify them for adverse clinical outcomes. Fibrocytes may represent a novel biomarker of outcome in this population.
INTRODUCTION
Patients who present under the umbrella term of acute coronary syndrome (ACS) have a wide range of clinical outcomes, including recurrent ischemia, need for revascularization, and death. While prognostic factors are well-established for patients with ST elevation myocardial infarction (STEMI) and non-STEMI1, identification of patients with unstable angina (UA) who are at increased risk of adverse outcomes has proven more elusive. Risk stratification of UA patients is clinically important, because this population has high rates of recurrent cardiovascular events over the year after their initial presentation.2 Compared to their troponin-positive counterparts, patients with UA have lower rates of death and MI at one year, but half develop recurrent ischemia requiring revascularization3, resulting in substantial morbidity and cost. The ability to identify UA patients during the index hospitalization who are at increased risk for adverse events has the potential to improve the outcomes of this subgroup, for example by targeting them for more aggressive therapy. Several biomarkers have been assessed in this population including brain natriuretic peptide (BNP), high sensitivity cardiac troponin T, and C-reactive protein3, 4–6. These biomarkers have been associated with increased short-term3, 4 and long-term5, 6 mortality, but not predictive of recurrent ischemic events.
Fibrocytes are circulating bone marrow-derived progenitor cells that are identified by the co-expression of the common leukocyte antigen CD45, and the fibroblast marker collagen-1. Fibrocytes can home to injured tissues, differentiate into fibroblasts and myofibroblasts, and contribute to fibrogenesis. Fibrocytes represent <0.5% of nucleated cells in the peripheral blood of healthy individuals, but in fibrotic diseases blood concentrations can increase up to 100-fold and correlate with severity of tissue injury.7–10 As fibrocytes differentiate into fibroblasts and myofibroblasts under the influence of the fibrogenic cytokine transforming growth factor (TGF)-β, they progressively lose the progenitor marker CD34 and CD45, retain the expression of collagens -1 and -3, and gain the expression of the myofibroblast marker, α-smooth muscle actin (α-SMA).11–14 Activated subsets of fibrocytes can thus be identified on the basis of expression of phosphorylated SMAD-2 and -3 downstream of the TGF-β receptor15, α-SMA, and discoidin domain receptor (DDR)-2, a cell surface receptor for extracellular fibrillar collagens that mediates up-regulation of matrix metalloproteinases and collagen turnover.16
In the context of coronary artery disease (CAD), fibrocytes have been detected in the fibrous cap of atherosclerotic plaques17, in areas of intimal hyperplasia18, in postmortem myocardial samples taken from the infarct zone of patients with MI19, and in the circulation of patients with acute MI.20 Given that the release of fibrocytes from the bone marrow to the peripheral blood is a conserved response to tissue injury, we reasoned that the extent of myocardial ischemia in UA may be reflected in the expansion and activation of the pool of circulating fibrocytes. We therefore tested the hypothesis that, in patients with UA, the number and phenotype of circulating fibrocytes predicts subsequent development of adverse clinical events.
METHODS
Patient recruitment and blood collection
Adult patients referred for coronary angiography were recruited into an observational cohort study at the University of Virginia between May 2010 and June 2012. Exclusion criteria were: (1) inability to provide informed consent; (2) known fibrotic disease; (3) active infection or malignancy; (4) trauma or a surgical procedure in the prior 6 weeks, and (5) expected survival less than one year. The research was carried out according to the principles of the Declaration of Helsinki. All patients provided written informed consent. The study was approved by the University of Virginia’s human ethics research committee.
Chronic stable angina (CSA) was defined as chest discomfort symptoms provoked by exertion or emotional stress and relieved by rest or nitroglycerin.21 UA was defined as having ≥ 2 negative troponin levels at least 12 hours prior to coronary angiography and rest, new onset, or worsening chest discomfort.22 Subjects without CAD as documented on coronary angiography prior to valve surgery served as controls. Results of coronary angiography were retrieved from the cardiac catheterization electronic database reporting system. Obstructive CAD was defined as the presence of ≥ 75% angiographic luminal diameter stenosis in at least one major epicardial artery or ≥ 50% luminal diameter stenosis of the left main coronary artery. Following arterial access and prior to angiography or heparin administration, 10ml of peripheral blood was drawn from the arterial sheath, anticoagulated with heparin, and immediately placed on ice. The samples were centrifuged (135 g, 10 minutes at 4 °C) and the plasma was stored at −80°C. Patients with unstable angina underwent coronary angiography and blood sample collection within 24 hours of their hospital admission.
Measurements
Total circulating fibrocytes and fibrocyte subsets were identified by flow cytometry without ex vivo manipulations as previously described.13, 23 Briefly, the blood samples were centrifuged and the buffy coat layer collected. Contaminating red blood cells were lysed and were washed, re-suspended and passed through 100 μm nylon mesh filters to remove clumps. The concentration of leukocytes in each sample was quantified by enumerating live cells under a hemacytometer using trypan blue exclusion. Cells were then brought to a concentration of 107 per mL and 100 μl of suspension used for staining. The cells were stained with surface markers anti-CD45-V500 (clone H130), anti-CXCR4 APC (clone 12G5), anti-CD34 PerCP (clone 8G12) (BD Biosciences), anti-DDR2 PerCP (Santa Cruz Biotech), or isotype controls. The cells were then permeabilized with a BD Cytofix/Cytoperm kit (BD Biosciences) and stained with anti5α-SMA-PE (R&D Systems, Minneapolis, MN, USA), anti-collagen-1 (Col-1) DyLight 488 (Rockland, Gilbertsville, PA, USA), and anti-p-SMAD2/3 DyLight 650 (Santa Cruz Biotech), or isotype control antibodies. Anti-collagen, anti-DDR2, anti-p-SMAD2/3, and respective control antibodies were conjugated to PerCP, DyLight 650 or 488 using Lightning-Link (Innova Biosciences) or DyLight conjugation kits (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. Samples were analyzed on a FACS Canto-II using Diva software (version 5.0.3, BD Biosciences). Compensation beads and the BD FACSDiva software algorithm were used for compensation. Forward- and side-scatter gates were used to exclude clumps and debris and data from 30,000 events of the remaining population were collected. Cells were analyzed by first gating on the CD45+ population and then collagen-1+ population, with negative control thresholds set at 0.5% using matched Ig control. The CD45+Col1+ population was then analyzed for staining for other antigens, using respective isotype controls. We identified the total fibrocyte population as CD45+Col1+ cells and subsets as activated CD45+ Col1+ α-SMA+ cells, fibrocytes expressing phosphorylated SMAD-2/3 (CD45+ Col1+ p-SMAD-2/3+), DDR2-expressing fibrocytes (CD45+ Col1+ DDR2+) and CD34-expressing fibrocytes (CD45+ CD34+ Col-1+). Representative flow cytometric plots show our gating strategy (Figure 1). Absolute concentration of each cell type was determined as the product of the ratio of that population to parent CD45+ population and the concentration of live leukocytes in the sample.
Figure 1.
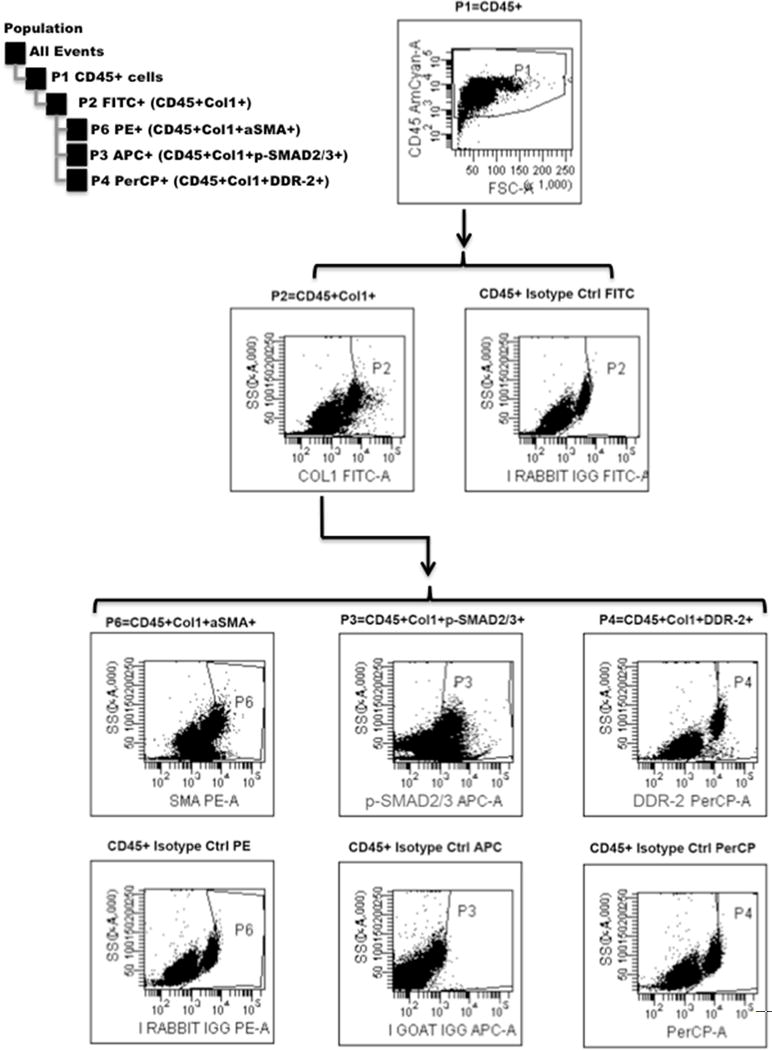
Representative gating scheme for flow cytometric analysis. FSC-A, forward scatter; SSC-A, side scatter.
Plasma TGF-β1 and N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels were measured using commercial ELISA kits according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN, USA).
Clinical events
Clinical follow-up for all subjects was collected by reviewing the electronic medical records and by telephone interview at 6 months and 1 year. Cardiac events including recurrent angina, MI, need for coronary revascularization either with percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG) surgery, and death were recorded. Reports of clinical outcomes were independently verified by chart review by two investigators (M.A.M., E.C.K.).
Statistical analysis
Chi-squared test was used to compare PCI rates and extent of CAD at baseline between UA and CSA groups. Comparisons among two or more groups were performed the using the Kruskal-Wallis test. Comparisons between subjects with and without recurrent events were performed using the Mann-Whitney test. Time dependent receiver operating characteristic curve analysis was done to evaluate the performance of total fibrocytes and their activated subset as a predictor of clinical events. Probability values (two-sided) were considered statistically significant if <0.05.
Two separate Cox proportional hazards models were used to assess the association of circulating fibrocyte levels with adverse events in subjects with UA independent of currently known predictors of poor outcome. The first model included the total fibrocyte concentration and the Thrombolysis in Myocardial Infarction (TIMI) percentage of all-cause mortality, new or recurrent MI, or severe recurrent ischemia requiring urgent revascularization.24 The second model included age, prior MI, diabetes, and smoking. Data were analyzed using Prism statistical software (Version 6.0, GraphPad Software, La Jolla, California, USA) or R (version 3.1.0; open source, www.R-project.org).25
RESULTS
We prospectively enrolled 95 consecutive subjects (55 with UA, 18 with CSA and 22 controls without CAD). The three groups were similar with respect to baseline demographics (Table 1). Compared with subjects without CAD, patients with UA and CSA had higher rates of chronic aspirin and beta-blocker use and prior coronary revascularization.
Table 1.
Clinical characteristics of patients and controls
| Variable | Controls (n=22) | CSA (n=18) | UA (n=55) | p value |
|---|---|---|---|---|
| Age (years) mean +/− SD | 67 +/− 11 | 66 +/− 11 | 63 +/− 11 | 0.236 |
| Sex (male) | 10 (45) | 11 (61) | 32 (58) | 0.493 |
| Caucasian | 20 (91) | 16 (89) | 50 (91) | 0.882 |
| Cardiovascular risk factors | ||||
| Hypertension | 13 (59) | 15 (83) | 44 (80) | 0.085 |
| Hyperlipidemia | 18 (82) | 15 (83) | 43 (78) | 0.934 |
| Diabetes mellitus | 3 (14) | 5 (28) | 20 (36) | 0.127 |
| Current smoker | 1 (5) | 2 (11) | 7 (13) | 0.557 |
| Family history of CAD | 4 (18) | 6 (33) | 20 (36) | 0.151 |
| Prior myocardial infarction | 0 | 6 (33) | 18 (33) | 0.250 |
| Prior stroke | 1 (5) | 1 (5) | 3 (5) | 0.983 |
| Prior PCI | 0 | 6 (33) | 17 (31) | 0.009 |
| Prior CABG | 0 | 6 (33) | 10 (18) | 0.018 |
| Ejection fraction ≥50% | 16 (73) | 10 (56) | 34 (62) | 0.521 |
| PCI during index procedure | 0 (0) | 8 (44%) | 17 (31%) | 0.263 |
| Medications | ||||
| Aspirin | 5 (23) | 16 (89) | 41 (75) | <0.0001 |
| Clopidogrel | 1 (5) | 5 (28) | 14 (25) | 0.089 |
| Beta-blocker | 10 (45) | 10 (56) | 41 (75) | 0.027 |
| ACE-inhibitor | 8 (36) | 10 (56) | 24 (44) | 0.478 |
| Statin | 16 (73) | 10 (56) | 41 (75) | 0.079 |
| Insulin | 2 (9) | 2 (11) | 7 (13) | 0.889 |
| Oral hypoglycemic agent | 2 (9) | 3 (17) | 11 (20) | 0.494 |
Values are expressed as either mean ± standard deviation (SD) or number (%). ACE, angiotensin converting enzyme; CABG, coronary artery bypass graft surgery; CAD, coronary artery disease; CSA, chronic stable angina; PCI, percutaneous coronary intervention; UA, unstable angina.
Compared to both the control subjects and subjects with CSA, subjects with UA had a 2-fold higher median concentration of total fibrocytes (Figure 2A). The median concentration of fibrocyte subsets expressing phosphorylated SMAD-2/3 (indicating response to TGF-β), α-SMA (indicating an activated phenotype), and DDR2 (indicating upregulation of collagen receptors) were also elevated in subjects with UA (Figure 2B–D). In contrast, the concentration of CD34+ fibrocytes (defined as CD45+ Col1+ CD34+ cells) represented only 12% of the total fibrocyte concentration in the no CAD group, 13% in subjects with CSA, and 11% in subjects with UA, and did not differ significantly between groups (Supplemental figure 1). This suggests that CD34 is not an optimal marker for circulating fibrocytes in this population. Plasma levels of the profibrotic cytokine TGF-β1, in UA subjects in the middle and highest tertiles for the activated (α-SMA+) subset of fibrocytes were significantly higher than those in the lowest tertile (Figure 3).
Figure 2.
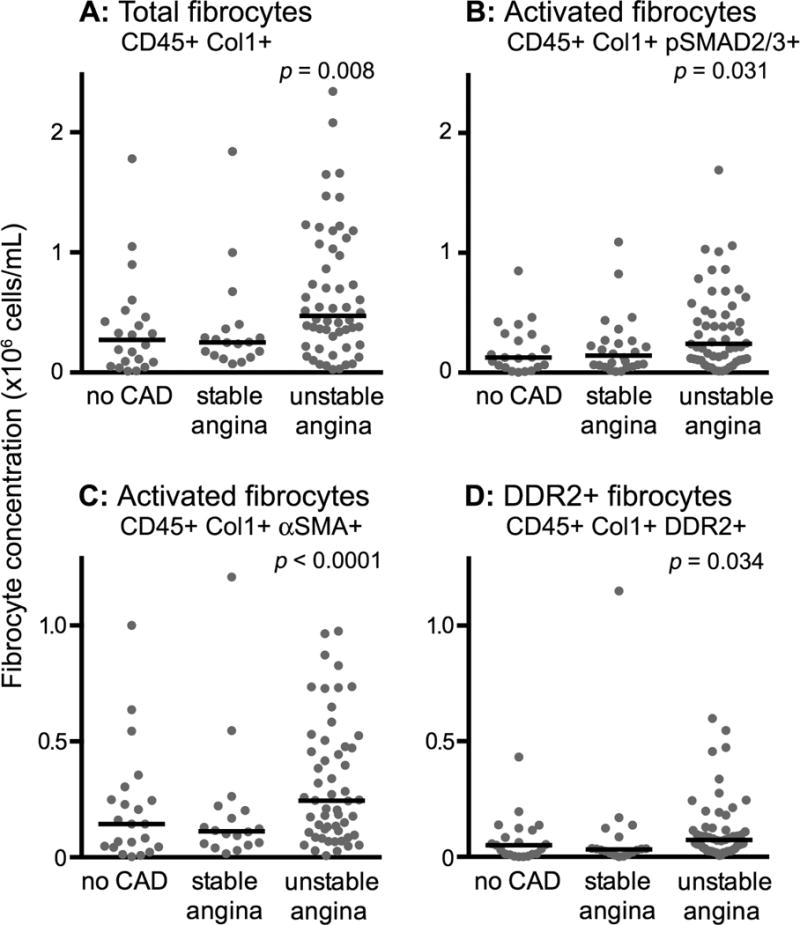
Cross-sectional comparison of peripheral blood fibrocytes in subjects without coronary artery disease, with chronic stable angina, and with unstable angina. Total fibrocytes (panel A), SMAD 2/3+ fibrocytes (panel B), α-SMA+ fibrocytes (panel C) and DDR2+ fibrocytes (panel D) are shown. Each data point represents one subject; horizontal lines designate the medians. α-SMA, alpha-smooth muscle actin; CAD, coronary artery disease; Col1, collagen-1; DDR2, discoidin domain receptor-2.
Figure 3.
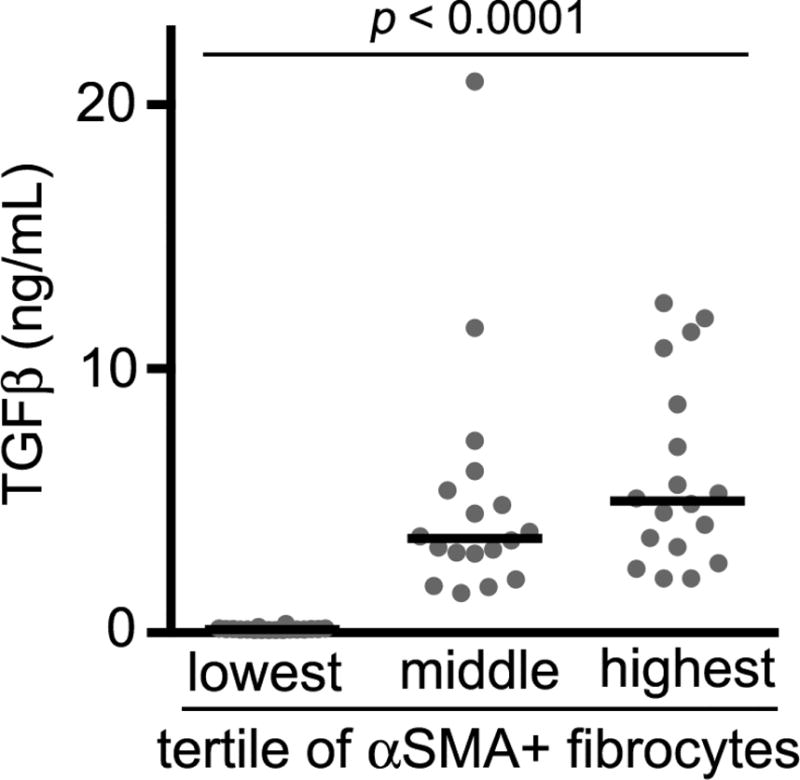
Transforming growth factor-β1 plasma concentration in subjects with unstable angina in the lowest, middle and highest tertile of α-SMA+ fibrocytes. Each data point represents one subject; horizontal lines designate the medians. TGF, transforming growth factor.
One year clinical follow-up was obtained for all 95 subjects. Recurrent angina requiring revascularization occurred in 15 (27.3%) of the subjects with UA and 3 (16.7%) of those with CSA. There were no MIs or deaths. Among the subjects with UA, those who had recurrent angina were predominately men (Table 2); otherwise there were no significant differences. Among patients with UA, however, those who required revascularization had significantly higher concentrations of circulating fibrocytes at initial presentation compared to those who did not (Figure 4A–D). Moreover, the concentration of total fibrocytes and their activated subset on first presentation identified those patients who developed recurrent angina requiring revascularization over the subsequent year [time dependent AUC at 1 year 0.85 and 0.84, respectively] (Figure 5A–B) and was superior to risk stratification using the TIMI risk score24 (0.85 vs. 0.53, p<0.001) (Figure 5C). Similar to the TIMI risk score, NT pro-BNP had minimal discriminatory value in predicting recurrent angina at one year (AUC 0.56) compared to circulating fibrocyte concentrations (Figure 5D). Among patients with UA whose total circulating fibrocyte concentration exceeded a threshold value of 9.2 × 106 cells/mL on initial presentation, 53% required revascularization within a year, whereas none of the patients with fibrocyte counts below the threshold required a procedure.
Table 2.
Clinical characteristics of unstable angina subjects with and without recurrent angina
| Variable | UA with recurrent angina (n=15) |
UA without recurrent angina (n=40) |
p value |
|---|---|---|---|
| Age (years) mean +/− SD | 70 [62–77] | 62 [53–69] | 0.049 |
| Sex (male) | 14 (93) | 19 (48) | 0.002 |
| Cardiovascular risk factors | |||
| Hypertension | 13 (87) | 32 (80) | 0.710 |
| Hyperlipidemia | 12 (80) | 32 (80) | 1.000 |
| Diabetes mellitus | 6 (40) | 14 (35) | 0.761 |
| Smoking | 2 (13) | 5 (13) | 1.000 |
| Prior CABG | 2 (13) | 9 (23) | 0.708 |
| Congestive heart failure | 1 (7) | 10 (25) | 0.708 |
| Medications | |||
| Aspirin | 13 (87) | 29 (73) | 0.477 |
| Beta-blocker | 10 (67) | 31 (78) | 0.493 |
| ACE-inhibitor | 7 (87) | 29 (73) | 0.477 |
| Statin | 12 (80) | 30 (75) | 1.000 |
Values are expressed as either mean ± standard deviation (SD) or number (%). ACE, angiotensin converting enzyme; CABG, coronary artery bypass graft surgery; UA, unstable angina.
Figure 4.
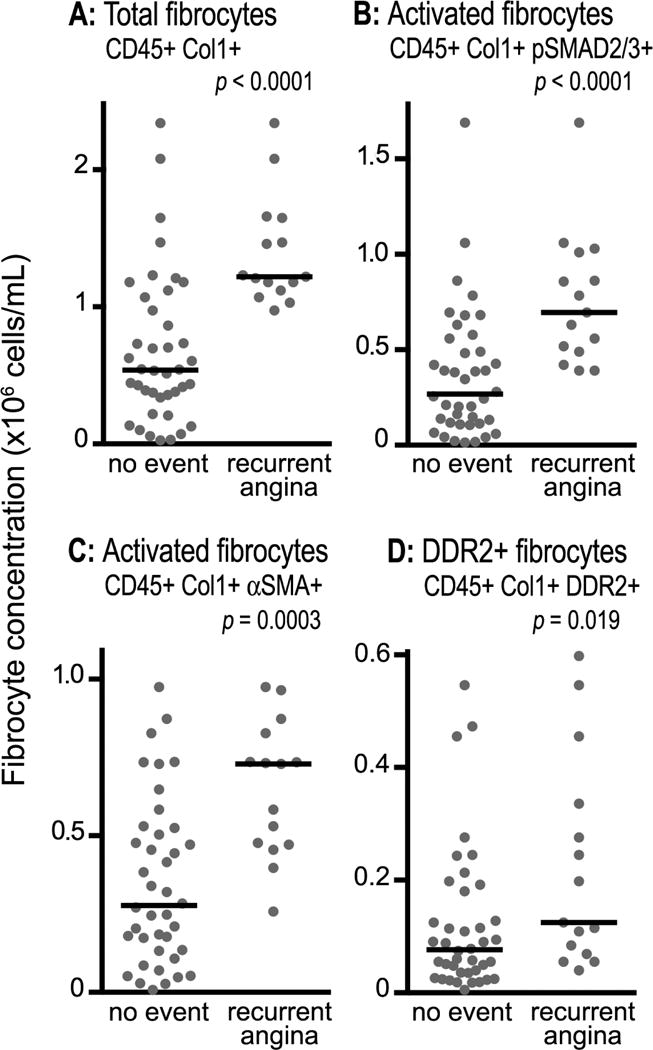
Cross-sectional comparison of peripheral blood fibrocytes in subjects with unstable angina who did and did not develop recurrent angina at one year follow-up. Total fibrocytes (panel A), SMAD 2/3+ fibrocytes (panel B), α-SMA+ fibrocytes (panel C) and DDR2+ fibrocytes (panel D) are shown. Each data point represents one subject and horizontal bars designate the medians. α-SMA, alpha-smooth muscle actin; Col1, collagen-1; DDR2, discoidin domain receptor-2.
Figure 5.
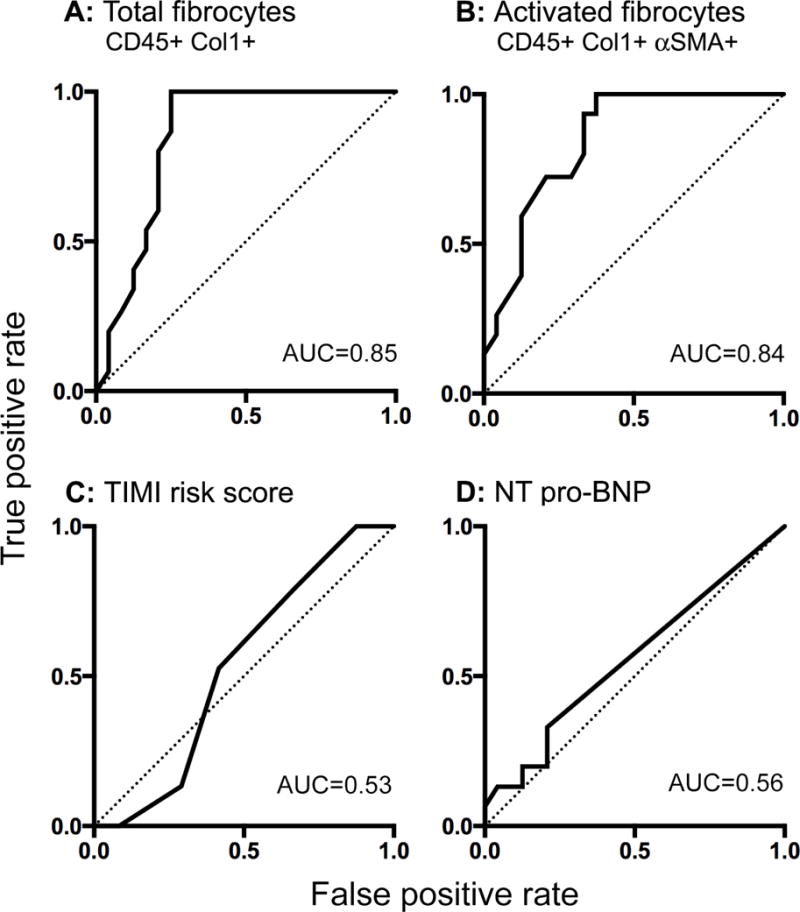
Receiver operating characteristic curves of total fibrocytes (panel A), α-smooth muscle actin + fibrocytes (panel B), Thrombolysis in Myocardial Infarction (TIMI) risk score (panel C), and N-terminal of the pro-hormone brain natriuretic peptide (NT pro-BNP, panel D) to predict recurrent angina at one year in subjects with unstable angina. A-SMA, alpha-smooth muscle actin; AUC, area under the curve; Col1, collagen-1.
Finally, we assessed the predictive value of fibrocytes after adjustment for the TIMI predicted percentage of all-cause mortality, new or recurrent MI, or severe recurrent ischemia requiring urgent revascularization. Total fibrocyte level remained strongly associated with recurrent angina (HR 1.016 per 10,000 cells/mL increase in circulating fibrocytes, 95% CI 1.007 to 1.024; p <0.001). Similar results were found when adjusted for age, prior MI, diabetes, and smoking (HR 1.016 per 10,000 cells/mL increase in circulating fibrocytes, 95% CI 1.006 to 1.027; p= 0.003). These data suggest that, in our cohort, circulating fibrocytes were strongly associated with future adverse events independent of conventional risk factors.
DISCUSSION
Our findings are unique in that we found circulating fibrocyte concentrations to be strongly predictive of recurrent ischemic events requiring revascularization. Although the mechanism is unknown, we speculate that it may reflect the role fibrocytes play in tissue repair and fibrosis.
Fibrocytes have not been studied extensively in the context of human heart disease, but have been causally linked to myocardial fibrosis in mouse models of acute MI and ischemic cardiomyopathy.26–28 In humans, the concentration of circulating fibrocytes are elevated in subjects with hypertensive cardiomyopathy29, and in the myocardium in patients who died of MI.19 Of specific relevance to unstable angina, experimental data suggest a role of fibrocytes in atherosclerotic plaque repair following injury: In human carotid endarterectomy specimens, fibrocytes accumulate in regions of plaque growth and repair, co-localized with TGF-β, and contributed to formation of the fibrous cap.30 In animal models, collagen 1 and α-SMA-expressing bone marrow progenitor cells have been shown to traffic to areas of intimal hyperplasia after endovascular injury31,32 and in a model of transplant vasculopathy, higher numbers of circulating fibrocytes were associated with more severe vasculopathy.33 Together these studies suggest that fibrocytes play an important role in vascular remodeling following injury.
In the context of troponin-positive ACS, circulating fibrocytes were previously reported to comprise a smaller proportion of total leukocytes in subjects with acute MI as compared to those with CSA or healthy controls than we report here20. There are several possible explanations for this discrepancy, including differences in the patient population under study and differences in the timing of the acquisition of fibrocytes in relationship to symptom onset. Most importantly, however, differences in the definition of fibrocytes explain the discrepant results. Specifically, we identified fibrocytes as CD45+ collagen-1+ cells and provide data that, in the context of unstable angina, CD45+ CD34+ collagen-1+ cells constitute a small subset (~10%) of this population, whereas most fibrocytes express the myofibroblast marker, α-SMA (Figure 1 and Supplemental figure 1). Other studies have also reported that only a small minority of circulating and tissue fibrocytes express CD34 in vivo8,13, consistent with the observation that, during in vitro culture, fibrocytes progressively lose expression of CD34 as they acquire the expression of α-SMA, a process that is accelerated by exposure to TGF-β.34 In contrast, the prior study reported only the CD45+ CD34+ collagen-1+ population, and not the CD45+ collagen-1+ parent population nor the CD45+ α-SMA collagen-1+ activated subset, thus likely significantly underestimating the concentration of total and activated fibrocytes.
An interesting finding in our study is the correlation between plasma TGF-β1 with concentrations of fibrocytes expressing α-SMA, evidence of myofibroblast-like differentiation. This finding provides in vivo support for the hypothesis that the profibrotic cytokine, TGF-β, drives fibrocyte-to-myofibroblast differentiation via phosphorylation of SMAD-2/3 downstream of the TGF-β receptor, which in turn stimulates proliferation, myofibroblast differentiation, and increased synthesis of extracellular matrix proteins.14,15 Similarly, DDR2, a receptor tyrosine kinase that is activated when bound to extracellular collagen triple helix, mediates fibroblast migration/proliferation and is required for the attachment of fibroblasts to collagen matrices.35 We interpret the expansion of DDR2+ fibrocytes as further evidence of an increased pool of circulating fibrocytes with activated phenotypes. Our findings are similar to those of investigators studying other inflammatory diseases, in which the population of circulating fibrocytes has been shown to expand, display an activated phenotype, and home to the site of injury.36–38
Our study has limitations. First, it is a small study without a validation cohort. Nonetheless, our subjects were well-characterized and one-year follow-up was obtained in all of them. Second, all patients underwent coronary angiography, potentially making the generalizability of our findings to low-risk UA patients who do not undergo angiography uncertain. Third, our results reflect an association but do not establish a causal relationship between circulating fibrocytes and prognosis in UA.
CONCLUSIONS
Our data indicate that the absolute concentration of circulating fibrocytes as well as their activated subsets are significantly elevated in patients with UA compared to those with CSA and controls. In subjects with UA, elevated fibrocyte levels during the index presentation were associated with recurrent angina over the subsequent year.
Speculations
Circulating fibrocytes may represent a novel biomarker of prognosis in UA. Additional studies are needed in order to validate these findings.
Supplementary Material
Supplemental figure 1 Cross-sectional comparison of CD34+ peripheral blood fibrocytes in subjects without coronary artery disease, with chronic stable angina, and with unstable angina. Each data point represents one subject; horizontal lines designate the medians. CAD, coronary artery disease; Col1, collagen-1.
Background.
Unstable angina is among the most common presentations of acute coronary syndrome, but our current ability to identify patients at high risk of recurrent ischemic events is very limited. Fibrocytes are circulating bone marrow-derived cells that home to sites of tissue injury, differentiate into fibroblasts and myofibroblasts, and contribute to scar formation. We hypothesized that in patients with unstable angina, the number of circulating fibrocytes correlates with subsequent adverse events.
Translational Significance.
This study found that fibrocytes and their activated subsets were significantly elevated in the peripheral blood of subjects with unstable angina compared to those with stable angina and controls. Moreover, in subjects with unstable angina, elevated fibrocyte levels during the index presentation were associated with recurrent angina over the subsequent year. Circulating fibrocytes may represent a novel biomarker of prognosis in patients with unstable angina and may be involved in atherosclerotic plaque growth.
Acknowledgments
This study was funded by the NIH HL97074 (to E.C.K.) and HL098329 (to B.M.) and the American Heart Association (13IRG14560018 to E.C.K.).
All authors have read the journal’s authorship agreement and the manuscript has been reviewed by and approved by all the authors.
ABBREVIATIONS
- ACS
acute coronary syndrome
- AUC
area under the curve
- CABG
coronary artery bypass surgery
- CAD
coronary artery disease
- CSA
chronic stable angina
- DDR
discoidin domain receptor
- FACS
fluorescence-activated cell sorting
- NT pro-BNP
N-terminal of the prohormone brain natriuretic peptide
- PCI
percutaneous coronary intervention
- SMA
smooth muscle actin
- SMAD
mothers against decapentaplegic homolog
- STEMI
ST elevation myocardial infarction
- TGF
transforming growth factor
- TIMI
Thrombolysis in Myocardial Infarction
- UA
unstable angina
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: All authors have read the journal’s policy on disclosure of potential conflicts of interest and have none to declare.
References
- 1.Heidenreich PA, Alloggiamento T, Melsop K, McDonald KM, Go AS, Hlatky MA. The prognostic value of troponin in patients with non-ST elevation acute coronary syndromes: a meta-analysis. J Am Coll Cardiol. 2001;38:478–485. doi: 10.1016/s0735-1097(01)01388-2. [DOI] [PubMed] [Google Scholar]
- 2.Meune C, Balmelli C, Twerenbold R, et al. Patients with acute coronary syndrome and normal high-sensitivity troponin. Am J Med. 2011;124:1151–1157. doi: 10.1016/j.amjmed.2011.07.032. [DOI] [PubMed] [Google Scholar]
- 3.Weber M, Bazzino O, Navarro Estrada JL, et al. N-terminal B-type natriuretic peptide assessment provides incremental prognostic information in patients with acute coronary syndromes and normal troponin T values upon admission. J Am Coll Cardiol. 2008;51:1188–1195. doi: 10.1016/j.jacc.2007.11.054. [DOI] [PubMed] [Google Scholar]
- 4.de Lemos JA, Morrow DA, Bentley JH, et al. The prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. N Engl J Med. 2001;345:1014–1021. doi: 10.1056/NEJMoa011053. [DOI] [PubMed] [Google Scholar]
- 5.Haverkate F, Thompson SG, Pyke SDM, Gallimore JR, Pepys MB, for the European concerted action on thrombosis and disabilities angina pectoris study group Production of C-reactive protein and risk of coronary events in stable and unstable angina. Lancet. 1997;349:462–466. doi: 10.1016/s0140-6736(96)07591-5. [DOI] [PubMed] [Google Scholar]
- 6.Ndreppa G, Braun S, Mehilli J, et al. Prognostic value of sensitive troponin T in patients with stable and unstable angina and undetectable conventional troponin. Am Heart J. 2011;161:68–75. doi: 10.1016/j.ahj.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 7.Phillips RJ, Burdick MD, Hong K, et al. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438–446. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol. 2004;36:598–606. doi: 10.1016/j.biocel.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol. 2003;171:380–389. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- 10.Keeley EC, Mehrad B, Strieter RM. The role of circulating mesenchymal progenitor cells (fibrocytes) in the pathogenesis of fibrotic disorders. Thromb Haemost. 2009;101:613–618. [PMC free article] [PubMed] [Google Scholar]
- 11.Andersson-Sjoland A, de Alba CG, Nihlberg K, et al. Fibrocytes are a potential source of lung fibroblasts in idiopathic pulmonary fibrosis. Int J Biochem Cell Biol. 2008;40:2129–2140. doi: 10.1016/j.biocel.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Mehrad B, Burdick MD, Zisman DA, Keane MP, Belperio JA, Strieter RM. Circulating peripheral blood fibrocytes in human fibrotic interstitial lung disease. Biochem Biophys Res Commun. 2007;353:104–108. doi: 10.1016/j.bbrc.2006.11.149. [DOI] [PubMed] [Google Scholar]
- 13.Trimble A, Gochuico BR, Markello TC, et al. Circulating fibrocytes as biomarker of prognosis in Hermansky-Pudlak syndrome. Am J Respir Crit Care Med. 2014;190:1395–1401. doi: 10.1164/rccm.201407-1287OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong KM, Belperio JA, Keane MP, Burdick MD, Strieter RM. Differentiation of human circulating fibrocytes as mediated by transforming growth factor-beta and peroxisome proliferator-activated receptor gamma. J Biol Chem. 2007;282:22910–22920. doi: 10.1074/jbc.M703597200. [DOI] [PubMed] [Google Scholar]
- 15.Schiller M, Javelaud D, Mauviel A. TGF-beta-induced SMAD signaling and gene regulation: consequences for extracellular matrix remodeling and wound healing. J Dermatol Sci. 2004;35:83–92. doi: 10.1016/j.jdermsci.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Ferri N, Carragher NO, Raines EW. Role of discoidin domain receptors 1 and 2 in human smooth muscle cell-mediated collagen remodeling: potential implications in atherosclerosis and lymphangioleiomyomatosis. Am J Pathol. 2004;164:1575–1585. doi: 10.1016/S0002-9440(10)63716-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medbury HJ, Tarran SL, Guiffre AK, et al. Monocytes contribute to the atherosclerotic cap by transformation into fibrocytes. Int Angiol. 2008;27:114–123. [PubMed] [Google Scholar]
- 18.Chen D, Ma L, Tham EL, et al. Fibrocytes mediate intimal hyperplasia post-vascular injury and are regulated by two tissue factor-dependent mechanisms. J Thromb Haemost. 2013;11:963–974. doi: 10.1111/jth.12198. [DOI] [PubMed] [Google Scholar]
- 19.Lei PP, Qu YQ, Shuai Q, et al. Fibrocytes are associated with the fibrosis of coronary heart disease. Pathol Res Pract. 2013;209:36–43. doi: 10.1016/j.prp.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 20.Fang L, Moore XL, Chan W, Whilte DA, Chin-Dusting J, Dart AM. Decreased fibrocyte number is associated with atherosclerotic plaque instability in man. Cardiovasc Res. 2012;95:124–133. doi: 10.1093/cvr/cvs156. [DOI] [PubMed] [Google Scholar]
- 21.Abrams J. Clinical practice. Chronic stable angina N Engl J Med. 2005;352:2524–2533. doi: 10.1056/NEJMcp042317. [DOI] [PubMed] [Google Scholar]
- 22.Braunwald E. Unstable angina. A classification Circulation. 1989;80:410–414. doi: 10.1161/01.cir.80.2.410. [DOI] [PubMed] [Google Scholar]
- 23.Shipe R, Burdick MD, Strieter BA, et al. Number, activation, and differentiation of circlulating fibrocytes correlate with asthma severity. J Allergy Clin Immunol. 2015 Sep 11; doi: 10.1016/j.jaci.2015.07.037. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA. 2000;284:835–842. doi: 10.1001/jama.284.7.835. [DOI] [PubMed] [Google Scholar]
- 25.R Core Team. R: A Language and Environment for Statistical Computing [Internet] Vienna, Austria: R Foundation for Statistical Computing; 2013. Available from: http://www.R-project.org/ [Google Scholar]
- 26.Chu PY, Mariani J, Finch S, et al. Bone marrow-derived cells contribute to fibrosis in the chronically failing heart. Am J Pathol. 2010;176:1735–1742. doi: 10.2353/ajpath.2010.090574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Amerongen MJ, Bou-Gharios G, Popa E, et al. Bone marrow-derived myofibroblasts contribute functionally to scar formation after myocardial infarction. J Pathol. 2008;214:377–386. doi: 10.1002/path.2281. [DOI] [PubMed] [Google Scholar]
- 28.Mollmann H, Nef HM, Kostin S, et al. Bone marrow-derived cells contribute to infarct remodelling. Cardiovasc Res. 2006;71:661–671. doi: 10.1016/j.cardiores.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 29.Keeley EC, Mehrad B, Janardhanan R, et al. Elevated circulating fibrocyte levels in patients with hypertensive heart disease. J Hypertens. 2012;30:1856–1861. doi: 10.1097/HJH.0b013e32835639bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Medbury HJ, Tarran SL, Guiffre AK, et al. Monocytes contribute to the atheroscleriotc cap by trnasformation into fibrocytes. Int Angiol. 2008;27:114–123. [PubMed] [Google Scholar]
- 31.Varcoe RL, Mikhail M, Guiffre AK, et al. The role of the fibrocyte in intimal hyperplasia. J Thromb Haemost. 2006;4:1125–1133. doi: 10.1111/j.1538-7836.2006.01924.x. [DOI] [PubMed] [Google Scholar]
- 32.Chen D, Ma L, Tham EL, et al. Fibrocytes mediate intimal hyperplasia post-vascular injury and are regulated by two tissue factor-dependent mechanisms. J Thromb Haemost. 2013;11:963–974. doi: 10.1111/jth.12198. [DOI] [PubMed] [Google Scholar]
- 33.Onuta G, van Ark J, Rienstra H, et al. Development of transplant vasculopathy in aortic allografts correlates with neointimal smooth muscle cell proliferative capacity and fibrocyte frequency. Atherosclerosis. 2010;209:393–402. doi: 10.1016/j.atherosclerosis.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol. 2003;171:380–389. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- 35.Vogel W, Gish GD, Alves F, Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell. 1997;1:13–23. doi: 10.1016/s1097-2765(00)80003-9. [DOI] [PubMed] [Google Scholar]
- 36.Galligan CL, Fish EN. Circulating fibrocytes contribute to the pathogenesis of collagen antibody-induced arthritis. Arthritis Rheum. 2012;64:3583–3593. doi: 10.1002/art.34589. [DOI] [PubMed] [Google Scholar]
- 37.Field JJ, Burdick MD, DeBaun MR, et al. The role of fibrocytes in sickle cell lung disease. PLoS One. 2012;7:e33702. doi: 10.1371/journal.pone.0033702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mehrad B, Burdick MD, Strieter RM. Fibrocyte CXCR4 regulation as a therapeutic target in pulmonary fibrosis. Int J Biochem Cell Biol. 2009;41:1708–1718. doi: 10.1016/j.biocel.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figure 1 Cross-sectional comparison of CD34+ peripheral blood fibrocytes in subjects without coronary artery disease, with chronic stable angina, and with unstable angina. Each data point represents one subject; horizontal lines designate the medians. CAD, coronary artery disease; Col1, collagen-1.


