Abstract
Objective
While vascular dysfunction is well-defined in HF patients with reduced ejection fraction (HFrEF), disease-related alterations in the peripheral vasculature of HF patients with preserved ejection fraction (HFpEF) are not well characterized. Thus, we sought test the hypothesis that HFpEF patients would demonstrate reduced vascular function, at both the conduit artery and microvascular levels, compared to controls.
Methods
We examined both conduit artery function via brachial artery flow-mediated dilation (FMD) and microvascular function via reactive hyperemia (RH) following 5 min of ischemia in 24 Class II–IV HFpEF patients and 24 healthy controls matched for age, sex, and brachial artery diameter.
Results
FMD was reduced in HFpEF patients compared to controls (HFpEF: 3.1 ± 0.7%; Controls: 5.1 ± 0.5%; P = 0.03). However, shear rate at time of peak brachial artery dilation was lower in HFpEF patients compared to controls (HFpEF: 42,070 ± 4,018 s−1; Controls: 69,018 ± 9,509 s−1; P = 0.01), and when brachial artery FMD was normalized for the shear stimulus, cumulative area-under-the-curve (AUC) at peak dilation, the between-group differences were eliminated (HFpEF: 0.11 ± 0.03 %/AUC; Controls: 0.09 ± 0.01 %/AUC; P = 0.58). RH, assessed as AUC, was lower in HFpEF patients (HFpEF: 454 ± 35 mL; Controls: 660 ± 63 mL; P < 0.01).
Conclusions
Collectively, these data suggest that maladaptations at the microvascular level contribute to the pathophysiology of HFpEF, while conduit artery vascular function is not diminished beyond that which occurs with healthy aging.
Keywords: HFpEF, normal ejection fraction, vascular function, flow-mediated dilation
INTRODUCTION
In many disease states, global reductions in vascular function impact the health of the vascular tree, including both conduit vessels and the microcirculation. This is particularly evident in heart failure (HF), a clinical syndrome that presents with numerous symptoms, including impaired cardiac contractile function and systemic vascular dysfunction, which collectively result in the prominent characteristic of severely reduced exercise tolerance and subsequently a reduced quality of life. While HF has classically been characterized by a reduction in ejection fraction, it is now recognized that approximately one-half of HF patients present with normal or “preserved” ejection fraction (HFpEF) [1, 2]. Importantly, the prognosis for HFpEF is similar to that of HF patients with reduced ejection fraction (HFrEF) [1, 3], yet the vascular pathophysiology of this clinical syndrome remains poorly understood.
Vascular dysfunction has been well documented in HFrEF patients [4, 5, 6, 7]. In contrast, very few studies have sought to evaluate vascular function in HFpEF patients, and in the studies that have been undertaken, the results are equivocal. Using a magnetic resonance imaging (MRI) approach, Hundley et al. [8] reported that flow-mediated dilation (FMD) of the superficial femoral artery was similar between HFpEF and age-matched controls. Subsequent to this, in one of the only studies to assess vascular function using conventional FMD testing, Haykowsky et al. [9] reported a similar brachial artery FMD in HFpEF patients compared to healthy, older controls. In contrast, a recent investigation by Farrero and colleagues [10] demonstrated reduced brachial artery FMD in HFpEF patients compared to hypertensive controls without HF. Unfortunately, none of these studies appear to have evaluated the shear stimulus that provokes brachial artery FMD, which is viewed as an important consideration to appropriately interpret the vasodilatory response [11]. Thus, whether HFpEF patients exhibit vascular dysfunction, as assessed by standardized, up-to-date FMD testing guidelines [12], remains uncertain in this patient population.
Though FMD testing has been established as a valuable research tool for non-invasive assessment of vascular function in the conduit vessels, the test provides limited information about vascular function at the level of the microcirculation. Determination of reactive hyperemia (RH) subsequent to a period of cuff occlusion fills this void, providing an index of microvascular function that is complimentary to conduit vascular function assessed via FMD. There is emerging evidence that RH, assessed via peripheral arterial tonometry (PAT), is reduced in HFpEF patients [13, 14], and that this disease-related reduction in RH-PAT is independently correlated with incidence of future cardiovascular events [15] and predictive of poor prognosis [14]. However, to date, there has not been a study that assessed both conduit artery and microvascular function in HFpEF patients to comprehensively assess peripheral vascular dysfunction in this ever-growing patient population.
Therefore, we sought to determine conduit artery and microvascular function in HFpEF patients compared to healthy controls using FMD and RH, respectively. We tested the hypothesis that HFpEF patients would demonstrate reduced vascular function, at both the conduit artery and microvascular levels, compared to controls.
METHODS
Subjects
Twenty four Class II–IV HFpEF patients and twenty four healthy control subjects matched for age, sex, and brachial diameter volunteered for this study. Patients were recruited from the University of Utah HFpEF Clinic. Within this clinic, patients were screened and included in a manner consistent with the inclusion criteria from the TOPCAT trial, which included the following criteria: (1) heart failure defined by the presence of ≥1 symptom at the time of screening (PND, orthopnea, dyspnea on exertion) and 1 sign (edema, elevation in JVD) in the previous 12 months; (2) LVEF ≥45%, (3) controlled systolic blood pressure, and (4) either ≥1 hospitalization in the previous 12 months for which heart failure was a major component of hospitalization, or B-type natriuretic peptide (BNP) in the previous 60 days ≥100 pg/mL. Diastolic dysfunction on echocardiogram was diagnosed using a lateral wall E/e’ of >10 with a lateral wall e’ of <10. Exclusion criteria for the HFpEF group included significant valvular heart disease, acute atrial fibrillation, and BMI > 45. All subjects were current non-smokers. The healthy controls were normotensive, free from overt cardiovascular disease, and were not taking any prescription medications. Protocol approval and written informed consent were obtained according to University of Utah and Salt Lake City Veterans Affairs Medical Center Institutional Review Board requirements. Subjects reported to the laboratory fasted (overnight; >10hrs) and had not exercised or consumed caffeine in the 24 hrs preceding the study. Data collection occurred in a thermoneutral laboratory with subjects resting in the supine position.
Resting Hemodynamic and Cardiovascular Assessment
Prior to the FMD and RH tests, supine resting systolic and diastolic arterial blood pressure were determined by an automated blood pressure monitor (Tango+, Suntech, Morrisville, NC) and mean arterial pressure (MAP) was calculated as diastolic arterial pressure plus one-third arterial pulse pressure. Heart rate was monitored from a standard three-lead ECG interfaced with a data acquisition device (Biopac, Goleta, CA, U.S.A.).
Measurements of Brachial Artery Flow-Mediated Dilation and Reactive Hyperemia
All FMD procedures were performed according to current guidelines [12]. Following 20 min of supine rest, baseline measurements of brachial artery diameter and blood velocity were taken for one minute. Immediately following baseline measurements a blood pressure cuff, placed on the right arm proximal to the elbow and distal to the Doppler probe measurement site, was inflated to a supra-systolic pressure (>250 mmHg) for five minutes. The cuff was then rapidly deflated and brachial artery diameter and blood velocity measures were obtained continuously for two minutes. RH was quantified as the cumulative brachial artery blood flow (i.e. AUC) for the two minute period post cuff release.
Blood velocity and vessel diameter were assessed with a Logiq 7 ultrasound Doppler system (GE Medical Systems, Milwaukee, WI) operating in duplex mode. The Logiq 7 was equipped with a linear array transducer operating at an imaging frequency of 14 MHz. Blood velocity was collected with the same transducer at a Doppler frequency of 5 MHz in high-pulsed repetition frequency mode (2 to 25 kHz). Sample volume was optimized in relation to vessel diameter and centered within the vessel. An angle of insonation of ≤ 60 degrees [16] was achieved for all measurements of blood velocity.
Analyses
Commercially available software (Logiq 7) was used to calculate angle-corrected, time-averaged, and intensity-weighted mean blood velocity (Vmean). Brachial artery vasodilation was determined offline from end-diastolic, ECG R-wave triggered images collected from the Logiq 7 using automated edge-detection software (Medical Imaging Applications, Coraville, IA). Brachial blood flow was calculated based on the formula: brachial blood flow (mL * min−1) = (Vmean × π (vessel diameter/2)2 × 60). FMD was quantified using the greatest increase in brachial artery diameter during the two minute period following cuff release. Shear rate was calculated as: shear rate (s−1) = 8 Vmean / arterial diameter. Cumulative area-under-the-curve (AUC) values for blood flow and shear rate were integrated via the trapezoid rule and calculated as: (Σ{yi[x(i + 1) − xi] + (1/2)[y(i + 1) − yi][x(i + 1) − xi]}).
Blood Analysis
A fasting glucose and lipid panel was performed on blood drawn from an antecubital vein in all subjects using standard methods.
Data Analysis and Statistical Approach
Statistical analysis was performed with commercially available software (SigmaStat 3.10, Systat Software, Point Richmond, CA). A Student’s unpaired t-test was used to determine mean differences for subject characteristics, resting MAP and heart rate, as well as FMD and RH AUC. A two-way ANOVA (group × time) was used to determine differences between groups for RH following cuff release. When a significant main effect was observed, a Holm-Sidak post hoc analysis was performed. Statistical significance was accepted at α ≤ 0.05. Group data are presented as mean ± SEM, and exact P-values are given unless otherwise noted.
RESULTS
Characteristics of HFpEF Patients and Age-matched Controls
Anthropometric data and general characteristics for both patients and controls are shown in Table 1. Disease related characteristics and pharmacological information for the HFpEF patients are shown in Table 2.
Table 1.
Subject characteristics
| Controls | HFpEF | P-value | |
|---|---|---|---|
| Subjects, N (males:females) | 24 (10:14) | 24 (10:14) | --- |
| Age, yrs | 69 ± 2 | 69 ± 2 | 0.87 |
| Body mass, kg | 76 ± 3 | 101 ± 6 * | <0.001 |
| Stature, cm | 170 ± 2 | 169 ± 3 | 0.83 |
| Body mass index, kg/m2 | 28 ± 3 | 35 ± 1 * | 0.03 |
| Body surface area, m2 | 1.90 ± 0.04 | 2.21 ± 0.07 * | <0.001 |
| Heart rate, beats/min | 62 ± 2 | 65 ± 2 | 0.25 |
| Mean arterial blood pressure, mmHg | 98 ± 2 | 90 ± 2 * | 0.01 |
| Glucose, mg/dL | 84 ± 2 | 120 ± 10 * | <0.001 |
| Cholesterol, mg/dL | 201 ± 9 | 163 ± 10 * | 0.01 |
| High-density lipoprotein, mg/dL | 51 ± 3 | 49 ± 4 | 0.98 |
| Low-density lipoprotein, mg/dL | 125 ± 6 | 91 ± 9 * | <0.01 |
| Triglycerides, mg/dL | 149 ± 17 | 145 ± 20 | 0.89 |
HFpEF, heart failure with preserved ejection fraction. Data are mean ± SEM.
Significantly different from Controls.
Table 2.
HFpEF characteristics and medications
| Disease related characteristics | ||
| NYHA class II | 10 (42%) | |
| NYHA class III | 10 (42%) | |
| NYHA class IV | 4 (17%) | |
| Six min walk distance, m | 402 ± 42 | |
| B-Type natriuretic peptide, pg/mL | 150 ± 26 | |
| Diabetes | 10 (42%) | |
| COPD | 2 (8%) | |
| CAD | 6 (25%) | |
| Hypertensive | 20 (83%) | |
| Echocardiography | Value | Reference Range |
| Ejection fraction, % | 62 ± 1 | ≥55 |
| LV IVSD, cm | 1.1 ± 0.1 | 0.6 – 1.1 |
| LV PWD, cm | 1.1 ± 0.1 | 0.6 – 0.9 |
| LV ID diastole, cm | 4.5 ± 0.2 | 3.9 – 5.3 |
| LV ID systole, cm | 3.0 ± 0.1 | 2.0 – 4.0 |
| Peak E wave, cm/s | 84 ± 7 | -- |
| Peak A wave, cm/s | 96 ± 11 | -- |
| E/A ratio | 1.1 ± 0.2 | 0.6 – 1.32 |
| E' lateral wall, cm/s | 7 ± 1 | 13–28 |
| E/E' ratio | 13 ± 2 | ≤ 8 |
| Mitral E-wave deceleration time, ms | 246 ± 14 | 142–258 |
| Medications | ||
| Beta receptor blocker | 11 (46%) | |
| ACEi or ARB | 15 (63%) | |
| Loop diuretics | 22 (92%) | |
| Aldosterone antagonist | 16 (67%) | |
| Statin | 18 (75%) | |
| Nitrates | 5 (21%) | |
| Calcium channel blockers | 3 (13%) |
HFpEF, heart failure with preserved ejection fraction; NYHA, New York Heart Association; LV IVSD, left ventricle interventricular septum diameter; LV PWD, left ventricle posterior wall diameter; LV ID, left ventricle internal diameter; COPD, chronic obstructive pulmonary disease; CAD, coronary artery disease; ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker. Data are mean ± SEM or % of group.
Brachial Artery Flow-Mediated Dilation
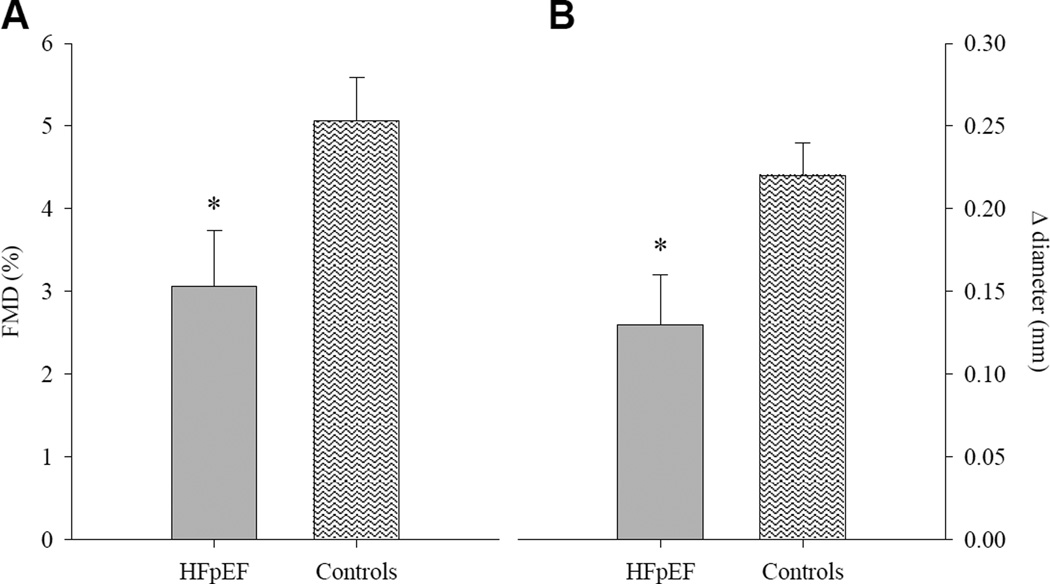
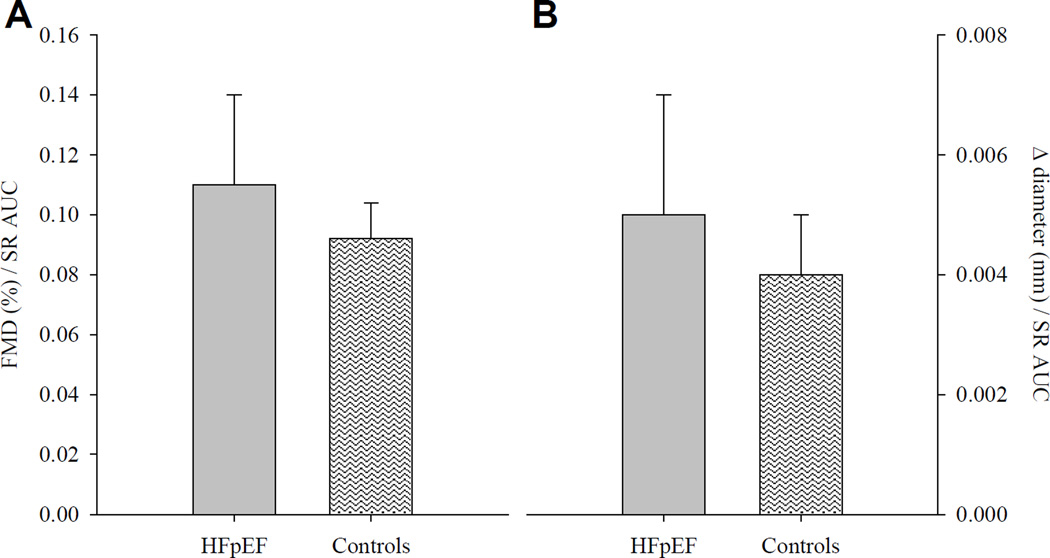
Patients and healthy controls were well matched for baseline brachial artery diameter (P = 0.44; Table 3). Peak brachial artery diameter following cuff release was not different between groups (P = 0.71); however, time to peak was longer in HFpEF compared to controls (P = 0.03; Table 3). HFpEF patients demonstrated a reduction in brachial artery FMD, assessed by both the peak percentage (HFpEF: 3.06 ± 0.68 % [range −5.9 – 10.7 %, median = 3.03 %]; Control: 5.06 ± 0.53 % [range 0.16 – 10.49 %, median = 4.30 %], P = 0.03; Figure 1A) and absolute (HFpEF: 0.13 ± 0.03 mm [range −0.23 – 0.35 mm, median = 0.16 mm]; Control: 0.23 ± 0.12 mm [range 0.01 – 0.48 mm, median = 0.20 mm], P = 0.02; Figure 1B) change in brachial artery diameter. However, cumulative shear rate at peak dilation was markedly lower in patients compared to controls (P = 0.01; Table 3), and when brachial artery FMD was normalized for shear rate the differences between groups were eliminated for both peak percentage (HFpEF: 0.11 ± 0.03 [range −0.14 – 0.77, median = 0.10]; Control: 0.09 ± 0.01 [range 0.01 – 0.20, median = 0.07], P = 0.58; Figure 2A) and absolute (HFpEF: 0.005 ± 0.002 [range −0.008 – 0.420, median = 0.004; Control: 0.004 ± 0.001 [range 0.0005 – 0.011, median = 0.003], P = 0.58; Figure 2B) change in brachial artery diameter.
Table 3.
Brachial artery flow-mediated dilation
| Controls | HFpEF | P-value | |
|---|---|---|---|
| Baseline brachial artery diameter, mm | 4.43 ± 0.14 | 4.61 ± 0.18 | 0.44 |
| Peak brachial artery diameter, mm | 4.65 ± 0.15 | 4.74 ± 0.19 | 0.71 |
| Time to peak dilation, s | 61 ± 5 | 78 ± 6 * | 0.03 |
| Cumulative shear rate at time of peak brachial artery dilation, s−1 | 69,018 ± 9,508 | 42,070 ± 4,018 * | 0.01 |
HFpEF, heart failure with preserved ejection fraction. Data are mean ± SEM.
Significantly different from Controls.
FIGURE 1.
Brachial artery flow-mediated dilation (FMD), expressed as percent (panel A) and absolute (panel B) change from baseline in heart failure patients with preserved ejection fraction (HFpEF) and healthy individuals (Controls). Data are presented as mean ± SE. * Significantly different from Controls, P ≤ 0.03.
FIGURE 2.
Brachial artery flow-mediated dilation (FMD) normalized for shear rate area-under-the-curve (SR AUC), expressed as percent (panel A) and absolute (panel B) change from baseline in heart failure patients with preserved ejection fraction (HFpEF) and healthy individuals (Controls). Data are presented as mean ± SE.
Reactive Hyperemia
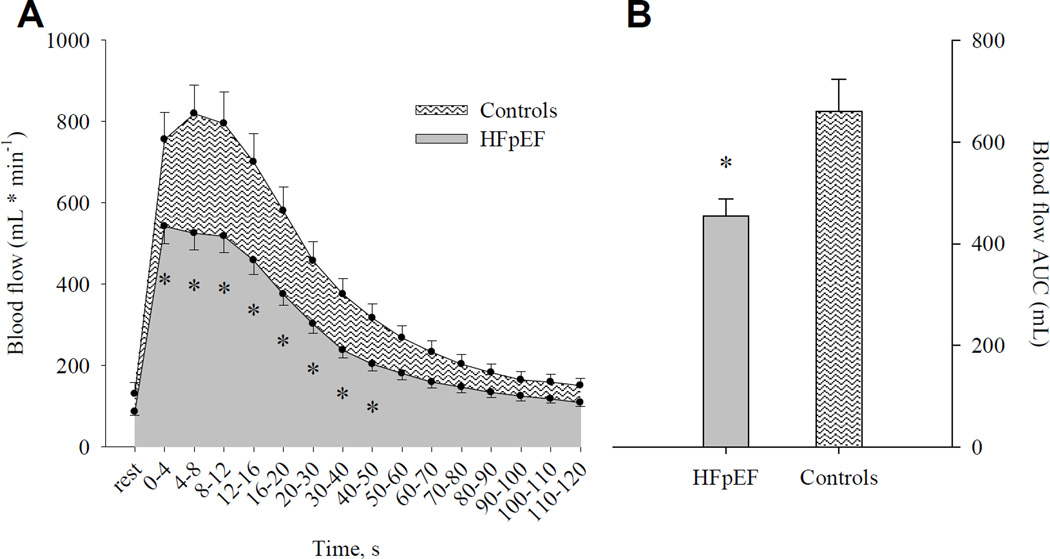
Although baseline brachial artery blood flow was not different between HFpEF patients and healthy controls (HFpEF: 86 ± 9 mL * min−1; Controls: 130 ± 28 mL * min−1; P = 0.15; Figure 3A), RH was blunted in HFpEF patients during the initial 50 s following cuff release (P ≤ 0.03; Figure 3A). Likewise, RH assessed as AUC was markedly (≈30%) lower in HFpEF patients relative to controls (HFpEF: 454 ± 35 ml/min [range 169 – 798 ml/min, median = 446 ml/min]; Control: 659 ± 63 ml/min [range 210 – 798 ml/min, median = 631 ml/min], Figure 3B).
FIGURE 3.
Post-occlusion reactive hyperemia, expressed as both absolute blood flow (panel A) and as blood flow area-under-the-curve (AUC; panel B) in heart failure patients with preserved ejection fraction (HFpEF) and healthy individuals (Controls). Data are presented as mean ± SE. * Significantly different from Controls, P ≤ 0.02.
DISCUSSION
The present investigation sought to comprehensively evaluate peripheral vascular function, at both the conduit and microvascular level, in HFpEF patients compared to healthy controls. With respect to conduit vessel vascular function, we identified a reduction in brachial artery FMD in HFpEF patients compared to controls. However, in the HFpEF group, this apparent impairment in FMD was accompanied by a marked (≈40%) reduction in post-occlusion shear rate, a hemodynamic variable that is widely viewed as the stimulus for the FMD response. When FMD was normalized for the shear stimulus, the between-group differences were no longer evident, suggesting an absence of overt vascular dysfunction at the conduit level in HFpEF patients. In contrast, RH, an index of microvascular function, was significantly (≈30%) reduced in HFpEF patients compared to controls, suggestive of abnormal function in the microcirculation in these patients. In combination, these findings provide new evidence in support of the concept that vascular dysfunction in the conduit vessels is not a requisite feature in the pathophysiology of HFpEF, implicating instead a reduction in peripheral microvascular function that may contribute significantly to clinical status and disease progression in this patient group.
Role of the Peripheral Vasculature in the Pathophysiology of HFpEF
Despite the fact that half of all patients with HF fall into the category of HFpEF, current understanding of the pathophysiology of this pervasive disease remains limited [17]. The guidelines for clinical diagnosis of HFpEF continue to evolve, with a focus on echocardiographic evidence of abnormal left ventricular relaxation, filling, and diastolic stiffness [18]. However, the classic clinical presentation of HFpEF continues to be defined by dyspnea upon exertion and severe exercise intolerance [19], symptoms that are likely attributable to deficits in both cardiac mechanics and peripheral vascular function [20]. With respect to the latter, a recent study examining “global cardiovascular reserve” in HFpEF patients identified a significant correlation between microvascular function (RH) and both peak exercise capacity (peak oxygen consumption) and subjective symptoms of fatigue and dyspnea (Borg scores) during exercise [13], highlighting the relationship between peripheral vasculature function and exercise intolerance in this cohort. Further evidence supporting the concept that disease-related changes in the peripheral circulation may contribute significantly to symptom status in HFpEF come from Dhakal et al. [21], who recently reported significant reductions in peripheral O2 extraction during incremental cycling exercise in HFpEF patients compared to HFrEF and control groups that the authors attributed to abnormalities in skeletal muscle or peripheral microvascular function. It is from this framework that the present study was undertaken, with the goal of comprehensively evaluating peripheral vascular function in HFpEF patients in an effort to better define the vascular pathophysiology in this growing patient group.
Conduit Vessel Vascular Function in HFpEF
Although vascular dysfunction, assessed by FMD, has been well documented in HFrEF patients [4, 5, 6, 7], and is an independent risk factor for an increased risk of clinical events and poor prognosis in this cohort [4], much less is known about vascular function in the HFpEF population. In the current study, we observed a reduction in brachial artery FMD in HFpEF patients compared to controls (Figure 1), which initially seemed to indicate impaired conduit vascular function in this patient group. However, assessing the FMD response in this manner does not take into account shear rate, which represents the laminar shear force across the vascular endothelial cells that initiates the FMD response [22]. The significance of considering this aspect of the stimulus-response relationship should not be underestimated. Indeed, it has been reported that much of the between-subject variability in FMD testing can be significantly reduced by correcting for the shear stimulus [11], and current guidelines for FMD testing recommend inclusion of this variable to provide a complete characterization of the FMD response [12]. Thus, in view of the substantial reduction in shear rate AUC in HFpEF patients at the time of peak vasodilation (Table 3), we deemed it appropriate to normalize FMD for wall shear rate. Using this approach, the decrement in FMD observed in the HFpEF patient group was no longer evident (Figure 2), suggesting that the ostensible reduction in %FMD (Figure 1) was, at least in part, the consequence of a reduced shear stimulus.
The current finding that FMD is not attenuated in HFpEF patients adds to a very small group of studies that have examined conduit vessel vascular function in this cohort. Using a magnetic resonance imaging (MRI) approach, Hundley et al. [8] evaluated changes in superficial femoral artery circumference after a 5 min supra-systolic cuff occlusion, and reported that FMD was similar between HFpEF and age-matched controls. Using the more traditional FMD methodology to explore vascular function, Haykowsky et al. [9] identified similar brachial artery %FMD in HFpEF patients compared to age-matched, healthy controls, which is in contrast to more recent work from Ferrero et al. [10] that identified impaired FMD in HFpEF patients compared to hypertensive controls. With strict adherence to current guidelines regarding FMD testing and careful matching of HFpEF patients with healthy controls, the present study may be viewed as both confirming and extending this previous work, providing new evidence in support of the concept that vascular dysfunction in conduit vessels is not a requisite feature in the pathophysiology of HFpEF.
Microvascular Function in HFpEF
Determination of RH subsequent to a period of cuff occlusion provides an index of microvascular function that is complimentary to conduit vascular function assessed via FMD. This hemodynamic assessment has been utilized in a number of patient groups, and has been identified as an independent predictor of prognosis in HFrEF [23], and HFpEF [14] patients, suggesting that RH responses may provide important information for risk stratification and determination of disease progression. In the present study, we determined RH by quantifying the blood flow AUC following a 5 min cuff occlusion of the lower arm, and identified a profound (≈30%) reduction in RH in HFpEF patients relative to healthy controls (Figure 3). This attenuation in the RH response provides clear evidence for the presence of microvascular dysfunction in this patient group that is well beyond the decrement that exists as a consequence of the aging process, which is in agreement with recent work that has utilized peripheral arterial tonometry (PAT) in HFpEF patients to evaluate RH. Indeed, a recent study by Borlaug et al. [13] reported a clear reduction in RH index in both HFpEF and hypertensive patients compared to healthy age-matched controls, which the authors interpreted as evidence of endothelial dysfunction in these patient groups. Using the more conventional forearm RH technique, findings from the present study build on this previous report, adding additional evidence indicating a clear reduction in microvascular function in HFpEF patients compared to older, healthy controls.
The observed decrement in peripheral microvascular function in HFpEF may be of particular relevance in the context of the coronary circulation, as there is recent evidence implicating coronary microvascular inflammation as a key player in the etiology of HFpEF. Paulus et al. [24] identified a new paradigm for the etiology of myocardial remodeling and dysfunction in HFpEF that relates inflammation, perhaps as a consequence of comorbidities such as diabetes, to the subsequent production of reactive oxygen species and reduction in NO bioavailability in the coronary microcirculation. According to this proposed model, a shift in myocardial redox balance creates an environment that promotes cardiomyocyte hypertrophy and increased collagen deposition, with the functional consequence of diastolic left ventricular dysfunction. In the same manner that flow-mediated vasodilation in the brachial artery been demonstrated to correlate with coronary artery vasomotor responses [25], it is tempting to speculate that the observed impairment in peripheral microvascular function may provide a surrogate measure for disease-related changes in the coronary microcirculation, and as such, serve as a diagnostic biomarker in this patient group. Additional studies are needed to explore this intriguing possibility.
The Distinct Vascular Pathology of HFpEF
The present findings identifying dysfunction at different sites along the arterial tree not only helps to further characterize vascular dysfunction in this cohort, but also presents another aspect of the HFpEF pathophysiology that differs significantly from HFrEF. Our group has recently demonstrated that HFrEF patients have reduced FMD, but not post occlusion RH, compared to healthy age-matched controls [7]. These data suggest that, in contrast to our current findings in HFpEF, conduit artery impairments alone contribute to the vascular dysfunction in HFrEF. This discrepancy between disease-related changes in the conduit and microvascular segments of the peripheral circulation may partially explain why many of the therapeutic approaches that have proven so successful in HFrEF patients are somewhat less efficacious in the HFpEF patient group. Indeed, previous studies in HFrEF have identified the ability of drugs targeting the renin-angiotensin-aldosterone system and HMG-CoA reductase inhibition to improve conduit vascular function [26, 27]. In contrast, the majority of clinical trials targeting these pathways in HFpEF have proven unsuccessful [28, 29, 30], though it should be noted that vascular function was not a primary endpoint in these studies. Thus, while the disparate response to pharmacologic treatment is likely due to a host of factors that differ between HFrEF and HFpEF, the distinct manner in which vascular dysfunction manifests in these two cohorts may be worthy of consideration as clinical care of HFpEF continues to evolve.
Experimental Considerations
In the present study, we enrolled HFpEF patients on optimized pharmacotherapy, and no medications were withheld on experimental days. Thus, we cannot exclude the possibility that existing drug therapy may have affected our measurements of vascular function. We also enrolled patients without regard to existing comorbidities such as hypertension, diabetes, and coronary artery disease. While this approach may introduce some heterogeneity in terms of baseline parameters, it provided an opportunity to study the pathophysiology of HFpEF in a manner that fairly represents the diverse nature of this patient population. It is acknowledged that the sample size of the present study was relatively small, though a sufficient number of subjects were enrolled to achieve adequate statistical power in the major variables. Finally, we recognize the known variability that is associated with FMD testing, an issue that was somewhat mitigated by the use of standardized testing procedures that conformed to current published guidelines [12].
Conclusions
This investigation documents attenuated vascular function in the microcirculation despite an apparent preservation of conduit artery function in HFpEF patients compared to well-matched, healthy controls. These novel findings highlight a specific site of peripheral vascular dysfunction in this patient group that further characterizes this disease.
KEY MESSAGES.
What is already known about this subject?
Vascular function in the peripheral circulation, assessed by FMD, has been well documented in HFrEF patients. However, there is currently no consensus regarding the presence of vascular dysfunction in HFpEF.
What does this study add?
By investigating vascular function in HFpEF patients at both the conduit and microvascular level, we have identified a distinct pattern of vascular dysfunction that is specific to the microvasculature. Brachial artery FMD normalized for shear rate was similar between HFpEF patients and controls, suggesting an absence of overt vascular dysfunction at the conduit level in this cohort. In contrast, RH, an index of microvascular function, was significantly (≈30%) reduced in HFpEF patients compared to controls, indicative of abnormal function in the peripheral microcirculation.
How might this impact on clinical practice?
The observed discrepancy between disease-related changes in the conduit and microvascular segments of the peripheral circulation may partially explain why many of the therapeutic approaches that have proven so successful in HFrEF patients are somewhat less efficacious in the HFpEF patient group. The results from this study may thus serve to guide the ongoing development of diagnostic algorithms and biomarkers related to vascular function in this patient group.
Acknowledgments
Funding Sources. Funded in part by NIH PO1 HL091830, VA RR&D I01RX000182, VA RR&D I21 RX001433, VA RR&D I21 RX001418, and NIH R01 HL118313.
Footnotes
Disclosures. None
Licensing Statement. The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive license (or non-exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article (if accepted) to be published in HEART editions and any other BMJPGL products to exploit all subsidiary rights"
REFERENCES
- 1.Bhatia R, Tu J, Lee D, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 2.Bursi F, Weston S, Redfield M, et al. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209–2216. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 3.Owan T, Hodge D, Herges R, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 4.Fischer D, Rossa S, Landmesser U, et al. Endothelial dysfunction in patients with chronic heart failure is independently associated with increased incidence of hospitalization, cardiac transplantation, or death. European heart journal. 2005;26:65–69. doi: 10.1093/eurheartj/ehi001. [DOI] [PubMed] [Google Scholar]
- 5.Katz SD, Biasucci L, Sabba C, et al. Impaired endothelium-mediated vasodilation in the peripheral vasculature of patients with congestive heart failure. Journal of the American College of Cardiology. 1992;19:918–925. doi: 10.1016/0735-1097(92)90271-n. [DOI] [PubMed] [Google Scholar]
- 6.Kubo SH, Rector TS, Bank AJ, et al. Endothelium-dependent vasodilation is attenuated in patients with heart failure. Circulation. 1991;84:1589–1596. doi: 10.1161/01.cir.84.4.1589. [DOI] [PubMed] [Google Scholar]
- 7.Witman MAH, Fjeldstad AS, McDaniel J, et al. Vascular Function and the Role of Oxidative Stress in Heart Failure, Heart Transplant, and Beyond. Hypertension. 2012;60:659–668. doi: 10.1161/HYPERTENSIONAHA.112.193318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hundley WG, Bayram E, Hamilton CA, et al. Leg flow-mediated arterial dilation in elderly patients with heart failure and normal left ventricular ejection fraction. Am J Physiol Heart Circ Physiol. 2007;292:H1427–H1434. doi: 10.1152/ajpheart.00567.2006. [DOI] [PubMed] [Google Scholar]
- 9.Haykowsky MJ, Herrington DM, Brubaker PH, et al. Relationship of flow-mediated arterial dilation and exercise capacity in older patients with heart failure and preserved ejection fraction. The journals of gerontology Series A, Biological sciences and medical sciences. 2013;68:161–167. doi: 10.1093/gerona/gls099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farrero M, Blanco I, Batlle M, et al. Pulmonary Hypertension Is Related to Peripheral Endothelial Dysfunction in Heart Failure With Preserved Ejection Fraction. Circ Heart Fail. 2014;7:791–798. doi: 10.1161/CIRCHEARTFAILURE.113.000942. [DOI] [PubMed] [Google Scholar]
- 11.Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol. 2005;568:357–369. doi: 10.1113/jphysiol.2005.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris RA, Nishiyama SK, Wray DW, et al. Ultrasound Assessment of Flow-Mediated Dilation. Hypertension. 2010;55:1075–1085. doi: 10.1161/HYPERTENSIONAHA.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borlaug BA, Olson TP, Lam CS, et al. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. Journal of the American College of Cardiology. 2010;56:845–854. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsue Y, Suzuki M, Nagahori W, et al. Endothelial dysfunction measured by peripheral arterial tonometry predicts prognosis in patients with heart failure with preserved ejection fraction. International journal of cardiology. 2013;168:36–40. doi: 10.1016/j.ijcard.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 15.Akiyama E, Sugiyama S, Matsuzawa Y, et al. Incremental Prognostic Significance of Peripheral Endothelial Dysfunction in Patients With Heart Failure With Normal Left Ventricular Ejection Fraction. Journal of the American College of Cardiology. 2012;60:1778–1786. doi: 10.1016/j.jacc.2012.07.036. [DOI] [PubMed] [Google Scholar]
- 16.Logason K, Barlin T, Jonsson ML, et al. The importance of Doppler angle of insonation on differentiation between 50–69% and 70–99% carotid artery stenosis. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2001;21:311–313. doi: 10.1053/ejvs.2001.1331. [DOI] [PubMed] [Google Scholar]
- 17.Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2014;11:507–515. doi: 10.1038/nrcardio.2014.83. [DOI] [PubMed] [Google Scholar]
- 18.Wachter R, Edelmann F. Diagnosis of heart failure with preserved ejection fraction. Heart Fail Clin. 2014;10:399–406. doi: 10.1016/j.hfc.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Kitzman DW, Little WC, Brubaker PH, et al. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA : the journal of the American Medical Association. 2002;288:2144–2150. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 20.Borlaug BA. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction. Circ J. 2014;78:20–32. doi: 10.1253/circj.cj-13-1103. [DOI] [PubMed] [Google Scholar]
- 21.Dhakal BP, Malhotra R, Murphy RM, et al. Mechanisms of Exercise Intolerance in Heart Failure with Preserved Ejection Fraction: The Role of Abnormal Peripheral Oxygen Extraction. Circulation Heart failure. 2014 doi: 10.1161/CIRCHEARTFAILURE.114.001825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melkumyants AM, Balashov SA, Khayutin VM. Endothelium dependent control of arterial diameter by blood viscosity. Cardiovasc Res. 1989;23:741–747. doi: 10.1093/cvr/23.9.741. [DOI] [PubMed] [Google Scholar]
- 23.de Berrazueta JR, Guerra-Ruiz A, Garcia-Unzueta MT, et al. Endothelial dysfunction, measured by reactive hyperaemia using strain-gauge plethysmography, is an independent predictor of adverse outcome in heart failure. European journal of heart failure. 2010;12:477–483. doi: 10.1093/eurjhf/hfq036. [DOI] [PubMed] [Google Scholar]
- 24.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. Journal of the American College of Cardiology. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 25.Anderson TJ, Uehata A, Gerhard MD, et al. Close relation of endothelial function in the human coronary and peripheral circulations. Journal of the American College of Cardiology. 1995;26:1235–1241. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 26.Varin R, Mulder P, Tamion F, et al. Improvement of endothelial function by chronic angiotensin-converting enzyme inhibition in heart failure : role of nitric oxide, prostanoids, oxidant stress, and bradykinin. Circulation. 2000;102:351–356. doi: 10.1161/01.cir.102.3.351. [DOI] [PubMed] [Google Scholar]
- 27.Tousoulis D, Oikonomou E, Siasos G, et al. Dose-dependent effects of short term atorvastatin treatment on arterial wall properties and on indices of left ventricular remodeling in ischemic heart failure. Atherosclerosis. 2013;227:367–372. doi: 10.1016/j.atherosclerosis.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 28.Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777–781. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 29.Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 30.Fu M, Zhou J, Sun A, et al. Efficacy of ACE inhibitors in chronic heart failure with preserved ejection fraction--a meta analysis of 7 prospective clinical studies. International journal of cardiology. 2012;155:33–38. doi: 10.1016/j.ijcard.2011.01.081. [DOI] [PubMed] [Google Scholar]