Abstract
Insects secrete antimicrobial peptides as part of the innate immune response. Most antimicrobial peptides from insects have antibacterial but not antifungal activity. We have characterized an antifungal peptide, diapausin-1 from hemolymph of a lepidopteran insect, Manduca sexta (tobacco hornworm). Diapausin-1 was isolated by size exclusion chromatography from hemolymph plasma of larvae that were previously injected with a yeast, Saccharomyces cerevisiae. Fractions containing activity against S. cerevisiae were analyzed by SDS-PAGE and MALDI-TOF MS/MS and found to contain a 45-residue peptide that was encoded by sequences identified in M. sexta transcriptome and genome databases. A cDNA for diapausin-1 was cloned from cDNA prepared from fat body RNA. Diapausin-1 is a member of the diapausin family of peptides, which includes members known to have antifungal activity. The M. sexta genome contains 14 genes with high similarity to diapausin-1, each with 6 conserved Cys residues. Diapausin-1 was produced as a recombinant protein in Escherichia coli. Purified recombinant diapausin-1 was active against S. cerevisiae, with IC50 of 12 μM, but had no detectable activity against bacteria. Spores of some plant fungal pathogens treated with diapausin-1 had curled germination tubes or reduced and branched hyphal growth. Diapausin-1 mRNA level in fat body strongly increased after larvae were injected with yeast or with Micrococcus luteus. In addition, diapausin-1 mRNA levels increased in midgut and fat body at the wandering larval stage prior to pupation, suggesting developmental regulation of the gene. Our results indicate that synthesis of diapausin-1 is part of an antifungal innate immune response to infection in M. sexta.
1. Introduction
Insects synthesize antimicrobial peptides as part of the immune response to infection by microorganisms (Casanova-Torres and Goodrich-Blair, 2013; Jiang et al., 2010; Hultmark et al., 1980; Yazdi et al., 2013; Zhang et al., 2014). Some small α-helical peptides from insect hemolymph have antibacterial and antifungal activity. These include some cecropins (Boulanger et al., 2002; Cavallarin et al., 1998; Delucca et al., 1997; Ekengren and Hultmark, 1999; Kim et al., 2010; Vizioli et al., 2000; Xia et al., 2013) and moricins (Brown et al., 2008; Dia et al., 2008; Hemmi et al., 2002). Proline-rich peptides with antifungal activity include lebocins (Chowdhury et al., 1995; Rao et al., 2012) and metchnikowin (Levashina et al., 1995; Rahnamaeian et al., 2009; Rahnamaeian and Vilcinskas, 2012). Glycine-rich antifungal peptides include Manduca sexta gloverins (Xu et al., 2012), attacin B from Hyphantria cunea (Kwon et al., 2008), holotricin from Holotrichia diomphalia (Lee et al., 1995), and tenecin-3 from Tenebrio molitor (Kim et al., 1998).
Most insect antifungal peptides characterized so far are 4-6 kDa cysteine-stabilized molecules, with structures consisting of an α-helix connected to a β-sheet, triple-stranded antiparallel β-sheets, or two-strand hairpin-like β-sheet (Bulet and Stocklin, 2005). Insect defensins have in common an α-helical structure connected to a β-sheet, stabilized by three disulfide bridges, including two that connect the α-helix to the β-sheet, forming a cysteine-stabilized alpha beta structure (CSαβ) (Landon et al., 1997). Drosomycin, from Drosophila melanogaster contains four disulfide bridges and differs from the defensins in the pattern of disulfide connectivity (Landon et al., 1997). Drosomycin and some defensins are strictly antifungal (Da Silva et al., 2003; Fehlbaum et al., 1994; Lamberty et al., 1999; Lamberty et al., 2001; Landon et al., 2004; Landon et al., 1997; Lee et al., 1995), whereas some defensins have both antifungal and antibacterial activity (Rees et al., 1997; Vizioli et al., 2001; Hwang et al., 2009; Wang et al., 2009). An antifungal peptide named diapause specific peptide isolated from hemolymph of a leaf beetle, Gastrophysa atrocyanea, has three disulfide bridges, but its fold and disulfide connectivity differ from the defensins and drosomycin (Kouno et al., 2007; Tanaka et al., 2003). cDNA sequences similar in sequence to the beetle diapause specific peptide have been identified from some Lepidopteran species (Wan et al., 2012), and this group of peptides is known as the diapausin family (Tanaka et al., 2003; Tanaka and Suzuki, 2005; Kouno et al., 2007).
Several antibacterial proteins from the tobacco hornworm, Manduca sexta have been investigated, including: lysozyme (Mulnix et al., 1994), attacins (Kanost et al., 1990), cecropins (Dickinson et al., 1988), a lebocin-related precursor protein (Rayaprolu et al., 2010), moricin (Dai et al., 2008), and gloverin (Zhu et al., 2003). Among these, gloverin and lebocin C have both antifungal and antibacterial activities (Xia et al., 2012; Rao et al., 2012). Genomic and transcriptomic studies have identified large numbers of putative antimicrobial peptides in M. sexta (Gunaratna and Jiang, 2013; He et al., 2015), but most lack experimental verification.
In this study we investigated antifungal activity in hemolymph of the caterpillar, Manduca sexta, which has been used as a model system for biochemical studies of insect immunity (Kanost et al., 2004; Kanost and Nardi, 2010; Ragan et al., 2009). We found that antifungal activity is induced in hemolymph after microbial exposure, and we isolated and characterized a 5 kDa antifungal peptide from the diapausin family. This peptide, M. sexta diapausin-1, affected the growth and morphology of yeast and fungal hyphae. Diapausin-1 mRNA level increased after injection of larvae with microorganisms and during normal development at the wandering larval stage prior to pupation.
2. Materials and methods
2.1. Insects
M. sexta larvae were fed with artificial diet and reared at 25 °C as described by Dunn and Drake (1983).
2.2. Sequence analysis
Sequence similarity searches were performed using Blast software (http://www.blast.ncbi.nlm.nih.gov/Blast.cgi) from the National Center for Biotechnology Information. Searches of the Manduca sexta genome sequence were carried out using a blast server at the Manducabase web site (http://agripestbase.org/manduca/?q=blast). Multiple sequence alignment was done using Clustal Omega (http://www.ebi.ac.uk/Tools/msa/clustalo/) (Sievers et al., 2011). Signal peptides were predicted using the SignalP 4.1 server (http://www.cbs.dtu.dk/services/SignalP/) (Petersen et al., 2011).
2.3. Yeast injection and hemolymph collection
Saccharomyces cerevisiae strain SUB62 was grown in suspension in YPD medium (1% yeast extract, 2% peptone, 2% dextrose) at 30 °C with shaking at 200 rpm to OD600 nm of ~ 0.6. Day two fifth instar M. sexta larvae were injected with 1.5×106 cells of S. cerevisiae. Uninjected larvae were used as control. After 24 h, hemolymph was collected from a cut proleg into a tube containing few crystals of diethyldithiocarbamate (DETC). The hemolymph was centrifuged at 7000×g for 25 min at 4 °C to remove hemocytes. The supernatant (plasma) was stored at −80 °C until use in protein preparations or assays.
2.4. Protein analysis
Diapausin concentration was measured by absorbance at 280 nm (calculated ε = 7815 M−1 cm−1). Hemolymph and recombinant protein samples were analyzed by SDS polyacrylamide gel electrophoresis (SDS-PAGE), using 16% acrylamide gels and tricine buffer and stained with Coomassie brilliant blue R-250 (Invitrogen, USA). Recombinant proteins were analyzed by SDS-PAGE with NuPAGE 4-12% polyacrylamide Bis-Tris gels (Invitrogen, USA), and stained with Coomassie blue. Immunoblotting analysis was performed with mouse anti-His antibody as primary antibody (1: 2000 dilution) (Bio- Rad, USA), and goat-anti mouse conjugated to alkaline phosphatase (1: 3000 dilution) (Bio- Rad) as the secondary antibody.
2.5. Antimicrobial activity assays
2.5.1. Anti-yeast activity assay
Measurement of activity against yeast was performed as described by Fai and Grant (2009) with minor modifications. Briefly, S. cerevisiae grown in YPD medium at 30 °C to OD600 of about 0.6. The cultures were diluted with YPD to OD600 of 0.1. Samples (100 μl) of the diluted culture were added to wells of a 96-well plate. Samples of hemolymph or recombinant protein were added, and the final volume was brought to 200 μl with YPD medium. The plates were incubated 16 h at 30 °C with shaking at 200 rpm, and then OD600 was measured using a microplate reader (BioTek, USA).
2.5.2. Bacterial strains and antibacterial activity assays
Listeria fleischmannii (DSM24998), Listeria grayi (DSM 20601), Listeria marthii (DSM 23813), Staphylococcus aureus (DSM 2569), Escherichia coli (D31) and Pseudomonas aeruginosa (DSM50071) were used to assess the antibacterial properties of M. sexta recombinant diapausin-1 peptide. Growth inhibition assays were performed as described by Rahnamaeian et al ( 2015) and Tonk et al (2015) in diapausin-1 concentrations ranging from 0.03 μM to 250 μM using 384-well plates (Griener Bio One, Germany). Bacterial strains in mid-logarithmic phase were used for these assays. The initial OD600 nm of the bacterial culture was set to 0.01 for Listeria spp. and 0.001 for other strains. We used Brain Heart Infusion Broth (BHIB) medium for Listeria spp. and Tryptic Soy Broth (TSB) (Roth, Germany) for other strains (Tonk et al, 2014). The assays lasted 16 h and the OD600 was recorded every 20 min using an Eon™ Microplate Spectrophotometer (BioTek Instruments, USA). Each assay included an untreated control bacterial culture.
2.5.3. Antifungal activity assays
Fusarium culmorum and Fusarium graminerum were grown on Spezieller Nahrstoffarmer agar (SNA) medium for 7 days, and conidia were washed with 1 mL of sterile water. To assay antifungal activity ~50 conidia in 30 μl of sterile water were supplemented with 4 μM of diapausin-1, and the germination was examined after 24 h by phase contrast microscopy. To assess the effect of diapausin-1 against Magnaporthe oryzae spores, 1×105 spores were incubated with 15 μM of diapausin-1 for 4 h, then the spores were examined by the phase contrast microscopy. For Beauveria bassiana, 1×103 spores/well in a 96-well plate were incubated with 0, 10, 20 or 30 μM diapausin-1 in 200 μl B-medium (4% glucose, 2% yeast extract). The plates were incubated at 25 °C, 200 rpm for 24 h, and then OD600 was measured using the microplate reader and wells were examined using an inverted phase contrast microscope.
2.6. Size exclusion chromatography
A 20 mL sample of M. sexta plasma collected 24 h after injection of larvae with S. cerevisiae as described above was subjected to size exclusion chromatography on a column (120 × 2.5 cm) of Sephacryl S-200 (GE healthcare) and eluted with 20 mM Tris, 150 mM NaCl, pH 7.5 at 2 mL/min, with collection of 8 mL fractions. Protein content of the fractions was assessed by absorbance at 280 nm. Samples of each fraction (100 μL) were assayed for anti-yeast activity as described above.
2.7. Peptide mass fingerprinting
Size exclusion chromatography fractions 56-58, containing peptides with activity against yeast, were pooled and concentrated from 24 ml to 3.5 ml by vacuum centrifugation. Then, 10 μL was added to 22 μL 5 mM dithiothreitol, 20 mM ammonium bicarbonate. The mixture was heated at 95 °C for 10 min, and then after reduced cystein residues were alkylated by iodoacetate, 250 ng of proteomics-grade Trypsin Gold (Promega) was added, and incubated at 30 °C overnight. The digestion products were analyzed using a Bruker Daltonics Ultraflex III MALDI TOF/TOF Mass Spectrometer in MS mode (Dittmer et al., 2012). The peak lists were analyzed using Mascot software v 2.2.04 (Matrix Science Ltd.) and compared with protein sequences from the M. sexta official gene set 1.0 obtained from genome sequencing (http://agripestbase.org/manduca/), and with the NCBI nonredundant database (NCBInr 2008.10.24.08, restricted to Metazoa).
2.8. Preparation of recombinant diapausin-1
cDNA made to a sample of RNA isolated from fat body of M. sexta larvae collected 24 h after injection with 100 μg M. luteus was used as a template for PCR using forward (5′-GCCATGGCTATCAACAACTG-3′) and reverse (5′-AAGCTTTTAACGTCTGTAACA-3′) primers containing NcoI and HindIII restriction sites, respectively, to amplify the sequence encoding the diapausin-1. The diapausin-1 cDNA and pET32a (Novagen, USA) were digested with NcoI and Hind III (New England Biolabs, USA). The digested fragments were separated by electrophoresis on a 1% agarose gel, purified using a QIAquick Gel Extraction Kit (Qaigen, USA), and then fragments were ligated using T4 DNA ligase (New England Biolabs, USA) and used to transform E. coli strain Rosetta gami (Novagen, USA). A single colony containing the expected sequence was grown in 1 L of LB medium, and recombinant protein expression was induced with 1mM isopropyl β-D-1-thiogalactopyranoside for 4 h. Bacteria were pelleted at 4000 × g for 20 min at 4 °C. The pellet was suspended in lysis buffer (0.05 M sodium phosphate buffer containing 10 mM imidazole and 300 mM NaCl, pH 8.0) and 50 μl inhibitor cocktail (P 8849, Sigma) per gram of cell pellet. Cells were lysed by sonication, and then centrifuged at 4000 × g for 20 min at 4 °C. The fusion protein consisting of diapausin-1 with an amino-terminal thioredoxin and 6-His tag was purified from the supernatant by nickel affinity chromatography. The supernatant was applied to Econo-column® (1.5 cm ID, 7 cm long) (Bio- Rad, USA). The column was washed three times with 5 mL 20 mM imidazole, 300 mM NaCl in 0.05 M sodium phosphate buffer, pH 8.0. The fusion protein was eluted with 5 ml of 250 mM imidazole in 0.05 M sodium phosphate buffer, pH 8.0. Fractions were analyzed by SDS-PAGE with NuPAGE 4-12% polyacrylamide Bis-Tris gels (Invitrogen, USA). Fractions contain the fusion protein were pooled and dialyzed against 10 mM sodium phosphate buffer, pH 7.4. The amino-terminal thioredoxin tag was removed by incubating 100 μg of fusion protein with one unit of recombinant enterokinase (Novagen, USA) at room temperature for 16 h according to the manufacturer’s instructions. Then, nickel affinity chromatography was used to separate the His and thioredoxin tags sequence from the mature diapausin-1, which flowed through the column. Fractions containing diapausin-1 were concentrated and buffer was exchanged to 10 mM Sodium Phosphate buffer pH 7.4, using Amicon centrifugal filters with 3000 Da molecular weight cutoff (Millipore, USA). The diapausin-1 was further purified by reverse phase chromatography on a C18 capillary column (75 μm inner diameter × 15 cm; PepMap, Dionex) using a linear acetonitrile gradient using buffer A (0.1% formic acid, 2% acetonitrile) and buffer B (0.1% formic acid, 80% acetonitrile) starting from 10% buffer B to 90% over 45 min at 2 mL/min.
2.9. Reverse transcription-polymerase chain reaction (RT-PCR) and quantitative PCR (qPCR)
Day 2 fifth instar larvae were injected with 100 μg of dried M. luteus (Sigma, USA) suspended in PBS or with 1.5×106 cells of S. cerevisiae or left without injection as controls. After 24 h, fat body was collected from each larva and washed three times with anti-coagulant saline (4 mM NaCl,40 mM KCl, 8 mM EDTA, 9.5 mM citric acid monohydrate, 27 mM sodium citrate, 5% sucrose, 0.1% polyvinylpyrollidone, 1.7 mM PIPES). Total RNA was isolated using TRI reagent (Sigma, USA). RNA (120 ng) was a template for reverse transcription using Oligo-dT primer and SuperScriptTM II RT (Invitrogen, USA) following the manufacturer’s instructions. Samples of cDNA (1 μL) were used in RT-PCR reactions for 30 cycles of 94 °C for 30 s, 48 °C for 30 s, 72 °C for 30 s followed by incubation at 72 °C for 4 min. The forward and reverse primers for diapausin were -:5′- GCCATGGCTATCAACAACTG -3′, and 5′- AAGCTTTTAACGTCTGTAACA -3′), respectively. Ribosomal protein small subunit 3 (rps3) was used as a control with forward and reverse primers 5′ - GCCGTTCTTGCCCGTTT -3′, and 5′- CGCGAGTTG ACTTCGGT- 3′) respectively. The PCR products were analyzed by electrophoresis on 1% agarose. For naive larvae in fifth instar feeding stage (day 2 and day 4) or wandering stage (days 0, 1, 2, 3) RNA was isolated from fat body and midgut. The cDNA, RT-PCR and analysis were performed as mentioned above. Quantitative RT-PCR (qPCR) was performed using IQTM SYBER Green supermix (Bio- Rad, USA) in a CFX96 thermo cycler (Bio- Rad, USA). The cDNA template was diluted 25 fold (v/v) with nuclease-free water (Ambion, USA), then 25 μL reaction volume (12.5 μL 2 × SYBER Green master mix, 1 μL forward primer, 1 μL reverse primer and 10.5 μL cDNA template) was incubated at 95 °C for 3 min, followed be 40 amplification cycles with 3 steps: 95 °C 10 s, 52 °C for 30 s, 70 °C for 30 s. The primers for diapausin-1 were 5′-ACCATGGGCATCAACAACTGG-3′ and 5′-CTCGAGACGTCGTAACAGTT-3′ for both forward and reverse primers, respectively. The rps3 primers were the same as mentioned above. Relative expression of diapausin-1 mRNA levels were calculated using delta delta Ct (ΔΔCt) method (Livak and Schmittgen, 2001). Statistical analysis was carried out using GraphPad Prism software (GraphPad, USA).
2.10. Fluorescein isothiocyanate conjugated proteins
Diapausin-1 and bovine serum albumin (BSA) were conjugated with fluorescein isothiocyanate (FITC; Sigma, USA) as described in the manufacturer’s instructions. The labeled proteins were isolated using PD 10 desalting column (GE healthcare, USA), and then the FITC-conjugated proteins were dialyzed against 10 mM sodium phosphate buffer, pH 7.4. S. cerevisiae cells were incubated with the FITC-labeled proteins each at 5 μM, for 8 h. After washing with 10 mM sodium phosphate buffer, pH 7.4, cells were examined by confocal laser microscopy.
3. Results
3.1. Purification of an antifungal peptide from M. sexta hemolymph
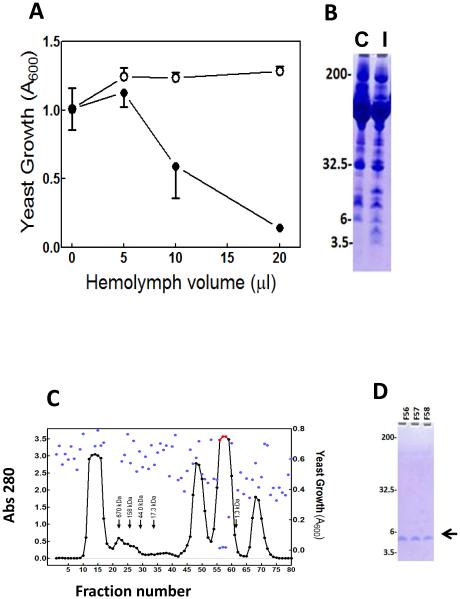
To investigate the presence of antifungal molecules in hemolymph of M. sexta larvae, we assayed activity against yeast in hemolymph from control larvae and from larvae 24 h after injection of S. cerevisiae. Plasma from larvae that had been injected with yeast had significant activity against growth of yeast in our bioassay (Fig. 1A), whereas plasma from control larvae did not. In SDS-PAGE analysis of these samples, plasma from larvae injected with yeast contained several protein bands at higher intensity than in controls, including a band at ~5 kDa (Fig. 1B).
Fig. 1. M. sexta larval hemolymph contains inducible anti-yeast activity.
Day 2 fifth instar M. sexta larvae were injected with 1.5 ×106 cells of S. cerevisiae (induced); control larvae were uninjected. Hemolymph plasma was collected 24 h after injection. (A) Control (open circles) and induced (closed circles) plasma samples at different volumes were tested for anti-yeast activity as described in Materials and Methods. Error bars indicate standard deviation of technical replicates (n = 3). (B) Analysis of control (C) and induced (I) plasma samples (1.5 μL) by SDS-PAGE. (C) Separation of plasma by size exclusion chromatography. Hemolymph plasma (20 mL) from larvae collected 24 h after injection with S. cerevisiae was separated by size exclusion chromatography on a column of Sephacryl-S200, as described in Materials and Methods. Protein content of each fraction was monitored by measuring absorbance at 280 nm (black or red points). Activity of each fraction against yeast was assayed as described in Materials and Methods, with growth of yeast measured as turbidity, using absorbance at 600 nm (blue points). Arrows indicate the elution volumes of standard proteins. (D) Analysis of the fractions 56-58 with highest anti-yeast activity, by SDS-PAGE using a 16% acrylamide tricine gel. Size and positions of the molecular weight markers are indicated on the left. The arrow indicates the position of the band that was analyzed by mass spectrometry of tryptic fractions.
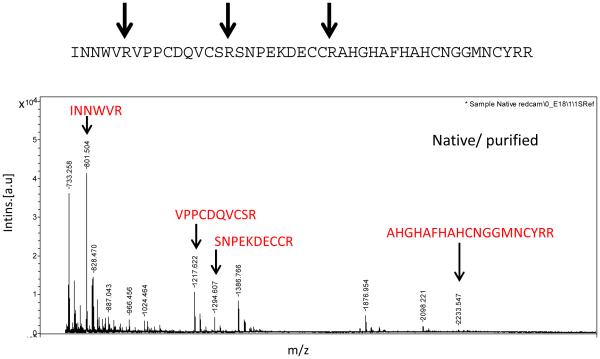
To isolate and identify antifungal molecules, 20 mL of plasma from larvae collected 24 h after injection with yeast was separated by size exclusion column chromatography, and fractions were tested for activity against S. cerevisiae (Fig. 1C). Three fractions (56-58) had strong anti-yeast activity. In analysis of these fractions by SDS-PAGE, a single band was visible at ~ 5 kDa (Fig. 1D). These fractions were pooled, concentrated, and analyzed by mass spectrometry. The MALDI-TOF analysis of purified ~ 5 kDa peptide showed the sharp ms peak at (m/z) 5165.5, which matched with SDS-PAGE analysis (supplement). Proteomics analysis was performed to identify 5 kDa peptide using MALDI-TOF MS/MS technology. Following MASCOT peptide search against NCBInr database, the spectra of tryptic fragments revealed significant matches with N-terminal region including INNWVRVPPCDQVCSR from psychimicin (NCBI accession P83421), a peptide isolated from a lepidopteran insect, Oiketicus kirbyi (Figs. 2 and 3). Psychimicin is a member of the diapausin family of antimicrobial peptides (Pfam PF08036), named for its first representative, diapause-specific peptide from a beetle, Gastrophysa atrocyanea, which also has antifungal activity (Tanaka et al., 2003;Tanaka et al., 1998).
Fig. 2. Identification of diapausin-1 by peptide mass fingerprinting.
The purified diapausin-1 peptide was digested with trypsin and then analyzed by MADLI-TOF MS/MS. The mass spectrum is labeled to show sequences of peptides represented by peaks with masses and fragmentation patterns that match predicted tryptic peptides from the mature diapausin-1 protein encoded in M. sexta gene Msex2.15011 found in the M. sexta genome sequence.
Fig. 3. Alignment of the M. sexta diapausin-1 amino acid sequence with other members of the diapausin family from M. sexta and other insect species.
The sequence encoded by M. sexta gene Msex2.15011, diapausin-1 (NCBI accession number KT222965), after removing the secretion signal sequence, was aligned with mature peptide sequences of other members of the diapausin family present in the M. sexta genome (He et al., 2014) and several diapausin family members from lepidopteran species as well as the original member of this gene family, G. atrocyanea diapause specific peptide. Conserved Cys residues are highlighted in yellow. Other residues conserved with M. sexta diapausin-1 are highlighted in blue. Accession numbers for the sequences retrieved from NCBI are: Gastrophysa atrocyanea diapause-specific peptide (GaDSP), Q8T0W8; Spodoptera litura diapausin (SlDiapausin), ABU96713; Spodoptera exigua diapause-specific peptide (SeDSP), ADM72854; Spodoptera littoralis spodomicin, P83411; Oiketicus kirbyi psychimicin, P83421.
We used the psychimicin sequence for blast searching of official gene set 1.0 from the M. sexta genome sequencing project (http://agripestbase.org/manduca/), but no gene models had significant matches. However, when we searched using tblastn against the DNA sequences of the assembled M. sexta genomic scaffolds, we identified a region on scaffold 6084 with a strong match to the psychimicin sequence. MASCOT peptide analysis against scaffold 6084 showed that all of the predicted tryptic peptides were found from the tryptic mass spectra with high MS/MS matching probability (supplemental data). This region contained a single exon encoding a predicted signal peptide of 23 residues, followed by a mature peptide of 45 residues, with mass of 5160.7 Da and theoretical pI of 8.5 (Fig. 3). This gene, named M. sexta diapausin-1 (synonym antifungal peptide-1, manually annotated by Haobo Jiang, Oklahoma State University) is designated as Msex2.15011 in official gene set 2.0 (http://agripestbase.org/manduca;https://i5k.nal.usda.gov/Manduca_sexta).
Additional tblastn searching of the scaffold sequences revealed 13 additional single-exon genes in the diapausin family, which were not recognized as genes in official gene set 1 (Fig. 3). Genes 2-10 form a cluster on scaffold 1204 and have very similar sequences (He et al., 2015). Members of the diapausin family have six absolutely conserved Cys residues. An alignment of the M. sexta diapausin-1 amino acid sequence with the original diapause-specific peptide and some homologous lepidopteran sequences available in transcriptome databases is presented in Fig. 3. M. sexta diapausin sequence similarity ranges from 91% identity with psychimicin to 49% identity with G. atrocyanea diapause specific peptide. Calculated molecular weight of Msex2.15011 is 5169.8, which is slightly larger than the MALDI-TOF analysis of purified diapausin-1 (m/z 5165.5), probably due to formation of threedisulfide bonds in the hemolymph peptide.
3.2. cDNA cloning and antimicrobial activity of recombinant diapausin-1.
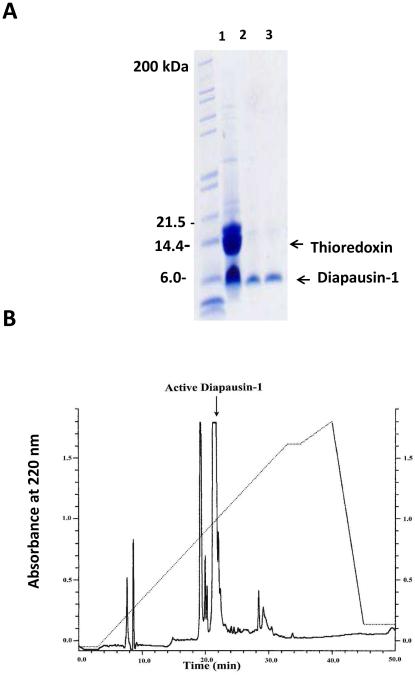
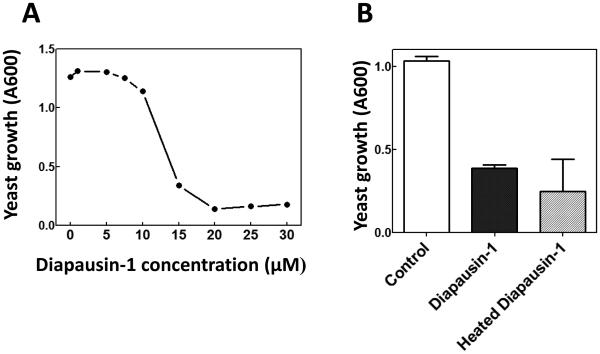
Primers for PCR amplification of the region encoding the mature form of diapausin-1 (after removal of the secretion signal peptide) were designed based on the genomic sequence of gene Msex2.15011 and used to amplify the cDNA from RNA prepared from fifth instar larval fat body. The resulting cDNA (Genbank accession KT222965) matched exactly with the genomic sequence. It was cloned into expression vector pET32a, and a fusion protein of an amino-terminal 6-His tag followed by thioredoxin, fused with carboxyl-terminal diapausin-1 was expressed in E. coli. The 22.5 kDa fusion protein was purified by nickel-affinity chromatography and then cleaved with enterokinase to remove the fusion tag. Diapausin-1 was separated from the 6-His-thioredoxin by passage through a nickel column, which retained the thioredoxin, and the 5 kDa diapausin-1 was collected in flow-through fractions (Fig. 4A). Diapausin-1 was further purified by reversed phase HPLC (Fig. 4B), and fractions were assayed for activity against S. cerevisiae. The major peak, representing ~50 % of the sample, was active against yeast. The sequence and total molecular weight of the expressed recombinant Diapausin-1 was confirmed by proteomics analysis. This recombinant diapausin-1 blocked yeast growth in a concentration dependent manner, with 50% inhibition at ~12 μM diapausin-1 (Fig. 5A). The activity of diapausin-1 was stable to heating at 90 °C for 10 min (Fig. 5B), perhaps due to structural stability provided by the three predicted disulfide bonds.
Fig. 4. Isolation of recombinant diapausin-1.
(A) The 6-His-thioredoxin-diapausin fusion protein was cleaved with enterokinase, and 6-His-thioredoxin was removed by nickel-affinity chromatography. Lane 1: reaction mixture after enterokinase cleavage; Lanes 2 and 3: first two flow-through fractions containing diapausin-1. (B) Reverse HPLC separation of the Diapausin-1 after cleavage with enterokinase and purification with Ni-NTA chromatography. The arrow indicates the active diapausin-1 which confirmed with anti-yeast activity test (data not shown).
Fig. 5. Activity of recombinant diapausin-1 against yeast.
(A) The concentration dependent activity of recombinant diapausin-1 in decreasing growth of S. cerevisiae was assayed by measuring turbidity at OD600 after a 12 hour incubation of cultures in a 96 well plate, containing different concentrations of diapausin-1, as described in Materials and Methods. The Inhibitory concentration 50% (IC50%) of recombinant diapausin-1 against S. cerevisiae was ~12 μM. (B) Heat stability of recombinant diapausin-1 was tested after treatment of the peptide at 90 °C or 25 °C for 10 min prior to assay against yeast at 12 μM diapausin-1. Bars represent mean ± standard deviation (n = 3). There was no significant difference in the activity of heated diapausin-1 compared with the unheated control (t-test, t > 0.05), indicating heat stability of this antifungal peptide.
To examine binding of diapausin-1 to yeast cells, we incubated S. cerevisiae with fluorescently labeled (FITC) recombinant diapausin-1 or with FITC labeled BSA as a control. Cells were pelleted, washed with 10 mM phosphate buffer, pH7.4, and then visualized by confocal laser scanning microscopy. FITC-diapausin was detected at the surface of yeast cells, but not in their interior (Fig. 6). In contrast, FITC-BSA did not bind to the yeast cells at a detectable level. This result indicates that diapausin-1 may affect yeast by binding with a surface component, such as the cell wall or cell membrane. Recombinant diapausin-1 lacked detectable activity against tested bacterial species (Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, Listeria fleischmannii, Listeria grayi, Listeria marthii, Listeria innocua, Listeria welshimeri, Listeria seeligeri, Listeria rocourtiae, Micrococcus luteus) (data not shown).
Fig. 6. Binding of diapausin-1 to S. cerevisiae.
FITC-labeled diapausin-1 (A) or FITC-labeled bovine serum albumin (B), each at 5 μM incubated with S. cerevisiae for 8 h and then examined by confocal laser scanning microscopy (left panels) or phase contrast microscopy (right panels) to detect fluorescent protein associated with the yeast.
3.3. Effects of diapausin-1 on other fungi.
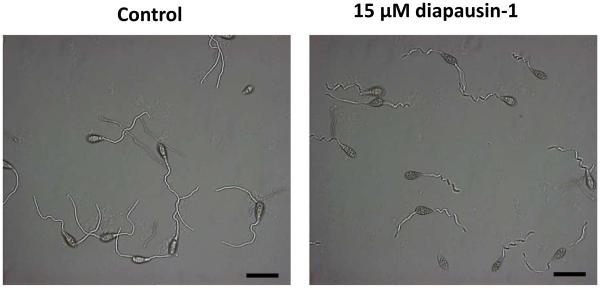
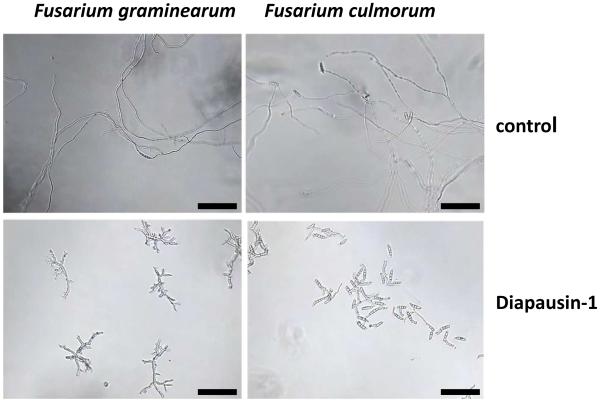
We carried out assays to test the activity of diapausin-1 on growth and morphology in some species of ascomycete fungi. Diapausin-1 had no apparent activity against growth of the insect fungal pathogen Beauvaria bassiana (data not shown). However, diapausin-1 did affect morphology and growth of some plant pathogenic fungi. When spores of Magnaporthe oryzae were treated with 15 μM diapausin-1and then allowed to germinate for 4 h, we observed that germination tubes were curled, with a corkscrew-like appearance, compared with the gently curved shape of the germ tubes from control spores not treated with diapausin-1 (Fig. 7). We examined the hyphal growth of two Fusarium species: Fusarium culmorum and Fusarium graminearum after treatment with diapausin-1. At 15 μM diapausin-1, no hyphal growth was apparent after 24 h (data not shown). Lower diapausin-1 concentration of 4 μM caused abnormal hyphal development after 24 h. In F. culmorum, germination was almost completely inhibited and few, very small germ tubes with constriction were observed. In F. graminearum, either strong constriction or destruction of septa was observed, which correlated with disintegration of conidia and loss of cell contents. In addition to ineffective germination, the hyphal length was significantly reduced compared with the controls, with frequent branching and changing in morphology (Fig. 8).
Fig. 7. Diapausin-1 alters morphology of M. oryzae germination tubes.
M. oryzae spores were incubated with 15 μM diapausin-1 for 4 h, and then examined by phase contrast microscopy. Scale bars indicate 50 μm.
Fig.8. Diapausin-1 alters Fusarium hyphal morphology.
Spores of F. graminearum or F.culmorum were incubated in medium with 4 μM diapausin-1 for 48 h or in medium alone as a control. Resulting hyphae were visualized by phase contrast microscopy. Scale bars indicate 100 μm.
3.4. Change in diapausin-1 mRNA level after exposure to microorganisms and during development.
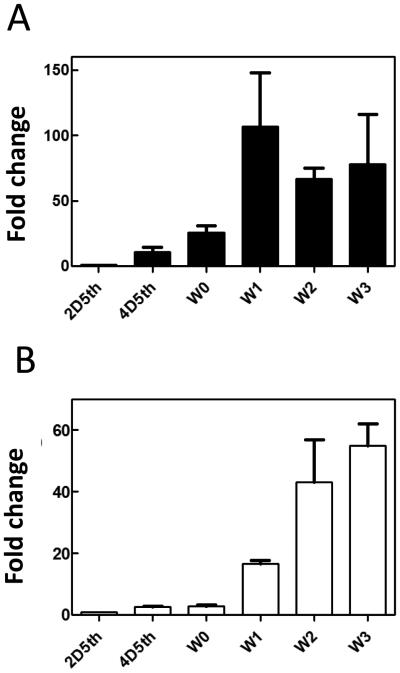
Because we found that activity against yeast increased dramatically in hemolymph after injection of larvae with yeast, we carried out experiments to test whether this change is correlated with increased level of diapausin-1 mRNA. We analyzed RNA extracted from feeding stage fifth instar larval fat body 24 h after injection with S. cerevisiae, M. luteus, or uninjected control larvae. cDNA samples prepared from these RNA templates were analyzed by quantitative PCR (qPCR). Diapausin-1 mRNA levels increased about 18-fold compared with control after injection with M. luteus and increased more than 100-fold after injection of yeast (Fig. 9).
Fig. 9. Diapausin-1 levels in the fat body of M. sexta after injection with Micrococcus luteus or S. cerevisiae.
Total RNA isolated from the fat body of three individual larvae for each treatment was used to synthesize cDNA, which was analyzed by qPCR, relative to the level of mRNA for ribosomal protein subunit 3 (RPS3). The bars represent the mean ± standard error of the mean, n=3.
Because some hemolymph proteins involved in M. sexta immune responses are known to be expressed in the wandering larval stage just prior to pupation in the absence of an immune challenge (Russell and Dunn, 1996), we tested whether diapausin-1 may also be expressed with a similar developmental pattern. We analyzed RNA extracted from the fat body and the midgut of naïve larvae during the feeding stage of the fifth instar and on the first four days of the wandering larval stage. Diapausin-1 expression level was low in fat body and midgut of feeding stage larvae, but increased dramatically by day one of the wandering stage (Fig. 10), with about 100-fold increase in fat body and 18-fold increase in midgut. The diapausin-1 mRNA level increased further in midgut on wandering days 2 and 3, reaching ~50-fold greater than the level in fifth instar day 2.
Fig.10. Diapausin-1 mRNA levels in fat body and midgut of naïve larvae increase at the wandering stage before pupation.
Total RNA isolated from the fat body (A) or midgut (B) of three individual larvae at each time point was used to synthesize cDNA, which was analyzed by qPCR to determine level of diapausin-1mRNA relative to ribosomal protein S3 mRNA. The relative expression level on fifth instar day 2 was set as 1.0. 2D5th= day 2 of fifth instar; 4D5th = day 4 of fifth instar, W0 to W3 indicates wandering larval stage days 0 to 3. The bars represent the mean ± standard error of the mean for three individual larvae.
4. Discussion
Insects can respond to infections by synthesizing antimicrobial peptides with activity against bacteria and/or fungi (Bulet et al., 2004; Casanova-Torres and Goodrich-Blair, 2013; Kanost et al., 2004; Yi et al., 2014; Rahnamaeian and Vilcinskas, 2015). Most known antimicrobial peptides from insect hemolymph are antibacterial, but some antifungal peptides have been discovered and characterized (Barbault et al., 2003; Fehlbaum et al., 1996; Kouno et al., 2007; Landon et al., 1997). M. sexta has been investigated extensively as a model organism for studies of innate immunity in insects (Kanost et al., 2004; Kanost and Nardi, 2010). Although many antibacterial peptides have been identified from M. sexta (Dai et al., 2008; Dickinson et al., 1988; Gorman et al., 2004; He et al., 2015; Zhu et al., 2003), gloverin and lebocin C are the only M. sexta plasma proteins so far shown experimentally to have antifungal activity (Rao et al., 2012; Xu et al., 2012)
In this study, we present the isolation, cDNA cloning, and characterization of an antifungal peptide, diapausin-1, isolated from M. sexta larval hemolymph after injection of the larvae with the yeast, S. cerevisiae. Diapausin-1, a slightly basic peptide of 45 amino acid residues, is a member of the diapausin family. The diapausin family includes antifungal peptides of 40-45 residues, with six conserved cysteines (Tanaka et al., 2003). The NMR structure of a beetle diapause-specific peptide, the first member of this family, contains a CSαβ - like motif stabilized by three disulfide bridges, which differ from the arrangement of disulfide bonds in insect defensins (Kouno et al., 2007). A search of the M. sexta genome revealed the presence of a family of genes that encode peptides similar to diapausin-1, all containing a predicted secretion signal peptide.
Diapausin-1 purified from hemolymph and produced as a recombinant protein had activity against S. cerevisiae. It was also active against some ascomycete filamentous fungi, although not the entomopathogen B. bassiana, which has perhaps adapted to lack susceptibility to insect antifungal defenses. Diapausin-1 lacked activity against all Gram-negative and Gram-positive bacteria that were tested in vitro. This specificity is similar to the well characterized insect antifungal peptide like drosomycin and few other insect antifungal peptides, which do not affect bacteria (Fehlbaum et al., 1994; Iijima et al., 1993). However, our data do not rule out the possibility that diapausin-1 contributes to antibacterial defenses by displaying combinatorial activities with other AMPs in M. sexta. Recent findings from bumble bees show at the mechanistic level that co-occurring AMPs can cooperate to fulfill immunity-related functions (Rahnamaeian et al, 2015).
Diapausin-1 appears to alter morphology when it restricts growth of fungal species. In germinating M. oryzae, the germination tube acquired a curled appearance not present in controls. With two Fusarium species, conidia germination was totally inhibited by 15 μM diapausin-1, and after treatment with only 4 μM of peptide, hyphal growth was severely reduced, and the hyphae became much more branched and constricted with abnormal morphology. Abnormal hyphae and delayed growth were observed when low concentrations of drosomycin were tested against Botrytis cinerea, and extruded cytoplasm observed along the hyphae may indicate that drosomycin caused a partial lysis of the cells (Fehlbaum et al., 1994). In plants, antifungal defensins are grouped based on their effect on hyphal morphology into morphogenic or nonmorphogenic. Morphogenic peptides reduce hyphal growth and cause morphological change, whereas the nonmorphogenic peptides affect only hyphal growth (Ramamoorthy et al., 2007; Sagaram et al., 2011). Diapausin-1 bound to the surface of S. cerevisiae (Fig. 6). Perhaps interaction of diapausin-1 with some component of the fungal cell surface disrupts growth while causing distortion of cell shape.
Diapausin-1 mRNA levels increased dramatically in the fat body after larvae were injected with M. luteus or S. cerevisiae, with particularly strong induction of expression after exposure to the yeast (Fig. 9). The elevated expression of diapausin-1 is correlated with the appearance of anti-yeast activity in hemolymph after immune stimulation by injection of yeast (Fig. 1A). Because diapausin was first discovered in a leaf beetle as a hemolymph protein expressed during a quiescent stage, we tested whether M. sexta diapausin-1 might be expressed during development in the absence of microbial challenge, particularly at the time of pupation, when the insects might be vulnerable to infections. We found that diapausin-1 was strongly expressed in fat body and midgut during the wandering stage, just prior to pupation. This expression pattern is similar to other M. sexta immune proteins, which are upregulated in hemolymph and in midgut fluid, beginning at the wandering stage and persisting into the pupa. These include hemolin, lysozyme, and phenoloxidase, antimicrobial proteins and peptides (Russell and Dunn, 1996; Yu and Kanost, 1999) and a β- 1,3 - glucan recognition protein that can bind to fungal cell walls (Jiang et al., 2004 ; Takahashi et al., 2014). Expression of diapausin-1 in the midgut and fat body at pupation may be part of a developmentally upregulated immune protection, to help block infections during metamorphosis. D. melanogaster drosomycin mRNA level is increased during pre-pupal and pupal stages (Attrill et al., 2016). Regarding structure and transient expression before pupation diapausin-1 displays similarity with the antifungal peptide gallerimycin from the greater wax moth Galleria mellonella (Langen et al., 2006). This model host has also been used to explore the induction of immune responses prior to pupation in Lepidoptera. The expression of a matrix metalloproteinases that mediates digestion of the extracellular matrix during metamorphosis has been found to produce fragments of collagen IV, which in turn function as danger signals that elicit immune responses (Altincicek and Vilcinskas, 2006, Altincicek and Vilcinskas, 2008).
We interpret the results of this study to conclude that diapausin-1 is an antifungal hemolymph peptide, which functions in the immune system of M. sexta larvae, likely providing protection against fungal infections. Diapausin-1 expression is strongly expressed after immune challenge and prior to metamorphosis. It is active against S. cerevisiae and some ascomycete fungi, disrupting growth and affecting fungal morphology. Further studies are needed to determine the molecular target of diapausin-1 and its mode of action.
Supplementary Material
An antifungal peptide, diapausin-1 was isolated from hemolymph of Manduca sexta larvae
Recombinant diapausin-1 was active against yeast and some ascomycete fungi.
Diapausin-1 expression in fat body was strongly increased after larvae were injected with yeast
Acknowledgments
We thank Dan Boyle for help with confocal microscopy, and Prof. Haobo Jiang for help in searching his M. sexta transcriptome data. This research was supported by National Institutes of Health grant GM41247. Contribution number 15-192-J from the Kansas Agricultural Experiment Station. AV acknowledges funding by the Hessen State Ministry of Higher Education, Research and the Arts (HMWK) via the “LOEWE Center for Insect Biotechnology and Bioresources”
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altincicek B, Vilcinskas A. Metamorphosis and collagen-IV-fragments stimulate innate immune response in the greater wax moth Galleria mellonella. Dev. Comp. Immunol. 2006;30:1108–1118. doi: 10.1016/j.dci.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Altincicek B, Vilcinskas A. Identification of a lepidopteran matrix metalloproteinase with dual roles in metamorphosis and innate immunity. Dev. Comp. Immunol. 2008;32:400–409. doi: 10.1016/j.dci.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Attrill H, Falls K, Goodman JL, Millburn GH, Antonazzo G, Rey AJ, Marygold SJ, the FlyBase Consortium FlyBase: establishing a Gene Group resource for Drosophila melanogaster. Nucleic Acids Res. 2016;44:D786–D792. doi: 10.1093/nar/gkv1046. doi:10.1093/nar/gkv1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbault F, Landon C, Guenneugues M, Meyer JP, Schott V, Dimarcq JL, Vovelle F. Solution structure of Alo-3: a new knottin-type antifungal peptide from the insect Acrocinus longimanus. Biochem. 2003;42:14434–42. doi: 10.1021/bi035400o. [DOI] [PubMed] [Google Scholar]
- Boulanger N, Brun R, Ehret-Sabatier L, Kunz C, Bulet P. Immunopeptides in the defense reactions of Glossina morsitans to bacterial and Trypanosoma brucei brucei infections. Insect Biochem. Mol. Biol. 2002;32:369–375. doi: 10.1016/s0965-1748(02)00029-2. [DOI] [PubMed] [Google Scholar]
- Brown SE, Howard A, Kasprzak AB, Karl H, Gordon KH, East PD. The discovery and analysis of a diverged family of novel antifungal moricin-like peptides in the wax moth Galleria mellonella. Insect Biochem. Mol. Biol. 2008;38:201–212. doi: 10.1016/j.ibmb.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Bulet P, Stocklin R. Insect antimicrobial peptides: Structures, properties and gene regulation. Protein Pept. Lett. 2005;12:3–11. doi: 10.2174/0929866053406011. [DOI] [PubMed] [Google Scholar]
- Bulet P, Stöcklin R, Menin L. Anti-microbial peptides: From invertebrates to vertebrates. Immunol. Rev. 2004;198:169–184. doi: 10.1111/j.0105-2896.2004.0124.x. [DOI] [PubMed] [Google Scholar]
- Casanova-Torres AM, Goodrich-Blair H. Immune signaling and antimicrobial peptide expression in lepidoptera. Insects. 2013;4:320–338. doi: 10.3390/insects4030320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallarin L, Andreu D, San Segundo B. Cecropin A-derived peptides are potent inhibitors of fungal plant pathogens. Mol. Plant Microbe. Interact. 1998;11:218–227. doi: 10.1094/MPMI.1998.11.3.218. [DOI] [PubMed] [Google Scholar]
- Chowdhury S, Taniai K, Hara S, Kadonookuda K, Kato Y, Yamamoto M, Xu J, Choi SK, Debnath NC, Choi HK, Miyanoshita A, Sugiyama M, Asaoka A, Yamakawa M. cDNA cloning and gene expression of lebocin, a novel member of antibacterial peptides from the silkworm, Bombyx mori. Biochem. Biophys. Res. Commun. 1995;214:271–278. doi: 10.1006/bbrc.1995.2284. [DOI] [PubMed] [Google Scholar]
- Da Silva P, Jouvensal L, Lamberty M, Bulet P, Caille A, Vovelle F. Solution structure of termicin, an antimicrobial peptide from the termite Pseudacan-thotermes spiniger. Protein Sci. 2003;12:438–446. doi: 10.1110/ps.0228303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai H, Rayaprolu S, Gong Y, Huang R, Prakash O, Jiang H. Solution structure, antibacterial activity, and expression profile of Manduca sexta moricin. J. Pept. Sci. 2008;14:855–863. doi: 10.1002/psc.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLucca AJ, Bland JM, Jacks TJ, Grimm C, Cleveland TE, Walsh TJ. Fungicidal activity of cecropin A. Antimicrob. Agents. Chemother. 1997;41:481–483. doi: 10.1128/aac.41.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson L, Russell V, Dunn PE. A family of bacteria-regulated, cecropin d-like peptides from Manduca sexta. J. Biol. Chem. 1988;263:19424–19429. [PubMed] [Google Scholar]
- Dittmer NT, Hiromasa Y, Tomich JM, Lu N, Beeman RW, Kramer KJ, Kanost MR. Proteomic and transcriptomic analysis of rigid and membranous cuticles and epidermis from elytra hindwings of the red beetle, Tribolium castaneum. J. Proteome. Res. 2012;11:269–278. doi: 10.1021/pr2009803. [DOI] [PubMed] [Google Scholar]
- Dunn PE, Drake DR. Fate of bacteria injected into naive and immunized larvae of the tobacco hornworm Manduca sexta. J. Invertebr. Pathol. 1983;41:77–85. [Google Scholar]
- Ekengren S, Hultmark D. Drosophila cecropin as an antifungal agent. Insect Biochem. Mol. Biol. 1999;29:965–972. doi: 10.1016/s0965-1748(99)00071-5. [DOI] [PubMed] [Google Scholar]
- Fai PB, Grant A. A comparative study of Saccharomyces cerevisiae sensitivity against eight yeast species sensitivities to a range of toxicants. Chemosphere. 2009;75:289–296. doi: 10.1016/j.chemosphere.2008.12.059. [DOI] [PubMed] [Google Scholar]
- Fehlbaum P, Bulet P, Michaut L, Lagueux M, Broekaert WF, Hetru C, Hoffmann JA. Septic injury of Drosophila induces the synthesis of a potent antifungal peptide with sequence homology to plant antifungal peptides. J. Biol. Chem. 1994;269:33159–63. [PubMed] [Google Scholar]
- Fehlbaum P, Bulet P, Chernysh S, Briand JP, Roussel JP, Letellier L, Hetru C, Hoffmann JA. Structure-activity analysis of thanatin, a 21-residue inducible insect defense peptide with sequence homology to frog skin antimicrobial peptides. Proc. Natl. Acad. Sci. U. S. A. 1996;93:1221–1225. doi: 10.1073/pnas.93.3.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman MJ, Kankanala P, Kanost MR. Bacterial challenge stimulates innate immune responses in extra-embryonic tissues of tobacco hornworm eggs. Insect Mol. Biol. 2004;13:19–24. doi: 10.1111/j.1365-2583.2004.00454.x. [DOI] [PubMed] [Google Scholar]
- Gunaratna RT, Jiang H. A comprehensive analysis of the Manduca sexta immunotranscriptome. Dev Comp Immunol. 2013;39:388–398. doi: 10.1016/j.dci.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Cao X, Li K, Hu Y, Chen Y, Blissard G, Kanost MR, Jiang H. A genome-wide analysis of antimicrobial effector genes and their transcriptional pattern in Manduca sexta. Insect Biochem. Mol. Biol. 2015;62:23–37. doi: 10.1016/j.ibmb.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmi H, Ishibashi J, Hara S, Yamakawa M. Solution structure of moricin, an antibacterial peptide, isolated from the silkworm Bombyx mori. FEEBS. Lett. 2002;518:33–38. doi: 10.1016/s0014-5793(02)02637-6. [DOI] [PubMed] [Google Scholar]
- Hultmark D, Steiner H, Rasmuson T, Boman HG. Insect immunity. Purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia. Eur. J. Biochem. 1980;106:7–16. doi: 10.1111/j.1432-1033.1980.tb05991.x. [DOI] [PubMed] [Google Scholar]
- Hwang JS, Lee J, Kim YJ, Bang HS, Yun EY, Kim SR, Suh HJ, Kang BR, Nam SH, Jeon JP, Kim I, Lee DG. Isolation and characterization of a defensin-like peptide (coprisin) from the dung beetle, Copris tripartitus. Int. J. Pept. 2009 doi: 10.1155/2009/136284. doi:10.1155/2009/136284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima R, Kurata S, Natori S. Purification, characterization, and cDNA cloning of an antifungal protein from the hemolymph of Sarcophaga peregrina (flesh fly) larvae. J. Biol. Chem. 1993;268:12055–1261. [PubMed] [Google Scholar]
- Jiang H, Vilcinskas A, Kanost MR. Immunity in lepidopteran insects. Adv. Exp. Med. Bio. 2010;708:181–204. doi: 10.1007/978-1-4419-8059-5_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Ma C, Lu ZQ, Kanost MR. Beta -1, 3-glucan recognition protein-2 (betaGRP-2) from Manduca sexta; an acute-phase protein that binds beta-1, 3-glucan and lipoteichoic acid to aggregate fungi and bacteria and stimulate prophenoloxidase activation. Insect Biochem. Mol. Biol. 2004;34:89–100. doi: 10.1016/j.ibmb.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Kanost MR, Jiang H, Yu XQ. Innate immune responses of a Lepidopteran insect, Manduca sexta. Immunol. Revs. 2004;198:97–105. doi: 10.1111/j.0105-2896.2004.0121.x. [DOI] [PubMed] [Google Scholar]
- Kanost MR, Kawooya JK, Law JH, Ryan RO, Van Heusden MC, Ziegler R. Insect Haemolymph Proteins. Adv. Insect Physiol. 1990;22:299–396. [Google Scholar]
- Kanost MR, Nardi JB. In: Innate immune responses of Manduca sexta. Goldsmith MR, Marec F, editors. Molecular Biology and Genetics of Lepidoptera, CRC Press; 2010. pp. 271–291. [Google Scholar]
- Kim DH, Lee YT, Lee YJ, Chung JH, Lee BL, Choi BS, Lee Y. Bacterial expression of tenecin 3, an insect antifungal protein isolated from Tenebrio molitor, and its efficient purification. Mol. Cells. 1998;8:786–789. [PubMed] [Google Scholar]
- Kim SR, Hong MY, Park SW, Choi KH, Yun EY, Goo TW, Kang SW, Suh HJ, Kim I, Hwang JS. Characterization and cDNA cloning of a cecropin-like antimicrobial peptide, papiliocin, from the swallowtail butterfly, Papilio xuthus. Mol. Cells. 2010;29:419–423. doi: 10.1007/s10059-010-0050-y. [DOI] [PubMed] [Google Scholar]
- Kouno T, Mizuguchi M, Tanaka H, Yang P, Mori Y, Shinoda H, Unoki K, Aizawa T, Makoto Demura M, Suzuki K, Kawano K. The structure of a novel insect peptide explains its Ca+2 channel blocking and antifungal activities. Biochem. 2007;46:13733–13741. doi: 10.1021/bi701319t. [DOI] [PubMed] [Google Scholar]
- Kwon YM, Kim HJ, Kim YI, Kang YJ, Lee IH, Jin BR, Han YS, Cheon HM, Ha NG, Seo SJ. Comparative analysis of two attacin genes from Hyphantria cunea. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 2008;151:213–220. doi: 10.1016/j.cbpb.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Lamberty M, Caille A, Landon C, Moindrot ST, Hetru C, Bulet P, Vovelle F. Solution structures of the antifungal heliomicin and a selected variant with both antibacterial and antifungal activities. Biochem. 2001;40:11995–12003. doi: 10.1021/bi0103563. [DOI] [PubMed] [Google Scholar]
- Lamberty M, Ades S, Uttenweiler-Joseph S, Brookhart G, Bushey D, Hoffmann JA, Bulet P. Isolation from the lepidopteran Heliothis virescens of a novel insect defensin with potent antifungal activity. J. Biol. Chem. 1999;274:9320–9326. doi: 10.1074/jbc.274.14.9320. [DOI] [PubMed] [Google Scholar]
- Landon C, Sodano P, Hetru C, Hoffmann J, Ptak M. Solution structure of drosomycin, the first inducible antifungal protein from insects. Protein Sci. 1997;6:1878–1884. doi: 10.1002/pro.5560060908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landon C, Barbault F, Legrain M, Menin L, Guenneugues M, Schott V, Vovelle F, Dimarcq JL. Lead optimization of antifungal peptides with 3D NMR structures analysis. Protein Sci. 2004;13:703–713. doi: 10.1110/ps.03404404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langen G, Imani J, Altincicek B, Kieseritzky G, Kogel KH, Vilcinskas A. Transgenic expression of gallerimycin, a novel antifungal insect defensin from the greater wax moth Galleria mellonella, confers resistance to pathogenic fungi in tobacco. Biol. Chem. 2006;387:549–557. doi: 10.1515/BC.2006.071. [DOI] [PubMed] [Google Scholar]
- Lee SY, Moon HJ, Kurata S, Natori S, Lee BL. Purification and cDNA cloning of an antifungal protein from the hemolymph of Holotrichia diomphalia larvae. Biol. Pharm. Bull. 1995;18:1049–1052. doi: 10.1248/bpb.18.1049. [DOI] [PubMed] [Google Scholar]
- Levashina EA, Ohresser S, Bulet P, Reichhart JM, Hetru C, Hoffmann JA. Metchnikowin, a novel immune-inducible proline-rich peptide from drosophila with antibacterial and antifungal properties. Eur. J. Biochem. 1995;233:694–700. doi: 10.1111/j.1432-1033.1995.694_2.x. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C (T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mulnix AB, Dunn PE. Structure and induction of a lysozyme gene from the tabacco hornworm, Manduca sexta. Insect Biochem. Mol. Bio. 1994;24:271–281. doi: 10.1016/0965-1748(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Petersen TN, Brunak S, Von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nature Methods. 2011;8:785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- Ragan EJ, An C, Jiang H, Kanost MR. Roles of hemolymph proteins in antimicrobial defences of Manduca sexta. In: Reynolds S, Rolff J, editors. Insect Infection and Immunity. Oxford University Press; 2009. pp. 34–48. [Google Scholar]
- Rahnamaeian M, Langen G, Imani J, Khalifa W, Altincicek B, Von Wettstein D, Koge KH, Vilcinska A. Insect peptide metchnikowin confers on barley a selective capacity for resistance to fungal ascomycetes pathogens. J. Exp. Bot. 2009;60:4105–4114. doi: 10.1093/jxb/erp240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahnamaeian M, Vilcinskas A. Defense gene expression is potentiated in transgenic barley expressing antifungal peptide Metchnikowin throughout powdery mildew challenge. J. Plant. Res. 2012;125:115–124. doi: 10.1007/s10265-011-0420-3. [DOI] [PubMed] [Google Scholar]
- Rahnamaeian M, Cytryńska M, Zdybicka-Barabas A, Dobslaff K, Wiesner J, Twyman RM, Zuchner T, Sadd BM, Regoes RR, Schmid-Hempel P, Vilcinskas A. Insect antimicrobial peptides show potentiating functional interactions against Gram-negative bacteria. Proc. Biol. Sci. 2015:282. doi: 10.1098/rspb.2015.0293. doi: 10.1098/rspb.2015.0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahnamaeian M, Vilcinskas A. Short antimicrobial peptides as cosmetic ingredients to deter dermatological pathogens. Appl. Microbiol. Biotechnol. 2015 doi: 10.1007/s00253-015-6926-1. doi:10.1007/s00253-015-6926-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy V, Zhao X, Snyder AK, Xu JR, Shah DM. Two mitogen-activated protein kinase signalling cascades mediate basal resistance to antifungal plant defensins in Fusarium graminearum. Cell Microbiol. 2007;9:1491–1506. doi: 10.1111/j.1462-5822.2006.00887.x. [DOI] [PubMed] [Google Scholar]
- Rao XJ, Xu XX, Yu XQ. Functional analysis of two lebocin-related proteins from Manduca sexta. Insect Biochem. Mol. Biol. 2012;42:231–239. doi: 10.1016/j.ibmb.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayaprolu S, Wang Y, Kanost MR, Hartson S, Jiang H. Functional analysis of four processing products from multiple precursors encoded by a lebocin-related gene from Manduca sexta. Comp. Immunol. 2010;34:638–647. doi: 10.1016/j.dci.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees JA, Moniatte M, Bulet P. Novel antibacterial peptides isolated from a European bumblebee, Bombus pascuorum (Hymenoptera, Apoidea) Insect Biochem. Mol. Biol. 1997;27:413–22. doi: 10.1016/s0965-1748(97)00013-1. [DOI] [PubMed] [Google Scholar]
- Russell V, Peter E, Dunn PE. Antibacterial proteins in the midgut of Manduca sexta during metamorphosis. J. Insect Physiol. 1996;42:65–71. [Google Scholar]
- Sagaram US, Pandurangi R, Kaur J, Smith TJ, Shah DM. Structure-activity determinants in antifungal plant defensins MsDef1 and MtDef4 with different modes of action against fusarium graminearum. PLoS ONE. 2011:6. doi: 10.1371/journal.pone.0018550. doi:10.1371/journal.pone.0018550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Soding J, Thompson JD, Higgins D. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Sys. Bio. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi D, Dai H, Hiromasa Y, Krishnamoortthi R, Kanost MR. Self-association of an insect β-1,3-glucan recognition protein upon binding laminarin stimulates prophenoloxidase activation as an innate immunity response. J. Bio. Chem. 2014;289:28399–28410. doi: 10.1074/jbc.M114.583971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Suzuki K. Expression profiling of a diapause-specific peptide (DSP) of the leaf beetle Gastrophysa atrocyanea and silencing of DSP by double-strand RNA. J. Insect Physiol. 2005;51:701–707. doi: 10.1016/j.jinsphys.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Satob K, Saitoc Y, Yamashitaa T, Agohd M, Okunishid J, Tachikawae E, Koichi Suzuki K. Insect diapause-specific peptide from the leaf beetle has consensus with a putative iridovirus peptide. Peptides. 2003;24:1327–1333. doi: 10.1016/j.peptides.2003.07.021. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Sudo C, An Y, Yamashita T, Sato K, Kurihara M, Suzuki K. A specific peptide produced during adult diapause of the leaf beetle, Gastrophysa atrocyanea Motschulsky (Coleoptera: Chrysomelidae) Appl. Entomol. Zool. 1998;33:535–543. [Google Scholar]
- Tonk M, Cabezas-Cruz A, Valdés JJ, Rego RO, Chrudimská T, Strnad M, Íma R, Bell-Sakyi L, Franta ZK, Vilcinskas A, Grubhoffer L, Rahnamaeian M. Defensins from the tick Ixodes scapularis are effective against phytopathogenic fungi and the human bacterial pathogen Listeria grayi. Parasites. & Vectors. 2014;7:554. doi: 10.1186/s13071-014-0554-y. Doi: 10.1186/s13071-014-0554-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonk M, Cabezas-Cruz A, Valdés JJ, Rego RO, Grubhoffer L, Estrada-Peña A, Vilcinskas A, Kotsyfakis M, Rahnamaeian M. Ixodes ricinus defensins attack distantly-related pathogens. Dev. Comp Immunol. 2015;53:358–65. doi: 10.1016/j.dci.2015.08.001. doi:10.1016/j.dci.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Vizioli J, Bulet P, Hoffmann JA, Kafatos FC, Müller HM, Dimopoulos G. Gambicin: a novel immune responsive antimicrobial peptide from the malaria vector Anopheles gambiae. Proc. Natl. Acad. Sci. U. S. A. 2001;22:12630–12635. doi: 10.1073/pnas.221466798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizioli J, Bulet P, Charlet M, Lowenberger C, Blass C, Müller HM, Dimopoulos G, Hoffmann J, Kafatos FC, Richman A. Cloning and analysis of a cecropin gene from the malaria vector mosquito, Anopheles gambiae. Insect Mol. Biol. 2000;9:75–84. doi: 10.1046/j.1365-2583.2000.00164.x. [DOI] [PubMed] [Google Scholar]
- Wan H, Yuan M, Youa H, Li J, Jin BR. Molecular cloning and characterization of a diapause-specific peptide in the beet armyworm, Spodoptera exigua. J. Asia. Pacific. Ento. 2012;15:369–373. [Google Scholar]
- Wang G, Li X, Wang Z. APD2: The updated antimicrobial peptide database and its application in peptide design. Nucleic. Acids. Res. 2009;37:D933–D937. doi: 10.1093/nar/gkn823. Database issue. doi:10.1093/nar/gkn823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, Zhang F, Liu Z, Ma J, Yang J. Expression and characterization of cecropin XJ, a bioactive antimicrobial peptide from Bombyx mori (Bombycidae, Lepidoptera) in Escherichia coli. Exp. Ther. Med. 2013;5:1745–1751. doi: 10.3892/etm.2013.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XO, Kanost MR. Developmental expression of Manduca sexta hemolin. Arch. Insect. Biochem.Physiol. 1999;42:198–212. doi: 10.1002/(SICI)1520-6327(199911)42:3<198::AID-ARCH4>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Xu XX, Zhong X, Yi HY, Yu XQ. Manduca sexta gloverin binds microbial components and is active against bacteria and fungi. Dev. Comp. Immunol. 2012;38:275–284. doi: 10.1016/j.dci.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdi MM, Zardini HZ, Asoodeh A. A novel antimicrobial peptide derived from the insect Paederus dermatitis. Int. J. Pept. Res. Ther. 2013;19:99–108. [Google Scholar]
- Yi HY, Chowdhury M, Huang YD, Yu XQ. Insect antimicrobial peptides and their applications. Appl. Microbiol. Biotechnol. 2014;98:5807–22. doi: 10.1007/s00253-014-5792-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Cao X, He Y, Hartson S, Jiang H. Semi-quantitative analysis of changes in the plasma peptidome of Manduca sexta larvae and their correlation with the transcriptome variations upon immune challenge. Insect Biochem. Mol. Biol. 2014;47:46–54. doi: 10.1016/j.ibmb.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Johnson TJ, Myers AA, Kanost MR. Identification by subtractive suppression hybridization of bacteria-induced genes expressed in Manduca sexta fat body. Insect Biochem. Mol Biol. 2003;33:541–559. doi: 10.1016/s0965-1748(03)00028-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.