Abstract
This review details methods for utilizing D & C suction abortus specimens as a source of human fetal organs to study the morphogenetic and molecular mechanisms of human fetal organ development. By this means it is possible to design experiments elucidating the molecular mechanisms of human fetal organ development and to compare and contrast human developmental mechanisms with that of laboratory animals. Finally human fetal organs can be grown in vivo as grafts to athymic mice, thus allowing ethical analysis of potential adverse effects of environmental toxicants.
Keywords: Human development, male and female external genitalia, internal genitalia
Introduction
The two most widely employed animal models for human development are the mouse and rat. On the whole, the overall organogenetic process is similar in animal models and in humans, and thus the tacit (but unproven) assumption is that mice and rats are valid models of human organogenesis from both a morphologic and molecular perspective. While this tacit assumption may be justified in many cases, there are notable exceptions with dramatic mouse/human differences in morphogenetic processes in certain organs. For example, studies demonstrate substantial differences in development of the mouse versus the human penile urethra. Most of the mouse penile urethra forms by direct canalization of the urethral plate within the genital tubercle (Hynes and Fraher, 2004; Seifert et al., 2008), whereas development of the human penile urethra involves “opening and closing zippers”, i.e., canalization of the urethral plate to form a widely open urethral groove and subsequent fusion of the urethral folds to form a tubular urethra (Li et al., 2014). Likewise, the male mouse has two prepuces, while the human has one (Blaschko et al., 2013; Cunha et al., 2015). Prostatic lobar patterning is completely different in mice versus human (McNeal, 1976; 1981; Sugimura et al., 1986). Uterine morphology and thus morphogenesis is vastly different in humans versus mice and rats (Kurita and Nakamura, 2008; O'Rahilly, 1973). Whether these morphologic/morphogenetic differences in laboratory animal versus human development are due to differences in molecular mechanisms remains to be explored.
Resolution of whether laboratory animals provide valid models of human development can only come from a detailed morphogenetic and molecular examination of human organogenesis so that similarities/differences between mouse/rat versus human organogenesis can be compared, and the underlying molecular mechanisms revealed in laboratory animals versus humans. Moreover, based upon examination of textbooks of human embryology, it is evident that our understanding of human development is derived for the most part on classical histologic studies decades old (Koff, 1933; Felix, 1912; Jirasek et al., 1968; Lowsley, 1912; Hart, 1908; Jones, 1910; Johnson, 1920; Hunter, 1935; Andrews, 1951). It is timely to re-examine organogenesis in humans using modern morphological techniques. The goal of this paper is to demonstrate how research on developing human organs can be achieved.
The first hurdle to be addressed is authorization to obtain human fetal specimens for research purposes. Such authorization must be consistent with national and state laws as well as local (University, College, etc.) and national agency (NIH, MRC, etc.) guidelines. For all human fetal samples, informed consent must be obtained from the patient, and the most important issue is that the specimens must be obtained without patient identifiers to preserve confidentiality. Of course, the decision to terminate pregnancy is made solely by the patient in consultation with her physician and without any contact whatsoever with the investigators.
Facilities performing therapeutic abortions have their own protocol for handling specimens, which in most cases involves formalin fixation. For research purposes this is to be avoided so that fresh specimens can be obtained from which viable cells and tissues can be isolated.
Most abortions occur via the D&C suction procedure in which the embryo/fetus is completely disrupted. Many assume that it is difficult (if not impossible) to find human fetal organs from such specimens, which contain placental villi, endometrial tissue as well as fetal tissues. We consistently find a multitude of human fetal organs in each D&C specimen. Success requires basic anatomical knowledge and experience for what human fetal organs look like at various stages of development. Knowledge of adult human anatomy is extremely useful, since developing human fetal organs will resemble their adult counterparts at some point in development. Knowledge of the anatomy of fetal mouse/rat organ development is also useful in recognizing what an undifferentiated organ looks like. The trick is to be able to recognize and isolate developing human organs from the specimen containing clotted blood, placental villi, endometrial tissue as well as fetal parts. The goal of this paper is to demonstrate how this can be achieved.
Materials and Methods and Results
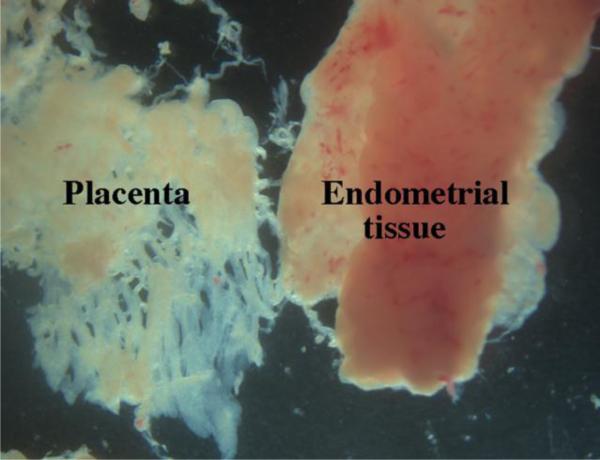
For safety, it is imperative that gloves are used by the investigator in handling abortus specimens. The D&C suction procedure is used for pregnancies in the ~6 to ~20 week age range, with most falling into the 9 to 14 week group. During the D&C suction procedure the specimen is collected in a cloth “sock”, which is emptied into a 9 by 9 inch Pyrex dish containing PBS or culture medium. Forceps are used to “spread out” the specimen thus breaking up clumps of tissue. A magnification aid is useful once the specimen is fully dispersed into individual pieces. Placental villi have a characteristic morphology (Fig. 1) and can be discarded. Likewise, endometrial tissue (Fig. 1) will be recognized as intact amorphous sheets and discarded. Endometrial and placental tissue have a fleshy appearance, are pink in color and have ragged edges. In contrast, many human fetal organs (depending of the organ) will have smooth edges (see Figs 2-5), a distinctive morphology and typically are light in color (an exception being the liver and kidney which are red). The following account describes the isolation of human fetal male and female internal and external genitalia, which are perhaps the most difficult to isolate.
Figure 1.
Photos of human placental villi and endometrial tissue. Note their characteristic morphology.
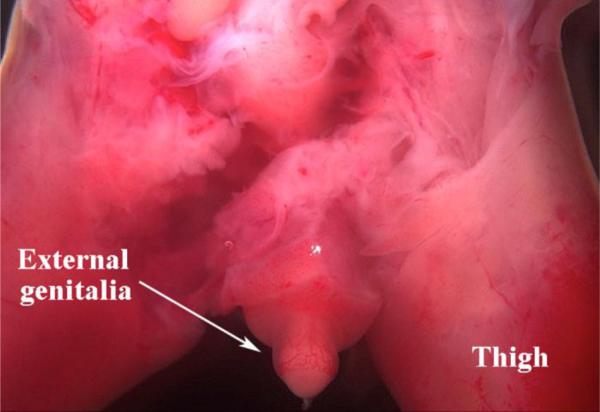
Figure 2.
A rare specimen (14 weeks of gestation) in which the legs are still attached to the pelvis. In this male specimen the external genitalia are easily identified and dissected (see prostate from same specimen in Figure 4).
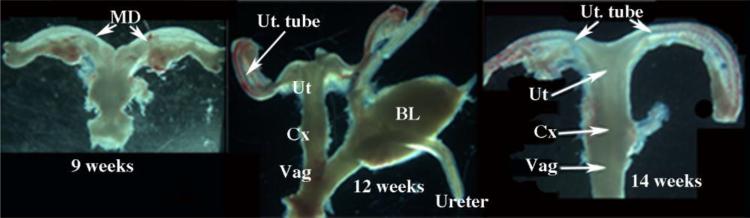
Figure 5.
Human fetal female reproductive tracts at 9 to 14 weeks of gestation. In all cases the Mullerian duct (MD) or uterine tube (Ut. Tube) join the midline uterovaginal canal, composed of the uterine corpus (Ut), uterine cervix (Cx) and vagina (Vag) whose respective boundries are indistinct. Ovaries were absent in all specimens. Bl=bladder.
The first “organ” to identify and isolate is an intact foot, from which heal-toe length will be determined and used to estimate age of the specimen (Taguchi et al., 1983; Drey et al., 2005). Information on “last menstrual period” or clinical staging of gestational age is usually unreliable. Gender is determined by reproductive tract gross appearance (see Figs. 4-5), and verified by PCR (Cui et al., 1994).
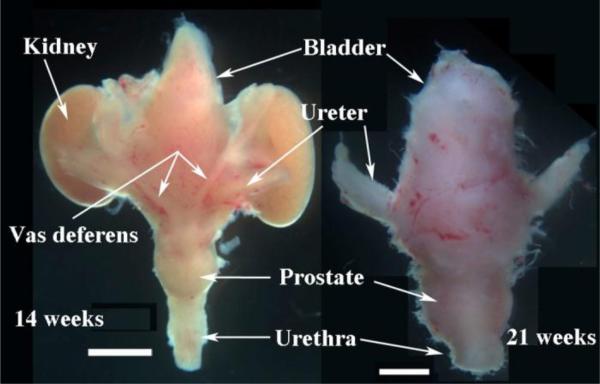
Figure 4.
Human fetal prostates at 14 and 21 weeks of gestation with associated organs. Dorsal view of the 14-week specimen (left). The vas deferens are subtle, but can be recognized. Ventral view of the 21-week specimen (right). In both cases note the prostate, which is a bulge in the urethra below the bladder. Scale bars = 2mm.
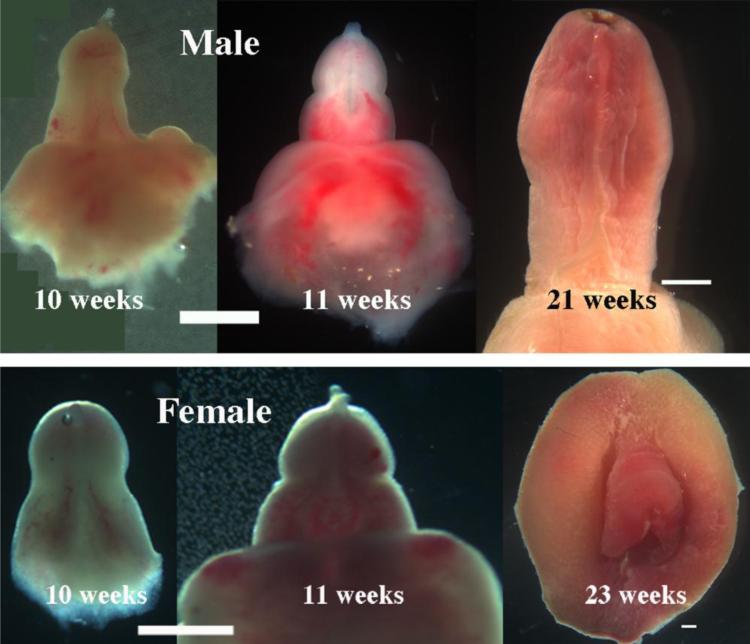
External genitalia are typically attached to a leg or to the pelvis and are easily identified and dissected (Fig. 2). Figure 3 presents a series of photographs of male and female external genitalia at various gestation ages. Note that size and morphology of the external genitalia cannot be used for determination of sex as male and female external genitalia are remarkably similar until very late stages.
Figure 3.
Photographs of male and female external genitalia at various gestational ages as indicated. Note that at early stages the size and gross morphology of male and female genital tubercles are remarkably similar. All images are ventral views except for the 23 week female specimen, which is a frontal view. Scale bar= 2mm.
Male and female internal genitalia are more difficult to find. One method is to isolate all tissue pieces that are light in color (large and small). Place these in a Petri dish with PBS or tissue culture medium for subsequent viewing with a dissecting microscope having capability of viewing with reflected light from above as well as transmitted light from below the specimen stage. The fetal pelvis frequently remains intact during the surgical procedure. Internal genitalia may be present within the pelvis, in which case knowledge of the dorsal-ventral positioning of male and female organs is immensely helpful. In males, the bladder, prostate and urethra are ventrally situated with the rectum dorsally positioned. However, in some cases the internal genitalia may have been dislodged from their original anatomical position. Whether internal genitalia remain within the pelvis or are free floating elsewhere, for males there are two sets of associated small bilateral tubes: (a) two ureters attached to the bladder and (b) two vas deferens or Wolffian ducts attached to the posterior aspect of the bladder or urogenital sinus. All 4 small ducts may not always be present, but using anatomic principals one should try to identify them within the specimen (Fig. 4). Testes may or may not be attached to the upper ends of the vas deferens.
In some cases, the pelvis may not contain reproductive tract organs, in which case one should try to identify the free floating umbilical cord. The umbilical arteries reach the umbilical cord by passing within the pelvis along both sides of the urinary bladder to which they are firmly fused. Due to torque on the umbilical cord during the operative procedure, the bladder and closely associated male internal genitalia may be found attached to the proximal portion of the umbilical cord. The prostate is recognizable as a bulge in the urethra immediately below the bladder. Prostates are more prominent in older specimens (Fig. 4).
Female internal genitalia are frequently located in their normal position within the pelvis dorsal to the bladder and ventral to the rectum/colon. In females there are 2 sets of small bilateral tubes in the pelvis: (a) two ureters and (b) two uterine tubes. The ureters are attached to the bladder, and the uterine tubes are attached to the cranial aspect of the uterovaginal canal at the uterotubal junction. Ideally all 4 tubes are present, but this is not always the case (Fig. 5). Ovaries are attached to the upper ends of the uterine tubes, but are frequently absent. Using large landmarks (the bladder and rectum/colon), the pelvis can be carefully dissected to isolate the entire female reproductive tract intact, and thus identity of the ureters and uterine tubes may be determined. Be aware of the normal dorsal-ventral positioning of organs in females, the bladder and urethra are the most ventral structures, and the utero-vaginal canal and rectum are situated dorsally in that order. Figure 5 shows human fetal female reproductive tracts at 9 to 14 weeks of gestation.
For all organs, the goal is to cleanly isolate organs by dissection free of extraneous tissues. This is done using small scissors, forceps, and/or scalpels. Spring-loaded Vannas scissors can be useful. Once organs are cleanly dissected, the investigator has many experimental choices. Organs can be fixed, embedded in paraffin and sectioned for H&E histology, immunohistochemistry (IHC) or in situ hybridization ISH). Thick vibrotome sections can also be prepared for IHC or ISH. Alternatively, the specimen can be processed for isolation of RNA, DNA or protein by standard procedures.
As stated above, the preferred method is to obtain fresh specimens whose organs and tissues are viable. Accordingly, isolated human fetal organs can be used to prepare cell cultures, or organ rudiments can be transplanted under the renal capsule of athymic mouse hosts (See review on renal capsule transplantation in this issue). Development of human fetal organs will proceed normally as grafts under the renal capsule (Cunha et al., 1988; Cunha et al., 1987; Robboy et al., 1982; Taguchi et al., 1984; Taguchi et al., 1983; Taguchi et al., 1986). Once a human fetal organ is growing in an athymic mouse host, the host can be treated with a multitude of exogenous agents based upon the goal of the experiment. In this regard, we have treated grafts of human fetal reproductive tracts with a variety of hormonal agents: diethylstilbestrol, Clomid, dihydrotesterone, bisphenol A, etc.
In the course of isolating male and female internal and external genitalia, we frequently find many other human fetal organs such as kidney, heart, supra-renal gland, liver, lung, intestine, and stomach. The cartilaginous vertebral column and ribs are often found as is the lower jaw containing tooth germs. Thus, many human fetal organs can be found in D&C suction specimens.
Discussion
Animal models such a mice and rats have been used for decades as models of human development, and for the most part developmental processes are similar in animals and humans, but with notable exceptions as discussed above. Due to similarities in human versus animal development, the tacit (but unproven) assumption is that laboratory animals provide valid models of human development. A significant information gap is whether the molecular mechanisms of human development are the similar or different from that in laboratory animals. One observation strongly favoring similar molecular mechanisms involves heterospecific tissue recombinants composed of rat or mouse urogenital sinus mesenchyme (UGM) and human fetal or adult urinary bladder epithelium (BLE). In such UGM+BLE tissue recombinants the rodent UGM induced the human bladder epithelium to form glands resembling prostate which express human prostatic specific antigen (PSA) (Aboseif et al., 1999; Cunha et al., 1983). Such data have profound implications and indicate that the signaling molecules involved in prostatic development are sufficiently conserved that a rodent mesenchymal inductor can reprogram differentiation of a human epithelium. While many of the molecules involved in mouse prostatic development have been revealed [fibroblast growth factor 10 (Kuslak and Marker, 2007; Thomson and Cunha, 1999; Donjacour et al., 2003), homeobox genes (Podlasek et al., 1997; Warot et al., 1997), hedgehog pathway genes (Doles et al., 2006), the retinoic acid pathway (Vezina et al., 2008), bone morphogenetic proteins (Cook et al., 2007; Grishina et al., 2005), and the wingless integration site (WNT5A) gene (Allgeier et al., 2008)], it is unknown whether the same molecules are expressed during human prostatic development. Likewise, it is unclear whether the signaling molecules are similar/different in developing internal genitalia in laboratory animals versus humans.
The prevalent (but unproven) idea that mice are valid models of human development has been extended to the investigation of agents that may elicit adverse developmental effects. A case in point is the plastic component, bisphenol A (BPA). Concern that BPA may adversely affect human development rests on a substantial literature of BPA-induced developmental defects in male and female laboratory animals (Rochester, 2013; Vandenberg, 2012; Calhoun et al., 2014; vom Saal et al., 1998; Vandenberg et al., 2013). These effects are consistent with BPA's known estrogenicity (Gray et al., 2004). Despite this large body of animal research on BPA, direct evidence that BPA adversely affects development of human fetal organs at any dose is wanting and challenged in several reports (Milman et al., 2002; Goodman et al., 2006; Goodman et al., 2009; Gray et al., 2004). The inferred adverse health effect of prenatal BPA exposure in humans is based solely on animal studies and correlational (epidemiological) studies of human populations. The sad reality is that there is NO direct proof of adverse effects of BPA on development of the developing human prostate and the developing human female reproductive tract at any dose. Treatment of host mice bearing grafts of human fetal reproductive tracts with BPA at high (fully estrogenic) as well as at extremely low environmental doses could resolve the issue of whether BPA can adversely affect development of human fetal reproductive tract organs in vivo.
In summary, this review emphasizes the relative ease in which human fetal organs can be isolated from D&C abortus specimens and used for a variety of experimental purposes, especially in determining the morphogenetic and molecular mechanisms of human development and determining the extent to which laboratory animals are valid models of human development. Such information certainly extends to agents such as the many estrogenic substances and other hormonally active agents in our environment (known to be teratogenic in laboratory animals) (McLachlan, 1981; 1985). Of course, the converse, that such hormonally active agents are without adverse effects on human organ development at environmental doses, is an equally important question that can be resolved one way or the other by human fetal organ research.
Acknowledgments
This work was supported by NIH grant RO1 DK0581050.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aboseif S, El-Sakka A, Young P, Cunha G. Mesenchymal reprogramming of adult human epithelial differentiation. Differentiation; research in biological diversity. 1999;65:113–118. doi: 10.1046/j.1432-0436.1999.6520113.x. [DOI] [PubMed] [Google Scholar]
- Allgeier SH, Lin TM, Vezina CM, Moore RW, Fritz WA, Chiu SY, Zhang C, Peterson RE. WNT5A selectively inhibits mouse ventral prostate development. Developmental biology. 2008;324:10–17. doi: 10.1016/j.ydbio.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews GS. The histology of the human foetal and prepubertal prostates. J. Anat. 1951;85:44–54. [PMC free article] [PubMed] [Google Scholar]
- Blaschko SD, Mahawong P, Ferretti M, Cunha TJ, Sinclair A, Wang H, Schlomer BJ, Risbridger G, Baskin LS, Cunha GR. Analysis of the effect of estrogen/androgen perturbation on penile development in transgenic and diethylstilbestrol-treated mice. Anatomical record. 2013;296:1127–1141. doi: 10.1002/ar.22708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun KC, Padilla-Banks E, Jefferson WN, Liu L, Gerrish KE, Young SL, Wood CE, Hunt PA, Vandevoort CA, Williams CJ. Bisphenol a exposure alters developmental gene expression in the fetal rhesus macaque uterus. PloS one. 2014;9:e85894. doi: 10.1371/journal.pone.0085894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook C, Vezina CM, Allgeier SH, Shaw A, Yu M, Peterson RE, Bushman W. Noggin is required for normal lobe patterning and ductal budding in the mouse prostate. Developmental biology. 2007;312:217–230. doi: 10.1016/j.ydbio.2007.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui KH, Warnes GM, Jeffrey R, Matthews CD. Sex determination of preimplantation embryos by human testis-determining-gene amplification. Lancet. 1994;343:79–82. doi: 10.1016/s0140-6736(94)90815-x. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Sekkingstad M, Meloy BA. Heterospecific induction of prostatic development in tissue recombinants prepared with mouse, rat, rabbit, and human tissues. Differentiation; research in biological diversity. 1983;24:174–180. doi: 10.1111/j.1432-0436.1983.tb01317.x. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Sinclair A, Risbridger G, Hutson J, Baskin LS. Current Understanding of Hypospadias: Relevance of Animal Models. Nature Reviews. 2015 doi: 10.1038/nrurol.2015.57. In Press. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Taguchi O, Lawrence WD, Robboy SJ. Absence of teratogenic effects of progesterone on the developing genital tract of the human fetus. Human Path. 1988;19:777–783. doi: 10.1016/s0046-8177(88)80260-0. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Taguchi O, Namikawa R, Nishizuka Y, Robboy SJ. Teratogenic effects of Clomid, tamoxifen, and diethylstilbestrol on the developing human female genital tract. Human Pathol. 1987;18:1132–1143. doi: 10.1016/s0046-8177(87)80381-7. [DOI] [PubMed] [Google Scholar]
- Doles J, Cook C, Shi X, Valosky J, Lipinski R, Bushman W. Functional compensation in Hedgehog signaling during mouse prostate development. Developmental biology. 2006;295:13–25. doi: 10.1016/j.ydbio.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Donjacour AA, Thomson AA, Cunha GR. FGF-10 plays an essential role in the growth of the fetal prostate. Developmental biology. 2003;261:39–54. doi: 10.1016/s0012-1606(03)00250-1. [DOI] [PubMed] [Google Scholar]
- Drey EA, Kang MS, McFarland W, Darney PD. Improving the accuracy of fetal foot length to confirm gestational duration. Obstet Gynecol. 2005;105:773–778. doi: 10.1097/01.AOG.0000154159.75022.11. [DOI] [PubMed] [Google Scholar]
- Felix W. The development of the urogenital organs. In: Kiebel R, Mall FP, editors. Manual of Human Embryology. Lippincott; Philadelphia: 1912. pp. 869–972. [Google Scholar]
- Goodman JE, McConnell EE, Sipes IG, Witorsch RJ, Slayton TM, Yu CJ, Lewis AS, Rhomberg LR. Updated weight of the evidence evaluation of reproductive and developmental effects of low doses of bisphenol A. Crit. Rev. Toxicol. 2006;36:387–457. doi: 10.1080/10408440600758317. [DOI] [PubMed] [Google Scholar]
- Goodman JE, Witorsch RJ, McConnell EE, Sipes IG, Slayton TM, Yu CJ, Franz AM, Rhomberg LR. Weight-of-Evidence Evaluation of Reproductive and Developmental Effects of Low Doses of Bisphenol A. Critical Reviews in Toxicology. 2009;39:1–75. doi: 10.3109/10408440903279946. [DOI] [PubMed] [Google Scholar]
- Gray GM, Cohen JT, Cunha G, Hughes C, McConnell EE, Rhomberg L, Sipes IG, Mattison D. Weight of the Evidence Evaluation of Low-Dose Reproductive and Developmental Effects of Bisphenol A. Human and Ecological Risk Assessment. 2004;10:875–921. [Google Scholar]
- Grishina IB, Kim SY, Ferrara C, Makarenkova HP, Walden PD. BMP7 inhibits branching morphogenesis in the prostate gland and interferes with Notch signaling. Developmental biology. 2005;288:334–347. doi: 10.1016/j.ydbio.2005.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart D. On the role of the developing epidernis in forming sheaths and lumina to organs, illustrated specially in the development of the prepuce and urethra. J. Anat. Lond. 1908;42:50–56. [PMC free article] [PubMed] [Google Scholar]
- Hunter RH. Notes on the Development of the Prepuce. J Anat. 1935;70:68–75. [PMC free article] [PubMed] [Google Scholar]
- Hynes PJ, Fraher JP. The development of the male genitourinary system: III. The formation of the spongiose and glandar urethra. Br J Plast Surg. 2004;57:203–214. doi: 10.1016/j.bjps.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Jirasek JE, Raboch J, Uher J. The relationship between the development of gonads and external genitals in human fetuses. Am J Obstet Gynecol. 1968;101:830–833. doi: 10.1016/0002-9378(68)90039-2. [DOI] [PubMed] [Google Scholar]
- Johnson FP. The later development of the urethra in the male. J. Urol. 1920;4:447–501. [Google Scholar]
- Jones F. The development and malformations of the glans and prepuce. Brit. Med. J. 1910;1:137–138. doi: 10.1136/bmj.1.2559.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koff AK. Development of the vagina in the human fetus. Contrib. Embryol. Carnegie Inst. Wash. 1933;24:59–90. [PubMed] [Google Scholar]
- Kurita T, Nakamura H. Embryology of the uterus. In: Aplin JD, Fazleabas AT, Glasser SR, Giudice LC, editors. Endometrium. Informa UK Ltd; London: 2008. pp. 1–18. [Google Scholar]
- Kuslak SL, Marker PC. Fibroblast growth factor receptor signaling through MEK-ERK is required for prostate bud induction. Differentiation; research in biological diversity. 2007;75:638–651. doi: 10.1111/j.1432-0436.2006.00161.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Sinclair A, Cao M, Shen J, Choudhry S, Cunha GR, Baskin L. Canalization of the urethral plate precedes fusion of the urethral folds during male penile urethral development: the double zipper hypothesis. J. Urology. 2014;193:1353–1359. doi: 10.1016/j.juro.2014.09.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowsley OS. The development of the human prostate gland with reference to the development of other structures at the neck of the urinary bladder. Am. J. Anat. 1912;13:299–349. [Google Scholar]
- McLachlan JA. Estrogens in the Environment. Elsevier; North Holland, New York: 1981. [Google Scholar]
- McLachlan JA. Estrogens in the Environment II: Influences on Development. Elsevier; New York: 1985. [Google Scholar]
- McNeal JE. Developmental and comparative anatomy of the prostate. In: Grayhack JT, Wilson JD, Scherbenske MJ, editors. Benign Prostatic Hyperplasia. US Govt. Printing Office; Washington, DC: 1976. pp. 1–7. [Google Scholar]
- McNeal JE. The zonal anatomy of the prostate. The Prostate. 1981;2:35–49. doi: 10.1002/pros.2990020105. [DOI] [PubMed] [Google Scholar]
- Milman HA, Bosland MC, Walden PD, Heinze JE. Evaluation of the adequacy of published studies of low-dose effects of bisphenol A on the rodent prostate for use in human risk assessment. Regulatory toxicology and pharmacology : RTP. 2002;35:338–346. doi: 10.1006/rtph.2002.1553. [DOI] [PubMed] [Google Scholar]
- O'Rahilly R. The embryology and anatomy of the uterus. In: Norris HJ, Hertig AT, Abell MR, editors. The Uterus. Williams and Wilkins Co.; Baltimore: 1973. pp. 17–39. [Google Scholar]
- Podlasek CA, Duboule D, Bushman W. Male accessory sex organ morphogenesis is altered by loss of function of Hoxd-13. Developmental dynamics : an official publication of the American Association of Anatomists. 1997;208:454–465. doi: 10.1002/(SICI)1097-0177(199704)208:4<454::AID-AJA2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Robboy SJ, Taguchi O, Cunha GR. Normal development of the human female reproductive tract and alterations resulting from experimental exposure to diethylstilbestrol. Human Pathol. 1982;13:190–198. doi: 10.1016/s0046-8177(82)80177-9. [DOI] [PubMed] [Google Scholar]
- Rochester JR. Bisphenol A and human health: a review of the literature. Reproductive toxicology. 2013;42:132–155. doi: 10.1016/j.reprotox.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Seifert AW, Harfe BD, Cohn MJ. Cell lineage analysis demonstrates an endodermal origin of the distal urethra and perineum. Developmental biology. 2008;318:143–152. doi: 10.1016/j.ydbio.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimura Y, Cunha GR, Donjacour AA. Morphogenesis of ductal networks in the mouse prostate. Biol. Reprod. 1986;34:961–971. doi: 10.1095/biolreprod34.5.961. [DOI] [PubMed] [Google Scholar]
- Taguchi O, Cunha GR, Lawrence WD, Robboy SJ. Timing and irreversibility of Mullerian duct inhibition in the embryonic reproductive tract of the human male. Developmental biology. 1984;106:394–398. doi: 10.1016/0012-1606(84)90238-0. [DOI] [PubMed] [Google Scholar]
- Taguchi O, Cunha GR, Robboy SJ. Experimental study of the effect of diethylstilbestrol on the development of the human female reproductive tract. International J. Biological Res. in Pregnancy. 1983;4:56–70. [PubMed] [Google Scholar]
- Taguchi O, Cunha GR, Robboy SJ. Expression of nuclear estrogen-binding sites within developing human fetal vagina and urogenital sinus. Am. J. Anat. 1986;177:473–480. doi: 10.1002/aja.1001770405. [DOI] [PubMed] [Google Scholar]
- Thomson AA, Cunha GR. Prostatic growth and development are regulated by FGF10. Development. 1999;126:3693–3701. doi: 10.1242/dev.126.16.3693. [DOI] [PubMed] [Google Scholar]
- Vandenberg L. Low-dose effects of endocrine disruptors, with Laura Vandenberg. Interview by Ashley Ahearn. Environmental health perspectives. 2012;120:1. p preceding A228. [PubMed] [Google Scholar]
- Vandenberg LN, Ehrlich S, Belcher SM, Ben-Jonathan N, Dolinoy DC, Hugo ER, Hunt PA, Newbold RR, Rubin BS, Saili KS, Soto AM, Wang H-S, vom Saal FS. Low dose effects of bisphenol A. Endocrine Disruptors. 2013;1:1–20. [Google Scholar]
- Vezina CM, Allgeier SH, Fritz WA, Moore RW, Strerath M, Bushman W, Peterson RE. Retinoic acid induces prostatic bud formation. Developmental dynamics : an official publication of the American Association of Anatomists. 2008;237:1321–1333. doi: 10.1002/dvdy.21526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Saal FS, Cooke PS, Buchanan DL, Palanza P, Thayer KA, Nagel SC, Parmigiani S, Welshons WV. A physiologically based approach to the study of bisphenol A and other estrogenic chemicals on the size of reproductive organs, daily sperm production, and behavior. Toxicol Ind Health. 1998;14:239–260. doi: 10.1177/074823379801400115. [DOI] [PubMed] [Google Scholar]
- Warot X, Fromental-Ramain C, Fraulob V, Chambon R, Dolle P. Gene dosage-dependent effects of the Hoxa-13 and Hoxd-13 mutations on morphogenesis of the terminal parts of the digestive and urogenital tracts. Development. 1997;124:4781–4797. doi: 10.1242/dev.124.23.4781. [DOI] [PubMed] [Google Scholar]