Supplemental Digital Content is Available in the Text.
Key Words: HIV-1, drug resistance, transmitted, integrase inhibitors, outcomes
Abstract
Background:
Nonnucleoside reverse transcriptase inhibitor (NNRTI)–associated transmitted drug resistance (TDR) is the most common type of TDR. Few data guide the selection of antiretroviral therapy (ART) for patients with such resistance.
Methods:
We reviewed treatment outcomes in a cohort of HIV-1–infected patients with isolated NNRTI TDR who initiated ART between April 2002 and May 2014. In an as-treated analysis, virological failure (VF) was defined as not reaching undetectable virus levels within 24 weeks, virological rebound, or switching regimens during viremia. In an intention-to-treat analysis, failure was defined more broadly as VF, loss to follow-up, and switching during virological suppression.
Results:
Of 3245 patients, 131 (4.0%) had isolated NNRTI TDR; 122 received a standard regimen comprising 2 NRTIs plus a boosted protease inhibitor (bPI; n = 54), an integrase strand transfer inhibitor (INSTI; n = 52), or an NNRTI (n = 16). The median follow-up was 100 weeks. In the as-treated analysis, VF occurred in 15% (n = 8), 2% (n = 1), and 25% (n = 4) of patients in the bPI, INSTI, and NNRTI groups, respectively. In multivariate regression, there was a trend toward a lower risk of VF with INSTIs than with bPIs (hazard ratio: 0.14; 95% confidence interval: 0.02 to 1.1; P = 0.07). In intention-to-treat multivariate regression, INSTIs had a lower risk of failure than bPIs (hazard ratio: 0.38; 95% confidence interval: 0.18 to 0.82; P = 0.01).
Conclusions:
Patients with isolated NNRTI TDR experienced low VF rates with INSTIs and bPIs. INSTIs were noninferior to bPIs in an analysis of VF but superior to bPIs when frequency of switching and loss to follow-up were also considered.
BACKGROUND
The reported prevalence of transmitted drug resistance (TDR) in the United States is between 15% and 19%.1–3 Transmitted nonnucleoside reverse transcriptase inhibitor (NNRTI) resistance has been increasing, and it is now the most common form of TDR.4 Despite the fact that a significant proportion of patients starting antiretroviral therapy (ART) have NNRTI-associated TDR, no studies have compared responses to different ART regimens in this population.
In the absence of studies informing the selection of ART in patients with transmitted NNRTI resistance, these patients have historically been treated with boosted protease inhibitors (bPIs) because of their high genetic barrier to resistance.5,6 Outcomes in these predominantly bPI-treated patients have been comparable to or slightly worse than those infected with wild-type virus.5–8 Although integrase strand transfer inhibitors (INSTIs) are now the most commonly recommended drug class due to their favorable tolerability, safety, and efficacy profile, there are few published data on the use of INSTIs in patients with NNRTI TDR.9 One clinical trial, in which 31 patients with NNRTI TDR started an INSTI-based regimen, showed favorable outcomes; however, no direct comparisons to other regimens were made.10
NNRTI TDR will not directly compromise INSTIs, but there is concern that their lower genetic barrier to resistance relative to bPIs could lead to higher rates of virological failure (VF).9,11 Patients with NNRTI TDR may have a higher prevalence of minority variant nucleoside reverse transcriptase inhibitor (NRTI) TDR,8,9,11–13 and such minority variants are able to render less robust regimens ineffective.14 Therefore, we sought to evaluate outcomes in patients with NNRTI TDR initiating a variety of regimens, with a particular focus on INSTIs.
METHODS
Patients and Treatments
All HIV-1–infected, ART-naive adult patients in the Kaiser Permanente Northern California (KPNC) Medical Care Program undergoing standard genotypic resistance testing (SGRT) by population sequencing before ART initiation between April 2002 and May 2014 were screened for inclusion. The inclusion criterion were (1) isolated NNRTI resistance defined as an initial genotype containing 1 or more NNRTI-associated surveillance drug-resistance mutations (SDRMs) without any NRTI or protease inhibitor (PI)–associated SDRMs and (2) treatment with a standard regimen defined as a dual NRTI backbone plus a bPI, INSTI, or NNRTI received by 2 or more patients with isolated NNRTI resistance.15 The NNRTI-associated SDRMs are L100I, K101E/P, K103N/S, V106A/M, V179F, Y181C/I/V, Y188L/H/C, and G190S/A/E, P225H, and M230L. Patient demographics, antiretroviral (ARV) treatment histories, plasma HIV-1 RNA levels, and CD4 counts were obtained from the KPNC HIV Registry. Between the beginning of the study period and January 2014, the Versant HIV-1 RNA bDNA v 3.0 assay was used (LLQ: 75 copies per milliliter; Bayer Diagnostic, Tarrytown, NY), and from January 2014 until the end of the study period, the COBAS HIV-1 RNA PCR v 2.0 assay was used (LLQ: 48 copies per milliliter; Roche Molecular Diagnostics, Pleasanton, CA). Additional data such as reasons for loss to follow-up (LTFU) or treatment failure were obtained from the review of provider notes. The Stanford University and KPNC Institutional Review Boards approved this study.
Virological Responses to ART
Virological responses to therapy were monitored for up to 2 years after ART initiation. To describe the effect of the anchor-drug class on the clinical outcomes in patients with isolated NNRTI-associated TDR, we performed 2 types of analyses: (1) an as-treated analysis focused on VF and (2) an intention-to-treat (ITT) analysis including not only VF but also changes in therapy and LTFU. In both the analyses, virological suppression (VS) was defined as having an HIV-1 RNA level below the limit of quantification. VF was defined as (1) failure to achieve VS by 24 weeks of ART, (2) ≥2 consecutive (confirmed) HIV-1 RNA levels ≥200 copies per milliliter following VS, or (3) changing therapy in patients with an elevated virus load. In the as-treated analysis, patients who changed therapy with a suppressed virus load were censored, whereas these patients were considered to have experienced failure events at the time of switch in the ITT analysis. Additionally, patients who were LTFU were censored in the as-treated analysis but considered to have developed failure events in the ITT analysis. All patients who did not develop a failure event were censored at 2 years or at the end of the observation period in March of 2015, whichever came first.
Statistical Analysis
We compared patient demographics, pretherapy CD4 counts, plasma HIV-1 RNA levels, year of ART initiation, NRTI backbone, and frequency of virological monitoring among the 3 anchor-drug classes. Kruskal–Wallis testing was used to compare continuous variables among the 3 anchor-drug classes, and Wilcoxon rank sum testing was used in 2-group comparisons to determine which groups were driving observed differences. Fisher exact testing was used for comparisons of categorical variables.16 We performed univariate Cox regression and Kaplan–Meier analyses to compare the effect of anchor-drug class on failure outcomes. We also performed univariate Cox regression analysis to identify additional explanatory variables significantly associated with outcome defined as those having a P value of ≤ 0.05. We performed multivariate Cox regression analyses that included anchor-drug class and those additional explanatory variables were found to be significantly associated with outcome in our univariate analyses.
Complementary Analysis of Virological Outcomes in ARV-Naive Patients Without TDR
To evaluate the impact of NNRTI TDR on virological responses to ART, we performed a complementary analysis in ART-naive patients from the KPNC population who did not have NNRTI, NRTI, or PI-associated SDRMs. This cohort included patients undergoing genotypic resistance testing before ART initiation between October 2002 and March 2014. Patients initiating nonstandard regimens were excluded as in our main analysis. Patients who did not develop VF were censored at 2 years of follow-up, LTFU, or at the end of the observation period. An as-treated analysis of overall and treatment group–specific cumulative risks of VF during the follow-up period was performed using the definitions in the NNRTI TDR analysis. Fisher exact testing was used to compare the risks of VF to the NNRTI TDR cohort. Because our analysis of this cohort was limited to the KPNC HIV registry, it did not include provider notes, confirmation of LTFU, or the reasons for LTFU and changing therapy. Therefore, we did not perform a time-dependent or ITT analysis for this complementary analysis.
RESULTS
Patient and ART Characteristics
Of 3245 ART-naive patients undergoing SGRT before ART initiation, 165 (5.1%) had NNRTI-associated TDR. Of these 165, 131 (79%; or 4.0% of all patients) had isolated NNRTI-associated TDR. Nine patients (7%) were excluded from analysis because they received nonstandard regimens as defined in the Methods (see Table S1, Supplemental Digital Content, http://links.lww.com/QAI/A786). The NNRTI-resistance mutations among the remaining 122 patients are listed in Table 1. The most common NNRTI SDRM was K103N, which was present in 95 patients (78%), followed by Y188L present in 9 patients (7%), Y181C present in 7 patients (6%), and G190A in 5 patients (4%). Two patients (2%) had multiple NNRTI SDRMs. Several non-SDRM NNRTI drug-resistance mutation (DRMs) were also present, occurring in combination with NNRTI SDRMs, including V108I in 2 patients (2%), K238T in 1 patient (1%), and A98G in 1 patient (1%).
TABLE 1.
Prevalence of SDRM Patterns
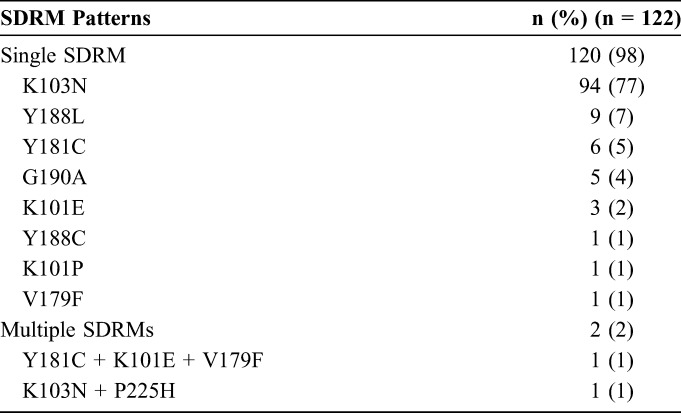
Forty-four percent of patients (n = 54) received a bPI including 25 who received atazanavir, 20 who received darunavir, and 9 who received lopinavir. Forty-three percent (n = 52) received an INSTI, including 31 who received raltegravir and 21 who received elvitegravir (EVG). Thirteen percent (n = 16) received an NNRTI including 9 who received rilpivirine (RPV), 5 who received efavirenz (EFV), and 2 who received etravirine. The median duration of follow-up was 104 weeks in the bPI group, 72 weeks in the INSTI group, and 65 weeks in the NNRTI group. Ninety percent (n = 110) of the 122-patient cohort had complete monitoring (either 2 years of follow-up or ongoing monitoring at the end of the follow-up period). Ten percent (n = 12) were LTFU, including 9 (17%) in the PI group, 2 (4%) in the INSTI group, and 1 (6%) in the NNRTI group.
Table 2 shows the baseline demographic characteristics, CD4 counts, and plasma HIV-1 RNA levels of the analysis cohort according to the anchor-drug class of their initial ART regimen. The patients were predominantly male (87%) and had a median age of 39 years. Forty-five percent were white, 22% were Hispanic, and 17% were black. There was a significant difference in race/ethnicity by anchor-drug class that appeared to be explained in part by a greater proportion of black patients receiving a bPI. The overall median baseline HIV-1 RNA was 4.5 log copies per milliliter, and the overall median baseline CD4 count was 343 cells per cubic millimeter. There was a difference in CD4 counts between the groups that appeared to be largely explained by a lower median CD4 count among those receiving bPIs compared with those receiving INSTIs (283 vs. 401; P = 0.006).
TABLE 2.
Patient Characteristics by Anchor-Drug Class
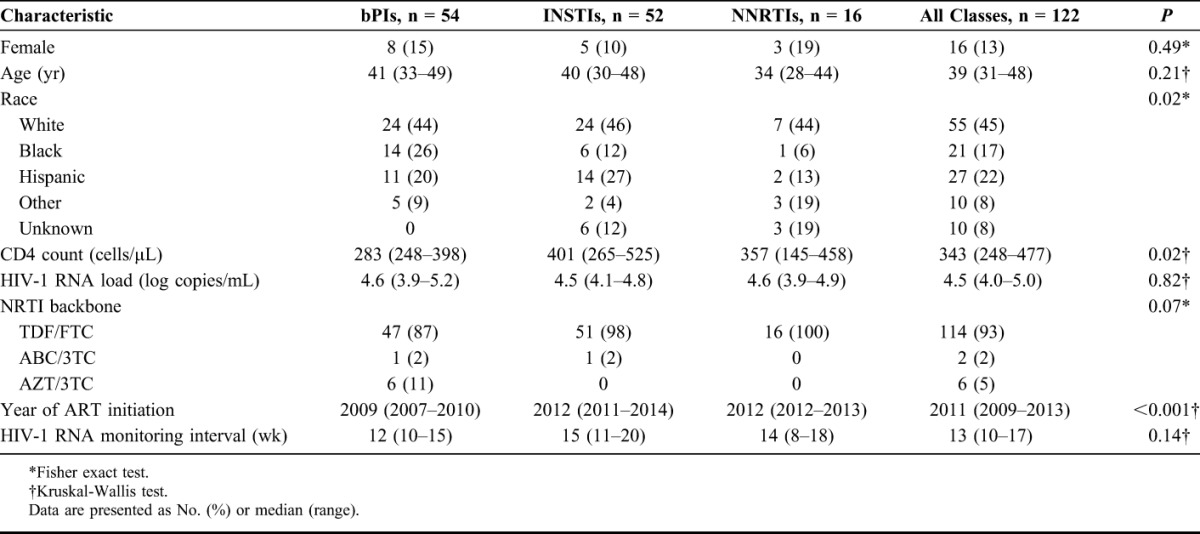
The median year of ART initiation was earlier for those receiving bPIs (2009) compared with those receiving INSTIs (2012; P < 0.001) or NNRTIs (2012; P < 0.001). Virus loads were measured a median of every 13 weeks, with no significant differences in monitoring between the groups. The NNRTI backbones used in each group were predominantly emtricitabine (FTC)/tenofovir (TDF) (93%) and were similar between the groups. However, each of the 6 patients receiving zidovudine (AZT)/lamivudine (3TC) also received lopinavir (Table 2).
Patient Outcomes According to Treatment: As treated
Overall 13 patients (11%) developed VF, including 8 patients (15%) in the bPI group, 1 patient (2%) in the INSTI group, and 4 patients (25%) in the NNRTI group. A log-rank test demonstrated significant differences in the survival curves between the 3 anchor-drug classes (P = 0.005; Kaplan–Meier survival curves shown in Fig. 1). Table 3 shows the results of the Cox proportional hazard univariate analyses comparing the risk of VF associated with each pair of anchor-drug classes. There was a trend toward a lower VF rate in the INSTI group compared with the bPI group [hazard ratio (HR): 0.15; 95% confidence interval (CI): 0.02 to 1.20; P = 0.07] and a lower risk of VF in the INSTI group compared with the NNRTI group (HR: 0.06; 95% CI: 0.01 to 0.50; P = 0.01). The risk of VF was higher in the NNRTI group compared with the bPI group (HR: 2.7; 95% CI: 0.80 to 9.0; P = 0.11), but this difference did not reach statistical significance.
FIGURE 1.
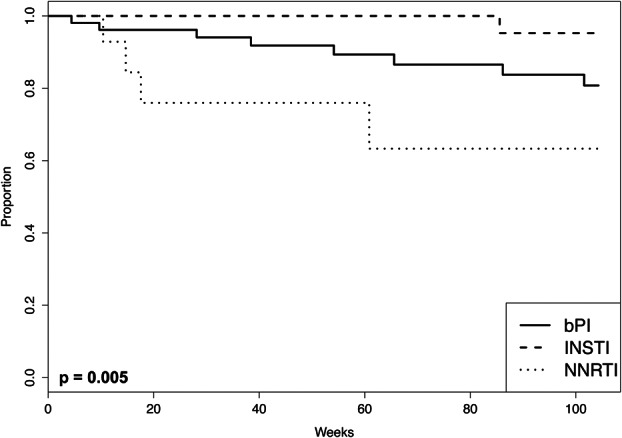
The Kaplan–Meier plot shows the cumulative incidence of patients free of failure events per the as-treated analysis according to the anchor-drug class. The as-treated analysis failure outcome was VF, defined as not reaching an undetectable HIV-1 RNA level by 24 weeks, virological rebound, and regimen switching during viremia. The P value was calculated by the log-rank test.
TABLE 3.
Univariate and Multivariate As-Treated Analysis
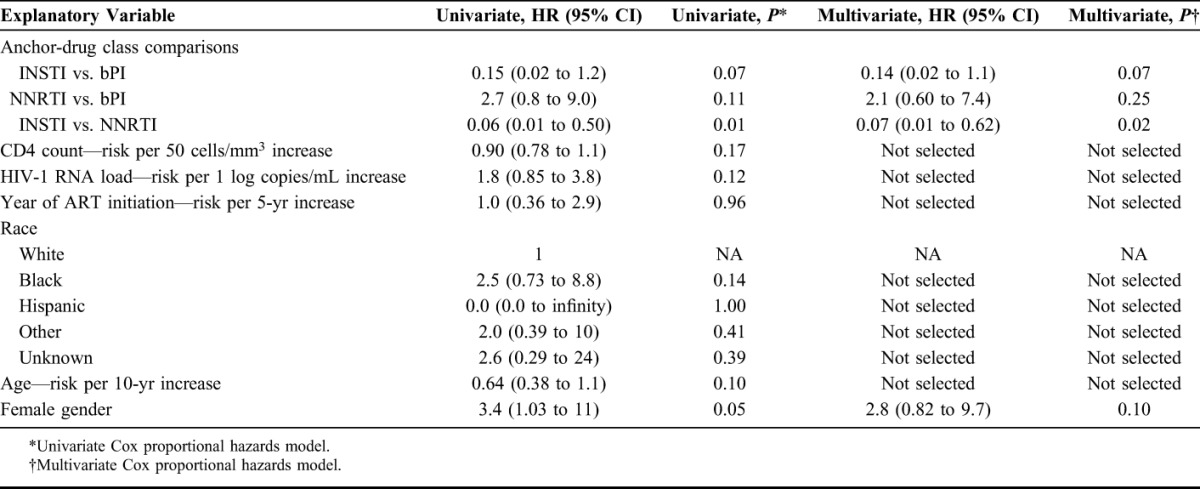
A univariate screen of variables other than the anchor-drug class using Cox proportional hazard analysis identified female gender (HR: 3.4; 95% CI: 1.03 to 11; P = 0.05; Table 3) as being associated with VF. In a multivariate analysis that included anchor-drug class and gender, the trend toward a reduced risk of VF associated with INSTIs compared with bPIs was still present (HR: 0.14; 95% CI: 0.02 to 1.1; P = 0.07). However, gender was no longer associated with VF (Table 3).
Of the 8 patients with VF on a bPI-based regimen, 6 had documented reduced adherence and 2 had PI-associated adverse reactions requiring a change in therapy. One patient underwent repeat genotypic testing, which did not contain additional drug-resistance mutations. Three of the nonadherent patients continued the same regimen after VF, each of whom attained virological suppression. The patients who developed VF in the bPI group had a median viral load (VL) of 4.4 log copies per milliliter and a median CD4 count of 282 cells per cubic millimeter—which were not significantly different from the bPI group as a whole. None of the patients received AZT/3TC.
Of the 4 patients with VF on an NNRTI, none had documented reduced adherence. Each of these 4 patients had repeat genotypes, which in 2 patients demonstrated new drug-resistance mutations. An EFV-treated patient with a baseline Y181C mutation developed the NRTI-associated DRMs M184I and K219N and an additional NNRTI-associated mutation Y188L. An RPV-treated patient with a baseline K103N mutation developed the NRTI-associated mutations D67G and M184I and the additional NNRTI-associated mutation L100I. Interestingly, only 2 of the 5 patients treated with EFV developed VF including 1 patient with baseline Y188L, 1 of 2 patients with baseline Y181C, but none of 2 patients with K103N. Comparisons of VL and CD4 count among patients with VF to the overall NNRTI means was not performed due to the low sample size in this group.
The 1 patient with VF on an INSTI received TDF/FTC/EVG/cobicistat and was not reported to have reduced adherence. This patient had a VL of 5.5 log copies per milliliter and a CD4 count of 434 cells per cubic millimeter, which were not significantly different from the overall INSTI group means. Genotypic testing was not repeated, and the patient resuppressed with no change in regimen.
Patient Outcomes According to Treatment: ITT
Overall, 42 patients (34%) had a failure event within the follow-up period per the ITT analysis including 25 patients (46%) in the bPI group, 9 (17%) in the INSTI group, and 8 (50%) in the NNRTI group. In the bPI group, there were 8 patients with VF events, 9 patients who were LTFU, and 8 patients who underwent treatment changes despite having sustained virological suppression. In the INSTI group, there was 1 VF event, 2 LTFU, and 6 switches during suppression, and in the NNRTI group, there were 4 VFs, 1 LTFU, and 3 switches during suppression. Kaplan–Meier survival curves are shown in Figure S1 (see Supplemental Digital Content, http://links.lww.com/QAI/A7860) and a log-rank testing showed significant differences by anchor-drug class (P = 0.009). The univariate Cox proportional hazard analysis demonstrated a significantly lower risk of failure in the INSTI group relative to the bPI group (HR: 0.43; 95% CI: 0.20 to 0.92; P = 0.03) (see Table S2, Supplemental Digital Content, http://links.lww.com/QAI/A786). The risk of failure was higher in the NNRTI group compared with the bPI group, but the difference did not reach statistical significance (HR: 1.7; 95% CI: 0.78 to 3.9; P = 0.18).
In contrast to the univariate as-treated analysis, the univariate ITT analysis identified older age (but not gender) as associated with a lower risk of failure (HR: 0.65 per 10 year increase; 95% CI: 0.49 to 0.87; P = 0.004) (see Table S2, Supplemental Digital Content, http://links.lww.com/QAI/A786). Table S2 (Supplemental Digital Content, http://links.lww.com/QAI/A786) shows that both anchor-drug class and age remained associated with VF in the multivariate ITT analysis. Relative to bPIs, INSTIs were associated with a lower risk of failure (HR: 0.38; 95% CI: 0.18 to 0.82; P = 0.01). NNRTIs had a higher HR than bPIs, but the increased risk was not statistically significant (HR: 1.66; 95% CI: 0.74 to 3.7; P = 0.22).
Complementary Analysis of Response to ART in Patients Without TDR
Of 1939 ART-naive patients without TDR initiating a standard regimen, 536 patients (28%) received bPIs, 180 (9%) received INSTIs, and 1223 (63%) received NNRTIs. Overall, 176 patients (9%) developed VF during the follow-up period: 85 patients (16%) in the bPI group, 6 (3%) in the INSTI group, and 85 (7%) in the NNRTI group. The overall relative risk of VF compared with those in our main analysis of patients with isolated NNRTI-associated TDR was 0.82 (P = 0.5). By treatment group, the relative risk was 1.07 in those receiving bPIs (P = 1.0), 1.50 in those receiving INSTIs (P = 1.0), and 0.28 in those receiving NNRTIs (P = 0.02).
DISCUSSION
NNRTI DRMs reduce viral fitness less than do most NRTI DRMs and are less likely than NRTI DRMs to fade below the 20%–30% detection threshold of SGRT in patients infected with viruses containing both the types.17–19 Because bPIs are usually successful in treating patients with accumulated NRTI resistance,20–22 they are expected to retain activity in settings in which minority variant NRTI TDR may also be present. Indeed, bPIs have been the mainstay in the treatment of patients with TDR. In contrast, it has been uncertain whether raltegravir and elvitegravir, INSTIs with lower genetic barriers to resistance, would remain active in these settings.23–25
The high response rates to therapy in this study among patients receiving both bPI- and INSTI-containing regimens provides the first empirical data suggesting that INSTI-containing regimens are an effective option with at least equal efficacy compared with bPIs for patients with isolated NNRTI TDR. In the as-treated analysis, which was sufficiently powered to detect a margin of inferiority greater than 1.2% with 95% confidence, INSTIs were noninferior to bPIs.
The relatively worse performance of bPIs in the ITT analysis was driven by higher rates of LTFU and switching, with the latter possibly due to more frequent problems with tolerability. NNRTIs were also noninferior to bPIs in both the as-treated and ITT analyses, but the low numbers in this group impeded the ability to detect a statistically significant degree of inferiority. The higher VF rates in the NNRTI group (as-treated analysis: 25% of all NNRTI-treated patients; 40% of EFV-treated patients) suggest that NNRTIs are likely a suboptimal choice in these patients and emphasize the importance of genotype-guided treatment selection.
Our complementary analysis that compared the overall responses to SGRT-guided therapy between patients with isolated NNRTI-associated TDR and those without any TDR showed similarly high responses for patients receiving both bPIs and INSTIs. However, not surprisingly, the response rate to a first-line NNRTI-containing regimen was significantly lower among those with isolated NNRTI resistance.
Our study has 2 main limitations. First, differences between anchor-drug groups may have caused confounding. The small number of observed VFs may have interfered with our ability to detect all significant confounders. The INSTI and bPI groups were found to differ in their year of ART initiation and their CD4 count. Although these variables were not found to be significantly associated with VF, the extent of overlap between these variables in the INSTI and bPI groups may have been insufficient to exclude the possibility of residual confounding. Additionally, the common practice of prescribing bPIs to patients predicted to be less adherent may be a further confounder.
Second, the vulnerability of INSTI-containing regimens to minority variant NRTI-resistance mutations depends on the likelihood that these variants are present within a patient. This in turn depends on the regional dynamics of TDR within the population studied. In a region in which most TDR is stably transmitted among ARV-naive patients, the prevalence of minority variant NRTI-resistance would be expected to be lower compared with a region in which the major source of TDR is treated patients who develop VF.17,18 Therefore, studies of INSTI-containing regimens for the treatment of transmitted NNRTI resistance in populations with different TDR dynamics remain necessary to confirm the effectiveness of INSTI-containing regimens for treating isolated NNRTI-associated TDR.
Supplementary Material
Footnotes
D.S.C. was supported by a grant for this work (T32 AI052073) from the National Institutes of Health. S.-Y.R. and R.W.S. were also supported by a grant (R01 AI068581) from the National Institutes of Health. D.S.C. to receive research funding through the Bristol-Myers Squibb Virology Fellows Research Program in March 2016. R.W.S. is a consultant for Celera, and receives research funding from Roche Molecular, Gilead Sciences, Bristol-Myers Squibb, and Merck. The other authors have no funding or conflicts of interest to disclose.
Part of the data in this article was presented in poster format at the XXIV International HIV Drug Resistance Workshop, February 21, 2015, Seattle. Data from this article was also presented in poster format at IDWeek, October 09, 2015, San Diego.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
REFERENCES
- 1.Wheeler WH, Ziebell RA, Zabina H, et al. Prevalence of transmitted drug resistance associated mutations and HIV-1 subtypes in new HIV-1 diagnoses, U.S.-2006. AIDS. 2010;24:1203–1212. [DOI] [PubMed] [Google Scholar]
- 2.Li J, Kim D, Linley L, et al. Sensitive screening reveals widesperad underestimation of transmitted HIV drug resistance [87]. Presented at: Conference on Retroviruses and Opportunistic Infections; March 3–6, 2014; Boston, MA.
- 3.Ocfemia MCB. Epidemiology of HIV-1 transmitted drug resistance among men who have sex with men in the United States [81]. Presented at: XXIV International HIV Drug Resistance Workshop; February 21–22, 2015; Seattle, WA.
- 4.Rhee SY, Blanco JL, Jordan MR, et al. Geographic and temporal trends in the molecular epidemiology and genetic mechanisms of transmitted HIV-1 drug resistance: an individual-patient- and sequence-level meta-analysis. PLoS Med. 2015;12:e1001810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oette M, Kaiser R, Daumer M, et al. Primary HIV drug resistance and efficacy of first-line antiretroviral therapy guided by resistance testing. J Acquir Immune Defic Syndr. 2006;41:573–581. [DOI] [PubMed] [Google Scholar]
- 6.Zu Knyphausen F, Scheufele R, Kucherer C, et al. First line treatment response in patients with transmitted HIV drug resistance and well defined time point of HIV infection: updated results from the German HIV-1 seroconverter study. PLoS One. 2014;9:e95956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shet A, Berry L, Mohri H, et al. Tracking the prevalence of transmitted antiretroviral drug-resistant HIV-1: a decade of experience. J Acquir Immune Defic Syndr. 2006;41:439–446. [DOI] [PubMed] [Google Scholar]
- 8.Taniguchi T, Nurutdinova D, Grubb JR, et al. Transmitted drug-resistant HIV type 1 remains prevalent and impacts virologic outcomes despite genotype-guided antiretroviral therapy. AIDS Res Hum Retroviruses. 2012;28:259–264. [DOI] [PubMed] [Google Scholar]
- 9.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescentes. Washington D.C.: U.S. Department of Health and Human Services; 2015. Available at: https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed September 1, 2015. [Google Scholar]
- 10.Kulkarni R, Abram ME, McColl DJ, et al. Week 144 resistance analysis of elvitegravir/cobicistat/emtricitabine/tenofovir DF versus atazanavir+ritonavir+emtricitabine/tenofovir DF in antiretroviral-naive patients. HIV Clin Trials. 2014;15:218–230. [DOI] [PubMed] [Google Scholar]
- 11.Tang MW, Shafer RW. HIV-1 antiretroviral resistance: scientific principles and clinical applications. Drugs. 2012;72:e1–e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toni TA, Asahchop EL, Moisi D, et al. Detection of human immunodeficiency virus (HIV) type 1 M184V and K103N minority variants in patients with primary HIV infection. Antimicrob Agents Chemother. 2009;53:1670–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varghese V, Shahriar R, Rhee SY, et al. Minority variants associated with transmitted and acquired HIV-1 nonnucleoside reverse transcriptase inhibitor resistance: implications for the use of second-generation nonnucleoside reverse transcriptase inhibitors. J Acquir Immune Defic Syndr. 2009;52:309–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li JZ, Paredes R, Ribaudo HJ, et al. Low-frequency HIV-1 drug resistance mutations and risk of NNRTI-based antiretroviral treatment failure: a systematic review and pooled analysis. JAMA. 2011;305:1327–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett DE, Camacho RJ, Otelea D, et al. Drug resistance mutations for surveillance of transmitted HIV-1 drug-resistance: 2009 update. PLoS One. 2009;4:e4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. Available at: http://www.R-project.org/. Accessed September 1, 2015. [Google Scholar]
- 17.Castro H, Pillay D, Cane P, et al. Persistence of HIV-1 transmitted drug resistance mutations. J Infect Dis. 2013;208:1459–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jain V, Sucupira MC, Bacchetti P, et al. Differential persistence of transmitted HIV-1 drug resistance mutation classes. J Infect Dis. 2011;203:1174–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buckton AJ, Prabhu D, Motamed C, et al. Increased detection of the HIV-1 reverse transcriptase M184V mutation using mutation-specific minority assays in a UK surveillance study suggests evidence of unrecognized transmitted drug resistance. HIV Med. 2011;12:250–254. [DOI] [PubMed] [Google Scholar]
- 20.Paton N, Kityo C, Hoppe A, et al. Assessment of second-line antiretroviral regimens for HIV therapy in Africa. N Engl J Med. 2014;371:234–247. [DOI] [PubMed] [Google Scholar]
- 21.Boyd M, Moore C, Molina J, et al. Baseline HIV-1 resistance, virological outcomes, and emergent resistance in the SECOND-LINE trial: and exploratory analysis. Lancet HIV. 2015;2:e42–e51. [DOI] [PubMed] [Google Scholar]
- 22.Paton N, Kityo C, Thompson J, et al. Impact of NRTI cross-resistance on second-line PI + NRTI therapy outcomes in Africa [119]. Presented at: Conference on Retroviruses and Opportunistic Infections; February 23–26, 2015; Seattle, WA.
- 23.Demarest J, Underwood M, St Clair M, et al. DTG-containing regimens are active in INI-naive patients with a history of NRTI resistance [TUAB0104]. Presented at: 20th International AIDS Conference; July 20–25, 2014; Melbourne, Australia.
- 24.Blanco JL, Varghese V, Rhee SY, et al. HIV-1 integrase inhibitor resistance and its clinical implications. J Infect Dis. 2011;203:1204–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winters MA, Lloyd RM, Jr, Shafer RW, et al. Development of elvitegravir resistance and linkage of integrase inhibitor mutations with protease and reverse transcriptase resistance mutations. PLoS One. 2012;7:e40514. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


