Abstract
Objective
Postmortem studies have reported Purkinje cell loss in essential tremor (ET), and we recently demonstrated a significant increase in the mean distance between Purkinje cell bodies (i.e., a larger gap length distance) in ET cases vs. controls, likely reflecting a disease-associated reduction in Purkinje cells. We now analyze the regularity of distribution of Purkinje cells along the Purkinje cell layer to determine whether there is greater disorganization in ET cases than age-matched controls.
Methods
A standard parasagittal, formalin-fixed, tissue block was harvested from the neocerebellum of 50 ET cases and 25 age-matched controls. The gap length distance (μm) between Purkinje cells was quantified using a nearest neighbor analysis in which the distance between each Purkinje cell body was measured in Open Lab software, version 5 (Improvision, Waltham, MA) by drawing a freehand line between adjacent Purkinje cell bodies along the entirety of the Purkinje cell layer within a given image. We analyzed the subject-specific variation in the organization of Purkinje cells along the Purkinje cell layer.
Results
The 50 ET cases and 25 controls were similar in age at death, gender and brain weight. Overall, greater variation in gap length distance (i.e., more disorganization) was associated with greater gap length distance (p <0.001) and younger age (p = 0.020). However, the variation in the Purkinje cell gap length distance (i.e., Purkinje cell organization) did not differ in ET cases and controls (p = 0.330).
Interpretation
We observed that the regularity of the distribution of Purkinje cells along the Purkinje cell layer did not differ between ET cases and controls. Several alternative biological interpretations for this finding are discussed.
Introduction
Essential tremor (ET) is a chronic, progressive neurological disease [1, 2] that involves the cerebellum and cerebellar system [3–5]; it is among the most common neurological diseases. Controlled postmortem studies in recent years have documented a growing number of structural, degenerative changes in the ET cerebellum, involving the Purkinje cell and neighboring neuronal populations [6–10]. In addition to these changes, Purkinje cell loss has been documented in some [11, 12] although not all controlled studies [13, 14], with methodological differences between studies possibly explaining the discrepant results [10, 15, 16]. At present, we are just beginning to understand the nature and extent of Purkinje cell loss in ET, which when detected, is more subtle than that seen in patients with the spinocerebellar ataxias [17]. Our overarching scientific goal is to shed light on the anatomical and physiological properties of the changes in the Purkinje cell layer in ET.
In addition to counting Purkinje cells, a useful measure of Purkinje cell loss is a nearest neighbor analysis [18, 19], in which one quantifies the distance (i.e., the length of the gap) between Purkinje cells along the Purkinje cell layer. We recently demonstrated a significant increase in the mean distance between Purkinje cells in ET cases compared with age-matched controls, with this change likely reflecting a disease-associated reduction in Purkinje cells in ET cases [18].
Beyond this basic observation; however, we know nothing about the regularity of the distribution of Purkinje cells in ET and whether this differs from that of controls. Addressing this question is technically challenging; this is because there is considerable natural variation in normal controls in the distance between Purkinje cells. The question is an important one because it might shed light on the nature of Purkinje cell loss in ET. Is the drop out of these neurons random in ET, thereby leading to greater disorganization of Purkinje cells along the Purkinje cell layer, or is it a more patterned process that is oriented around specific functional cerebellar units, thereby not leading to greater disorganization?
Over the past eight years, we have prospectively assembled a large sample of 50 ET and 25 age-matched control brains. The current analyses specifically test whether, aside from an increase in gap length distance, the regularity of distribution of Purkinje cells along the Purkinje cell layer distinguishes ET cases from age-matched controls. To our knowledge, this issue has not been examined in ET nor has it been assessed more broadly in human cerebellar neurodegeneration.
Methods
Cases and Controls
This study was conducted at the Essential Tremor Centralized Brain Repository [6], an NIH-funded effort that involves the prospective collection of ET brains from study participants who reside throughout the United States and who have self-referred for brain donation. The ET cases were diagnosed as described below. Controls were normal elderly subjects from the New York Brain Bank (Columbia University Medical Center, New York, NY), Harvard Brain Tissue Resource Center (McLean Hospital, Belmont, MA) or the University of Kentucky Alzheimer’s Disease Center (Sanders-Brown Center on Aging, Lexington, KY). The controls were free of clinical diagnoses of Alzheimer’s disease, ET or Parkinson’s disease and without a neuropathological diagnosis of neurodegenerative disease [6]. All study subjects signed informed consent approved by the above-referenced University Ethics Boards.
There were 50 ET cases. Controls were frequency-matched to ET cases using a 2:1 scheme, reflecting the greater availability of ET tissue. The final sample comprised 50 ET cases and 25 age-matched controls.
Clinical Evaluation
During life, ET diagnoses were assigned using three sequential methods, as described in detail elsewhere [6]. ET cases were also assigned a total tremor score (range = 0 – 36), based on the neurologist’s 0 – 3 rating of postural and kinetic tremors on videotaped examination.
In ET cases, data on lifetime exposure to medications (e.g., lithium, diphenylhydantoin, chemotherapeutic agents) known to cause cerebellar damage were collected, and the average number of daily drinks of beer, liquor, or wine was collected. Heavy ethanol use was previously defined as consumption of an average of ≥4 standard drinks (15 mL of absolute ethanol) per day for a man or ≥3 drinks per day for a woman at any point in their lives [6, 20].
Every 6 months, a follow-up semi-structured telephone evaluation was performed on ET cases. At that point, 4 additional hand-drawn spirals were collected. The median time interval between last visit with their doctor (ET cases) and death was 4 months.
Neuropathological Assessment
As previously described [6], after death, brains were harvested by and underwent a complete neuropathological assessment at the New York Brain Bank. Standardized measurements of brain weight (in grams) were recorded. Paraffin embedded blocks from standardized brain regions were sectioned at 7-μm and stained respectively with Luxol fast blue and hematoxylin and eosin (LH&E) for general tissue survey and assessment of myelin, and the modified Bielschowsky silver stain for axons and neurofibrils. Additional sections were processed with peroxidase-antiperoxidase immunohistochemical methods for α-synuclein-, β-amyloid-, and for tauopathic-burdens.
A standard 3 x 20 x 25 mm parasagittal, formalin-fixed, tissue block was harvested from the cerebellar hemisphere. The block contained dentate nucleus, white matter and cerebellar cortex from anterior and posterior quadrangulate lobules (IV–VI), which are involved in motor control [21]. Using the standard LH&E stained 7-μm thick section [12], a senior neuropathologist (P.L.F.) blinded to clinical information quantified torpedoes in the entire section, also adjusting the count to the Purkinje layer length as described previously [22]. Using the same section, a trained research technician (M.C.), blinded to clinical information, quantified the distance between Purkinje cells (i.e., the length of the gap) using a nearest neighbor analysis [18, 19]. Each of the individual folia within the Purkinje cell layer was assigned a number in sequential order (approximately 40 – 50 folia per section). A random number generator was used to select ten folia per section for quantification. These ten folia were centered in a 5x objective microscopic field and imaged using a Zeiss Axioplan 2 microscope. The distance between each Purkinje cell body was measured in Open Lab software, version 5 (Improvision, Waltham, MA) by drawing a freehand line between adjacent Purkinje cell bodies along the entirety of the Purkinje cell layer within a given image. The average distance between Purkinje cell bodies was determined from each of the ten images and then averaged together to determine the mean nearest neighbor distance (μm) for each ET case and control.
Statistical Analyses
These analyses were performed in Statistical Analysis System (SAS, Version 9.4). First, we examined demographic and clinical differences between ET cases and controls using Student’s t tests and chi-square tests (Table 1). Torpedo count was not normally distributed; therefore, a Mann-Whitney test was used to compare ET cases to controls (Table 1).
Table 1.
Clinical and Pathological Data on Essential Tremor Cases and Controls
| Controls (n = 25) | Essential Tremor Cases (n = 50) | Significance | |
|---|---|---|---|
|
| |||
| Age (years) | 84.9 ± 5.4 | 86.8 ± 5.8 | p = 0.17 a |
|
| |||
| Women | 15 (60.0) | 31 (62.0) | p = 0.87 b |
|
| |||
| Total tremor score | Not applicable | 24.3 ± 6.4 | Not applicable |
|
| |||
| Brain weight (grams) | 1183 ± 150 | 1187 ± 154 | p = 0.92 a |
|
| |||
| Torpedo count | |||
| Raw Value | 4.2 ± 2.8 [3.0] | 18.5 ± 15.0 [15.0] | p < 0.001 c |
| Corrected Value | 2.2 ± 1.3 [1.7] | 8.3 ± 6.4 [6.7] | p < 0.001 c |
|
| |||
| Gap Length distance (μm) | 174.6 ± 41.4 | 209.3 ± 46.9 | p = 0.002 a |
All values represent mean ± standard deviation [median] or number (percentage).
Student’s t test.
Chi-square test.
Mann-Whitney test.
Estimation and testing focused on the organization of scale-adjusted distances between adjacent Purkinje cells. We assessed scale-adjusted organization by assessing triplets (i.e., three adjacent cells): the absolute distance between the middle cell in a triplet and the mid-point between the two cells flanking the middle cell was adjusted by dividing by the distance between the cells adjacent to the middle cell. If cells were perfectly regularly situated within a slice, the adjusted absolute difference would be uniformly zero, but if cells were randomly placed within a slice, according to a homogeneous Poisson process, for example, then the adjusted absolute difference would be 0.25. After estimating the adjusted absolute difference, both for ET cases and controls, we then used fixed-effects models to assess the extent to which diagnosis (ET vs. control) as well as age (years), brain weight (grams), gap length distance (μm), torpedo count, and, for subjects with ET, the total tremor score, explained subject-specific variation in the organization measure in the sample.
Raw torpedo counts were presented. We also corrected the torpedo count for the length of the Purkinje cell layer (i.e., torpedo count÷Purkinje cell layer length in microns). As described previously [22], because some values for the torpedo count were 0, we needed to add the value 1 to the numerator in all cases and controls to allow for mathematical division; we also multiplied the numerator by 105 in order to avoid the presentation of data with multiple decimal places (e.g., 2.1 rather than 0.000021). Hence, the final formula for corrected torpedo count=105 (raw value+1)/Purkinje layer length.
Results
The 50 ET cases and 25 controls were similar in age at death, gender and brain weight (Table 1). No cases were heavy ethanol users and none had lifetime exposure to cerebellar toxic medications. Age of onset was ≤ 65 years in 82% of ET cases and the mean tremor duration was 43.7 ± 23.1 years. Lewy bodies (alpha-synuclein: dorsal vagal nucleus, locus ceruleus, pars compacta of substantia nigra) were detected in none. The gap length distance was greater in ET cases than controls (Table 1).
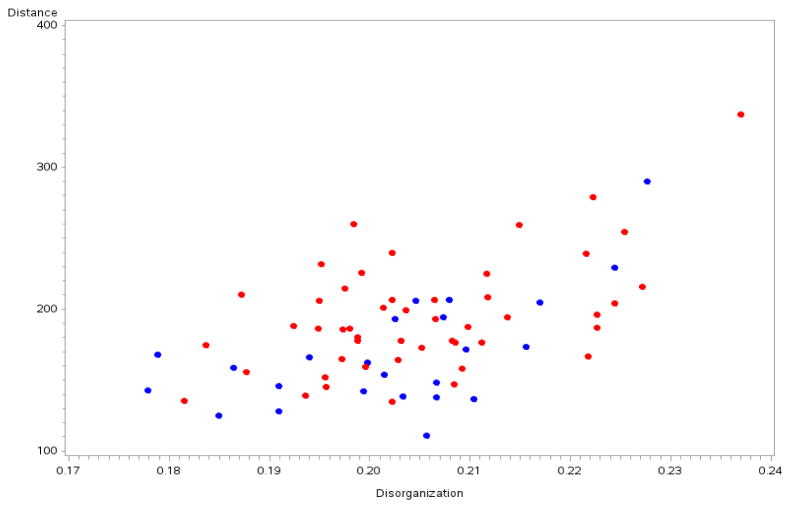
The adjusted absolute difference, which was our measure of organization, was nearly identical in ET cases (0.20 ± 0.12) and controls (0.20 ± 0.13) (p = 0.330), indicating that variation in Purkinje cell gap length distance did not differ in ET cases compared to age-matched controls (Figure). This value, 0.20, is substantially less than 0.25 (a random distribution), indicating a substantial measure of organization in the placement of Purkinje cells. However, the value of 0.20 was not close to zero, which indicates that Purkinje cells were not strictly uniformly placed along the Purkinje cell layer.
Figure.
Purkinje cell disorganization by Purkinje cell gap length distance in ET cases (red) vs. controls (blue). The disorganization is similar in both groups, although distance is greater in ET cases than controls and there is a positive association between distance and disorganization.
In five separate fixed effects models, we observed that diagnosis (ET patients vs. control) did not explain the subject-specific variation in our organization measure (p = 0.330), nor did the brain weight (p = 0.938); however, higher gap length distance was associated with greater subject-specific variation in our organization measure (p < 0.001) (Figure). Also, higher raw torpedo count (p = 0.002) and younger age (p = 0.035) were associated with greater subject-specific variation in our organization measure (Table 2). Inclusion of each of these five variables (diagnosis, brain weight, gap length distance, raw torpedo count and age) in the same model yielded similar results, with no association between diagnosis (p = 0.256), brain weight (p = 0.559), or raw torpedo count (beta = 0.00016, p = 0.11) and our organization measure, but a robust association between gap length distance (beta = 0.00015, p < 0.001) and younger age (beta = −0.0005, p = 0.020) and our organization measure. Within ET cases, the total tremor score was not associated with greater subject-specific variation in our organization measure (beta = −0.00003, p = 0.945).
Table 2.
Association Between Variables of Interest and Our Organization Measure
| Variable Assessed In Separate Fixed Effects Models | Estimate | p value |
|---|---|---|
| Diagnosis (ET vs. control) | 0.003 | 0.330 |
| Brain weight (grams) | 0.00000076 | 0.938 |
| Gap length distance (μm) | 0.00017 | <0.001 |
| Torpedo count | 0.0003 | 0.002 |
| Age (years) | −0.00054 | 0.035 |
Discussion
Although considerable variation in Purkinje cell placement along the Purkinje cell layer has been the subject of anecdotal remark in the past [23], we are unaware of a study that has attempted to quantify the regularity of Purkinje cell placement or to compare it across disease states. With this in mind, this study aimed to examine the regularity of the distribution of Purkinje cells in ET cases vs. controls. We questioned whether loss of Purkinje cells in ET occurs in a random manner, which one would expect might lead to greater disorganization of Purkinje cells along the Purkinje layer in comparison with control brains. However, we did not find such a pattern. There are a number of potential biological interpretations. One is that Purkinje cell loss in ET is not a completely random process but occurs in some sort of a patterned manner that does not result in greater disorganization. That is, the loss could involve specific functional cerebellar units. While intriguing, the problem with this interpretation is that it implies that there would be a patterned loss (i.e., a reduction in disorganization) in ET, which is not what we observed. Another interpretation is that the loss of Purkinje cells in ET, while greater than that seen in controls, follows a similar pattern and that Purkinje cell loss in ET is merely an acceleration of the same process that occurs to a lesser extent in normal aging [23]. A final interpretation is that because Purkinje cell loss is overall mild in ET, it is not accompanied by greater disorganization of the Purkinje cell layer. Further studies are needed to examine these different possibilities.
There is considerable natural variation even in normal controls in the distance between Purkinje cells [23]. In our study, in both cases and controls, the adjusted absolute difference, which was our measure of Purkinje cell organization, had a value of 0.20. While on the one hand, this value is substantially less than 0.25 (a random distribution), indicating a substantial measure of organization in the placement of Purkinje cells, on the other hand, the value of 0.20 is not close to zero, which indicates that Purkinje cells are not strictly uniformly placed along the Purkinje cell layer.
We also examined several predictors of Purkinje cell organization. Greater gap length distance, in both cases and controls, was associated with the greater subject-specific variation in our organization measure (p < 0.001). That is, in general, drop out of Purkinje cells seemed to result in an increase in variability of Purkinje cell placement along the Purkinje cell layer. However, the greater drop out of Purkinje cells in ET cases relative to controls did not result in a corresponding increase in variability in Purkinje cell placement. Younger age, independent of greater Purkinje cell loss, was also associated with greater subject-specific variation in our organization measure, although to less of an extent. Other predictors of this between-subject variation (e.g., genetic factors) are worthy of exploration.
Several genetic rodent models with Purkinje cell degeneration have demonstrated patterns of neurodegeneration within specific cerebellar cortical microdomains; these have been best studied in relation to parasagittal bands of zebrin II protein expression [24]. However, to our knowledge, the specific issue we address here, namely, variation in Purkinje cell placement along the Purkinje cell layer, has not been examined in ET or, more broadly, in any form of human cerebellar neurodegeneration.
This study should be interpreted within the context of certain limitations. Our analyses involved a single standardized section, localized to one region of the cerebellar hemisphere. We did not examine regional differences in Purkinje cell organization across regions of the cerebellum; future studies should undertake these analyses.
In summary, although a reduction in Purkinje cells and greater gap distance between Purkinje cells has been reported in ET cases vs. controls, we were unable to detect a difference in the regularity of the distribution of Purkinje cells in these two groups. That is, the organization of these cells did not differ. A number of alternative biological interpretations could account for finding. This observation sheds additional light on the anatomical properties of the changes in the Purkinje cell layer in ET, which is among the most common neurological diseases.
Acknowledgments
We thank the Harvard Brain Tissue Resource Center (supported in part by PHS grant number R24 MH068855) for providing control tissue. We similarly thank the University of Kentucky Alzheimer’s Disease Center Biobank (P30 AG028383) for providing control tissue, and the patients, staff, and clinicians who have contributed to their efforts.
Dr. Louis has received research support from the National Institutes of Health: NINDS #R01 NS042859 (principal investigator), NINDS #R01 NS086736 (principal investigator), NINDS #R01 NS085136 (principal investigator) and NINDS #R01 NS088257 (principal investigator). Dr. Kuo has received funding from International Essential Tremor Foundation, NINDS #K08 NS08738 (principal investigator), Louis V. Gerstner Jr. Scholar Award (principal investigator), Parkinson’s Disease Foundation (principal investigator), American Parkinson’s Disease Association (principal investigator), NIEHS pilot grant #ES009089 (principal investigator), NINDS #R01NS084948 (co-investigator), and NINDS #R01NS088257 (co-investigator). Dr. Faust has received funding from NINDS #R01 NS042859 (co-investigator), NINDS #R01 NS085136 (co-investigator) and NINDS #R01 NS088257 (co-investigator).
Footnotes
Author Contributions
Each of the listed authors participated in both the conception and design of the study, and in the data analysis and editing. All authors approved the final draft of the paper before it is submitted.
Conflicts of Interest
None of the listed authors has any conflicts of interest to declare.
References
- 1.Putzke JD, Whaley NR, Baba Y, Wszolek ZK, Uitti RJ. Essential tremor: predictors of disease progression in a clinical cohort. J Neurol Neurosurg Psychiatry. 2006;77:1235–1237. doi: 10.1136/jnnp.2006.086579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis ED, Agnew A, Gillman A, Gerbin M, Viner AS. Estimating annual rate of decline: prospective, longitudinal data on arm tremor severity in two groups of essential tremor cases. J Neurol Neurosurg Psychiatry. 2011;82:761–765. doi: 10.1136/jnnp.2010.229740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bares M, Husarova I, Lungu OV. Essential tremor, the cerebellum, and motor timing: towards integrating them into one complex entity. Tremor Other Hyperkinet Mov (N Y) 2012;2 pii: tre-02-93-653-1. [PMC free article] [PubMed] [Google Scholar]
- 4.Grimaldi G, Manto M. Is essential tremor a Purkinjopathy? The role of the cerebellar cortex in its pathogenesis. Mov Disord. 2013;28:1759–1761. doi: 10.1002/mds.25645. [DOI] [PubMed] [Google Scholar]
- 5.Passamonti L, Cerasa A, Quattrone A. Neuroimaging of essential tremor: What is the evidence for cerebellar involvement? Tremor Other Hyperkinet Mov (N Y) 2012;2 doi: 10.7916/D8F76B8G. pii: 02-67-421-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Babij R, Lee M, Cortes E, Vonsattel JP, Faust PL, Louis ED. Purkinje cell axonal anatomy: quantifying morphometric changes in essential tremor versus control brains. Brain. 2013;136:3051–3061. doi: 10.1093/brain/awt238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louis ED, Lee M, Babij R, Ma K, Cortes E, Vonsattel JP, et al. Reduced Purkinje cell dendritic arborization and loss of dendritic spines in essential tremor. Brain. 2014;137:3142–3148. doi: 10.1093/brain/awu314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erickson-Davis CR, Faust PL, Vonsattel JP, Gupta S, Honig LS, Louis ED. “Hairy baskets” associated with degenerative Purkinje cell changes in essential tremor. J Neuropathol Exp Neurol. 2010;69:262–271. doi: 10.1097/NEN.0b013e3181d1ad04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin CY, Louis ED, Faust PL, Koeppen AH, Vonsattel JP, Kuo SH. Abnormal climbing fibre-Purkinje cell synaptic connections in the essential tremor cerebellum. Brain. 2014;137:3149–3159. doi: 10.1093/brain/awu281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Louis ED. Essential Tremor: A Common Disorder of Purkinje Neurons? Neuroscientist. 2015 Jun 4; doi: 10.1177/1073858415590351. pii: 1073858415590351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Louis ED, Faust PL, Vonsattel JP, Honig LS, Rajput A, Robinson CA, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007;130:3297–3307. doi: 10.1093/brain/awm266. [DOI] [PubMed] [Google Scholar]
- 12.Louis ED, Babij R, Lee M, Cortes E, Vonsattel JP. Quantification of cerebellar hemispheric purkinje cell linear density: 32 ET cases versus 16 controls. Mov Disord. 2013;28:1854–1859. doi: 10.1002/mds.25629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Symanski C, Shill HA, Dugger B, Hentz JG, Adler CH, Jacobson SA, et al. Essential tremor is not associated with cerebellar Purkinje cell loss. Mov Disord. 2014;29:496–500. doi: 10.1002/mds.25845. [DOI] [PubMed] [Google Scholar]
- 14.Rajput AH, Robinson CA, Rajput ML, Robinson SL, Rajput A. Essential tremor is not dependent upon cerebellar Purkinje cell loss. Parkinsonism Relat Disord. 2012;18:626–628. doi: 10.1016/j.parkreldis.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Jellinger KA. Is there cerebellar pathology in essential tremor? Mov Disord. 2014;29:435–436. doi: 10.1002/mds.25852. [DOI] [PubMed] [Google Scholar]
- 16.Louis ED, Faust PL, Vonsattel JP. Purkinje cell loss is a characteristic of essential tremor: Towards a more mature understanding of pathogenesis. Parkinsonism Relat Disord. 2012;18:1003–1004. doi: 10.1016/j.parkreldis.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 17.Louis ED, Kuo SH, Vonsattel JP, Faust PL. Torpedo formation and purkinje cell loss: modeling their relationship in cerebellar disease. Cerebellum. 2014;14:433–439. doi: 10.1007/s12311-014-0556-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choe M, Cortés E, Vonsattel JPG, Kuo SH, Faust PL, Louis ED. Purkinje cell loss in essential tremor: Random sampling quantification and nearest neighbor analysis. Mov Disord. doi: 10.1002/mds.26490. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamling KR, Tobias ZJ, Weissman TA. Mapping the development of cerebellar Purkinje cells in zebrafish. Dev Neurobiol. 2015 Feb 4; doi: 10.1002/dneu.22275. [DOI] [PubMed] [Google Scholar]
- 20.Harasymiw JW, Bean P. Identification of heavy drinkers by using the early detection of alcohol consumption score. Alcohol Clin Exp Res. 2001;25:228–235. [PubMed] [Google Scholar]
- 21.Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 22.Louis ED, Faust PL, Ma KJ, Yu M, Cortes E, Vonsattel JPG. Torpedoes in the cerebellar vermis in essential tremor cases vs. controls Cerebellum. 2011;10:812–819. doi: 10.1007/s12311-011-0291-0. [DOI] [PubMed] [Google Scholar]
- 23.Hall TCMA, Corsellis JAN. Variations in the human Purkinje cell population according to age and sex. Neuropathol Appl Neurobiol. 1975;1:267–292. [Google Scholar]
- 24.Cerminara NL, Lang EJ, Sillitoe RV, Apps R. Redefining the cerebellar cortex as an assembly of non-uniform Purkinje cell microcircuits. Nat Rev Neurosci. 2015;16:79–93. doi: 10.1038/nrn3886. [DOI] [PMC free article] [PubMed] [Google Scholar]