Abstract
Wilms tumor (WT), a pediatric renal cancer, is the most common childhood kidney cancer. The etiology of WT is heterogeneous with multiple genes known to result in WT tumorigenesis. However, these genes are rarely associated with familial Wilms tumor (FWT). To identify mutations predisposing to FWT, we performed whole genome sequencing using genomic DNA from three affected/obligate carriers in a large WT family, followed by Sanger sequencing of candidate gene mutations in 47 additional WT families to determine their frequency in FWT. As a result, we identified two novel germline DICER1 mutations (G803R and R800Xfs5) co-segregating in two families, thus expanding the number of reported WT families with unique germline DICER1 mutations. The one large family was found to include individuals with multiple DICER1 Syndrome phenotypes, including 4 WT cases. Interestingly, carriers of the DICER1 mutation displayed a greatly increased frequency of WT development compared to the penetrance observed in previously published pedigrees. Also uniquely, in one tumor this DICER1 mutant allele (G803R) was reduced to homozygosity, in contrast to the somatic hotspot mutations typically observed in tumors in DICER1 families.
Keywords: Cancer genetics, microRNA
INTRODUCTION
Wilms tumor (WT) is a pediatric kidney cancer arising from undifferentiated embryonal mesenchyme. WT is diagnosed in 1 in 10,000 live births in the United States. Although the majority of these cases are sporadic, roughly 2% occur in patients who have an affected relative.[1] In these familial cases, WT predisposition displays an autosomal dominant inheritance pattern with incomplete penetrance estimated at 30%.[2–4] The age of onset for sporadic cases is 42–47 months compared to 30–33 months for familial WT cases, although this varies between families.[5] Familial predisposition genes have been localized by genetic linkage analysis to 17q12-21 and 19q13.3, although neither has been identified. The lack of linkage of some WT families to these regions indicates additional familial WT genes exist.[6–7] The development of WT is observed in several syndromes, including Beckwith-Wiedeman, Denys-Drash, WAGR, Perlman syndrome, and rarely, in DICER1 syndrome.[8–9] DICER1 syndrome is a pleiotropic cancer syndrome comprising a range of phenotypes, including but not limited to pleuropulmonary blastoma (PPB), cystic nephroma (CN), embryonal rhabdomyosarcoma (ERMS), ovarian sex cord-stromal tumors, multinodular goiter (MNG) and WT.[9–14] While PPB, CN, ERMS and MNG are common in DICER1 syndrome, the incidence of WT within reported families is low.[11] To date, few DICER1 families have been reported that each contain one WT case. Here we describe a family in which whole genome sequencing was used to identify the gene mutation contributing to the development of WT. As a result, we identified a novel germline DICER1 mutation that segregates with the affected or obligate carrier status within the family and shows loss of heterozygosity (LOH) in a tumor sample. In total, we identified novel germline DICER1 mutations in 2/48 (4.2%) WT families.
PATIENTS AND METHODS
Patients
Following informed consent, blood was drawn and DNA isolated from affected individuals and/or obligate carriers from 48 WT families. Of these, four families carried germline mutations in WT1, one of the most frequently mutated genes in WTs. The two families reported here did not carry WT1 mutations.
Family 1 consisted of four individuals diagnosed with unilateral WT, with the age of diagnosis ranging from 38–57 months. Additionally, thyroid colloid cysts, MNG, and kidney multi colloid cysts, and/or pulmonary cysts were noted upon further review of family medical records. Fresh-frozen Wilms tumor was available from one individual.
In the second family (Family 2) two individuals were diagnosed with WT, one at 3.5 years of age and the other at 13 years of age. The latter, the proband, relapsed after two years and was found by CT scan to have two small pulmonary nodules which, however, were too small to biopsy. Additionally the father of the proband had one kidney removed at 3 years of age due to a suspected, but poorly documented, premalignant condition.
Whole genome sequencing
Whole genome sequencing of peripheral blood DNA was carried out on two affected individuals and one obligate carrier individual in Family 1. Libraries were generated and sequenced using the Illumina HiSeq2000 platform, producing 100bp paired-end reads. Sequence reads were aligned to the hg19 reference genome and variants were identified using the Genome Analysis Toolkit (GATK). Variants were compared between family members to identify shared heterozygous alterations and filtered using dbSNP and 1000 Genomes databases to remove variants with a minor allele frequency greater than 1% in those databases. Novel variants in exonic regions which could potentially affect protein structure (nonsynonymous and frameshift variants) were identified and annotated using ANNOVAR. dbNSFP was used to predict whether or not the amino acid changes were damaging, and PhyloP was used to assess conservation at the variant site.
Sanger sequencing
Sanger sequencing was performed to confirm the DICER1 variant detected by WGS and to assess 47 additional families for mutations in the same DICER1 exon (exon 15) or seven other DICER1 exons (exons 8, 9, 13, 14, and 24–26) at which mutations have been reported in WT.
RESULTS
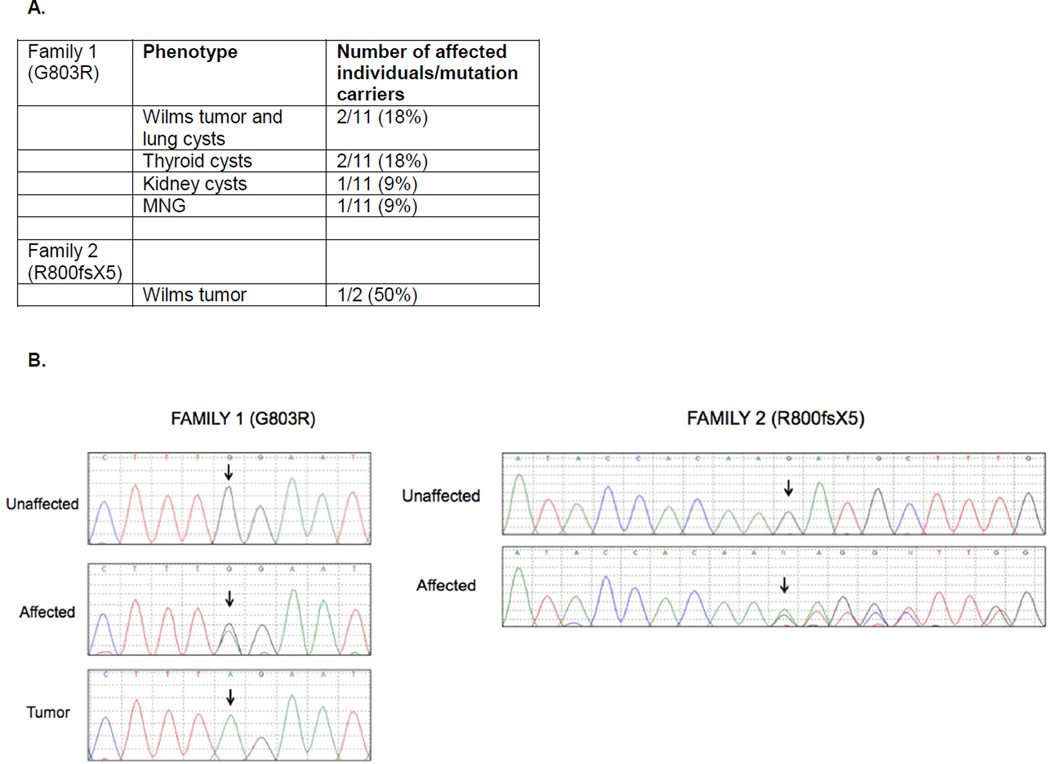
The mean coverage across all genes for the three individuals sequenced from Family 1 ranged from 32–45X, with 97% coverage of genes at >10X depth. Shared variants were identified and filtered based on their allele frequency reported in the general population (<1%), with novel variants occurring in protein coding regions assigned a higher priority for investigation. Of the 113 variants left after filtering, a novel missense mutation in DICER1 was of greatest interest because mutations in this gene had recently been found in DICER1 syndrome families which include a member with WT[14], and, upon closer examination of family history and medical records, it was noted that other phenotypes of DICER1 syndrome were present in some family members (Figure 1A). The identified mutation (c.2407G>A, p.G803R, chr14:95574690, hg19) occurred in exon 15 of DICER1 (Figure 1B). The variant was not present in the Single Nucleotide Polymorphism database (dbSNP), NHLBI Exome Variant Server, nor 1000 Genomes database. This alteration is predicted to be deleterious by dbNSFP. The mutation occurred at an evolutionary conserved amino acid residue (PhyloP – 0.999) within the platform domain of DICER1, which is involved in the recognition of the 5’ phosphate group of pre-miRNAs, and between residues that interact with the 5' phosphate of miRNAs.[15] We confirmed the alteration by Sanger sequencing and extended our analysis to additional family members to estimate the penetrance of the variant in the family. In total, we detected the G803R mutation in eleven family members, some of whom displayed one or more of the phenotypes associated with familial DICER1 syndrome, including lung cysts, thyroid cysts, MNG, kidney cysts, and WT. The one tumor available for study was found to be homozygous for this mutation. Such homozygosity for a germline DICER1 mutation has not previously been reported for Wilms tumors, although this has been observed in cases of DICER1-associated pineoblastoma and pituitary blastoma [16].
Figure 1.
A. Included in the table are the phenotypes found in each family, as well as the number of affected individuals and mutation carriers. B. DICER1 exon 15 Sanger sequences indicating the presence of a heterozygous G803R mutation (Family 1) and R800fsX5 (Family 2) in affected family members, as well as loss of the wildtype allele in a tumor sample in Family 1.
Sanger sequence analysis of one member from 47 additional WT families revealed that one carried a novel frameshift mutation (c.2399delG, p.R800fsX5) in the same exon (exon 15) as that in Family 1 (Figure 1B). This mutation also occurs in the evolutionarily conserved platform domain and segregates with the affected status in the family. It is predicted to encode a truncated protein lacking an intact platform domain and the remaining half of the protein, including the PAZ domain and the RNase IIIa and IIIb domains. Tumor samples were not available for this family; LOH could not be assessed.
No germline variants were observed in the other 46 families in our analysis of exon 15 and seven other exons in which DICER1 mutations have been previously identified in WT cases (exons 8, 9, 13, 14, 24, 25, 26).
DISCUSSION
Germline mutations in DICER1, an endoribonuclease critical for the generation of mature microRNAs, are associated with a pleiotropic tumor predisposition syndrome called DICER1 syndrome. In this syndrome, inherited truncating mutations and somatically acquired missense mutations occurring at metal-ion binding residues within the RNaseIIIb domain are typically found in families. The result of these biallelic mutations is impaired 5p miRNA biogenesis, including major miRNA families such as let-7, while 3p miRNA levels are unaltered.[17] This reduction of 5p miRNAs is thought to contribute to the development of DICER1-associated phenotypes through the deregulation of target genes.
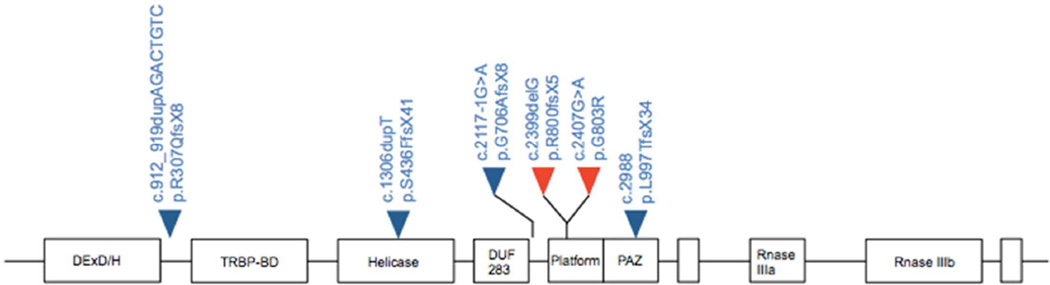
To date, few DICER1 families with a member diagnosed with WT have been reported in the literature and each of these has only one WT case. In these rare families, similar to non-WT DICER1 families, germline DICER1 nonsense mutations were observed, along with an additional somatic missense mutation occurring in trans in the tumor.[14] In our study, we identified two WT families with novel germline DICER1 mutations. Both germline mutations occurred in the platform domain, which has a role in recognizing the terminal phosphate group of the 5p strand of pre-miRNAs for correct positioning over the RNase III domains for cleavage (Figure 2). Previous studies have shown mutations in this domain result in reduced processing efficiency of mature miRNAs.[17] Consistent with what has been observed in other DICER1 families, the novel mutation (R800Xfs5) we identified in Family 2 is predicted to result in the premature truncation of the protein. Family 2 is a small WT family, and, while it is not known whether individuals in this family displayed phenotypes associated with DICER1 syndrome, pulmonary nodules were detected in the proband by CT scan. These nodules, however, were too small for biopsy and it is unknown if they were WT metastases or were similar to the cystic pulmonary phenotype that is observed in DICER1 Syndrome. Uniquely, multiple family members were diagnosed with WT in this small family; DICER1 families reported to date have only a single WT case.
Figure 2.
The germline mutations identified in Family 1 and 2 (red triangles) are located within the platform domain of DICER1. DICER1 mutations previously reported in Wilms tumor cases are shown (blue triangles).
The diagnosis, not only of WT, but also of MNG, lung cysts, thyroid and kidney cysts in Family 1 is consistent with this being a “DICER1 syndrome family”. In total, 11 individuals were found to carry the germline mutation, with 2/11 diagnosed with WT and lung cysts, 2/11 with thyroid cysts, 1/11 with MNG and 1/11 with kidney cysts. There are 2 other WT cases in the family, but DNA was not available from these individuals. In addition to the presence of multiple WT cases, Family 1 is unique on several counts. First, it carries a germline DICER1 missense mutation rather than a frameshift or nonsense mutation. Only one other family (in which only MNG was observed) is reported to have sustained a germline missense mutation. Interestingly, this mutation (Ser839Phe) also occurred in the platform domain. The G803R mutation we identified in Family 1 is predicted to be deleterious by a number of functional prediction algorithms and occurs at an evolutionarily conserved amino acid residue, suggesting this mutation is functionally significant.
Secondly, the germline G803R mutation in Family 1 was reduced to homozygosity in a tumor from a family member. In contrast, almost all the second, somatic mutations observed in tumors from individuals with germline DICER1 mutations are missense mutations that occur in the Rnase IIIb domain of DICER1. To date, LOH with retention of the germline mutation has only been reported in two DICER1-associated phenotypes – pineoblastoma and pituitary blastoma. LOH has not previously been reported in Wilms tumors with DICER1 mutations.
Thirdly, like Family 2, Family 1 represents a DICER1 Syndrome family with multiple individuals with WT. The penetrance of the G803R allele with respect to Wilms tumor is notably much higher than other germline DICER1 mutations (which, as noted above, are almost invariably truncating mutations). The basis for this increased penetrance is not known. Obviously, ascertainment bias is a factor as the families being studied were originally selected due to the occurrence of two or more WT cases. However, is it still curious that families ascertained for PPB and other, DICER1-associated phenotypes only rarely have a member with WT (much less two or more), while in both families reported here, the DICER1 mutations were relatively highly penetrant with respect to WT. One factor impacting penetrance could be the type of germline mutation present in the family. Published data indicate that biallelic mutation of DICER1 is necessary for WT development. Therefore the nature of the second, somatic alterations may affect penetrance.
Interestingly, while the penetrance of the two novel DICER1 mutations we identified in these two families was unusually high, the ages at which individuals in these families were diagnosed with WT was notably older than average. The median age of diagnosis for unilateral WT overall is ~39 months of age while that of unilateral cases in familial context is 35 months.[18–19] WT families, however, greatly vary with respect to age at WT diagnosis, with some families exhibiting typically younger ages, while other families consist of cases with later than average ages of onset.[20] The observation that both WT families carrying DICER1 mutation reported here have members diagnosed at late ages suggests that this may be a common feature of DICER1-related WT.
In conclusion, we identified two novel DICER1 germline mutations in 2/48 WT families, observed LOH in a WT sample with a germline DICER1 mutation, and presented a ‘DICER1 family’ with a higher incidence of WT than previously reported in the literature. Thus, we have further expanded the number of reported familial WT cases with unique germline DICER1 mutations.
Acknowledgments
The authors would like to thank the patients and their families for participation in the study, the many physicians and genetic counselors who assisted with recruiting participants, and Phyllis Begin for patient enrollment and sample collection. This study was supported by NIH grants CA34936, DK069599, NCI CCSG grant CA16672, CPRIT RP100329, CPRIT RP110324, and NIH/NCI T32 CA009299.
Contributor Information
Vicki Huff, The University of Texas MD Anderson Cancer Center, Department of Genetics Unit 1010, 1515 Holcombe Blvd., Houston, TX 77030, 713-834-6384, vhuff@mdanderson.org.
Timothy Blake Palculict, The University of Texas MD Anderson Cancer Center, Department of Genetics, Houston, TX, USA.
E. Cristy Ruteshouser, The University of Texas MD Anderson Cancer Center, Department of Genetics, Houston, TX, USA.
Yu Fan, The University of Texas MD Anderson Cancer Center, Department of Bioinformatics and Computational Biology, Houston, TX, USA.
Wenyi Wang, The University of Texas MD Anderson Cancer Center, Department of Bioinformatics and Computational Biology, Houston, TX, USA.
Louise Strong, The University of Texas MD Anderson Cancer Center, Department of Genetics, Houston, TX, USA.
REFERENCES
- 1.Huff V. Wilms tumor genetics. AM J Med Genet. 1998;79:260–267. doi: 10.1002/(sici)1096-8628(19981002)79:4<260::aid-ajmg6>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 2.Brown WT, Puranik SR, Altman DH, Hardin HC., Jr Wilms’ tumor in three successive generations. Surgery. 1972;72(5):756–761. [PubMed] [Google Scholar]
- 3.Knudson AG, Jr, Strong LB. Mutation and cancer: a model for Wilms’ tumor of the kidney. J Natl Cancer Inst. 1972;48(2):313–324. [PubMed] [Google Scholar]
- 4.Matsunaga E. Genetics of Wilms’ tumor. Hum Genet. 1981;57(3):231–246. doi: 10.1007/BF00278936. [DOI] [PubMed] [Google Scholar]
- 5.Breslow N, Olshan A, Beckwith JB, Green DM. Epidemiology of Wilms tumor. Med Pediatr Oncol. 1993;21(3):172–181. doi: 10.1002/mpo.2950210305. [DOI] [PubMed] [Google Scholar]
- 6.McDonald JM, Douglass EC, Fisher R, Geiser CF, Krill CE, Strong LC, Virshup D, Huff V. Linkage of familial Wilms’ tumor predisposition to chromosome 19 and a two-locus model for the etiology of familial tumors. Cancer Res. 1998;58:1387–1390. [PubMed] [Google Scholar]
- 7.Rahman N, Arbour L, Tonin P, Renshaw J, Pelletier J, Baruchel S, Pritchard-Jones K, Stratton MR, Narod SA. Evidence for a familial Wilms’ tumour gene (FWT1) on chromosome 17q12-q21. Nat Genet. 1996;13:461–463. doi: 10.1038/ng0896-461. [DOI] [PubMed] [Google Scholar]
- 8.Hohenstein P, Pritchard-Jones K, Charlton J. The yin and yang of kidney development and Wilms’ tumors. Genes & Dev. 2015;29:467–482. doi: 10.1101/gad.256396.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slade I, Bacchelli C, Davies H, Murray A, Abbaszadeh F, Hanks S, Barfoot R, Burke A, Chisholm J, Hewitt M, Jenkinson H, King D, Morland B, Pizer B, Prescott K, Saggar A, Side L, Traunecker H, Vaidya S, Ward P, Futreal PA, Vujanic G, Nicholson AG, Sebire N, Turnbull C, Priest JR, Pritchard-Jones K, Houlston R, Stiller C, Stratton MR, Douglas J, Rahman N. DICER1 syndrome: clarifying the diagnosis, clinical features and management implications of a pleiotropic tumour predisposition syndrome. J Med Genet. 2011;48(4):273–278. doi: 10.1136/jmg.2010.083790. [DOI] [PubMed] [Google Scholar]
- 10.Hill DA, Ivanovich J, Priest JR, Gurnett CA, Dehner LP, Desruisseau D, Jarzembowski JA, Wikenheiser-Brokamp KA, Suarez BK, Whelan AJ, Williams G, Bracamontes D, Messinger Y, Goodfellow PJ. DICER1 mutations in familial pleuropulmonary blastoma. Science. 2009;325(5943):965. doi: 10.1126/science.1174334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foulkes WD, Bahubeshi A, Hamel N, Pasini B, Asioli S, Baynam G, Choong CS, Charles A, Frieder RP, Dishop MK, Graf N, Ekim M, Bouron-Dal Soglio D, Arseneau J, Young RH, Sabbaghian N, Srivastava A, Tischkowitz MD, Priest JR. Extending the phenotypes associated with DICER1 mutations. Hum Mutat. 2011;32(12):1381–1384. doi: 10.1002/humu.21600. [DOI] [PubMed] [Google Scholar]
- 12.Doros L, Yang J, Dehner L, Rossi CT, Skiver K, Jarzembowski JA, Messinger Y, Schultz KA, Williams G, André N, Hill DA. DICER1 mutations in embryonal rhabdomyosarcomas from children with and without familial PPB-tumor predisposition syndrome. Pediatr Blood Cancer. 2012;59(3):558–560. doi: 10.1002/pbc.24020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bahubeshi A, Bal N, Rio Frio T, Hamel N, Pouchet C, Yilmaz A, Bouron-Dal Soglio D, Williams GM, Tischkowitz M, Priest JR, Foulkes WD. Germline DICER1 mutations and familial cystic nephroma. J Med Genet. 2010;47(12):863–866. doi: 10.1136/jmg.2010.081216. [DOI] [PubMed] [Google Scholar]
- 14.Wu MK, Sabbaghian N, Xu B, Addidou-Kalucki S, Bernard C, Zou D, Reeve AE, Eccles MR, Cole C, Choong CS, Charles A, Tan TY, Iglesias DM, Goodyer PR, Foulkes WD. Biallelic DICER1 mutations occur in Wilms tumours. J Pathol. 2013;230(2):154–164. doi: 10.1002/path.4196. [DOI] [PubMed] [Google Scholar]
- 15.Park JE, Heo I, Tian Y, Simanshu DK, Chang H, Jee D, Patel DJ, Kim VN. Dicer recognizes the 5’ end of RNA for efficient and accurate processing. Nature. 2011;475(7355):201–205. doi: 10.1038/nature10198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Kock L, Sabbaghian N, Druker H, Weber E, Hamel N, Miller S, Choong CS, Gottardo NG, Kees UR, Rednam SP, van Hest LP, Jongmans MC, Jhangiani S, Lupski JR, Zacharin M, Bouron-Dal Soglio D, Huang A, Priest JR, Perry A, Mueller S, Albrecht S, Malkin D, Grundy RG, Foulkes WD. Germ-line and somatic DICER1 mutations in pineoblastoma. Acta Neuropathol. 2014;128(4):583–595. doi: 10.1007/s00401-014-1318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anglesio MS, Wany Y, Yang W, Senz J, Wan A, Heravi-Moussavi A, Salamanca C, Maines-Bandiera S, Huntsman DG, Morin GB. Cancer-associated somatic DICER1 hotspot mutations cause defective miRNA processing and reverse-strand expression bias to predominantly mature 3p strands through loss of 5p strand cleavage. J Pathol. 2013;229(3):400–409. doi: 10.1002/path.4135. [DOI] [PubMed] [Google Scholar]
- 18.Breslow N, Beckwith JB, Ciol M, Sharples K. Age distribution of Wilms’ tumor: report from the National Wilms’ Tumor Study. Cancer Res. 1988;48(6):1653–1657. [PubMed] [Google Scholar]
- 19.Breslow NE, Olson J, Moksness J, Beckwith JB, Grundy P. Familial Wilms’ tumor: a descriptive study. Med Pediatri Oncol. 1996;27(5):398–403. doi: 10.1002/(SICI)1096-911X(199611)27:5<398::AID-MPO2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 20.Huff V, Amos CI, Douglass EC, Fisher EC, Geiser CF, Krill CE, Li FP, Strong LC, McDonald JM. Cancer Res. 1997;57(10):1859–1862. [PubMed] [Google Scholar]