Abstract
nab-paclitaxel, an albumin-stabilized paclitaxel formulation, demonstrates clinical activity when administered in combination with gemcitabine in patients with metastatic pancreatic ductal adenocarcinoma (PDA). The limited availability of patient tissue and exquisite sensitivity of xenografts to chemotherapeutics have limited our ability to address the mechanistic basis of this treatment regimen. Here, we used a mouse model of PDA to show that the co-administration of nab-paclitaxel and gemcitabine uniquely demonstrates evidence of tumor regression. Combination treatment increases intratumoral gemcitabine levels due to a marked decrease in the primary gemcitabine metabolizing enzyme, cytidine deaminase (Cda). Correspondingly, paclitaxel reduced Cda protein levels in cultured cells through reactive oxygen species-mediated degradation, resulting in the increased stabilization of gemcitabine. Our findings support the concept that suboptimal intratumoral concentrations of gemcitabine represent a crucial mechanism of therapeutic resistance in PDA and highlight the advantages of genetically engineered mouse models in preclinical therapeutic trials.
Keywords: pancreatic cancer, mouse model, chemotherapy, reactive oxygen species
Introduction
Pancreatic ductal adenocarcinoma (PDA) remains one of the most aggressive tumors in humans. A striking clinical feature of PDA is the innate resistance to available chemotherapies resulting in a 5-year survival rate of <5%. The standard systemic chemotherapy for PDA is gemcitabine, but treatment with gemcitabine only marginally extends survival and combinations with a second cytotoxic agent have so far proven largely ineffective (1, 2). Recent data in mice and humans suggest that poor drug delivery due to highly desmoplastic and hypovascular tumors and rapid metabolic inactivation of therapeutic agents may be at least partly responsible for this unusually poor response to treatment (3, 4).
Therefore, methods that can increase intratumoral gemcitabine levels in PDA are under active investigation. Recently, it was proposed that nab-paclitaxel, a water soluble albumin-bound formulation of paclitaxel, could disrupt the PDA stromal architecture in tumor xenografts and induce reactive angiogenesis resulting in increased perfusion and delivery of gemcitabine (5). nab-paclitaxel was initially developed to avoid toxicities associated with oil-based solvents required to solubilise paclitaxel, such as Cremophor (6). Preclinical and clinical data have demonstrated superior efficacy and safety of nab-paclitaxel over solvent-based paclitaxel (7, 8), thus leading to FDA approval in 2005 as a second-line therapy for metastatic breast cancer. The mechanism of delivery of nab-paclitaxel has been proposed to be mediated by active transport of albumin into the interstitial space via gp60-mediated transcytosis (9). In addition, secreted protein, acidic and rich in cysteine (SPARC), also known as osteonectin, is highly expressed and secreted by PDA peritumoral fibroblasts (10) and may serve as an albumin-binding protein that sequesters nab-paclitaxel to concentrate the drug intra-tumorally. Elevated expression of SPARC has been correlated with improved response to nab-paclitaxel, however this effect may be tumor type-specific (5, 11, 12). Given that PDA is a stromal rich tumor with abundant SPARC expression, a series of clinical trials are evaluating the combination of nab-paclitaxel and gemcitabine in patients with metastatic PDA. Initial results from a phase I/II trial in stage IV pancreatic cancer patients were recently reported demonstrating promising clinical activity in PDA patients (5). More recently, an international phase III trial was initiated for metastatic pancreatic cancer patients randomized to gemcitabine or gemcitabine plus nab-paclitaxel (13).
In clinical trials, the investigation of mechanisms of actions of novel drug combinations is often hampered by the paucity of available tumor tissue for detailed pharmacological, biochemical and histological analysis. Genetically engineered mouse models (GEMM) constitute a promising platform for preclinical testing of novel drugs since many GEMMs recapitulate the molecular and clinical features of the cognate human malignancy (14, 15). As tumor tissue can be readily obtained at predefined time points, GEMMs enable the direct correlation between drug levels, response to treatment and alterations at the cellular and molecular level. Thus, potential efficacy of drug combinations and also mechanisms of resistance can be identified guiding the selection and rapid translation of more effective therapies for human cancers.
We have previously described a GEMM of PDA that is based upon the pancreatic specific expression of endogenous mutant Kras and Trp53 alleles (16). Such mutant mice, termed “KPC” mice, develop primary pancreatic tumors that faithfully recapitulate the clinical, histopathologic, pharmacokinetic, and molecular features of the human disease (17). Furthermore, unlike many transplantation models of PDA, KPC mice demonstrate innate resistance to gemcitabine, (3). Here, we investigate the antitumor efficacy and the molecular mechanism of action of nab-paclitaxel and gemcitabine in KPC mice.
Results
Combination of nab-paclitaxel and gemcitabine causes tumor regression and reduces metastasis
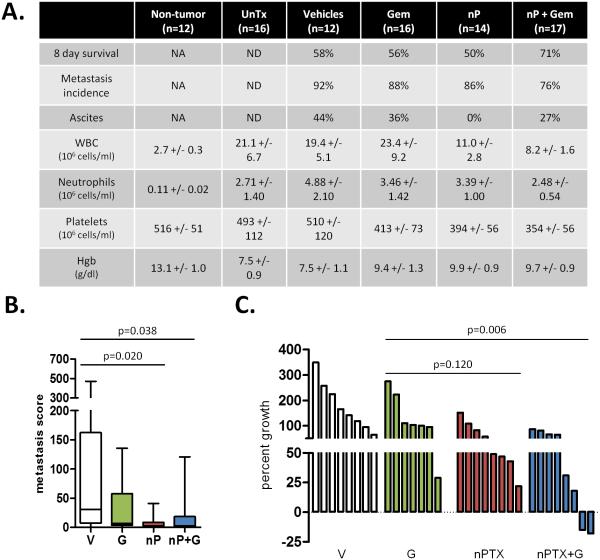
To test the efficacy of nab-paclitaxel in the KPC model, we treated mice with established tumors of comparable size for 8 days with vehicle, gemcitabine, nab-paclitaxel, or nab-paclitaxel/gemcitabine (Fig. S1A-B). Consistent with clinical reports (18, 19), both nab-paclitaxel monotherapy and nab-paclitaxel/gemcitabine treatments were well-tolerated with blood counts in acceptable ranges (Fig. 1A, S1C). Mice treated with combination therapy were more likely to survive the entire treatment regimen (Fig. 1A). Furthermore, combination treatment modestly reduced metastasis incidence and quantification of liver metastases revealed a significant decrease in metastatic burden in both the nab-paclitaxel cohort and the nab-paclitaxel/gemcitabine cohort when compared to the vehicle cohort (Fig. 1A-B). Consistent with clinical observations, gemcitabine treatment alone had no statistically significant effect on tumor growth. Tumors in mice treated with single agent nab-paclitaxel (mean: 170% ± 15) did not significantly differ from the gemcitabine cohort (p=0.12). Treatment with nab-paclitaxel/gemcitabine resulted in significantly smaller tumors (mean: 140% ± 15) as compared to gemcitabine (mean: 234% ± 32; p<0.01) and vehicle (mean: 278% ± 33). (Fig. S1D). Importantly, two tumors in the nab-paclitaxel/gemcitabine cohort regressed after only 8 days of treatment (Fig. 1C). As nab-paclitaxel is formulated with human serum albumin, we were unable to treat mice continuously to assess longer-term survival benefit due to the development of a mouse anti-human albumin humoral immune response (Fig. S1E).
Figure 1. nab-paclitaxel slows tumor growth, improves survival, and decreases metastasis.
A) The percentage of mice that survived for 8 days, exhibited at least one metastasis, or developed ascites was quantified. Analysis of terminal blood draws were used to measure white blood cell count, neutrophil/granulocyte count, platelet count, and hemoblogin. Normal ranges for healthy littermate non-tumor bearing mice as well as untreated KPC mice are listed. NA = not applicable, ND = not determined. B) Liver metastasis score was quantified by factoring the number and size of metastases throughout the liver. Please see materials and methods for additional information. (n≥9) C) Waterfall plot of tumor response of individual tumors from each cohort. nab-paclitaxel monotherapy is significantly better than vehicle, but not gemcitabine (p=0.006 and p=0.120, respectively).
nab-paclitaxel treatment targets tumor epithelial cells
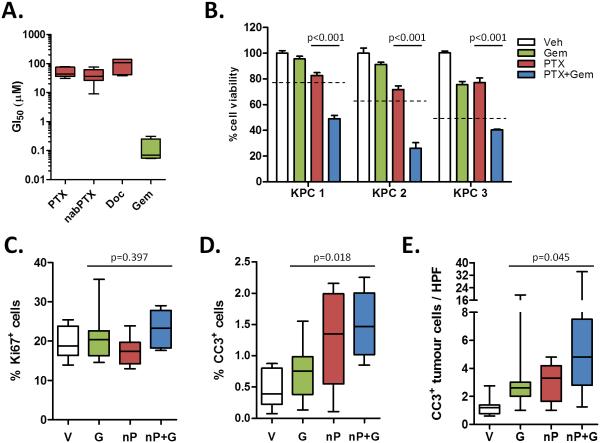
Gemcitabine and paclitaxel are chemotherapeutic agents that have been shown to elicit their anti-tumoral effects through induction of apoptosis or a cell cycle arrest in G1 or G2-M, respectively. Although KPC cells display similar sensitivity to paclitaxel and nab-paclitaxel in vitro, the elevated maximum tolerated dose in vivo permitted increased intratumoural paclitaxel levels in the nab-paclitaxel-treated cohort (Fig. 2A, S2A). In vitro, cells derived from KPC tumors are much more sensitive to gemcitabine than taxanes, and pre-treatment with paclitaxel sensitizes cells to gemcitabine (Fig. 2A-B). Similarly, treatment with both drugs yielded a significant increase in apoptosis in tumors treated with nab-paclitaxel/gemcitabine compared to gemcitabine alone, whereas there were no significant changes in proliferation (Fig. 2C-D). This correlated with the appearance of aberrant mitotic figures that contained an abundance of phosphorylated histone H3 (Fig. S2B-C). Necrotic areas were present in the majority of tumors but did not significantly differ among the treatment groups (Fig. S2D).
Figure 2. nab-paclitaxel targets the tumor epithelial cells.
A) 8 KPC cell lines were exposed to a dose range of paclitaxel, nab-paclitaxel, docetaxel, or gemcitabine for 3 days to determine the GI50 of each agent. Data is representative of four independent experiments. B) 3 KPC cell lines were exposed to sub-GI50 levels of agents. Cells were pre-treated with DMSO or 10μM paclitaxel for 24 hours and/or treated with 30nM gemcitabine for 2 days. Data is representative of two independent experiments. The dotted lines represent predicted additive effect of combination therapy. Proliferation (C) and apoptosis (D) in tumors was measured via quantitative immunohistochemistry for Ki67 and cleaved caspase 3, respectively. (n=8) E) 10-20 high powered fields per tumor were quantified by performing co-immunofluorescence for cleaved caspase 3 and E-cadherin. (n≥9)
Contrary to the observation that nab-paclitaxel promotes stromal disruption in a human xenograft model (5), histological assessment did not reveal any evidence for changes in stromal content or composition (Fig. S2E). The vast majority of apoptotic cells were E-cadherin-expressing neoplastic cells rather than αSMA-expressing stromal cells, and these apoptotic cells were significantly increased only in nab-paclitaxel/gemcitabine treated mice (Fig. 2E, S2F). Moreover, neither intratumoral αSMA content nor collagen density significantly changed upon treatment with nab-paclitaxel (Fig. S3A-D). In support of the lack of effect upon stromal cells in KPC tumors, SPARC levels remained unchanged upon treatment (Fig. S3E-F). Therefore, we conclude that the anti-tumor effect of nab-paclitaxel, in particular in combination with gemcitabine, is mediated by induction of apoptosis in tumor cells rather than stromal cells.
nab-paclitaxel promotes elevated intratumoral gemcitabine levels
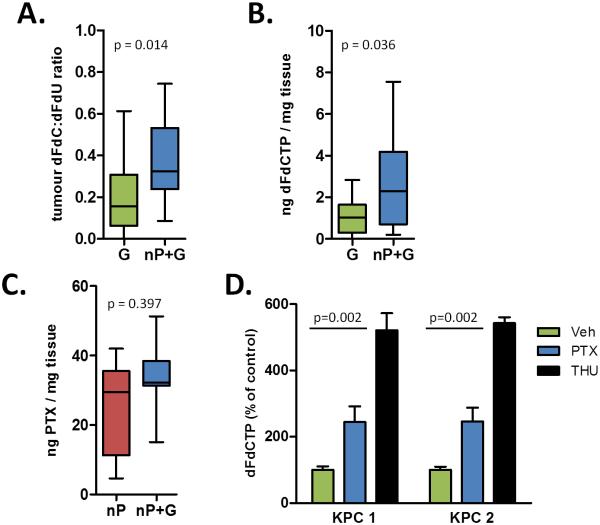
We have previously demonstrated that treatment with the hedgehog inhibitor IPI-926 promotes gemcitabine delivery, resulting in enhanced anti-tumor effects and a doubling of survival time (3). We therefore wanted to determine whether the enhanced anti-tumor activity of nab-paclitaxel/gemcitabine stemmed from increased drug delivery. Utilizing a highly sensitive method, we examined the intratumoral levels of the gemcitabine prodrug dFdC, as well as its inactivated and activated metabolites dFdU and dFdCTP, respectively (20). Notably, we found that combination treatment with nab-paclitaxel elevated the dFdC:dFdU ratio and increased the amount of dFdCTP in tumors (Fig. 3A-B, Table S1). Conversely, paclitaxel concentrations were not significantly different between the nab-paclitaxel/gemcitabine group and the single agent nab-paclitaxel cohort, suggesting that overall drug delivery was not affected (Fig. 3C). Unlike IPI-926, treatment with nab-paclitaxel did not affect vascular density or structure, as measured by microvascular density or mean vascular lumen area, respectively (Fig. S4A-B). Finally, we found that the treatment of cultured PDA cells with free paclitaxel significantly elevates dFdCTP levels, indicating that the chemotherapeutic component in nab-paclitaxel directly affects the metabolism of gemcitabine independent of any alterations in vascular delivery (Fig. 3D).
Figure 3. nab-paclitaxel promotes elevated intratumoral gemcitabine levels.
A) The dFdC:dFdU ratio in bulk tumor was quantified in mice 2 hours after the last dose of gemcitabine. (n≥12) B) Intratumoral levels of dFdCTP were measured in duplicate samples from mice in each cohort 2 hours after the last dose of gemcitabine. (n≥12) C) Intratumoral levels of paclitaxel were measured in samples from mice in each cohort 4 hours after the last dose of nab-paclitaxel. (n≥7) D) 2 KPC cell lines were pre-treated with 10μM paclitaxel or DMSO for 36 hours or 10μM THU (cytidine deaminase inhibitor) for 30 minutes as a positive control and incubated with 1μM gemcitabine for 2 hours. dFdCTP levels were then measured. Data is representative of three independent experiments.
nab-paclitaxel decreases cytidine deaminase protein levels
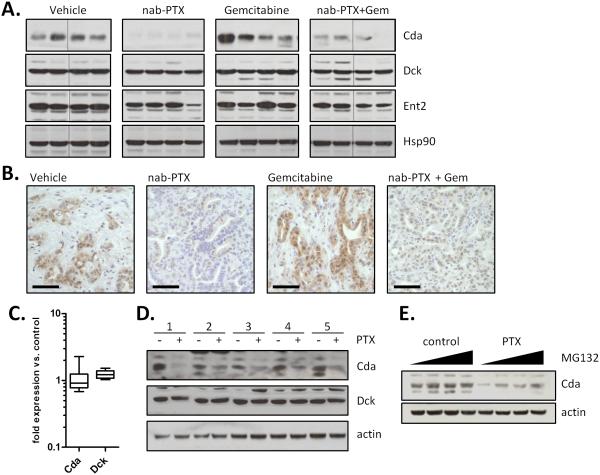
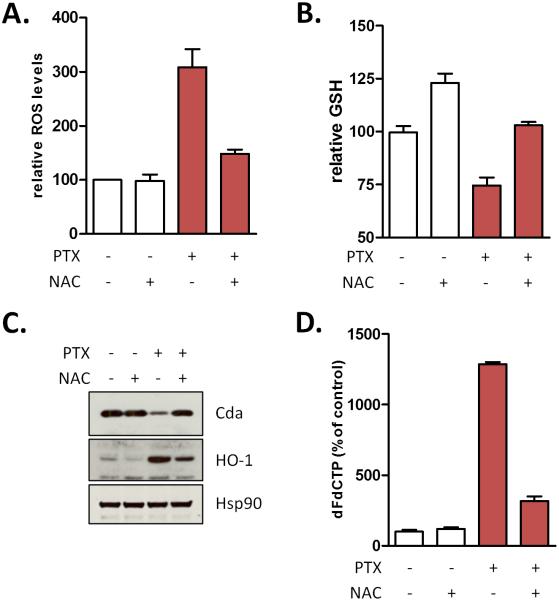
To assess the mechanism of increased levels of dFdCTP in tumors, we performed realtime PCR on RNA extracted from bulk tumor for a variety of enzymes involved in gemcitabine transport and metabolism. Among these only two genes were significantly downregulated (Ent2 and Tk2) and one gene was upregulated (Cnt3) (Fig. S5A), however decreased expression of Ent2 and Tk2 would be predicted to decrease rather than enhance the formation of dFdCTP. The unavailability of commercially available antibodies against murine Cnt3 has prevented us from further investigating this gene; however siRNA-mediated knockdown of Cnt3 had no effect on the sensitivity of tumor cells to gemcitabine (Fig. S5B). Conversely, knocking down Ent1, an established gemcitabine transporter, decreased sensitivity to gemcitabine, whereas depletion of Cda, the primary gemcitabine catabolic enzyme, increased sensitivity to gemcitabine (Fig. S5B). A subset of proteins for which reliable antibodies were available was examined in bulk tumor cell lysates. Strikingly, protein levels of Cda were lower in both the nab-paclitaxel and gemcitabine/nab-paclitaxel cohorts, whereas expression of deoxycytidine kinase and equilibrative nucleoside transporter 2 remained unchanged (Fig. 4A). Immunohistochemical analysis revealed that Cda is primarily expressed in the tumor epithelial cells and that treatment with nab-paclitaxel decreased its expression (Fig. 4B). To determine whether this phenotype was due to a direct effect on tumor cells or indirectly mediated through the microenvironment, we assessed the effects of paclitaxel on cultured KPC tumor cells in vitro. Whereas the mRNA levels of Cda were not altered by treatment with free paclitaxel in culture, protein levels were substantially reduced, indicating that paclitaxel can act directly on tumor cells (Fig. 4C-D). Treatment of cells with the proteasome inhibitor MG132 reversed the effects of paclitaxel on Cda, indicating that paclitaxel regulates Cda protein stability through a post-translational mechanism (Fig. 4E). Paclitaxel treatment generates reactive oxygen species (ROS) that result in a more oxidized intracellular environment that can be reverted with the free radical scavenger N-acetylcysteine (NAC) (Fig. 5A-B). Considering the relative abundance of cysteine residues in Cda and the finding that two of these cysteines are highly reactive (21), we determined whether paclitaxel-induced ROS had an effect on Cda. The paclitaxel-mediated decrease in Cda protein correlated with the induction of the antioxidant gene heme oxygenase (Fig. 5C). Conversely, treatment with NAC prevented the reduction in Cda protein levels. Importantly, NAC also inhibited the paclitaxel-mediated increase in dFdCTP, indicating that ROS is required for the effect of paclitaxel on gemcitabine activation (Fig. 5D). This observation was not restricted to paclitaxel since cisplatin, but not gefitinib, also reduced Cda levels, induced ROS, and elevated dFdCTP levels in cultured pancreatic cancer cells (Fig S6).
Figure 4. nab-paclitaxel and paclitaxel destabilizes cytidine deaminase protein.
A) 40μg of bulk tumor cell lysates were immunoblotted for indicated proteins. B) Immunohistochemistry for cytidine deaminase reveals reduced protein levels in tumour epithelial cells. Scale bar = 50 μm. (n=8) C) RNA isolated from 5 KPC cell lines treated for 36 hours with 10μM paclitaxel was subjected to qRT-PCR, revealing no alterations in mRNA levels compared to controls. RQ values were generated using actin as an endogenous control. D) Protein lysates were generated from the same 5 KPC cell lines treated for 36 hours with DMSO or 10μM paclitaxel and immunoblotted for indicated proteins. Data is representative of four independent experiments. E) Protein lysates were generated from KPC cells pre-treated for 36 hours with DMSO or 10μM paclitaxel followed by 10μM MG132 for 0, 3, 10, or 30 minutes.
Figure 5.
Paclitaxel inactivates cytidine deaminase through induction of reactive oxygen species (ROS). KPC cells were pretreated with 10μM paclitaxel and/or 5mM N-acetylcysteine (NAC) for 4 hours and A) incubated with CM-H2DCFDA to assess intracellular ROS via flow cytometry (n=3) or B) assessed for intracellular redox state via GSH-Glo. (n=3) C) Protein lysates were generated from KPC cells treated for 36 hours with 10μM paclitaxel and/or 5mM NAC and immunoblotted for indicated proteins. D) KPC cells were pretreated with 10μM paclitaxel and/or 5mM NAC for 36 hours and incubated with 1μM gemcitabine for 1 hour. Intracellular dFdCTP was measured (n=3).
Discussion
Although gemcitabine exhibits potent cytotoxicity against PDA cells in vitro, its short half-life may contribute to its relatively weak anti-tumor activity in vivo. Indeed, methods that increase gemcitabine delivery (3) or stability (22) have been proposed to circumvent this problem clinically. Taxanes are also active in pancreatic cancer xenografts and patients, although treatment is limited by systemic toxicity (23, 24). nab-paclitaxel is a solvent-free formulation composed of paclitaxel and human albumin with a mean particle size of 130 nm. It offers several advantages over solvent-based paclitaxel, including increased water solubility that obviates Cremophor-based toxicities. In addition, albumin is hypothesized to target paclitaxel to stromal rich tumors and thereby increase the local concentration. Although combinations of gemcitabine and taxanes have demonstrated anti-tumor activity in PDA patients, its toxicity has limited its use in the clinic (25). Accumulating clinical data support the combination of nab-paclitaxel and gemcitabine as an active regimen for patients with PDA, therefore understanding the mechanisms of sensitivity will be necessary to prevent eventual therapeutic relapse.
A recently published phase I/II clinical trial for stage IV patients demonstrated that the addition of nab-paclitaxel to gemcitabine has tolerable adverse effects and robust anti-tumor activity (5). Although the study was not designed to assess clinical efficacy, the median survival achieved with nab-paclitaxel/gemcitabine (12.2 months) is comparable to results for FOLFIRINOX (11.1 months) and substantially better than gemcitabine monotherapy (6.8 months) in a phase III trial with comparable patients (26). Our results indicate that nab-paclitaxel/gemcitabine treatment effectively prevents tumor growth and uniquely causes tumor regression in some mice. Conversely, tumors treated with gemcitabine more than doubled in size over this time period. Although nab-paclitaxel monotherapy elicits some anti-tumor activity, it fails to cause any tumor regression in the KPC model. Together these data suggest that nab-paclitaxel/gemcitabine combination therapy offers great potential for future use in the treatment of advanced pancreatic cancer.
Concurrent with our data, work with a xenograft model of PDA has shown that combination treatment with nab-paclitaxel and gemcitabine exhibits synergistic anti-tumor activity and improved drug delivery (5). Increased drug delivery was hypothesized to stem from stromal depletion and subsequent reactive angiogenesis through a mechanism similar to what has been described for IPI-926 (3). Conversely, we failed to demonstrate any measurable effect on the tumor stroma of KPC mice. One possible explanation for these disparate results may be the different dosing regimens. While we dosed at MTD, Von Hoff et. al. administered nab-paclitaxel as a low-dose metronomic therapy. Furthermore, stromal depletion occurred after 28 days of treatment, a time frame we could not assess due to the development of an acquired immune response to the human albumin component of nab-paclitaxel after 8 days of treatment. Another key difference is our use of a genetically engineered model that develops autochthonous tumors instead of a subcutaneous transplant model in which human tumor cells must interact with murine stromal cells in an immune compromised mouse. This aberrant microenvironment may create conditions that render the stroma more sensitive to chemotherapeutic treatment.
Although our study did not reveal stromal depletion, both studies concluded that treatment with nab-paclitaxel elevates intratumoral gemcitabine levels. Interestingly, paclitaxel has previously been shown to alter gemcitabine pharmacokinetics in both plasma samples and NSCLC cell lines (27-29), however the mechanism of action was not determined. Here, we reveal that nab-paclitaxel treatment decreased protein levels of cytidine deaminase. Native gemcitabine, 2’,2’-difluorodeoxcytidine (dFdC), is deaminated into the metabolite 2’,2’-difluorodeoxyuridine (dFdU), which accounts for about 80% of the administered dose with only 5% of native gemcitabine excreted unchanged in the urine within the first six hours (30, 31). Cda is ubiquitously expressed in mice and humans and can inactivate dFdC into dFdU in both plasma and cells (32, 33). Notably, recent in vitro and in vivo data have provided the first evidence that high Cda expression is associated with gemcitabine resistance and a small study in pancreatic cancer patients showed that Cda ultrametabolizers were five times more likely to progress after gemcitabine-based therapy (30, 34, 35). Conversely, patients with functionally deficient Cda were associated with an increased risk of experiencing severe or even lethal adverse effects (36, 37). In our model, we found Cda protein levels, but not RNA levels, to be decreased upon treatment with nab-paclitaxel paralleled by a significant increase in gemcitabine triphosphate (dFdCTP) and improved therapeutic efficacy. In vivo, Cda was primarily expressed by tumor cells and addition of paclitaxel to various KPC tumor cell lines consistently reduced Cda protein levels. Interestingly, the molecular mechanism for Cda degradation is mediated by paclitaxel-induced ROS. Upon ROS induction Cda destabilizes and ultimately results in increased levels of active cytotoxic dFdCTP, an effect that is reversed by the ROS scavenger N-acetylcysteine. Although we found that other chemotherapeutic agents such as cisplatin are also capable of inducing ROS in vitro, the dramatically reduced toxicity profile of the nab-paclitaxel formulation allowed us to administer higher doses in vivo to execute the synergistic effects on gemcitabine metabolism within the range of relatively mild side effects.
In conclusion, we have used a GEMM of pancreatic cancer to identify a mechanism for the synergistic anti-tumor effects of the combination of nab-paclitaxel and gemcitabine. nab-paclitaxel exhibits monotherapeutic anti-neoplastic effects, and simultaneously depresses Cda levels through induction of ROS to stabilize gemcitabine and thereby sensitize the PDA tumor to combination treatment. These data uncover novel insight into the anti-tumor activity of nab-paclitaxel and provide a distinct mechanism for improving gemcitabine delivery to pancreatic tumors that warrants further investigation in the clinical setting.
Materials and methods
Cell culture
Cell lines were derived from our murine KPC tumors as previously described (16) and maintained in DMEM (41966029, Invitrogen) + 10% FBS (SH30070.03, HyClone). Protein lysates were obtained using RIPA buffer with protease and phosphatase inhibitors (38). Tetrahydrouridine (Merck) was dissolved in PBS and used as a positive control for Cda inhibition. Paclitaxel (T7191, Sigma), Docetaxel (01885, Sigma), MG132 (474790, Merck), and Gefitinib (G-4408, LC Labs) were dissolved in DMSO, whereas cisplatin (P4394, Sigma), N-acetylcysteine (A9165, Sigma), and gemcitabine (Addenbrookes) were dissolved in saline, and used as indicated. Cell viability experiments were performed via Cell Titer-Glo (G7570, Promega) or MultiTox-Glo Multiplex Cytotoxicity Assays (G9270, Promega) according to manufacturers recommended protocols. Intracellular GSH levels were measured via GSH Glo (V6911, Promega) according to manufacturers recommended protocols.
Mouse strains
The LSL-KrasG12D, LSL-Trp53R172H, Pdx-1-Cre (KPC) mice have been described previously (16). KPC mice develop advanced and metastatic pancreatic ductal adenocarcinoma with 100% penetrance at an early age recapitulating the full spectrum of histopathological and clinical features of human PDA. Mice were housed at a 12 hr. light, 12 hr. dark cycle. All procedures were conducted in accordance to the institutional and national guidelines.
Quantitative PCR
Pancreatic tissue samples were immediately placed in an RNA later solution (Qiagen) and stored for at least 24 hours at 4°C and then snap-frozen until processing. Total RNA was isolated using the Qiagen TissueLyser and Qiagen RNeasy kit. cDNA was synthesized from 1 μg of RNA using the Applied Biosystems QPCR cDNA Synthesis Kit (Applied Biosystems) and analyzed by quantitative real-time PCR on a 7900HT Real-Time PCR system using relative quantification (ΔΔCt) with the Taqman gene expression assays (Applied Biosystems). FAM-labelled assays are listed in the supplementary section.
Western blot analysis
Western blots were performed as previously described (38). The following primary antibodies were used: Hsp90 (4874, Cell Signaling), phospho-ERK1/2 (4370, Cell Signaling), phospho-EGFR (4407, Cell Signaling), actin (I-19, Santa Cruz Biotechnologies), Cda (ab82346, Abcam), Ent2 (ab48595, Abcam), and Dck (ab96599, Abcam). Membranes were incubated with secondary HRP-antibodies (Jackson ImmunoResearch) and developed using the ECL detection system (GE Healthcare).
LC-MS/MS of gemcitabine and paclitaxel
dFdC, dFdU and dFdCTP
Fresh frozen tumor samples and cell pellets were processed and analyzed on LC-MS/MS as previously described (20). Briefly, LC-MS/MS was performed on a TSQ Vantage triple stage quadrupole mass spectrometer (Thermo Scientific, USA) fitted with a heated electrospray ionization (HESI-II) probe operated in positive and negative mode at a spray voltage of 2.5 KV, capillary temperature of 150°C. Quantitative data acquisition was done using LC Quan2.5.6 (Thermo Fisher Scientific, USA).
Paclitaxel
Fresh frozen tumor samples were processed and analysed for paclitaxel concentrations using LC-MS/MS. Briefly, samples were extracted with 90:10 acetonitrile:methanol, and LC-MS/MS was performed on a SCIEX API 4000TM mass spectrometer (Applied Biosystems/MDS SCIEX, USA). Deuterated paclitaxel (d5-paclitaxel, Moravek, USA) was used as the internal standard. Instrument control and quantitative data acquisition were performed by Analyst® Version 1.42 (Applied Biosystems-MDS Sciex, USA).
Histological examination
Tissues were fixed in 10% neutral buffered formalin for 24h and transferred to 70% ethanol. Tissues were embedded in paraffin, and 3-5μm sections were processed for H&E staining, immunohistochemistry and immunofluorescence using standard protocols as previously described (3). The following antibodies were used: SPARC (15274-1-AP, Proteintech), αSMA (1A4, Dako), Cda (ab82346, Abcam), E-cadherin (612130, BD Pharmingen), Cleaved Caspase-3 (9661, Cell Signaling Technology) and CD31 (553370, BD Pharmingen). Images were acquired on an Olympus BX51 microscope or Aperio XT automated scanning system and Imagescope 10 software (Aperio). More information can be found in supplementary material and methods.
ROS quantification
Reactive oxygen species were quantified essentially as described (39). Briefly, cells were treated as indicated and subsequently incubated with CM-H2DCFDA (C6827, Invitrogen) for 30 minutes in PBS, trypsinized, and analyzed via flow cytometry.
Statistical analysis
Statistical analysis was carried out using GraphPad Prism version 5.01 (GraphPad Software). The Mann-Whitney non-parametric t test was used and results are presented as mean ± SE. p< 0.05 was considered to be significant.
Supplementary Material
Statement of significance.
This study provides mechanistic insight into the clinical cooperation observed between gemcitabine and nab-paclitaxel in the treatment of pancreatic cancer.
Acknowledgements
We thank Dan Von Hoff for sharing his data prior to publication. We thank Frances Connor, Paul Mackin, and Lisa Young for maintenance and management of mouse colonies, as well staff from the Cambridge Research Institute BRU, histology core, and pharmacokinetics core. Paclitaxel concentrations were measured by Sherri Ci of Abraxis Bioscience. This research was supported by the University of Cambridge and Cancer Research UK, The Li Ka Shing Foundation and Hutchison Whampoa Limited and the NIHR Cambridge Biomedical Research Centre. KKF was supported under the NIH Ruth L. Kirschstein National Research Service Award F32CA123887-01, and KKF and DAT were supported by the European Community Grant EPC-TM-Net 256974. AN was supported by the Deutsche Krebshilfe Mildred Scheel Postdoctoral Fellowship. NC was supported by a Cancer Research UK Clinician Fellowship. M.P. Lolkema has received a Dutch Cancer Foundation Fellowship grant (UU2008-4380) to support this work. DAT and DIJ are Group Leaders in the Cancer Research UK Cambridge Research Institute. TB is supported by Cancer Research UK.
Footnotes
Competing financial interests: The authors declare no competing financial interests.
Author contributions
KKF, AN, and DAT conceived of and designed the experiments. AN, KKF, and MPL performed animal experiments. KKF performed cell culture experiments. KKF, NC, TB, and DIJ designed and carried out gemcitabine pharmacokinetic experiments. KKF, AN, and DAT wrote the manuscript. All authors reviewed the manuscript.
References
- 1.Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–13. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 2.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–17. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 3.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–61. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Komar G, Kauhanen S, Liukko K, Seppanen M, Kajander S, Ovaska J, et al. Decreased blood flow with increased metabolic activity: a novel sign of pancreatic tumor aggressiveness. Clin Cancer Res. 2009;15:5511–7. doi: 10.1158/1078-0432.CCR-09-0414. [DOI] [PubMed] [Google Scholar]
- 5.Von Hoff DD, Ramanathan RK, Borad MJ, Laheru DA, Smith LS, Wood TE, et al. Gemcitabine Plus nab-Paclitaxel Is an Active Regimen in Patients With Advanced Pancreatic Cancer: A Phase I/II Trial. J Clin Oncol. 2011;29:4548–54. doi: 10.1200/JCO.2011.36.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gradishar WJ. Albumin-bound paclitaxel: a next-generation taxane. Expert Opin Pharmacother. 2006;7:1041–53. doi: 10.1517/14656566.7.8.1041. [DOI] [PubMed] [Google Scholar]
- 7.Desai N, Trieu V, Yao Z, Louie L, Ci S, Yang A, et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res. 2006;12:1317–24. doi: 10.1158/1078-0432.CCR-05-1634. [DOI] [PubMed] [Google Scholar]
- 8.Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23:7794–803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 9.Vogel SM, Minshall RD, Pilipovic M, Tiruppathi C, Malik AB. Albumin uptake and transcytosis in endothelial cells in vivo induced by albumin-binding protein. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1512–22. doi: 10.1152/ajplung.2001.281.6.L1512. [DOI] [PubMed] [Google Scholar]
- 10.Infante JR, Matsubayashi H, Sato N, Tonascia J, Klein AP, Riall TA, et al. Peritumoral fibroblast SPARC expression and patient outcome with resectable pancreatic adenocarcinoma. J Clin Oncol. 2007;25:319–25. doi: 10.1200/JCO.2006.07.8824. [DOI] [PubMed] [Google Scholar]
- 11.Desai N, Trieu V, Damascelli B, Soon-Shiong P. SPARC Expression Correlates with Tumor Response to Albumin-Bound Paclitaxel in Head and Neck Cancer Patients. Transl Oncol. 2009;2:59–64. doi: 10.1593/tlo.09109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shao H, Tang H, Salavaggione OE, Yu C, Hylander B, Tan W, et al. Improved response to nab-paclitaxel compared with cremophor-solubilized paclitaxel is independent of secreted protein acidic and rich in cysteine expression in non-small cell lung cancer. J Thorac Oncol. 2011;6:998–1005. doi: 10.1097/JTO.0b013e318217b739. [DOI] [PubMed] [Google Scholar]
- 13.NIH A Trial of Patients With Metastatic Adenocarcinoma of the Pancreas. 2011 http://clinicaltrialsgov/ct2/show/NCT00844649.
- 14.Becher OJ, Holland EC. Genetically engineered models have advantages over xenografts for preclinical studies. Cancer Res. 2006;66:3355–8, discussion 8-9. doi: 10.1158/0008-5472.CAN-05-3827. [DOI] [PubMed] [Google Scholar]
- 15.Frese KK, Tuveson DA. Maximizing mouse cancer models. Nat Rev Cancer. 2007;7:645–58. doi: 10.1038/nrc2192. [DOI] [PubMed] [Google Scholar]
- 16.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–83. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 17.Hruban RH, Adsay NV, Albores-Saavedra J, Anver MR, Biankin AV, Boivin GP, et al. Pathology of genetically engineered mouse models of pancreatic exocrine cancer: consensus report and recommendations. Cancer Res. 2006;66:95–106. doi: 10.1158/0008-5472.CAN-05-2168. [DOI] [PubMed] [Google Scholar]
- 18.Roy V, LaPlant BR, Gross GG, Bane CL, Palmieri FM. Phase II trial of weekly nab (nanoparticle albumin-bound)-paclitaxel (nab-paclitaxel) (Abraxane) in combination with gemcitabine in patients with metastatic breast cancer (N0531) Ann Oncol. 2009;20:449–53. doi: 10.1093/annonc/mdn661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stinchcombe TE, Socinski MA, Lee CB, Hayes DN, Moore DT, Goldberg RM, et al. Phase I trial of nanoparticle albumin-bound paclitaxel in combination with gemcitabine in patients with thoracic malignancies. J Thorac Oncol. 2008;3:521–6. doi: 10.1097/JTO.0b013e31816de2a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bapiro TE, Richards FM, Goldgraben MA, Olive KP, Madhu B, Frese KK, et al. A novel method for quantification of gemcitabine and its metabolites 2',2'-difluorodeoxyuridine and gemcitabine triphosphate in tumour tissue by LC-MS/MS: comparison with (19)F NMR spectroscopy. Cancer Chemother Pharmacol. 2011;68:1243–53. doi: 10.1007/s00280-011-1613-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weerapana E, Wang C, Simon GM, Richter F, Khare S, Dillon MB, et al. Quantitative reactivity profiling predicts functional cysteines in proteomes. Nature. 2010;468:790–5. doi: 10.1038/nature09472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beumer JH, Eiseman JL, Parise RA, Joseph E, Covey JM, Egorin MJ. Modulation of gemcitabine (2',2'-difluoro-2'-deoxycytidine) pharmacokinetics, metabolism, and bioavailability in mice by 3,4,5,6-tetrahydrouridine. Clin Cancer Res. 2008;14:3529–35. doi: 10.1158/1078-0432.CCR-07-4885. [DOI] [PubMed] [Google Scholar]
- 23.Bissery MC, Guenard D, Gueritte-Voegelein F, Lavelle F. Experimental antitumor activity of taxotere (RP 56976, NSC 628503), a taxol analogue. Cancer Res. 1991;51:4845–52. [PubMed] [Google Scholar]
- 24.Lenzi R, Yalcin S, Evans DB, Abbruzzese JL. Phase II study of docetaxel in patients with pancreatic cancer previously untreated with cytotoxic chemotherapy. Cancer Invest. 2002;20:464–72. doi: 10.1081/cnv-120002146. [DOI] [PubMed] [Google Scholar]
- 25.Ryan DP, Kulke MH, Fuchs CS, Grossbard ML, Grossman SR, Morgan JA, et al. A Phase II study of gemcitabine and docetaxel in patients with metastatic pancreatic carcinoma. Cancer. 2002;94:97–103. doi: 10.1002/cncr.10202. [DOI] [PubMed] [Google Scholar]
- 26.Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 27.Kroep JR, Giaccone G, Tolis C, Voorn DA, Loves WJ, Groeningen CJ, et al. Sequence dependent effect of paclitaxel on gemcitabine metabolism in relation to cell cycle and cytotoxicity in non-small-cell lung cancer cell lines. Br J Cancer. 2000;83:1069–76. doi: 10.1054/bjoc.2000.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kroep JR, Giaccone G, Voorn DA, Smit EF, Beijnen JH, Rosing H, et al. Gemcitabine and paclitaxel: pharmacokinetic and pharmacodynamic interactions in patients with non-small-cell lung cancer. J Clin Oncol. 1999;17:2190–7. doi: 10.1200/JCO.1999.17.7.2190. [DOI] [PubMed] [Google Scholar]
- 29.Shord SS, Faucette SR, Gillenwater HH, Pescatore SL, Hawke RL, Socinski MA, et al. Gemcitabine pharmacokinetics and interaction with paclitaxel in patients with advanced non-small-cell lung cancer. Cancer Chemother Pharmacol. 2003;51:328–36. doi: 10.1007/s00280-002-0560-1. [DOI] [PubMed] [Google Scholar]
- 30.Ciccolini J, Mercier C, Dahan L, Andre N. Integrating pharmacogenetics into gemcitabine dosing-time for a change? Nat Rev Clin Oncol. 2011;8:439–44. doi: 10.1038/nrclinonc.2011.1. [DOI] [PubMed] [Google Scholar]
- 31.Abbruzzese JL, Grunewald R, Weeks EA, Gravel D, Adams T, Nowak B, et al. A phase I clinical, plasma, and cellular pharmacology study of gemcitabine. J Clin Oncol. 1991;9:491–8. doi: 10.1200/JCO.1991.9.3.491. [DOI] [PubMed] [Google Scholar]
- 32.Ruiz van Haperen VW, Veerman G, Boven E, Noordhuis P, Vermorken JB, Peters GJ. Schedule dependence of sensitivity to 2',2'-difluorodeoxycytidine (Gemcitabine) in relation to accumulation and retention of its triphosphate in solid tumour cell lines and solid tumours. Biochem Pharmacol. 1994;48:1327–39. doi: 10.1016/0006-2952(94)90554-1. [DOI] [PubMed] [Google Scholar]
- 33.Besnard T, Renee N, Etienne-Grimaldi MC, Francois E, Milano G. Optimized blood sampling with cytidine deaminase inhibitor for improved analysis of capecitabine metabolites. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;870:117–20. doi: 10.1016/j.jchromb.2008.05.040. [DOI] [PubMed] [Google Scholar]
- 34.Ogawa M, Hori H, Ohta T, Onozato K, Miyahara M, Komada Y. Sensitivity to gemcitabine and its metabolizing enzymes in neuroblastoma. Clin Cancer Res. 2005;11:3485–93. doi: 10.1158/1078-0432.CCR-04-1781. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida T, Endo Y, Obata T, Kosugi Y, Sakamoto K, Sasaki T. Influence of cytidine deaminase on antitumor activity of 2'-deoxycytidine analogs in vitro and in vivo. Drug Metab Dispos. 38:1814–9. doi: 10.1124/dmd.110.034397. [DOI] [PubMed] [Google Scholar]
- 36.Mercier C, Raynal C, Dahan L, Ortiz A, Evrard A, Dupuis C, et al. Toxic death case in a patient undergoing gemcitabine-based chemotherapy in relation with cytidine deaminase downregulation. Pharmacogenet Genomics. 2007;17:841–4. doi: 10.1097/FPC.0b013e32825ea6e3. [DOI] [PubMed] [Google Scholar]
- 37.Ciccolini J, Dahan L, Andre N, Evrard A, Duluc M, Blesius A, et al. Cytidine deaminase residual activity in serum is a predictive marker of early severe toxicities in adults after gemcitabine-based chemotherapies. J Clin Oncol. 28:160–5. doi: 10.1200/JCO.2009.24.4491. [DOI] [PubMed] [Google Scholar]
- 38.Karreth FA, DeNicola GM, Winter SP, Tuveson DA. C-Raf inhibits MAPK activation and transformation by B-Raf(V600E) Mol Cell. 2009;36:477–86. doi: 10.1016/j.molcel.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 39.DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K, et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–9. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.