Introduction
The World Health Organization (WHO) recommends starting antiretroviral therapy (ART) for all HIV-positive individuals regardless of CD4 cell count [1]. While 15 million people are already receiving ART, these new guidelines make nearly 20 million more people eligible for lifelong ART [1]. However, as ART coverage expands, successful treatment requires routine HIV viral load monitoring to ensure treatment adherence and control of drug resistance. Therefore, simple, cost-effective models of care need to focus on maintaining viral suppression and improving retention in care, while not increasing the burden on HIV care providers and laboratories [2].
Current WHO guidelines recommend plasma viral load monitoring at six months after ART initiation followed by annual testing [1], and define treatment failure as two consecutive viral load levels above 1000 copies/ml, despite continued adherence support interventions. The ambitious UNAIDS 90-90-90 target aims to achieve viral suppression in 90% of people on ART by 2020 [2]. In practice, performing routine laboratory-based viral load testing for the 15 million people receiving ART [3] has been a challenge, particularly in resource-limited settings. In areas hardest hit by the HIV epidemic, laboratory services are overburdened, results management systems are often weak, and some patients still lack access to viral load testing altogether. The consequences of weak health care systems are undiagnosed virological failure, late treatment switches, and the potential spread of HIV drug resistance.
A point-of-care HIV viral load test could broaden access to routine viral load monitoring, and decentralize HIV care, so that limited clinic resources can be prioritized to manage more complex patients. Clinic-based HIV viral load monitoring could allow same day adherence counseling, simplify stable patient management, and improve early detection of virological failure, all at a reduced cost. In order to assess clinic-based HIV viral load testing further, we conducted a cross-sectional validation study of two novel HIV viral load test within a South African HIV clinic.
Methods
We report on our early clinical experience with the Xpert® HIV-1 VL and Xpert® HIV-1 Qual assays, which are both processed on the GeneXpert® System (Cepheid, Sunnydale, California, USA). The Xpert® HIV-1 VL is a fully automated real-time molecular cartridge-based assay, measuring a linear range of 40 to 10 million copies/ml of HIV RNA, which received European CE-IVD regulatory approval in December 2014. The Xpert® HIV-1 Qual is a molecular cartridge-based assay that detects total nucleic acid (DNA and RNA) and provides a qualitative result (HIV detectable or undetectable). Both assays can be operated by a health care worker in a clinical setting and provide a result within 90 minutes.
We performed a total of 42 Xpert® HIV-1 VL assays on plasma samples and 20 Xpert® HIV-1 Qual assays on whole blood samples collected consecutively from known HIV positive South African women who attended for routine study visits in the CAPRISA 002 study. This study has been following HIV-positive women from acute infection through chronic infection, and while receiving ART since 2004 [4]. Of the 31/42 fresh (not frozen) plasma samples, five were obtained within one year of HIV acquisition, 12 during chronic infection from women not receiving ART, 11 were obtained from virologically suppressed women on ART, and three from women who were failing ART. We also evaluated an additional 11/42 frozen samples, of which two were collected from women with early infection not on ART, and nine from women failing ART.
For both assays, samples were collected in 5ml EDTA tubes. For the Xpert® HIV-1 VL assay, specimen were first centrifuged at 1200rpm for 10 minutes before transfer of 1ml of plasma into the assay's cartridge chamber using a sterile pipette. For the Xpert® HIV-1 Qual assay, 100 microliters of EDTA whole blood was transferred to the assay's cartridge chamber. Each assay was then loaded into the GeneXpert® System for analysis. We performed HIV viral load testing with the Roche Taqman version 2 assay (Roche Diagnostics, Switzerland) as the gold standard diagnostic test. To minimize bias, the Xpert® HIV-1 VL and Qual assays were performed in the clinic and the Taqman assays in a central laboratory by different technicians. We also stratified results between fresh versus frozen samples, and determined if the Xpert® HIV-1 VL assay misclassified any patients above or below a viral load threshold of 1000 copies/ml. Ethical approval for this evaluation was granted by the Biomedical Research Ethics Committee of the University of KwaZulu-Natal.
Results
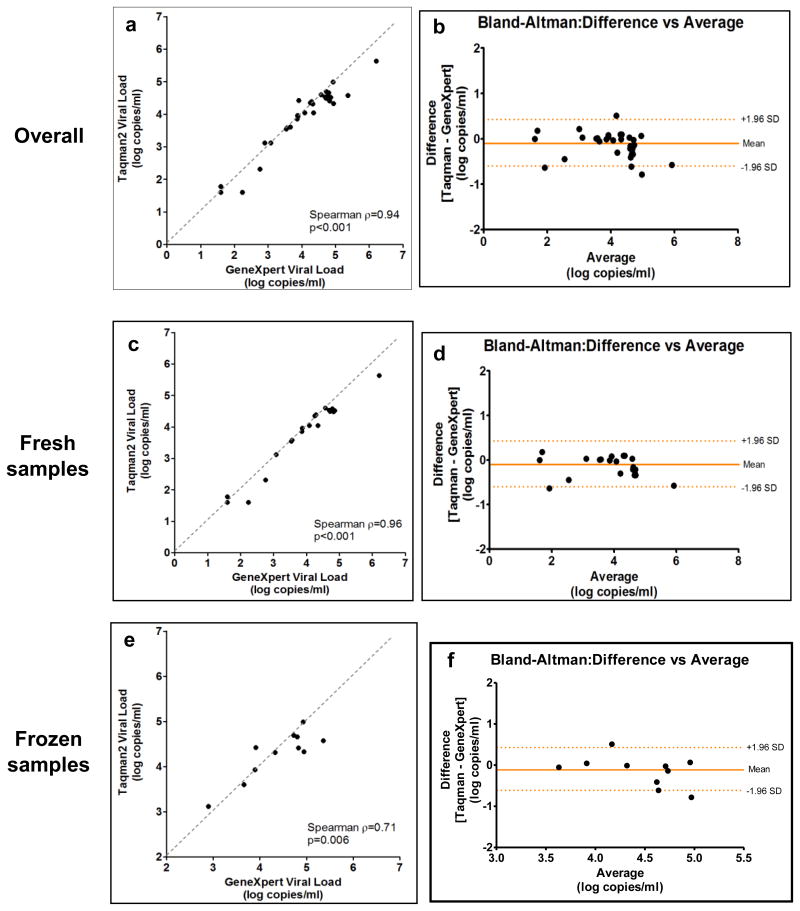
The median age of the 62 HIV-positive women sampled was 33 years (IQR 28 - 37) and their median CD4 cell count was 609 cells/mm3 (IQR 458 – 703). Overall, Xpert® HIV-1 VL and Taqman viral load results had excellent correlation across the viral load spectrum (Spearman ρ=0.94, p<0.001) (Figure 1a). A Bland-Altman plot showed a mean difference between Taqman and Xpert® results of -0.10 log copies/ml (95% limits of agreement -0.59 to 0.39) with slightly higher values on Xpert® (Figure 1b). The number and proportion of Taqman results correctly categorized by Xpert® HIV-1 VL into relevant viral load strata (<3, 3-4 and >4 log copies/ml) were 14/14 (100%), 7/8 (88%) and 18/20 (90%), respectively.
Figure 1. Correlation between Xpert® HIV-1 viral load and Roche Taqman version 2 assays using correlation curves and Bland-Altman plots.
Results were similar when stratified by fresh versus frozen samples. The correlation was strong for the 31 fresh samples [Spearman ρ=0.96, p<0.001; mean difference 0.10 log copies/ml (0.30 to -0.50)] (Figure 1c and 1d). The 11 frozen samples showed a lower coefficient, but similar mean difference [Spearman ρ= 0.71; mean difference 0.11 log copies/ml (0.61 to -0.83)] (Figure 1e and 1f).
Twelve women on ART had a detectable HIV viral load of >1000 copies/ml measured with the Taqman version 2 assay. The median viral load was 4.37 log copies/ml (range 3.11 – 4.99). All 12 of these women had detectable viral load results on the Xpert® HIV-1 VL (median viral load 4.52 log copies/ml, range 2.90 – 5.36). Amongst the 42 women tested, only one person was misclassified using a viral load threshold of 1000 copies/ml. This woman had a Taqman result of 1302 copies/ml and a Xpert® HIV-1 VL result of 792 copies/ml.
In addition, 20 samples were tested on the qualitative Xpert® HIV-1 Qual. Of these samples, 13 participants had detectable viral loads (median log viral load 3.83; IQR 2.98 – 4.66) and seven were virologically suppressed. All except one participant with a low viral load of 523 copies/ml on Taqman version 2 were correctly identified by the qualitative assay (N=19/20; 95% sensitivity). This misclassified person had a viral load of 200 copies/ml one month previous to testing and an undetectable (<40 copies/ml) viral load one month after testing.
Discussion
In summary, the Xpert® HIV-1 VL showed good correlation with an established laboratory-based viral load assay, and could be a reliable tool for clinic-based viral load monitoring. The strength of the correlation was slightly weaker when comparing stored specimen. The Xpert® HIV-1 Qual may play a role in the diagnosis of HIV, either in early infant diagnosis, as a confirmatory test after antibody based testing, or for the detection of acute HIV infection in antibody negative patients with symptoms indicative of acute infection [5]. Although Xpert® HIV-1 Qual requires lower blood volumes, our study adds evidence that it is not suitable for monitoring HIV infected patients, because it detects total nucleic acids.
Recent data from South Africa has revealed that many clinics are struggling with the large number of patients and the rapid ART scale-up [6]. For example, more than 25% of patients initiating ART are lost to follow-up after one year, and less than half of patients on ART have a recorded 12-month viral load result [6]. Moreover, of those patients who receive a viral load result, 25% do not achieve virological suppression, the ultimate goal of ART delivery. This high level of treatment failure suggests that clinic facilities are struggling to manage and retain patients, and care pathways for stable patients on ART may be too complex, consuming both patient and staff time and vital resources. The Xpert® HIV-1 VL and other point-of-care viral load assays could play an important role in streamlining pathways for stable patients on ART. Patients would be able to receive test results during the same visit, thereby increasing the number of patients on ART with viral load results. Furthermore, a more satisfactory patient experience could potentially improve retention in care, and prove to be cost-effective. The Xpert® HIV-1 VL is currently available for $16.90/cartridge ex-factory, which could rise up to ∼$20/cartridge, if taxes are added (personal communication with Cepheid). Similar to ART provisions in Africa in the early 2000s, reducing the cost further would allow public funds to maximize the number of tests performed and patient benefit. The successful scale-up of the Xpert® MTB/RIF assay and the existing infrastructure in South Africa could serve as a blueprint, if larger studies confirm its utility.
This first clinic-based validation of the Xpert® HIV-1 VL in a well-characterized cohort provides some early evidence that this tool may be able to fill an important gap in the rapid scale–up of ART globally. However, additional studies will be required to assess the strength of this assay in patients with low-level or rebounding viremia, where decisions to switch treatment are often made. In addition, implementation of this assay would require careful consideration of the potential drawbacks of point-of-care technology, such as the need for ongoing training of site staff, the ability to manage a large volume of tests in clinics with a large patient population, and the potential lack of quality control [7], especially when utilized in more remote settings, where the technology could have the greatest impact.
Nevertheless, the prospect of a potentially simpler, cheaper and more patient-centered care model is appealing, and studies looking at the implementation of Xpert® HIV-1 VL and other point-of-care viral load assays to replace traditional care pathways should be prioritized.
Acknowledgments
We thank all the CAPRISA 002 Study participants who are continuing to make an important personal contribution to HIV research. We are grateful to Marlene Venter, Keenan Govender, and Jessica Naidoo for processing the Xpert® HIV-1 viral load samples.
Funding: Over the last decade, the CAPRISA 002 study has received support from the National Institute of Allergy and infectious Disease (NIAID), National Institutes of Health (NIH) (grants # AI51794, #AI104387, #AI115981, #AI116086), from CONRAD (USAID co-operative grant #GP00-08-00005-00, subproject agreement # PPA-09-046), from the National Research Foundation (grant # 67385), the Technology Innovation Agency, and the Columbia University-Southern African Fogarty AIDS International Training and Research Programme (AITRP) funded by the Fogarty International Center, NIH (grant #D43TW00231).
Sources of support: Cepheid provided the Xpert® HIV-1 viral load cartridges to the authors free of charge. However, the company was not involved in the design and conduct of the study, or in the preparation of the manuscript.
Footnotes
Presentation of data: Preliminary results of this study were presented at the International AIDS Society Meeting in Vancouver on July 19, 2015.
Authors' contributions: NJG, PKD, LW, NS, and SSAK contributed to study design, data collection, analysis and interpretation of the data. NJG drafted the manuscript and all authors reviewed and approved the final version.
References
- 1.World Health Organization. [1 November 2015];Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. 2015 Sep; http://apps.who.int/iris/bitstream/10665/186275/1/9789241509565_eng.pdf. [PubMed]
- 2.UNAIDS. [1 November 2015];90-90-90 An ambitious treatment target to help end the AIDS epidemic. 2014 Oct; http://www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf.
- 3.UNAIDS report: UNAIDS. How AIDS changed everything. Geneva: UNAIDS; 2015. [1 November 2015]. http://www.unaids.org/sites/default/files/media_asset/MDG6Report_en.pdf. [Google Scholar]
- 4.van Loggerenberg F, Mlisana K, Williamson C, et al. Establishing a cohort at high risk of HIV infection in South Africa: challenges and experiences of the CAPRISA 002 acute infection study. PLoS ONE. 2008;3:e1954. doi: 10.1371/journal.pone.0001954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanders EJ, Wahome E, Powers KA, et al. Targeted screening of at-risk adults for acute HIV-1 infection in sub-Saharan Africa. AIDS. 2015 Dec;29(Suppl 3):S221–30. doi: 10.1097/QAD.0000000000000924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.South African National AIDS Council. [1 November 2015];Progress report on national strategic plan for HIV, TB and STIs (2012-2016) 2014 Nov; http://www.sanac.org.za/publications/reports/cat_view/7-publications/9-reports.
- 7.Stevens W, Gous N, Ford N, et al. Feasibility of HIV point-of-care tests for resource-limited settings: challenges and solutions. BMC Medicine. 2014 Sep 8;12:173. doi: 10.1186/s12916-014-0173-7. [DOI] [PMC free article] [PubMed] [Google Scholar]