Abstract
Aim
To determine the relationship of head turn preference in the preterm infant to: 1) perinatal medical factors, 2) neonatal neurobehavior, and/or 3) infant neurodevelopmental outcomes.
Methods
Seventy preterm infants born ≤30 weeks gestation were enrolled at birth. Detailed information regarding neonatal intensive care unit (NICU) medical course was compiled for each infant. Neurobehavioral testing was performed during NICU hospitalization. Head turn preference was quantified at term equivalent age using a newly developed scale. Infants returned at age two years for standardized developmental testing.
Results
All infants demonstrated a head turning preference, with most preferring the right side (n = 51, 77%). Fifty-five infants (79%) had moderate to severe head turn preference. Head turn preference was associated with 1) medical severity (hours of inotrope use, p = 0.02; oxygen requirement at 36 weeks postmenstrual age, p = 0.03), 2) worse neurobehavioral performance (decreased self-regulation, p = 0.007; more sub-optimal reflexes p = 0.006), and 3) worse developmental outcome at age two years (poorer fine motor, p = 0.02).
Interpretation
Medical factors in the NICU appear to be associated with the development of a head turn preference. Increased severity of head turn preference may be a marker for poor developmental outcome. Early identification may inform therapeutic interventions designed to minimize symptoms and optimize neurodevelopmental outcome.
Keywords: Head turn preference, Neonatal Intensive Care Unit, Preterm infant, Infant, Child development, Infant positioning
Head turn preference in the newborn infant is defined as preferred positioning of the head to one side, a strong push of the head into rotation to one side, and/or an inability to achieve or maintain the head in midline position. Head turning during the neonatal period has been described as a typical developmental phenomenon, and may also be implicated in the development of laterality and hand-preference [1–3]. Neonatal head turning to the right is more common, consistent with the larger proportion of people who are right handed [4]. The persistence of head turning to the right into adulthood, within various activities of daily living that do not utilize handedness, has also been reported [5].
While some head turning during the neonatal period and beyond may be normal and developmentally-regulated, significant head turn preference may negatively impact developmental progression. Head turn preference in high risk infants can impact function, as head rotation results in reflexive extension of the extremities on the side of the head turn and flexion on the opposite side, leading to asymmetric movement patterns [6,7]. Prolonged time with the head rotated to one side can impact reflex patterns, muscle tone, and movement for later function. In addition, severe head turn preferences may promote asymmetric movements in early infancy [8,9] and lead to future complications in posture [10–12]. Postural asymmetries, including infantile postural scoliosis, torticollis, and an asymmetrical skull shape including deformational plagiocephaly also result from prolonged head turn preference [13–17]. Visual orientation and social interaction also rely on midline head orientation [18,19].
It remains unclear what factors may be influencing severe head turn preference and when its presence can be related to altered developmental trajectory [20]. Neonatal head turning can be influenced by caretaking, specifically right-handed caregivers or those who approach predominately on the right side, which encourages head positioning to the right [21]. While this head turning may resolve as environmental interactions become balanced, this may not be the case with preterm infants who spend several months in an environment with imposed positioning. Medical interventions during the neonatal intensive care unit (NICU) hospitalization may also play a role in the development of head turn preference in preterm infants. Some interventions, such as endotracheal intubation, often result in passive rotation of the neck to one side for prolonged periods of time, contributing to development of head turn preference. These variables likely contribute to the higher rates of head turn preference present in preterm infants compared to their term-born peers [16,22,23]. While normal environmental factors can influence sidedness [24], it remains unclear how the early environment as well as medical complications, including cerebral injury, can influence severe head turn preference in preterm infants.
While head turn preference has been described using assessments of active and passive range of motion, there are few bedside clinical tools to assess head turn preference. To better understand head turn preference in the high-risk infant and discriminate mild head turning from more severe forms of head turn preference, we developed a new head turn preference scale. This tool was subsequently used to determine if head turn preference among preterm infants is: 1) associated with NICU medical factors, 2) related to early neurobehavior, and 3) a marker for adverse developmental outcome at two years of age.
1. Methods
1.1. Study site and participants
Infants were prospectively enrolled as part of an overarching study aimed at investigating longitudinal neurodevelopment of preterm infants. Infants were born at ≤30 weeks estimated gestational age (EGA) from 2007 to 2010, free of congenital anomalies, and enrolled within the first 72 h of life from a level III–IV NICU. Infants received routine care in the NICU and also underwent magnetic resonance imaging (MRI) and videotaped neurobehavioral testing during their NICU course. Participants returned for developmental testing at two years of age. This study used a subset of infants from the parent study, which included all infants who underwent videotaped neurobehavioral assessments that were of adequate quality to enable head turn preference assessment. This study was approved by the Human Research Protection Office at Washington University, and the parents of all participating infants provided informed consent.
1.2. Early medical factors
For all infants, information was collected from the electronic medical record on medical factors including: EGA at birth, birth weight, number of days on mechanical and high frequency oscillatory ventilation, number of days on continuous positive airway pressure (CPAP), hours of oxygen use (ventilation, CPAP, or oxygen delivered by nasal cannula), oxygen requirement at 36 weeks, hours of inotrope use, Clinical Risk Index for Babies score [25], number of days on total parental nutrition, patent ductus arteriosus (treated with indomethacin or surgical ligation), necrotizing enterocolitis (all stages), cerebral injury, and postmenstrual age (PMA) at discharge. Cerebral injury was identified using routine cranial ultrasound and MRI and defined as the presence of grade III or IV intraventricular hemorrhage, cystic periventricular leukomalacia, and/or cerebellar hemorrhage. A single trained neonatal neurologist (author TI) defined the presence or absence of cerebral injury based on imaging findings.
1.3. Neurobehavioral testing
Neurobehavioral testing was conducted at 34 weeks PMA and again at term equivalent age (37–41 weeks PMA), using the NICU Network Neurobehavioral Scale (NNNS). These videotaped assessments were conducted at the infant's bedside by a single certified examiner (author RG). The NNNS yields 13 summary scores including measures of habituation, orientation, arousal, self-regulation, hypertonia, hypotonia, stress, lethargy, excitability, sub-optimal reflexes, asymmetry, quality of movement, and tolerance of handling [26].
1.4. Head Turn Preference Scale Score
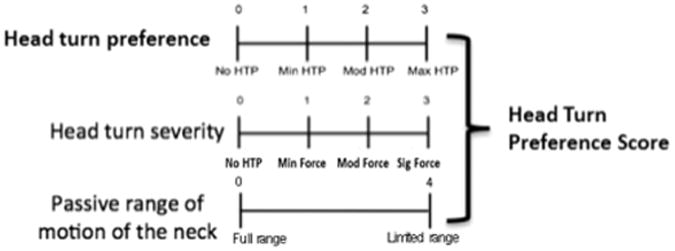
From the videotaped neurobehavioral evaluations, head turn preference was quantified using a newly developed scale (see Appendix A). Scores on the Head Turn Preference Scale range from 0 to 10, with higher scores indicating greater head turn preference. Numerical scores are then categorized into no (score of 0), minimal (scores of 1–3), moderate (scores of 4–6), or severe (scores of 7–10) head turn preference. The score measures head turn preference by identifying: 1) whether a head turn preference is present at rest, and if so, how much cervical rotation is entailed, 2) the severity of head turn preference by observing the force exerted by the head to move into the preferred position, and 3) whether there are restrictions in neck rotation during passive range of motion. The scale was developed in an attempt to quantify head turn preference while discriminating mild head turning from severe forms of head turn preference that result in strong pushing and decreased range of motion of neck rotation.
Reliability of the Head Turn Preference Scale was determined using four trained occupational therapists. The therapists engaged in a training session in which the scale was described and videos were reviewed and scored until agreement was reached (three videos). Following the training period, five videos were randomly selected from the cohort and presented in random order to the therapists. Inter-rater reliability was assessed using Fleiss' Kappa statistics. There was 100% agreement in defining the head turn preference category (none, minimal, moderate, severe), resulting in a Fleiss' Kappa value of 1. The Fleiss' Kappa value for Domain 1 was 0.01 (Item 1 in Domain 1: 0.55; Item 2 in Domain 1: 0.30; Item 3 in Domain 1: 0.08; Item 4 in Domain 1: 0.26); Domain 2 was 0.59; and Domain 3 was 0.38. This demonstrates good reliability using the categorical variable, but poor reliability of individual items. Therefore, the categorical variable was used for all analyses.
1.5. Developmental outcome at age two years
Infants returned for developmental testing at two years of age using the Bayley Scales of Infant and Toddler Development-Third Edition (Bayley-III) [27]. Bayley-III composite scores for cognitive, language, and motor outcome as well as subscale scores for expressive and receptive language and fine and gross motor outcome were used to determine the associations between head turn preference and developmental outcome at age two years.
1.6. Statistical analyses
Early medical factors were explored for associations with the Head Turn Preference Scale score with chi-square analysis and logistic regression using α = .05. Associations between Head Turn Preference Scale score and developmental outcomes (NNNS summary scores and composite and subscale scores on the Bayley-III at age two years) were investigated using logistic regression models. A multivariate model was also used to investigate relationships between head turn preference and developmental outcomes, while controlling for clinical factors related to head turn preference and known to affect developmental outcome. This was an exploratory study, aimed at defining relationships between head turn preference and medical factors and developmental outcome.
2. Results
Seventy infants in the cohort underwent videotaped neurobehavioral assessments and were used for this investigation. See Table 1 for sample descriptives and relationships between NICU factors and head turn preference.
Table 1.
Sample descriptives and relationships of NICU factors to head turn preference.
| Mean (±SD); or median (IQ); or N (%) for whole sample (n = 70) | Mean (±SD); or median (IQ); or N (%) among those with severe head turn preference (n = 38) | Mean (±SD); or median (IQ); or N (%) among those without severe head turn preference (n = 32) | *P value | |
|---|---|---|---|---|
| Gender, female | 38 (54.3%) | 19 (50.0%) | 19 (59.4%) | 0.06 |
| EGA (weeks) | 26.3 (±1.8) | 26.0 (±1.9) | 26.8 (±1.7) | 0.17 |
| Birth weight (grams) | 929.0 (±247.5) | 888.1 (±242.1) | 977.7 (±248.7) | 0.38 |
| Days on ventilation | 3.0 (0.0–89.0) | 5.0 (0.0–89.0) | 1.5 (0.0–47.0) | 0.06 |
| Days on HFOV | 0.0 (0.0–16.0) | 0.0 (0.0–16.0) | 0.0 (0.0–16.0) | 0.28 |
| Days on CPAP | 3.0 (0.0–68.0) | 3.5 (0.0–68.0) | 3.0 (0.0–45.0) | 0.47 |
| Hours of oxygen | 1639.1 (±958.8) | 1848.0 (±1030.8) | 1391.1 (±813.0) | 0.08 |
| Oxygen at 36 weeks | 42 (60.0%) | 27 (71.1%) | 15 (46.9%) | 0.03 |
| Hours of inotropes | 41.3 (±98.6) | 68.5 (±126.0) | 9.1 (±26.5) | 0.02 |
| CRIB score | 3.81 (±3.5) | 4.76 (±3.9) | 2.69 (±2.6) | 0.06 |
| Days on TPN | 18.5 (5.0–117.0) | 24.5 (5.0–117.0) | 14.0 (5.0–43.0) | 0.054 |
| PDA | 42 (60.0%) | 24 (63.2%) | 18 (56.3%) | 0.81 |
| NEC | 8 (11.4%) | 5 (13.1%) | 3 (9.4%) | 0.77 |
| Cerebral injury | 14 (20.3%) | 9 (23.7%) | 5 (16.1%) | 0.96 |
| PMA at discharge (weeks) | 39.9 (±3.5) | 40.3 (±4.0) | 39.5 (±2.7) | 0.72 |
| Length of stay (days) | 90.5 (50.0–232.0) | 99.5 (52.0–232.0) | 85.5 (50.0–140.0) | 0.33 |
EGA: estimated gestation age, HFOV: high frequency oscillatory ventilation, CPAP: continuous positive airway pressure, CRIB: Clinical Risk Index for Babies, TPN: total parenteral nutrition, PDA: patent ductus arteriosus, NEC: necrotizing enterocolitis, PMA: postmenstrual age.
p value investigating relationships between NICU factors and head turn preference severity using chi-square analysis and logistic regression.
2.1. Head turn preference
All participants demonstrated a head turn preference with 15 (21%) being mild, 17 (24%) being moderate, and 38 (54%) being severe. The Head Turn Preference Scale score ranged from 1 to 9 with a mean (standard deviation) of 5.9 (2.4). Fifty-one (77%) preferred the right, 15 (23%) preferred the left and 4 (18%) varied their preference with head turning to the right and left within the time of observation demonstrating poor midline orientation.
2.2. Medical factors
Greater Head Turn Preference Scale score was associated with more inotrope hours (p = .0.02; β = 32.8 [4.6–61.2]) and having an oxygen requirement at 36 weeks PMA (p = 0.03; β = 2.0 [1.1–3.6]). There were no other associations observed between Head Turn Preference Scale score and other medical factors investigated. Controlling for the direction of the head turn preference did not alter the findings.
2.3. Neurobehavioral outcomes
Greater Head Turn Preference Scale scores at term equivalent age were related to worse self-regulation (p = 0.007; β = −0.35 [−0.60 to −0.10] and more sub-optimal reflexes (p = 0.006; β = 1.0 [0.31 to 1.77]) at 34 weeks PMA. There were no other significant relationships between early neurobehavior and head turn preference (Table 2). Controlling for the direction of head turn preference and PMA at time of testing did not alter the findings.
Table 2.
Neurobehavioral factors and relationships to head turn preference.
| Mean (±SD); or median (IQ); or N (%) (n = 70) | Mean (±SD); or median (IQ); or N (%) among those with severe head turn preference (n = 38) | Mean (±SD); or median (IQ); or N (%) among those without severe Head turn preference (n = 32) | *p Value | |
|---|---|---|---|---|
| Neurobehavioral factors | ||||
| Orientation-34 weeks PMA | 3.3 (±1.2) | 3.1 (±1.0) | 3.5 (±1.5) | 0.18 |
| Arousal-34 weeks | 3.1 (±0.8) | 3.2 (±0.9) | 3.1 (±0.6) | 0.88 |
| Habituation-34 weeks PMA | 5.8 (±2.8) | 6.1 (±2.6) | 5.4 (±3.1) | 0.97 |
| Tolerance of handling-34 weeks PMA | 0.7 (±0.1) | 0.7 (±0.1) | 0.7 (±0.1) | 0.49 |
| Quality of movement-34 weeks PMA | 3.7 (±0.8) | 3.6 (±0.8) | 3.9 (±0.8) | 0.10 |
| Self-regulation-34 weeks PMA | 4.5 (±0.8) | 4.3 (±0.8) | 4.7 (±0.9) | 0.007 |
| Sub-optimal reflexes-34 weeks PMA | 7.3 (±2.5) | 7.9 (±2.2) | 6.6 (±2.6) | 0.006 |
| Stress-34 weeks PMA | 0.3 (±0.1) | 0.4 (±0.1) | 0.3 (±0.1) | 0.56 |
| Hypotonia-34 weeks PMA | 0.9 (±1.1) | 1.1 (±1.2) | 0.6 (±0.9) | 0.15 |
| Hypertonia-34 weeks PMA | 1.2 (±0.9) | 1.1 (±0.8) | 1.2 (±0.9) | 0.82 |
| Asymmetry-34 weeks PMA | 2.8 (±2.0) | 2.8 (±1.9) | 2.8 (±2.2) | 0.93 |
| Excitability-34 weeks PMA | 3.4 (±2.0) | 3.6 (±2.0) | 3.1 (±1.9) | 0.18 |
| Lethargy-34 weeks PMA | 9.1 (±3.0) | 9.1 (±2.8) | 9.1 (±3.3) | 0.60 |
| Orientation-term | 3.3 (±1.3) | 3.3 (±1.4) | 3.4 (±1.3) | 0.98 |
| Arousal-term | 4.1 (±0.9) | 4.1 (±0.9) | 4.2 (±0.9) | 0.84 |
| Habituation-term | 6.8 (±2.6) | 6.7 (±2.6) | 6.9 (±2.7) | 0.67 |
| Tolerance of handling-term | 0.7 (±0.2) | 0.7 (±0.2) | 0.7 (±0.1) | 0.84 |
| Quality of movement-term | 3.5 (±0.8) | 3.5 (±0.8) | 3.5 (±0.8) | 0.67 |
| Self-regulation-term | 4.4 (±0.8) | 4.3 (±0.8) | 4.4 (± 0.8) | 0.43 |
| Sub-optimal reflexes-term | 7.1 (±2.2) | 7.5 (±1.9) | 6.6 (±2.4) | 0.08 |
| Stress-term | 0.4 (±0.1) | 0.4 (±0.1) | 0.4 (±0.1) | 0.90 |
| Hypertonia-term | 1.8 (±1.2) | 2.0 (±1.3) | 1.5 (±1.1) | 0.18 |
| Hypotonia-term | 0.7 (±0.8) | 0.8 (±0.7) | 0.7 (±0.9) | 0.36 |
| Asymmetry-term | 2.5 (±2.0) | 2.3 (±1.9) | 2.7 (±2.1) | 0.78 |
| Excitability-term | 5.5 (±2.6) | 5.8 (±2.5) | 5.3 (±2.7) | 0.28 |
| Lethargy-term | 6.9 (±3.0) | 7.2 (±2.8) | 6.6 (±3.2) | 0.77 |
p value investigating relationships between neurobehavioral factors and head turn preference severity using linear regression.
2.4. Developmental outcomes
Greater Head Turn Preference Scale scores were associated with lower Bayley-III fine motor (p = 0.016; β = −0.83 [−1.5 to −.16] and expressive language (p = 0.049; β = −0.76 [−1.50 to −0.003]) scores at age two years. There were no other associations between Head Turn Preference Scale score and developmental outcomes (Table 3).
Table 3.
Developmental outcome at age 2 years and relationships to head turn preference.
| Mean (±SD); or median (IQ); or N (%) for whole sample (n = 70) | Mean (±SD); or median (IQ); or N (%) among those with severe head turn preference (n = 38) | Mean (±SD); or median (IQ); or N (%) among those without severe head turn preference (n = 32) | *P value | |
|---|---|---|---|---|
| Bayley cognitive | 86.8 (±10.0) | 85.2 (±9.6) | 89.0 (±10.2) | 0.10 |
| Bayley language | 89.1 (±12.9) | 86.7 (±13.7) | 92.4 (±11.1) | 0.052 |
| Receptive language | 7.9 (±2.3) | 7.5 (±2.4) | 8.6 (±2.0) | 0.06 |
| Expressive language | 8.4 (±2.4) | 7.9 (±2.6) | 9.0 (±1.8) | 0.049 |
| Bayley motor | 84.5 (±11.7) | 82.3 (±12.2) | 87.3 (±10.6) | 0.07 |
| Fine motor | 8.0 (±2.2) | 7.5 (±2.0) | 8.8 (±2.2) | 0.02 |
| Gross motor | 6.8 (±2.3) | 6.6 (±2.5) | 7.0 (±2.0) | 0.44 |
NNNS: NICU Network Neurobehavioral Scale.
p value from investigating relationships between the Head Turn Preference Scale categorical score and the developmental outcome measures using linear regression.
A multivariate model was employed to better understand the relationships between head turn preference and developmental outcome while controlling for oxygen requirement at 36 weeks PMA, which was related to head turn preference, and cerebral injury, a factor known to influence outcome. Inotrope use was not put in the model, as it was co-linear with oxygen requirement at 36 weeks PMA. Relationships between Head Turn Preference Scale score and fine motor, self-regulation and sub-optimal reflexes remained significant, but relationships between Head Turn Preference Scale score and expressive language were no longer significant.
3. Discussion
The key findings of this study were that head turn preference was: 1) common in preterm infants at term equivalent age and toward the right direction for most infants, 2) related to early medical factors, and 3) a marker for adverse developmental outcome. In addition, we were able to validate a new measure of head turn preference for infants.
Head turn preference is a common observation in the NICU. Mild head turning was present in all infants in this cohort, and moderate to severe head turn preference was more prevalent than previous reports (79% versus 45%) [15]. This may be attributed to the differences in methodology used to define head turn preference between the two studies, as well as the use of a younger, higher-risk cohort in the current study. A higher incidence of head turn preference to the right was observed in this cohort, consistent with other reports [17]. Liederman and Kinsbourne [28] suggest that head turning to the right may result from activation of the left side of the brain during the programming of the motor output and the degree of activation of each side of the brain during the processing of a sensory input. Some claim it is a sign of laterality and hand-preference [2]. Still others postulate that strong pushing of the head to one side is a sign of altered developmental progression [14,16]. Our findings support that some head turning, predominantly to the right, is common. However, when head turn preference results in significant rotation, strong pushing and loss of range of motion, it can be a marker for adverse developmental outcome.
Our findings complement others that describe handedness, which may be linked to head turn preference, as being reinforced by environmental influences [24]. Early medical factors appear to be a contributing factor related to the presence of head turn preference. We found persistent need for oxygen and inotropes to be related to more severe head turn preference. While these factors could be discriminating the infants who are medically comprised, the early environment may also play a role in head turn preference, as sicker infants may have greater clinical requirements necessitating forced head turning, such as tubing that rotates the head to the side or imposed positioning during periods when there are decreased spontaneous movements. It remains unclear if sicker infants were more likely to demonstrate head turn preference or if the medical interventions themselves promoted development of head turn preference. Additionally, we did not find a relationship between head turn preference and cerebral injury, which was inconsistent with other reports [29].
Our findings of relationships between head turn preference and poor outcome are suggestive of an altered developmental trajectory in affected infants. Among those with a significant head turn preference, poorer self-regulation and reflex development were present by 34 weeks PMA. These relationships were not evident at term age, however, the range in age at time of testing (37 weeks to 41 weeks) may have made relationships more difficult to isolate than at 34 weeks postmenstrual age, when all infants underwent neurobehavioral assessment in a one week window. Infants with early head turn preference also demonstrated alterations in development at age 2 years, with worse fine motor performance. However, we are unable to determine whether head turn preference is a marker for developmental impairment or whether midline head positioning is critical for subsequent development, as postural asymmetries can result from head rotation to the side resulting in subsequent patterns of postural deformity [14,30]. Due to the importance of midline orientation of the head in the first year of life, therapeutic and environmental interventions may be important for optimizing developmental outcomes, especially in infants who are at high risk of impairment.
Proper positioning and therapeutic interventions in the NICU can potentially reduce the effects of head turn preference [31]. The primary aims of positioning a neonate are to support posture and movement, optimize skeletal development and biomechanical alignment, provide controlled exposure to varied proprioceptive, tactile, and visual stimuli, and promote a calm, regulated behavioral state [16]. Current neonatal positioning practices include positioning the head in midline and changing the direction of the head of the bed to prevent environmental contributions to preference [30]. Our group demonstrated less asymmetry among infants in the NICU positioned with an alternative device aimed at maintaining the infant in a flexed, midline-oriented position [32]. Additionally, therapeutic interventions that can be conducted in the NICU to address head turn preference include neonatal positioning and passive range of motion to facilitate midline orientation of the head and neck.
This study was limited by a small sample size and used recorded videos of the NNNS, rather than direct assessment of head turn during clinical exam. The study used a sample of preterm infants with significant variability in medical course and interventions, and factors that could be contributing to head turn preference may not have been defined and investigated. Conducting the assessment of head turn preference on a sample of healthy controls could improve our understanding of normal head turning and laterality as opposed to use of solely a high-risk sample. In addition, this study did not capture the effect of therapeutic interventions received during the NICU stay. The NICU at the study site, St. Louis Children's Hospital, has a dynamic therapy program, and all of the infants in the cohort received physical and occupational therapy. Interventions for head turn preference in the study site NICU are common. Other early environmental factors, such as positioning of the bed and the direction that caregivers approached the bedside, were considered in the current study. However, it was evident that these factors were confounded by environmental adaptations made at the study site, such as therapist recommendations at the bedside indicating which way to position the infant to promote orientation away from the head turn preference. Thus, these factors were not reported as part of this study. At the study site, methods to promote midline orientation in the first week of life to potentially stabilize cerebral perfusion are current standard of care. However, these practices were not instituted until after this cohort was enrolled [33]. Finally, this was an exploratory study that investigated multiple outcome variables without correcting for multiple comparisons. Such work sets the stage for future inquiry.
4. Conclusion
Head turn preference is common in preterm infants who are at high risk of developmental impairment. Observation of a head turn preference, severity of the preference, and range of motion of the neck may suggest increased risk for altered developmental trajectory. By identifying head turn preference in the neonate, targeted interventions could potentially be implemented that may optimize developmental outcome. More research on head turn preference is warranted to understand normal and abnormal presentations, factors influencing it, effects on outcome, as well as potential treatments. The new scoring system for head turn preference developed for this study will pave the way for future inquiry.
Highlights.
Head turn preference is common in preterm infants.
Head turn preference to the right is more common.
NICU medical factors are associated with head turn preference.
Head turn preference is associated with poorer developmental outcome.
A scale has been developed to quantify head turn preference.
Acknowledgments
We express our appreciation to the following individuals who assisted with this project: Michael Wallendorf who provided biostatistical support; Karen Lukas, Anthony Barton, Jessica Conners, Claudine Vavasseur, Cynthia Rogers, Amit Mathur, Brad Schlaggar, Han Tjoeng, Divyen Shah, and Rachel Paul who provided team support related to this research project; Jim Alexopoulos, Joshua Shimony, Reginald Lee, Tara Smyser, and Joe Ackermann Jr. who worked on imaging data synthesis; Sarah Oberle, Lauren Reynolds, Joy Bender, and Jessica Roussin who provided editorial assistance, data management support, and participated with the head turn preference reliability testing; and Katie Ross, Kelsey Dewey, Felicia Foci, Justin Ryckman, Polly Durant, Rachel Harris, Elizabeth Heiny, Hayley Chrzastowski, and Odochi Nwabara who assisted with the formatting and early testing of the head turn preference scales. We thank Sarah Jossart, Michelle Tang and Elaine Ward for their assistance with head turn preference scoring. We express our sincere gratitude to Meredith Gronski and her son, Joshua Gronski, for being our model for the head turn preference scales. We also thank the families and infants who were participants and made this research possible.
Funding: This project was supported by the National Institute of Health (NIH/NCMRR, NICHD, NINDS K12 HD055931, R0I HD 057098, P30 HD062171, NIH/NINDS K02 NS089852, NIH UL1 TR000448, and KL2 TR000450).
Appendix A
References
- 1.Konishi Y, et al. Laterality of finger movements in preterm infants. Dev Med Child Neurol. 1997;39(4):248–252. doi: 10.1111/j.1469-8749.1997.tb07420.x. [DOI] [PubMed] [Google Scholar]
- 2.Michel GF. Right-handedness: a consequence of infant supine head-orientation preference? Science. 1981;212(4495):685–687. doi: 10.1126/science.7221558. [DOI] [PubMed] [Google Scholar]
- 3.Hepper PG, McCartney GR, Shannon EA. Lateralised behaviour in first trimester human foetuses. Neuropsychologia. 1998;36(6):531–534. doi: 10.1016/s0028-3932(97)00156-5. [DOI] [PubMed] [Google Scholar]
- 4.Ververs IA, et al. Prenatal head position from 12 to 38 weeks. II. The effects of fetal orientation and placental localization. Early Hum Dev. 1994;39(2):93–100. doi: 10.1016/0378-3782(94)90158-9. [DOI] [PubMed] [Google Scholar]
- 5.Gunturkun O. Human behaviour: Adult persistence of head-turning asymmetry. Nature. 2003;421(6924):711. doi: 10.1038/421711a. [DOI] [PubMed] [Google Scholar]
- 6.Domellof E, Hopkins B, Ronnqvist L. Upper and lower body functional asymmetries in the newborn: do they have the same lateral biases? Dev Psychobiol. 2005;46(2):133–140. doi: 10.1002/dev.20046. [DOI] [PubMed] [Google Scholar]
- 7.J V Neurology of the Newborn. Philadelphia: Saunders Elsevier; 2008. [Google Scholar]
- 8.Aucott S, et al. Neurodevelopmental care in the NICU. Ment Retard Dev Disabil Res Rev. 2002;8(4):298–308. doi: 10.1002/mrdd.10040. [DOI] [PubMed] [Google Scholar]
- 9.Grenier I, et al. Comparison of motor, self regulatory, and stress behaviors of preterm infants across body positions. Am J Occup Ther. 2003;57(3):289–97. doi: 10.5014/ajot.57.3.289. [DOI] [PubMed] [Google Scholar]
- 10.Yoo H, Rah D, Kim Y. Outcome analysis of cranial molding therapy in nonsynostotic plagiocephaly. Arch Plast Surg. 2012;39:338–44. doi: 10.5999/aps.2012.39.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Konishi Y, Mikawa H, Suzuki J. Asymmetrical head-turning of preterm infants: some effects on later postural and functional lateralities. Dev Med Child Neurol. 1986;28(4):450–457. doi: 10.1111/j.1469-8749.1986.tb14282.x. [DOI] [PubMed] [Google Scholar]
- 12.Konishi Y, et al. Effect of body position on later postural and functional lateralities of preterm infants. Dev Med Child Neurol. 1987;29(6):751–757. doi: 10.1111/j.1469-8749.1987.tb08820.x. [DOI] [PubMed] [Google Scholar]
- 13.Konishi Y, et al. Development of posture in prone and supine positions during the prenatal period in low risk preterm infants. Arch Dis Child. 1994;70:188–91. doi: 10.1136/fn.70.3.f188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porter D, Michael S, Kirkwood C. Is there a relationship between foetal position and both preferred lying posture after birth and pattern of subsequent postural deformity in nonambulant people with cerebral pasly? Child Care Health Dev. 2009;36(5):742–7. doi: 10.1111/j.1365-2214.2009.01035.x. [DOI] [PubMed] [Google Scholar]
- 15.Nuysink J, et al. Prevalence and predictors of idiopathic asymmetry in infants born preterm. Early Hum Dev. 2012;88:387–92. doi: 10.1016/j.earlhumdev.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Sweeney J, Gutierrez T. Musculoskeletal implications of preterm infant positioning in the NICU. J Perinat Neonatal Nurs. 2002;16(1):58–70. doi: 10.1097/00005237-200206000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Geerdink JJ, Hopkins B, Hoeksma JB. The development of head position preference in preterm infants beyond term age. Dev Psychobiol. 1994;27(3):153–168. doi: 10.1002/dev.420270303. [DOI] [PubMed] [Google Scholar]
- 18.Moors P, et al. Perceiving where another person is looking: the integration of head and body information in estimating another person's gaze. Front Psychol. 2015;6:909. doi: 10.3389/fpsyg.2015.00909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pomianowska I, et al. The role of social cues in the deployment of spatial attention: head-body relationships automatically activate directional spatial codes in a Simon task. Front Integr Neurosci. 2011;6:4. doi: 10.3389/fnint.2012.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.K P. Early infant asymmetries and handedness: a critical evaluation of the evidence. Dev Neuropsychol. 1992;8(4):325–65. [Google Scholar]
- 21.R B. Effects of infant head position on sides preference in adult handling. Infant Behav Dev. 1979;2:355–8. [Google Scholar]
- 22.Peitsch W, et al. Incidence of cranial asymmetry in healthy newbors. Pediatrics. 2002;110(6):e72. doi: 10.1542/peds.110.6.e72. [DOI] [PubMed] [Google Scholar]
- 23.Hutchison B, et al. Plagiocephaly and brachycephaly in the first two years of life: a prospective cohort study. Pediatrics. 2004;114(4):970–80. doi: 10.1542/peds.2003-0668-F. [DOI] [PubMed] [Google Scholar]
- 24.Fagard J. The nature and nurture of human infant hand preference. Ann N Y Acad Sci. 2013;1288:114–123. doi: 10.1111/nyas.12051. [DOI] [PubMed] [Google Scholar]
- 25.The CRIB (clinical risk index for babies) score: a tool for assessing initial neonatal risk and comparing performance of neonatal intensive care units The International Neonatal Network. Lancet. 1993;342(8865):193–198. [PubMed] [Google Scholar]
- 26.Lester B, Tronick E. History and description of the Neonatal Intensive Care Unit Network Neurobehavioral Scale. Pediatrics. 2004;113:634–40. [PubMed] [Google Scholar]
- 27.Bayley N. (Bayley-III) Motor Scale Kit. 3rd. Pearson Educational Inc; 2011. Bayley Scales of Infant and Toddler Development. [Google Scholar]
- 28.Liederman J, Kinsbourne M. The mechanism of neonatal rightward turning bias: a sensory or motor asymmetry? Infant Behav Dev. 1980;3:223–38. [Google Scholar]
- 29.Perez A, et al. Long-term neurodevelopmental outcome with hypoxic–ischemic encephalopathy. J Pediatr. 2013;163(2):454–9. doi: 10.1016/j.jpeds.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Nuysink J, et al. Clinical course of asymmetric motor perfromance and deformational plagiocephaly in very preterm infants. J Pediatr. 2013;163(3):658–65. doi: 10.1016/j.jpeds.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 31.de Lima-Alvarez CD, et al. Effects of postural manipulations on head movements from birth to 4 months of age. J Mot Behav. 2013;45(3):195–203. doi: 10.1080/00222895.2013.778814. [DOI] [PubMed] [Google Scholar]
- 32.Madlinger-Lewis L, et al. The effects of alternative positioning on preterm infants in the neonatal intensive care unit: a randomized clinical trial. Res Dev Disabil. 2014;35(2):490–7. doi: 10.1016/j.ridd.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malusky S, Donze A. Neutral head positioning in premature infants for intraventricular hemorrhage prevention: an evidence-based review. Neonatal Netw. 2011;30(6):381–396. doi: 10.1891/0730-0832.30.6.381. [DOI] [PubMed] [Google Scholar]


