Introduction
The mechanisms leading to the neurocognitive deficits in humans with immunodeficiency virus type 1 (HIV-1) are not well understood. Glutamate is the most abundant neurotransmitter in the brain and is necessary for learning, memory, and cognitive processing (Danbolt, 2001). Notably, glutamate’s accumulation in the extracellular space must be tightly regulated by glutamate transporters, which are necessary for maintaining fast neuronal glutamate transmission, synaptic plasticity, and preventing neurotoxicity (Huang and Bergles, 2004; Matsugami et al., 2008). The HIV-1 genome codes for a number of envelope proteins such as glycoprotein 120 (gp120) that cause major disturbances in neuronal function, often leading to extensive synaptodendritic injury (Kaul et al., 2001; Ellis et al, 2007). In particular, a number of studies using cell culture models have shown that gp120 significantly reduces the expression and function of glutamate transporters, resulting in excessive accumulation of extracellular glutamate, overstimulation of glutamate receptors, and neurotoxicity (Dreyer and Lipton, 1995; Vesce et al., 1997; Patton et al., 2000; Belmadani et al., 2001; Wang et al., 2003). Although considerable effort has been expended to understand the mechanisms responsible for glutamate transporter dysfunction in vitro, there are no studies available that have determined the effects of gp120 on glutamate uptake systems in vivo.
Two types of transporter systems are necessary for maintaining brain extracellular glutamate homeostasis. These include a family of sodium-dependent and sodium-independent glutamate transporters, referred to as systems XAG and xc-, respectively (Danbolt, 2001; Kanai and Hediger, 2003). System XAG accounts for the majority (~90%) of total glutamate uptake in the central nervous system (CNS). The primary excitatory amino acid transporter (EAAT) for system XAG is glutamate transporter subtype 1 (GLT-1/EAAT2), which is ubiquitously expressed in the brain and predominantly in astrocytes (Danbolt, 2001). System xc- however, acts as an obligate exchanger of intracellular glutamate for extracellular cystine, where cystine is transported into cells and reduced to cysteine for glutathione synthesis (Cho and Bannai, 1990; Bridges et al., 2012). Notably, disruption of system xc- could lead to elevated intracellular concentrations of glutamate, which may result in cell death due to oxidative stress (Murphy et al., 1989; Patel et al., 2004). Deficits in either of these glutamate uptake systems are implicated in a number of neurodegenerative and neurocognitive disorders ranging from Alzheimer’s disease to drug addiction (Choi, 1988; Robinson and Gonzales, 2005; Kalivas, 2009; Bridges et al., 2012).
In the present study, we characterized the kinetic properties of system XAG and xc- in striatum and hippocampus of HIV-gp120 transgenic mice, a well-established rodent model of HIV-associated neurocognitive deficits (Toggas et al., 2004). Gp120 mice are shown to manifest dendritic pruning and neuronal loss in various brain regions, particularly the striatum, which appears particularly vulnerable to gp120 (Toggas et al., 2004; Ellis et al., 2007). We revealed a significant deficit in the Vmax (maximal velocity) of both systems XAG and xc- in striatum of gp120 mice relative to wild type (WT) controls, and without altering the Km (affinity) for glutamate. There were no significant alterations observed in the hippocampus. In separate cohorts, we also demonstrated that the Vmax of system XAG and xc- were reduced in both glial and neuronal preparations from striatum of gp120 mice, extensive deficits that likely contribute to the onset and/or severity of HIV-associated neurocognitive disorders.
Materials and Methods
Subjects
A breeding colony of homozygous transgenic mice producing HIV-1 gp120 in their brains under the regulatory control of a modified murine glial fibrillary acidic protein (GFAP) gene (described in Toggas et al., 1994) were maintained in our animal facility along with B6SJLF mice (cross between C57BL/6J female x SJL/J male), which were used as wild-type (gp-120 background) controls. All breeder stocks were purchased from the Jackson Laboratories (Bar Harbor, ME). Following weaning, animals were housed in clear plastic ventilated cages, maintained in a temperature- and humidity-controlled room on a 12-h light/dark cycle with food and water provided ad libitum. Only adult male gp120 and WT mice were used for the experiments. For brain preparations, mice were removed from their home cages and killed by decapitation using sharp-ended scissors. The brains were rapidly extracted and coronal sections of ~2mm thickness was plated over ice. The striatum and hippocampus was bilaterally dissected using the stereotaxic coordinates derived from the Paxinos and Franklin (2013) mouse brain atlas. All protocols were approved by the Institutional Animal Care and Use Committee at the UPR-SOM, and in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and AVMA Guidelines for the Euthanasia of Animals (2013).
Crude synaptosomal preparations
For the first experiment, the striatum and hippocampus of gp120 and WT mice (n=4/strain/region) were each homogenized in 1 ml of ice-cold buffer containing 0.32 M sucrose, 1mM EDTA, and 4mM Tris (pH 7.4) using a glass homogenizer (8-10 strokes; Kontes, CA). Homogenates were centrifuged at 1000 x g for 10 min in a microcentrifuge at 4°C (Sorvall 23R, Thermo Scientific). The resulting pellet (P1) was discarded and the supernatant (S1) was centrifuged at 10,000 x g for 10 min at 4°C. The resulting pellet (P2) constituted the crude synaptosomal fraction, which contains a rich mixture of neurons and glial cells (Dunkley et al., 2008). This fraction was suspended to a final volume of 1 ml with SEDH solution (0.32 M Sucrose, 1 mM EDTA, 0.25 mM dithiothreitol (DTT), and 20 mM HEPES, pH 7.4) to give a protein concentration of ~0.5 mg/ml as determined by the Bradford/BSA Protein Assay (BioRad, CA). The total preparation time was approximately 1 h.
Glial plasmalemmal vesicles (GPV) and synaptosomes (SYN)
These glial and neuronal subcellular fractions allow for the direct comparison of glutamate uptake systems in preparations enriched with vesicles ‘pinched off’ from glial membranes (i.e., GPV) or purified nerve endings (i.e., SYN). This approach has been extensively validated using morphological, immunohistochemical, and pharmacological measures (Nakamura et al., 1993; Hirst et al., 1998; Suchak et al., 2003; Dunkley et al., 2008). As such, the striatum of gp120 and WT mice (n=4/group) was homogenized in 1 ml of homogenization buffer, and centrifuged at 1000 x g for 10 min at 4°C. The resulting S1 fraction (~0.5 ml) was carefully layered using a microsyringe pump (0.1 ml/min) onto a four-step discontinuous gradient composed of 20, 10, 6, and 2% Percoll (0.25 ml/gradient; GE Healthcare Biosciences) in SEDH solution. Each gradient was carefully layered the previous night using High-G force microcentrifuge tubes (VWR International, PA) and left to stand at 4°C. The tubes were centrifuged at 33,500 g x 5 min at 4°C. The GPV fraction was carefully collected from the interfacial layer of the 2% and 6% gradient, while synaptosomes were collected from the interface of the 10% and 20% gradient, as described previously (e.g., Suchak et al., 2003). Each fraction was washed twice in 1 ml of ice-cold SEDH by centrifugation at 18,500 g x 5 min at 4°C. The GPV and synaptosomal fractions were re-suspended to a final volume of 0.5 ml with SEDH to give a protein concentration of ~0.10 and 0.25 mg/ml, respectively (Bradford/BSA; Bio-Rad). The total preparation time was approximately 3.5 hours.
Glutamate Uptake Assay
The uptake reaction was started by the addition of 25 μl of the corresponding tissue fraction in test tubes containing L-[3H]-glutamate (100 nM; specific activity 49.0 Ci/mmol; Perkin Elmer, MA) and 0.1-100 μM of unlabeled glutamate (for saturation analysis) in 200 μl of uptake buffer containing (in mM) 140 NaCl, 5 KCl, 1.3 CaCl2, 1 MgCl2, 0.4 KH2PO4, 10 glucose, and 20 HEPES (pH 7.4) and incubated at 37°C for 2 min. System x-c uptake was measured similarly except by replacing sodium chloride with equal concentrations of choline chloride. Under this condition, the system mediates the uptake of cystine along with tritiated glutamate in exchange of unlabeled intracellular glutamate, as described previously (e.g., Patel et al., 2004; Melendez et al., 2005). Hence, this system utilizes normally high intracellular levels of glutamate to drive the import of cystine (Patel et al., 2004). Uptake was terminated by rapid filtration with ice-cold sodium-free buffer onto pre-wetted Whatman GF/B filter paper using a tissue harvester (Brandel, MD). Filters were dissolved overnight in scintillation fluid (EcoLite, MP Biomedicals, CA) and processed for scintillation counting (Beckman Instruments, USA).
Western Blot Analysis
Western blot analysis was performed on crude (mixed) synaptosomal preparations of the striatum and hippocampus (n=4-5/strain/region) to determine the expression of GLT-1 protein, which is the predominant glutamate transporter in the CNS (Danbolt, 2001). Proteins were separated using 10% SDS-PAGE and transferred electrophoretically from the gel onto a PVDF membrane (Bio-Rad, CA). Subsequently, the membrane was blocked in 5% nonfat dry milk in Tris-buffered saline Tween 20 at room temperature and probed overnight at 4°C with primary antibodies (Cell Signaling, MA) against rabbit GLT-1 (1:500) and mouse β-actin (1:1000), a ubiquitous cytoskeletal protein used as a loading (marker) control. Labeled proteins were detected with use of a Horseradish peroxidase conjugated antirabbit secondary immunoglobulin G diluted to 1:5000. The Odyssey (LI-COR Biosciences, US) photoimage scanner was used to capture the infrared fluorescent signal from GLT-1 and β-actin on the same Western blot, without film, a darkroom, or any chemiluminescent substrates. The relative levels of proteins were quantified with the use of Odyssey software (Li-COR) and analyzed as the ratio of GLT-1/ β-actin protein expression.
Statistical Analyses
The maximal velocity (Vmax) and the affinity (Km) constants for systems XAG and xc- glutamate uptake were determined by nonlinear regression analysis using Prism (GraphPad Software, CA) by applying the Michaelis Menten Equation (Y= Vmax *X/(Km + X) to the data. Significant differences in the estimated Vmax and Km values were determined by mixed-factorial analysis of variance (ANOVA) using the SPSS statistical software package (IBM, USA). For immunoblot analysis, differences in the ratio of GLT-1/ β-actin protein expression were analyzed by simple one-way ANOVAs. In all cases, p-values < 0.05 were considered significant.
Results
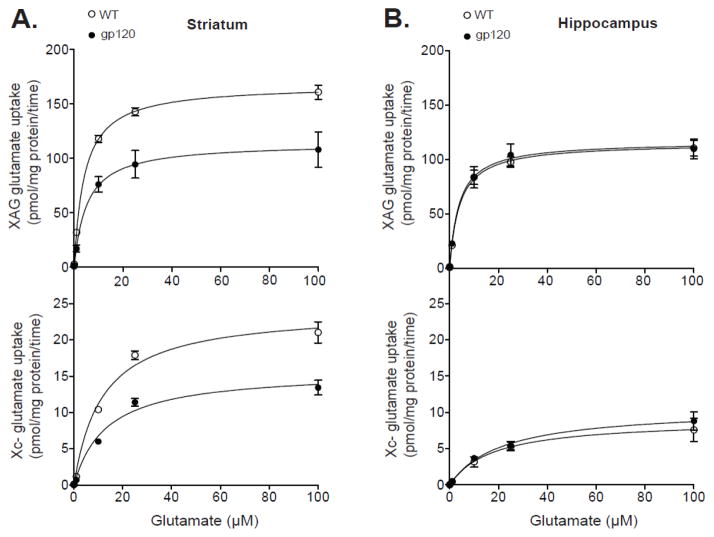
We initially determined the kinetic properties of glutamate uptake of systems XAG (sodium dependent) and xc- (sodium independent) in crude (mixed) synaptosomes from striatum and hippocampus of gp120 and WT mice. The kinetic constants Vmax and Km were used to distinguish between the number of glutamate transporters and affinity for glutamate, respectively. Figure 1 shows the saturation curves for systems XAG and xc- glutamate uptake in striatum and hippocampus. Analysis of striatum (Figure 1A) revealed a significant reduction in the Vmax of both systems XAG and xc- in gp120 mice compared to WT controls (F1,6 > 6.75; p < 0.05). There were no significant differences in the Km for neither system XAG nor xc- in either strain (F1,6 < 0.69; p > 0.43), suggesting a reduction in the number of XAG and xc- glutamate transporters in striatum of gp120 mice. Estimated Vmax constants for systems XAG and xc- were (in pmol/mg protein/time) 115.9 ± 19.1 and 15.7 ± 1.3 for gp120 mice and 168.5 ± 6.6 and 24.2 ± 1.9 for WT, respectively. Estimated Km constants for systems XAG and xc- were (in μM) 5.2 ± 1.3 and 13.2 ± 1.2 for gp120 mice and 4.3 ± 0.6 and 11.5 ± 1.5 for WT controls, respectively. Analysis of the kinetic parameters of glutamate uptake in hippocampus (Figure 1B) indicated no significant differences in neither the Vmax nor Km of systems XAG and xc- between gp-120 and WT mice (F1,6 < 0.37; p > 0.56), suggesting that glutamate uptake systems in hippocampus are less susceptible to HIV-1 gp120 exposure. Estimated Vmax constants for XAG and xc- glutamate uptake were (in pmol/mg protein/time) 118.2 ± 9.5 and 10.7 ± 1.7 for gp120 mice and 116.3 ± 6.2 and 9.1 ± 1.9 for WT, respectively. Estimated Km constants for systems XAG and xc- were (in μM) 4.2 ± 0.7 and 21.1 ± 2.5 for gp120 mice and 4.4 ± 0.7 and 18.4 ± 4.1 for WT controls, respectively.
Figure 1.
System XAG and xc- glutamate uptake in crude (mixed) synaptosomes from striatum and hippocampus of gp120 and wild-type (WT) mice. The maximal velocity of XAG (i.e., sodium-dependent) and xc- (i.e., sodium-independent) glutamate uptake (pmol/mg protein/time) was plotted as a function of the external unlabeled glutamate concentration. Saturation analysis was performed using 100 nM of L-[3H]-glutamate in the absence or presence of unlabeled L-glutamate (0-100 μM) to give the final concentrations shown (x-axis). The maximal velocity (Vmax) and the affinity (Km) constants were determined by nonlinear regression analysis by applying the Michaelis Menten Equation to the data. Significant differences in the estimated Vmax and Km values were determined by analysis of variance (ANOVA). A significant (p<0.05) reduction in the Vmax of both systems XAG and xc- glutamate uptake was observed in striatum (A) but not hippocampus (B) of gp120 mice compared to WT. There were no significant group differences in the Km (affinity) for glutamate in either glutamate uptake systems in striatum and hippocampus. Data are expressed as mean ± SEM (n=4/group).
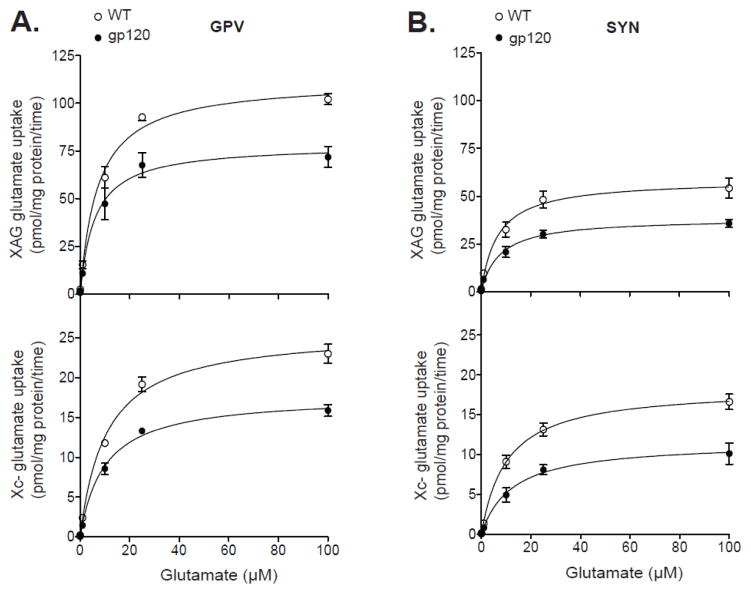
In separate cohorts of mice, we then determined whether gp120 inhibition of striatal glutamate uptake was specific to neurons versus glial cells. To do so, we employed a subcellular fractionation method that gives rise to both purified synaptosomes (SYN) and glial plasmalemmal vesicles (GPV) from the same sample. Figure 2 shows the saturation curves for glutamate uptake systems XAG and xc- in GPV (Figure 2a) and SYN (Figure 2b) fractions from gp120 and WT mice. Analysis of the estimated Vmax constants for systems XAG and xc- showed significant effects of strain (F1,12 > 32.8; p < 0.01) and fraction (F1,12 > 32.8; p < 0.01), but no strain x fraction interactions (F1,12 < 1.78; p > 0.20). Further analysis indicated a significant decrease in the Vmax of XAG and xc- glutamate uptake in both GPV (F1,6 > 17.4; p < 0.01) and SYN (F1,16 > 9.61; p < 0.05) fractions from striatum of gp120 mice relative to WT. Analysis of the estimated Km values for systems XAG and xc- showed no significant effects of strain (F1,6 < 2.49; p > 0.16) or fraction (F1,6 < 2.49; p > 0.16), nor a strain x fraction interaction (F1,6 < 2.49; p > 0.16). The estimated Vmax and Km constants for XAG and xc- glutamate uptake in GPV and SYN fractions are illustrated in Figure 3.
Figure 2.
Saturation analysis for system XAG and xc- glutamate uptake in glial and neuronal preparations from striatum of gp120 and wild-type (WT) mice. These glial (i.e., glial plasmalemmal vesicles; GPV) and neuronal (i.e., synaptosomes; SYN) preparations are enriched in small vesicles pinched off from glial membranes and nerve endings, respectively. Analysis revealed a significant (p<0.05) reduction in the Vmax of systems XAG and xc- in both (A) GPV and (B) SYN fractions from gp120 mice compared to WT. There were no significant group differences in the Km (affinity) for XAG or xc- glutamate uptake in GPV and SYN. Data are expressed as mean ± SEM (n=4/preparation/group).
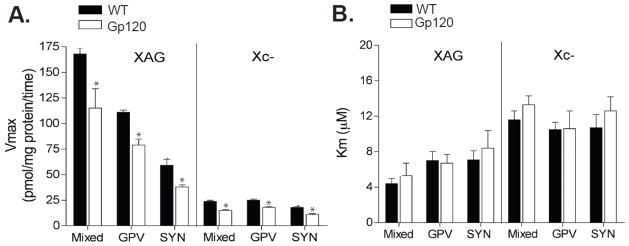
Figure 3.
Summary of the kinetic properties of system XAG and xc- glutamate uptake in striatum of gp120 and WT mice. Summary of results demonstrating a significant deficit in the Vmax both system XAG and xc- in neuronal and glial cells from striatum of gp120 mice. Moreover, several key properties regarding the specificity of high affinity and high capacity glutamate uptake were revealed. (A) The Vmax of system XAG was significantly greater than xc- in all striatal preparations (mixed, GPV, and SYN). Furthermore, the Vmax of systems XAG and xc- were significantly greater in mixed and GPV compared to SYN preparations. (B) The Km constants for glutamate were significantly lower in system XAG relative to system xc- (p < 0.05) across all preparations. * p<0.05 compared to glial preparations (i.e. crude or GPV).
Summaries of the kinetic parameters for glutamate uptake by systems XAG and xc- in all subcellular fractions from striatum are shown in Figure 3. Notably, several key properties underlying XAG and xc- uptake were observed in both strains of mice. First, as expected, the Vmax of system XAG was significantly greater than system xc- in all subcellular fractions (p < 0.01), indicating that the majority of glutamate uptake is sodium-dependent. Second, the Vmax of both systems XAG and xc- were significantly greater in mixed and GPV fractions compared to SYN (p < 0.05), which is consistent with an elevated number of glutamate transporters in glial cells compared to neurons (Danbolt, 2001). In final, the Km constants for glutamate were significantly lower in system XAG compared to xc- (p < 0.05), which is consistent with high affinity glutamate uptake under sodium-dependent conditions in both glial and neuronal cells (Danbolt, 2001).
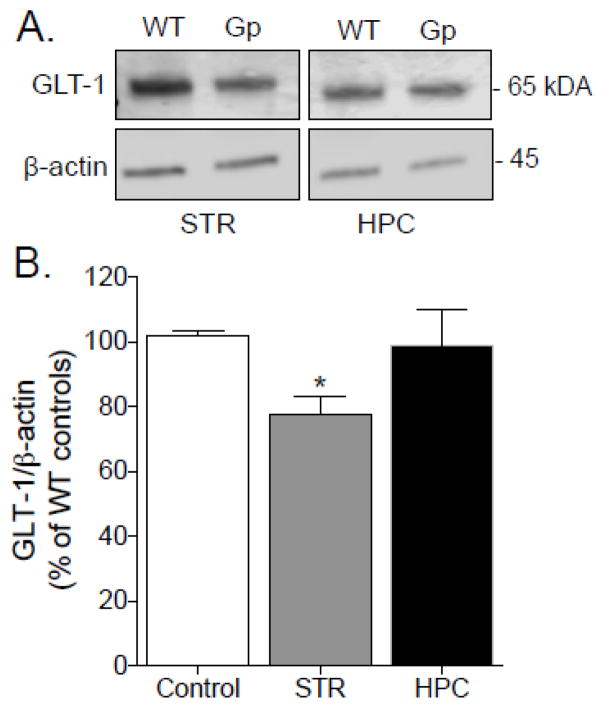
In final, we determined whether the observed gp120-inhibition of glutamate uptake correlates with the expression of GLT-1 protein, the primary glutamate transporter in CNS (Danbolt, 2001). As illustrated in Figure 4A, crude synaptosomal fractions from striatum and hippocampus of gp120 and WT mice were subjected to western blot analysis of GLT-1; standardized by the levels of β-actin. As shown in Figure 4B, GLT-1 protein levels were significantly reduced (75.6 ± 8% of WT-controls) in striatum but not hippocampus of gp120 mice relative to WT-controls (F2,12 = 3.47; p = 0.03), which is consistent with the kinetic profile observed in Figure 3. Together, these findings indicate that the expression and function of GLT-1 is particularly impaired in the striatum of gp120 mice.
Figure 4.
Western blots of GLT-1 protein derived from crude synaptosomal preparations from striatum (STR) and hippocampus (HPC) of gp120 (GP) and wild type (WT) mice. A. Representative immunoblots for GLT-1 and β-actin actin proteins yielded a band at approximately 65 and 45 kDa, respesctively. B. Mean density of GLT-1 and β-actin immunoreactivity was expressed as the difference in ratio of GLT-1/ β-actin protein expression and normalized to percent of WT-controls. Data were analyzed by simple A significant (p< 0.05) reduction in total protein levels of GLT-1 was observed in the STR but not HPC of gp120 mice compared to WT (p < 0.05 one-way ANOVA). Data are expressed as mean ± SEM (n=5/region/group).
Discussion
The present study is the first to characterize the kinetic properties of glutamate uptake in HIV-1 gp120 transgenic mice, an established model of HIV-associated neurocognitive deficits. Glutamate transporters play an indispensable role in maintaining extracellular glutamate homeostasis, which is necessary for learning, memory, and cognitive processing. The results presented here revealed a significant reduction in the Vmax of system XAG (sodium-dependent) and system xc- (sodium-independent) in both glial and neuronal preparations from striatum of gp120 mice relative to WT controls. There were no significant group differences in the Km for glutamate, suggesting that gp120 reduced the number of striatal glutamate transporters without affecting the affinity for glutamate. Consistent with the kinetic analysis, we also indicated a significant reduction in the total expression of GLT-1 protein in striatum of gp120 mice, which is likely involved in the development or severity of HIV-associated neurocognitive deficits.
Several investigators have previously shown that HIV-1 or gp120 inhibits glutamate uptake in cultured glial cells (Dryer et al., 1995; Vesce et al., 1997; Patton et al., 2000; Belmadani et al., 2001; Wang et al., 2003). It is also shown that gp120 alters the exchange of sodium and potassium in glial membranes (Benos et al., 1994; Holden et al., 1999), which typically leads to inhibition of glutamate uptake (Danbolt, 2001). Interestingly, Wang et al. (2003) further indicated that gp120-inhibition of glutamate uptake was linked to a significant reduction in the expression of GLT-1 protein (Wang et al., 2003), the predominant glutamate transporter in the brain (Danbolt, 2001). In the present study we also show significant decreases in both glutamate uptake and GLT-1 expression in glial preparations from striatum of gp120 mice. Taken together, these findings indicate that glial glutamate transporters are susceptible to gp120 exposure both in vitro and in vivo. The precise mechanisms, however, responsible for gp120-mediated down-regulation of GLT-1 expression and function remain to be investigated.
Apart from glia, few studies if any have determined the impact of gp120 exposure on neuronal glutamate transporters. In our study, neuronal (i.e., SYN) glutamate uptake accounted for approximately 35% of total glutamate uptake (Figure 3), which is in agreement with previous results (Hirst et al., 1998). Studies suggest that the majority of glutamate uptake in neurons is mediated in part between GLT-1 and EAAC1 (excitatory amino acid carrier subtype-1) transporters (Danbolt, 2001; Suchak et al., 2003). Here, similar to glial cells, we also show a significant deficit in neuronal glutamate uptake in striatum of gp120 mice, and without altering the Km for glutamate. Although EAAC1 was not measured for Western blot analysis, the possibility exists that gp120 may reduce both GLT-1 and EAAC1 protein expression in neuronal cells, which could lead to alterations in synaptic glutamate transmission and plasticity (Nieoullon et al., 2006; Waxman et al., 2007; Scimemi et al., 2009). Indeed, most neuronal glutamate transporters are localized primarily to dendritic spines, primary sites of synaptic plasticity that receive the majority of glutamatergic inputs (Rothstein et al., 1994; Danbolt, 2001), Interestingly, increased activity of N-methyl-D-aspartate (NMDA) receptor function, which is necessary for the induction of synaptic plasticity, is often associated with greater cell surface expression of EAAC1 compared to GLT-1 (Nieoullon et al., 2006; Waxman et al., 2007; Scimemi et al., 2009).
Beyond sodium dependent (XAG ) glutamate uptake, we also observed a significant deficit in the Vmax of sodium-independent (xc-) glutamate uptake in both glial and neuronal preparations from the striatum of gp120 mice, and also without altering the Km for glutamate. System xc- acts as a glutamate antiporter that mediates the exchange of extracellular cystine for intracellular glutamate (Bridges et al., 2012). The import of cystine through system xc- is critical to glutathione synthesis and oxidative protection in various cells both outside and inside the CNS (Murphy et al., 1989). In the CNS, the protein distribution of system xc- shows the antiporter to be localized in both neurons and glial cells (Bridges et al., 2012). Notably, disturbances in glutathione homeostasis may contribute to impaired immunity, which may lead to the progression of several immunological diseases, including HIV. For instance, HIV-1 infection induces oxidative stress in glial cells and neurons, which is often correlated with the neurocognitive deficits observed in some HIV patients (Shi et al., 1998; Pocernich et al., 2004). Thus, gp120-inhibition of system xc- may lead to major disruptions in glutathione synthesis and the emergence of excitotoxic neuropathology.
A major limitation to this study is the lack of data to demonstrate the impact of gp120 on oxidative stress. Nonetheless, it is worth noting that independent of oxidative stress, system xc- plays a crucial in providing a significant source of extracellular glutamate in striatum (Baker et al., 2002), which is important for activating glutamate receptors and modulating synaptic plasticity. Thus, the possibility exists that gp120 plays an important role in altering extracellular glutamate homeostasis, via disruption of glutamate uptake in the striatum in addition to promoting neurotoxicity. In any case, understanding the role of system xc- and other mechanisms that lead to alterations in glutamate and glutathione homeostasis in HIV-1 infection may advance the development of new therapeutic strategies to prevent synaptodendritic injury and improve cognitive function in HIV patients.
In the present study it was interesting to observe that the expression and function of glutamate transporters was unaltered in the hippocampus of gp120 mice. This suggests brain-region specific alterations by gp120, potentially similar to those found in brains of neurocognitive impaired, HIV-infected humans (Moore et al., 2006). Indeed, there is evidence suggesting that the striatum (made up of the putamen and caudate nucleus) shows one of the highest burdens of HIV proteins and viral RNA following infection in brain (Wiley et al., 1998). It is also possible that the perturbations observed in the striatum, but not the hippocampus may reflect disturbances in the balance between synaptodendritic injury and repair. For instance, virus- and host-derived toxic factors that are active during HIV infection may be counteracted in the hippocampus by host trophic factor production and neuronal plasticity (Ellis et al., 2007). Future studies will be necessary to address the functional and brain-region specificity of HIV-1 gp120 on glutamate uptake systems.
To conclude, the results of the present study suggest that restoring glutamate uptake systems in the striatum may be a potential mechanism for restoring memory and cognitive performance in HIV-infected individuals. Research to this end will be amenable to new glutamate- and neuroplasticity-based pharmacotherapies for the treatment of HIV-associated neurocognitive disorders.
Acknowledgments
All authors approved the final version of this article. The authors would like to thank L. Perez for his excellent technical assistance. This work was supported in part by National Institutes of Health Grants SNRP-U54N54301, 2R25GM061151, and NIGMS 1P20GM103642.
Footnotes
The authors declare that there is no conflict of interests.
References
- Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci. 2002;22:9134–41. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballester LY, Capo-Velez CM, Garcia-Beltran WF, Ramos FM, Vazquez-Rosa E, Rios R, Mercado JR, Melendez RI, Lasalde-Dominicci JA. Up-regulation of the neuronal nicotinic receptor alpha7 by HIV glycoprotein 120: potential implications for HIV-associated neurocognitive disorder. J Biol Chem. 2012;287 doi: 10.1074/jbc.M111.262543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmadani A, Zou JY, Schipma MJ, Neafsey EJ, Collins MA. Ethanol pre-exposure suppresses HIV-1 glycoprotein 120-induced neuronal degeneration by abrogating endogenous glutamate/Ca2+-mediated neurotoxicity. Neuroscience. 2001;104 doi: 10.1016/s0306-4522(01)00139-7. S0306-4522(01)00139-7. [DOI] [PubMed] [Google Scholar]
- Benos DJ, Hahn BH, Bubien JK, Ghosh SK, Mashburn NA, Chaikin MA, Shaw GM, Benveniste EN. Envelope glycoprotein gp120 of human immunodeficiency virus type 1 alters ion transport in astrocytes: implications for AIDS dementia complex. Proc Natl Acad Sci U S A. 1994;91:494–8. doi: 10.1073/pnas.91.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges RJ, Natale NR, Patel SA. System xc(-) cystine/glutamate antiporter: an update on molecular pharmacology and roles within the CNS. Br J Pharmacol. 2012;165 doi: 10.1111/j.1476-5381.2011.01480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y, Bannai S. Uptake of glutamate and cysteine in C-6 glioma cells and in cultured astrocytes. J Neurochem. 1990;55:2091–7. doi: 10.1111/j.1471-4159.1990.tb05800.x. [DOI] [PubMed] [Google Scholar]
- Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1 doi: 10.1016/0896-6273(88)90162-6. 0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Dreyer EB, Lipton SA. The coat protein gp120 of HIV-1 inhibits astrocyte uptake of excitatory amino acids via macrophage arachidonic acid. Eur J Neurosci. 1995;7:2502–7. doi: 10.1111/j.1460-9568.1995.tb01048.x. [DOI] [PubMed] [Google Scholar]
- Dunkley PR, Jarvie PE, Robinson PJ. A rapid Percoll gradient procedure for preparation of synaptosomes. Nat Protoc. 2008;3 doi: 10.1038/nprot.2008.171. [DOI] [PubMed] [Google Scholar]
- Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007;8 doi: 10.1038/nrn2040. nrn2040. [DOI] [PubMed] [Google Scholar]
- Gonzalez MI, Robinson MB. Neurotransmitter transporters: why dance with so many partners? Curr Opin Pharmacol. 2004;4 doi: 10.1016/j.coph.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Hirst WD, Price GW, Rattray M, Wilkin GP. Serotonin transporters in adult rat brain astrocytes revealed by [3H]5-HT uptake into glial plasmalemmal vesicles. Neurochem Int. 1998;33 doi: 10.1016/s0197-0186(05)80003-8. S0197-0186(98)00003-5. [DOI] [PubMed] [Google Scholar]
- Holden CP, Haughey NJ, Nath A, Geiger JD. Role of Na+/H+ exchangers, excitatory amino acid receptors and voltage-operated Ca2+ channels in human immunodeficiency virus type 1 gp120-mediated increases in intracellular Ca2+ in human neurons and astrocytes. Neuroscience. 1999;91 doi: 10.1016/s0306-4522(98)00714-3. S0306-4522(98)00714-3. [DOI] [PubMed] [Google Scholar]
- Huang YH, Bergles DE. Glutamate transporters bring competition to the synapse. Curr Opin Neurobiol. 2004;14 doi: 10.1016/j.conb.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10 doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Hediger MA. The glutamate and neutral amino acid transporter family: physiological and pharmacological implications. Eur J Pharmacol. 2003;479 doi: 10.1016/j.ejphar.2003.08.073. S0014299903023264. [DOI] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410 doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamatehomeostasis and prevents relapse to cocaine seeking. Biol Psychiatry. 2010;67 doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsugami TR, Tanemura K, Mieda M, Nakatomi R, Yamada K, Kondo T, Ogawa M, Obata K, Watanabe M, Hashikawa T, Tanaka K. From the Cover: Indispensability of the glutamate transporters GLAST and GLT1 to brain development. Proc Natl Acad Sci U S A. 2006;103 doi: 10.1073/pnas.0509144103. 0509144103 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI, Vuthiganon J, Kalivas PW. Regulation of extracellular glutamate in the prefrontal cortex: focus on the cystine glutamate exchanger and group I metabotropic glutamate receptors. J Pharmacol Exp Ther. 2005;314 doi: 10.1124/jpet.104.081521. jpet.104.081521. [DOI] [PubMed] [Google Scholar]
- Moore DJ, Masliah E, Rippeth JD, Gonzalez R, Carey CL, Cherner M, Ellis RJ, Achim CL, Marcotte TD, Heaton RK, Grant I HNRC Group. Cortical and subcortical neurodegeneration is associated with HIV neurocognitive impairment. AIDS. 2006;20 doi: 10.1097/01.aids.0000218552.69834.00. [DOI] [PubMed] [Google Scholar]
- Murphy TH, Miyamoto M, Sastre A, Schnaar RL, Coyle JT. Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress. Neuron. 1989;2 doi: 10.1016/0896-6273(89)90043-3. 0896-6273(89)90043-3. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Iga K, Shibata T, Shudo M, Kataoka K. Glial plasmalemmal vesicles: a subcellular fraction from rat hippocampal homogenate distinct from synaptosomes. Glia. 1993;9 doi: 10.1002/glia.440090107. [DOI] [PubMed] [Google Scholar]
- Nieoullon A, Canolle B, Masmejean F, Guillet B, Pisano P, Lortet S. The neuronal excitatory amino acid transporter EAAC1/EAAT3: does it represent a major actor at the brain excitatory synapse? J Neurochem. 2006;98 doi: 10.1111/j.1471-4159.2006.03978.x. JNC3978. [DOI] [PubMed] [Google Scholar]
- Patel SA, Warren BA, Rhoderick JF, Bridges RJ. Differentiation of substrate and non-substrate inhibitors of transport system xc(-): an obligate exchanger of L-glutamate and L-cystine. Neuropharmacology. 2004;46 doi: 10.1016/j.neuropharm.2003.08.006. S0028390803003460. [DOI] [PubMed] [Google Scholar]
- Patton HK, Zhou ZH, Bubien JK, Benveniste EN, Benos DJ. gp120-induced alterations of human astrocyte function: Na(+)/H(+) exchange, K(+) conductance, and glutamate flux. Am J Physiol Cell Physiol. 2000;279:C700–8. doi: 10.1152/ajpcell.2000.279.3.C700. [DOI] [PubMed] [Google Scholar]
- Pocernich CB, Sultana R, Hone E, Turchan J, Martins RN, Calabrese V, Nath A, Butterfield DA. Effects of apolipoprotein E on the human immunodeficiency virus protein Tat in neuronal cultures and synaptosomes. J Neurosci Res. 2004;77 doi: 10.1002/jnr.20182. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13 doi: 10.1016/0896-6273(94)90038-8. 0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Sato H, Tamba M, Okuno S, Sato K, Keino-Masu K, Masu M, Bannai S. Distribution of cystine/glutamate exchange transporter, system x(c)-, in the mouse brain. J Neurosci. 2002;22 doi: 10.1523/JNEUROSCI.22-18-08028.2002. 22/18/8028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scimemi A, Tian H, Diamond JS. Neuronal transporters regulate glutamate clearance, NMDA receptor activation, and synaptic plasticity in the hippocampus. J Neurosci. 2009;29 doi: 10.1523/JNEUROSCI.4845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi B, Raina J, Lorenzo A, Busciglio J, Gabuzda D. Neuronal apoptosis induced by HIV-1 Tat protein and TNF-alpha: potentiation of neurotoxicity mediated by oxidative stress and implications for HIV-1 dementia. J Neurovirol. 1998;4:281–90. doi: 10.3109/13550289809114529. [DOI] [PubMed] [Google Scholar]
- Suchak SK, Baloyianni NV, Perkinton MS, Williams RJ, Meldrum BS, Rattray M. The 'glial' glutamate transporter, EAAT2 (Glt-1) accounts for high affinity glutamate uptake into adult rodent nerve endings. J Neurochem. 2003;84 doi: 10.1046/j.1471-4159.2003.01553.x. 1553. [DOI] [PubMed] [Google Scholar]
- Toggas SM, Masliah E, Rockenstein EM, Rall GF, Abraham CR, Mucke L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994;367 doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- Vesce S, Bezzi P, Rossi D, Meldolesi J, Volterra A. HIV-1 gp120 glycoprotein affects the astrocyte control of extracellular glutamate by both inhibiting the uptake and stimulating the release of the amino acid. FEBS Lett. 1997;411 doi: 10.1016/s0014-5793(97)00674-1. S0014-5793(97)00674-1. [DOI] [PubMed] [Google Scholar]
- Wang Z, Pekarskaya O, Bencheikh M, Chao W, Gelbard HA, Ghorpade A, Rothstein JD, Volsky DJ. Reduced expression of glutamate transporter EAAT2 and impaired glutamate transport in human primary astrocytes exposed to HIV-1 or gp120. Virology. 2003;312 doi: 10.1016/s0042-6822(03)00181-8. S0042682203001818. [DOI] [PubMed] [Google Scholar]
- Waxman EA, Baconguis I, Lynch DR, Robinson MB. N-methyl-D-aspartate receptor-dependent regulation of the glutamate transporter excitatory amino acid carrier 1. J Biol Chem. 2007;282 doi: 10.1074/jbc.M702278200. M702278200. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Soontornniyomkij V, Radhakrishnan L, Masliah E, Mellors J, Hermann SA, Dailey P, Achim CL. Distribution of brain HIV load in AIDS. Brain Pathol. 1998;8:277–84. doi: 10.1111/j.1750-3639.1998.tb00153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]