Abstract
Belatacept is a B7-specific fusion protein used to prevent allograft rejection by blocking T cell costimulation. Generally efficacious, it fails to prevent acute rejection in a sizable minority of patients. In experimental models, memory T cells mediate costimulation blockade-resistant rejection (CoBRR), but this remains undefined in humans. To explore relationships between individuals’ immune cell phenotypes and CoBRR, we studied patients receiving belatacept or conventional calcineurin inhibitor-based immunosuppression. We identified a population of CD57+PD1− CD4 T cells present prior to transplantation that correlated with CoBRR. Contrary to data recognizing CD57 as a marker of senescence on CD8 T cells, we discovered a non-senescent, cytolytic phenotype associated with CD57 on CD4 T cells. Moreover, CD57+ CD4 T cells: expressed high levels of adhesion molecules implicated in experimental CoBRR; were CD28 negative; expressed a transcriptional phenotype broadly defining allograft rejection; and were shown to be present in rejecting human kidney allografts. These data implicate CD57+ CD4 T cells in clinical CoBRR and if prospectively validated, this characteristic could identify patients at higher risk for acute rejection on belatacept-based therapy.
Introduction
Kidney transplantation is a standard, life-saving therapy, but organ survival necessitates the use of immunosuppressive drugs. These drugs render a transplant recipient immune compromised, the degree of which in large part determines the clinical risk of the procedure. As such, clinical immunosuppression has evolved through the development of increasingly targeted drug therapies to successfully manipulate the immune response toward an allograft without overly impairing the recipient’s protective immune capacity. Following kidney transplantation most patients receive calcineurin inhibitors (CNIs: e.g. tacrolimus), which suppress T cell function through inhibition of a ubiquitous intracellular signaling pathway. This leads to very effective, non-specific, T cell immunosuppression and a substantial decrease in acute rejection rates that comes at the expense of impaired protective immunity, particularly to viruses and fungi (1–4); it also precipitates chronic CNI-associated non-immune nephrotoxic and metabolic side effects (5, 6). This dilemma has impelled the development of more specific, targeted therapeutics to prevent rejection without the complications observed with CNIs; the most prominent of which is belatacept.
Belatacept, a fusion protein targeting a specific extracellular costimulation pathway (the CD28-B7 receptor ligand pair), was developed as a potential replacement for CNIs. Belatacept binds to CD80 and CD86 with high affinity, preventing their binding to the critical T cell costimulatory receptor CD28; a mechanism now referred to as costimulation blockade (CoB)(7). TCR ligation in the absence of costimulation is generally ineffective in activating naïve, antigen-specific T cells, such that CoB substantially impairs de novo alloimmune responses. Antigen experienced T cells often have reduced requirements for costimulation, and thus, CoB can have a sustained inhibitory effect on new immune encounters without impairing previously established protective immunity.
Clinical trials evaluating the efficacy of belatacept-based immunosuppression demonstrated improved side effect profiles, graft function and patient and graft survival up to five years post transplant compared to patients receiving CNI-based immunosuppression(8–10). Unfortunately, patients treated with belatacept experienced more severe and higher rates of acute cellular rejection (ACR) compared to patients treated with CNIs(9, 10). This CoBRR has limited the clinical use of belatacept irrespective of its substantially reduced side-effect profile. Given the highly specific nature of belatacept’s action, we sought to investigate whether the immune cell profile of an individual at the time of transplant, particularly the degree to which they have progressed from a naïve to antigen experienced cell phenotype, can be assessed to identify patients at risk for CoBRR. We find that a particular cell type, the CD57+ CD4 T cell, which is atypical in healthy adults but frequent in patients with kidney failure, is associated with belatacept-resistant rejection (BRR) and worthy of further study.
Materials and Methods
Sample acquisition
Kidney allograft recipients receiving belatacept or tacrolimus according to labeled indication were enrolled in an IRB-approved tissue acquisition protocol at the Emory Transplant Center. Collection and use of patient blood samples for laboratory analysis was approved by the Institutional Review Board at Emory University (Approval No. IRB00006248). Written informed consent was received from participants prior to inclusion in each study. Peripheral blood mononuclear cells (PBMCs) were collected prior to transplantation and at multiple time points post-transplantation. Patients were followed clinically and segregated by outcome for analysis. For this study, we performed a retrospective analysis of stored patient samples. All belatacept-treated patients enrolled that that had not received a prior transplant and had baseline samples available for analysis were included in this study. Within 7 months of transplantation, of the 14 patients receiving belatacept-based therapy, 9 patients experienced ACR and 5 were rejection-free. 10 tacrolimus-treated patients, 5 that experienced ACR and 5 that were rejection-free, were selected with similar demographics to the belatacept-treated patients for comparison. Patient demographics and additional supporting information may be found in the online version of this article.
Flow cytometry and intracellular cytokine staining
PBMCs obtained prior to drug administration were analyzed by flow cytometry to characterize the immune cell repertoire at baseline, interrogating for markers of memory, differentiation, activation, exhaustion and senescence. Antibodies were used against CD2 (BD #562300), CD3 (BD #557943), CD4 (BioLegend #317435), CD8 (eBioscience #47-00878-42), LFA1 (BD #563936), CD28 (BioLegend #302930), VLA4 (BioLegend #304314), CD57 (BD #555619) and PD1 (BioLegend #329906). Intracellular staining for Ki67 (BD #556026), granzyme B (BD #562462) was done using BD Biosciences Fixation/Permeabilization Solution Kit. Intracellular staining for IFNγ (BD #562392), and TNFα (BD #340534) was done following a four-hour stimulation with phorbol 12-myristate 13-acetate (PMA) and ionomycin in the presence of brefeldin A (Sigma-Aldrich). All samples were run using a BD Biosciences LSRFortessa and analyzed using FlowJo software (Treestar).
PCR
Analysis of patient telomere length was performed on QuantStudio (Life Technologies) with iTaq Sybr (Biorad). Reference DNA was generated by the combination of five healthy controls and used to produce a standard curve to determine relative telomere/single copy gene ratio (11, 12).
Microarray
Peripheral blood obtained from 5 healthy controls was processed and sorted on a BD FACSAria cell sorter into CD3+CD4+CD8−CD57+PD1− and CD3+CD4+CD8−CD57−PD1− populations. Antibodies were used against CD3 (BD #560366), CD4 (BD #557852), CD8 (eBioscience #47-0088-42), CD57 (BD #555619) and PD1 (BioLegend #329906). Total RNA was extracted from the sorted populations and quality assessed. cDNA synthesis and amplification was performed, and fragmented and biotinylated samples were hybridized to the Affymetrix Human U133 plus 2.0 probe array. The arrays were scanned and probe intensity measurements were normalized across the samples using the robust multichip average (RMA) algorithm. GEO accession GSE64805.
Immunohistochemistry
Kidney allograft biopsies were obtained per protocol time point or suspected rejection. 36 biopsies were dual stained for anti-CD57 (DAKO, Carpinteria, CA) and anti-CD4 (DAKO, Carpinteria, CA). Blinded analysis was performed to determine cell density and localization.
Allogeneic mixed lymphocyte reaction
PBMCs were obtained by cytapheresis of healthy volunteers who gave their informed written consent. PBMCs were isolated by Ficoll Paque density-gradient centrifugation (GE Heathcare), monocytes and lymphocytes were isolated by positive CD14 or CD3 selection (Miltenyi Biotec, purity > 95%). Monocytes were then plated and cultured as previously described with 25 ng/ml recombinant human IL-4 (hIL-4; ImmunoTools) and 1000 IU/ml recombinant human granulocyte-macrophage colony stimulating factor (rhGM-CSF; Peprotech). At day 6, immature DCs were characterized by staining for CD14 and CD209 and stimulated for an additional 48 hr with lipopolysaccharide (LPS; 50 ng/ml, Sigma-aldrich) (activated DC). For proliferation assay, lymphocytes were stained with 1 μM Violet Proliferation Dye 450 (VPD450; BD Biosciences) in DPBS for 10 min at 37°C and washed 2 times in complete medium and added to activated allogeneic DC (DC/CD3+ T cells ratio : 1:3) with or without belatacept (25μg/ml). After 7 days of co-culture, VPD450 dilution and phenotype were analyzed by FACS. Antibodies were purchased from BD Biosciences (V450-labeled anti-CD14, APC-labeled anti-CD209, APC-Cy7-labeled anti-CD3, BUV395-labeled anti-CD4, PerCP-Cy5.5-labeled anti-CD8, BV605-labeled anti-CCR7, PE-labeled anti-CD57, APC-labeled anti-27, PE-Cy7-labeled anti-CD38) or from Miltenyi Biotec (FITC-labeled anti-CD45RO). Flow cytometry data were recorded with an LSR Fortessa cell analyser (BD Biosciences) and analyzed with Diva software (BD Biosciences).
Statistics
Differences in percentage of CD57+PD1− CD4 T cells were calculated using Mann-Whitney test due to the bimodal distribution of this cell subset. For additional flow cytometry analyses, P values were calculated using unpaired t tests, linear regressions and one-way and two-way ANOVA, and r values were computed using Pearson correlation coefficients. For the microarray, a paired t test was employed on the normalized expression values to find differentially expressed genes in CD57+ compared to CD57− cells. For immunohistochemistry, significant differences between stable and rejection outcomes were calculated using an unpaired t test. P values of less than 0.05 were considered statistically significant.
Results
High prevalence of CD57+PD1− CD4 T cells in peripheral blood prior to transplant is associated with belatacept-resistant rejection
It is known that the activation of antigen-experienced T cells is less costimulation-dependent relative to that of naïve cells(13, 14), and molecules associated with antigen experience have been related to CoBRR(15–17). We therefore explored pre-transplant expression of surface markers indicative of antigen experience and terminal differentiation, specifically PD1 and CD57. PD1 is upregulated following activation and maintains expression on exhausted T cells(18, 19), and CD57 is a carbohydrate epitope present on replicative senescent cells(20–23). While this has been well described in the context of CD8 T cells(24, 25) our study identified a population of CD4 T cells in the peripheral blood of transplant patients receiving belatacept-based immunosuppression that were CD57+PD1− and correlated with BRR, regardless of memory status (Figure 1B). No significant differences in outcome were observed in any subset of PD1 or CD57 expressing CD8 T cells (Figure 1C).
Figure 1. CD57+PD1− CD4 T cells in peripheral blood associate with belatacept-resistant rejection.
(A) Representative flow plot of the gating strategy to identify CD57+PD1− T cells. (B) The left panel shows baseline PBMCs from patients treated with belatacept who went on to experience acute cellular rejection had a significantly higher prevalence of CD57+PD1− CD4 T cells compared to patients with a stable outcome (p<0.03). The right panel shows CD57+PD1− CD4 T cells were not segregated to terminally differentiated effector memory cells, but were significantly increased in patients with rejection compared to patients with a stable outcome in all memory compartments based on CD45RA and CCR7 expression (p<0.03). (C) The left panel shows that the percentage of CD57+PD1− CD8 T cells did not segregate with outcome in belatacept-treated patients. The right panel demonstrates the lack of association is true regardless of memory subset. (D, E) Analysis of baseline PBMCs from patients treated with tacrolimus reveals no correlation between outcome and prevalence of CD57+PD1− CD4 or CD8 T cells. PBMC, peripheral blood mononuclear cell.
To distinguish whether this was specific to BRR, or characteristic of rejection in general, we studied patients treated with conventional tacrolimus-based immunosuppression. Using the same parameters, no significant difference between outcome and the expression of CD57 or PD1 was elucidated in CD4 or CD8 T cells (Figure 1D, E). These data demonstrate that CD57 expression on CD4 T cells in peripheral blood prior to transplant associates with rejection specifically in belatacept-treated patients. We found this population of cells to be rare in healthy controls, but significantly increased in patients with kidney failure (Figure 2), increasing its unique relevance to kidney transplantation.
Figure 2. CD57+PD1− CD4 T cells are atypical in healthy controls.
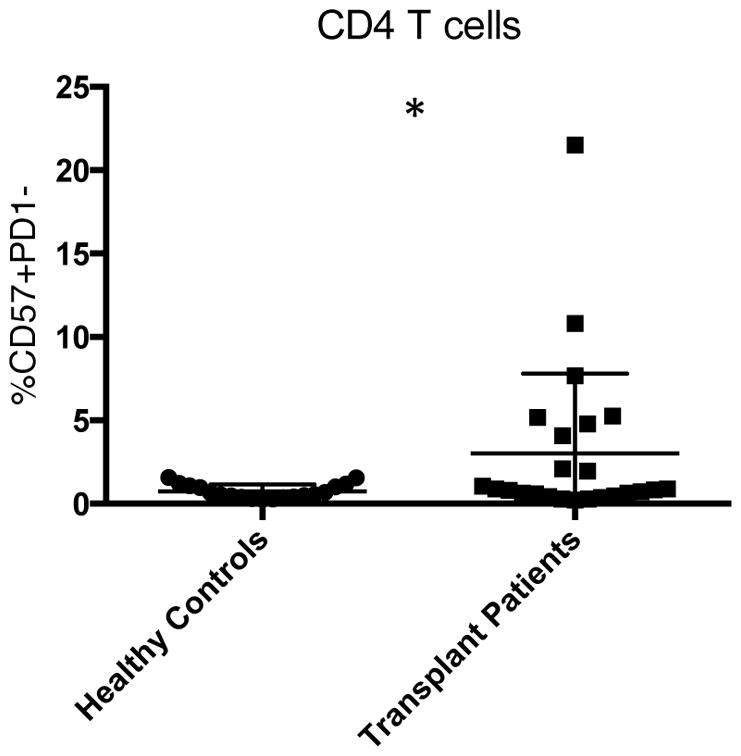
The frequency of CD57+PD1− CD4 T cells is significantly increased in PBMCs of patients with kidney failure awaiting transplant compared to PBMCs of healthy controls (p<0.05), highlighting their unique relevance to kidney transplantation. PBMC, peripheral blood mononuclear cell.
CD57+ CD4 T cells are not senescent by traditional indices
CD57 has been described as a marker of immune cell senescence, a phenotype that would be unlikely to segregate with an active process like allograft rejection. Therefore, we employed independent assays to ascertain whether this was true in our cell population of interest. First we performed PCR analysis of relative telomere length in the same patient cohort described above. Terminally differentiated cells that have undergone multiple divisions have shortened telomeres (26), which should correlate with increased CD57 expression. In the patients treated with belatacept, expression of CD57 on CD4 T cells did not correlate with relative telomere length (Figure 3A). This lack of correlation indicated a possible non-traditional role of CD57 on CD4 T cells. Secondly, we looked at expression of Ki67, a protein strictly associated with cell proliferation (27). Increased expression of CD57 on CD4 T cells in belatacept-treated patients significantly correlated with increased Ki67 expression (p < 0.01) (Figure 3B), also inconsistent with cell senescence. The non-senescent phenotype of CD57+ CD4 T cells in this setting contradicts theparadigm of CD57 expression and senescence.
Figure 3. CD57+ CD4 T Cells are not senescent based on traditional indices.
(A) Relative telomere length of belatacept-treated patients was determined by PCR and did not correlate with CD57 expression on CD4 T cells in the same belatacept-treated patients. (B) Ki67 expression significantly correlated with increased expression of CD57 on CD4 T cells in belatacept-treated patients (p<0.01, r=0.6704).
The gene profile of CD57+PD1− CD4 T cells is highly associated with allograft rejection
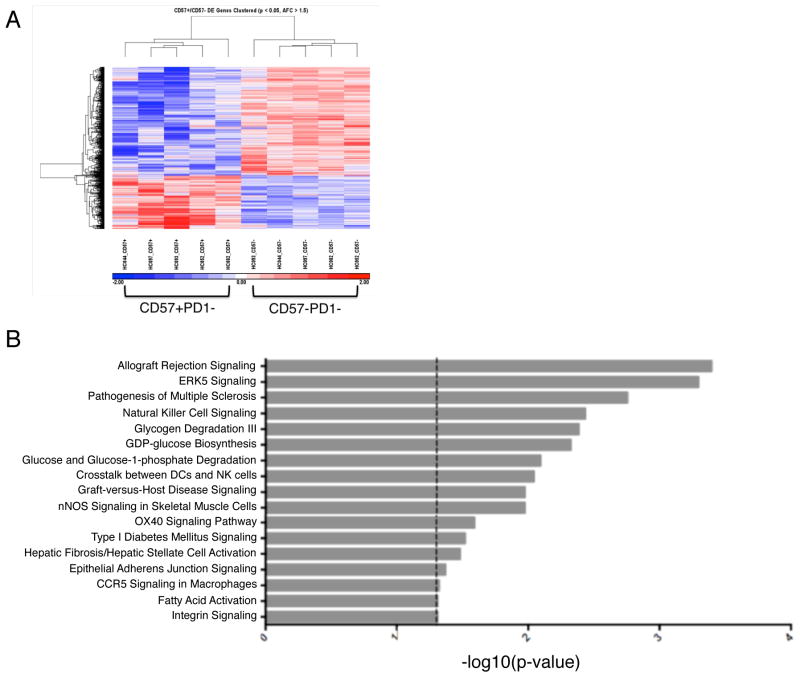
To gain insight into the function of CD57+PD1− CD4 T cells, we performed a microarray to examine their gene expression profile. PBMCs from healthy controls were sorted into CD57+PD1− and CD57−PD1− CD4 T cells and analyzed by microarray. A heat map of normalized gene expression revealed a distinct transcriptome associated with CD57+ compared to CD57− CD4 T cells (Figure 4A). The most upregulated genes were indicative of a highly cytotoxic profile and included molecules involved in activation, adhesion, cell trafficking and cytotoxicity. We then imported the gene expression profiles into an independent Ingenuity Pathway Analysis (IPA), an unsupervised analysis of over 600 biological pathways. IPA remarkably deemed ‘Allograft Rejection Signaling’ the most closely associated signaling pathway with the genes upregulated in CD57+ CD4 T cells (Figure 4B).
Figure 4. Genes Involved in Allograft Rejection are upregulated in CD57+ CD4 T cells.
(A) PBMCs from healthy controls (n=5) were sorted into CD57+PD1− and CD57−PD1− CD4 T cells. RNA was extracted, cDNA synthesis and amplification was performed and samples were hybridized to the Affymetrix Human U133 plus 2.0 probe array. Clear segregation of gene expression was observed in CD57 positive compared to CD57 negative CD4 T cells. (B) Ingenuity pathway analysis deemed ‘Allograft Rejection Signaling’ the most closely associated signaling pathway with the genes upregulated in CD57+ compared to CD57−CD4 T cells. PBMC, peripheral blood mononuclear cell.
CD57+ cells have increased expression of adhesion molecules
Adhesion molecules, such as CD2 and LFA1, have also been implicated in CoBRR. Indeed, their selective elimination has been shown to substantially reduce the risk of CoBRR in animal models(15–17, 28). To evaluate the relationship between expression of CD57 and CD2, LFA1, and VLA4, we performed additional phenotyping on baseline PBMCs from transplant patients. CD2hiCD4 T cells were shown to express high levels of LFA1 and VLA4, with CD57 present on cells that expressed high levels of these adhesion molecules (Figure 5A). Importantly, while all CD57+ cells expressed high levels of CD2, LFA1 and VLA4, the converse was not true, such that CD57 was a unifying marker of increased adhesion molecule expression (Figure 5B). These results suggested that CD57+PD1− CD4 T cells may not only serve as a marker of rejection risk in the periphery, but may play an active role in mediating rejection, prompting us to determine whether these cells were present in rejecting allografts.
Figure 5. CD57 is present on cells with increased expression of other adhesion molecules implicated in CoBRR.
(A) Representative flow cytometry plots of PBMCs from patients prior to transplantation demonstrate demonstrating CD2hi CD4 T cells also express high levels of adhesion molecules LFA1 and VLA4, whereas CD2lo CD4 T cells express low levels of LFA1 and VLA4. CD57+ CD4 T cells are found exclusively in the CD2hi LFA1hi VLA4hi subset. (B) Representative flow cytometry plots of PBMCs from the same patients prior to transplantation validate that all CD57+ CD4 T cells express high levels of CD2, LFA1 and VLA4. CoBRR, costimulation blockade-resistant rejection.
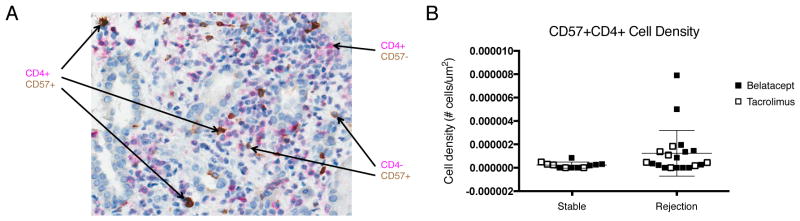
There is an increased density of CD57+ T cells in rejecting kidney allografts
Clinical kidney allograft biopsies obtained either for protocol surveillance or at the time of suspected rejection and were dual stained for CD4 and CD57 (Figure 6A). CD57+ CD4 T cells were present in rejecting kidney allografts at a higher density compared to stable kidney allografts (Figure 6B). In fact, patients with a higher percentage of circulating CD57+PD1− CD4 T cells prior to transplantation also had an increased density of CD57+ CD4 T cells in rejecting allografts. Thus, this population of cells is not only present in the peripheral blood, but they are capable of infiltrating the graft, likely due to the increased expression of adhesion molecules.
Figure 6. Increased density of CD57+ T cells in rejecting allografts.
(A) A representative kidney allograft biopsy dual stained to show CD4 (in pink) and CD57 (in brown) expression demonstrates that CD57+ CD4 T cells are able to infiltrate kidney allografts. (B) Summary data of all biopsies from patients treated with belatacept (closed squares) and patients treated with tacrolimus (open squares) demonstrate the density of CD57+ CD4 T cells in kidney biopsies collected at the time of rejection is greater compared to patient biopsies obtained at a stable protocol time point (p=0.08).
CD57+ CD4 T cells are CD28− and exhibit cytolytic properties
Multiple groups have shown that CD28 loss is associated with CoBRR(29, 30), but loss of CD28 did not statistically associate with BRR in our study. While we found an inverse correlation between CD57 and CD28 expression (Figure 7A), not all CD28− cells were CD57+ (Figure 7B). In vitro studies revealed that CD57+PD1− CD4 T cells are almost exclusively polyfunctional, producing both IFNγ and TNFα following stimulation with PMA and ionomycin (Figure 7C), while CD28-PD1−CD4 T cells were largely incapable of producing either IFNγ or TNFα (Figure 7D). Furthermore, all CD57+ CD4 T cells expressed high levels of Granzyme B compared to CD57− CD4 T cells, which express low levels of Granzyme B (Figure 7E). As such, while loss of CD28 perhaps identified cells indifferent to CD28-B7 blockade by belatacept, CD57 expression identified those CD28 negative cells capable of actively mediating BRR.
Figure 7. CD57+ CD4 T cells are polyfunctional with a cytolytic profile.
(A) Summary data for all patient samples, n=24, show an inverse correlation of CD57 and CD28 expression on CD4 T cells at baseline, prior to transplantation (p<0.01, r=0.7759). (B) Representative flow plot demonstrating CD57 and CD28 are not expressed in a mutually exclusive manner. (C) Baseline PBMCs from the same patients were stimulated with PMA and ionomycin. Following stimulation, most CD57+PD1− CD4 T cells produce both IFNγ and TNFα (p<0.01). (D) Following stimulation with PMA and ionomycin, most CD28+PD1− CD4 T cells from the same patients’ baseline PBMCs are incapable of producing either IFNγ or TNFα (p<0.01). (E) Representative flow and histogram demonstrating CD57+ CD4 T cells express high levels of Granzyme B (in red) while CD57− CD4 T cells express low levels of Granzyme B (in blue).
CD57+ CD4 T cells are resistant to the immunosuppressive effects of belatacept
To determine whether CD57+ CD4 T cells are indeed resistant to the immunosuppressive effects of belatacept, CD3 T cells from healthy volunteers were labeled with Violet Proliferative Dye 450 and co-cultured with activated allogeneic dendritic cells (DCs). After 7 days in culture in the presence or absence of belatacept, cell subset proliferation was assessed by flow cytometry. Both CD57+ and CD57− CD4 T cells were able to proliferate in response to activated DCs in the absence of belatacept (Figure 8A). While proliferation of CD57− CD4 T cells was significantly inhibited in the presence of belatacept, proliferation of CD57+ CD4 T cells remained uninhibited (Figure 8B, C), confirming that CD57+ CD4 T cells are activated in a costimulation independent fashion.
Figure 8. CD57+ CD4 T cells are resistant to in vitro immunosuppression with belatacept.
(A) CD57− (left panels) and CD57+ (right panels) CD4 T cells from healthy controls are able to proliferate in response to activated allogeneic dendritic cells in the absence of belatacept. (B) Proliferation of CD57− CD4 T cells is inhibited by belatacept while CD57+ CD4 T cells retain the ability to proliferate in the presence of belatacept. (C) Summary data shows proliferation of CD57− CD4 T cells is significantly inhibited (p<0.03) with the addition of belatacept, while no significant change in proliferation is observed in CD57+ CD4 T cells (n=4).
Discussion
The search for a suitable alternative to CNI-based immunosuppression has become increasingly relevant as long term outcomes and toxicities have gained prominence in clinical transplantation. Early results with belatacept, though encouraging with regard to graft function and off-target side effects, have been disappointing with regard to the ability to prevent early acute rejection. This has impelled an explicit look for mechanistic correlates of belatacept resistance and in particular a means of risk stratification for patients to guide immunosuppressive regimen choice. We chose to investigate variability in cell surface phenotype recognizing that individuals’ lymphocyte repertoire evolves considerably over time as a result of environmental pathogen exposure and that these changes markedly alter cells’ costimulation requirements including, but not limited to, expression of and dependence on CD28 (31–34). These characteristics have experimentally been shown to mollify the efficacy of CoB-based regimens. In this series of experiments, we show this to be potentially relevant to humans, and characterize a particular cell type that is prevalent in renal failure patients, the CD57+PD1− CD4 T cell, the presence of which correlates with the risk of CoBRR, is present within rejecting allografts, and whose characteristics relate to the known mechanisms of cellular allograft rejection and costimulation resistance. It is important to note that there are several mechanisms of allograft rejection and the prevalence of CD57+PD1− CD4 T cells demonstrates a potential additional, and not exclusive, mechanism of CoBRR to be aware of in this population.
Although CD57+ CD4 T cells have previously been associated with cell senescence in HIV infected patients (23, 35), we have demonstrated that CD57 is a unique, unifying marker of human CD4 T cells that are enriched in patients with kidney failure and have a cytolytic profile characterized by high levels of adhesion molecules previously implicated in CoBRR. CD57+CD4 T cells are capable of infiltrating allografts and producing multiple cytokines upon stimulation. In this context, CD57 appears not to be a marker of senescence, but rather a potential surrogate indicator of patients’ immune history as it relates to BRR. This population is consistent with that described previously as being prevalent in the kidney transplant population(36). Their enrichment in patients with renal failure is an additional novel observation of these studies, the mechanism of which deserves further study. Similarly, additional studies are necessary to completely define the mechanism by which these cells mediate CoBRR. Nevertheless, their association with and proximity to rejecting kidneys is clearly demonstrated in our studies.
CD57+ CD4 T cells have been previously described in the context of transplantation. Legendre, et al described a higher percentage of CD57+ (then, Leu7+) CD4 T cells in patients that experienced rejection compared to stable patients(36). This study, predating the tacrolimus era, combined with studies reported herein, strongly implicates this cell type as worthy of attention. Collectively, these data support the finding that CD57+ CD4 T cells play a role in allograft rejection, particularly in the absence of CNIs, and that prospective assessment of this population could be used to identify patients at particular risk for ACR, especially in costimulation blockade-based regimens.
Graft survival rates have improved in the past 20 years largely due to the development of targeted immunosuppressant drugs. Traditional immunosuppression management consists of universalized algorithms of serum drug levels and dosing regimens that are generally applied to transplant populations. With increasingly specific forms of immune manipulation, the opportunity for more personalized immune management becomes more evident and indeed, may become increasingly required. This is particularly true when selectively targeting a molecule that progressively diminishes in expression over a lifetime of immune perturbation. Though the increased risk of rejection associated with belatacept-based immunosuppression has proved a significant challenge for its clinical implementation, CD57+PD1− CD4 T cells represent a potential therapeutic target, as well as a practical screening tool to identify patients at higher risk for acute cellular rejection on belatacept-based therapy. The ability to clearly and accurately define markers of risk of rejection will help tailor immunosuppression therapies on an individual basis.
We acknowledge that these studies have been performed on a small group of patients, albeit one that has been very carefully monitored and controlled. Clinical intolerance for high rejection rates has prevented us from expanding the clinical cohort substantially. As our clinical goal has been to provide adjuvant therapies to mitigate BRR, expanding this cohort has not been feasible. Additional studies are necessary to elucidate associations between CD57 and other costimulation molecules to potentially target and inhibit the activation of CD57+ CD4 T cells, as well as phenotype these cells following transplantation. Nevertheless, we believe these observations to be sufficiently statistically supported and mechanistically plausible to warrant their reporting and to stimulate further study.
In summary, we find that expression of CD57 identifies a population of polyfunctional CD28 negative cells with cytolytic potential. While CD28 loss is intuitively necessary, it does not appear to be sufficient for BRR. Thus, CD28 loss may define belatacept indifference, but CD57 expression appears to identify cells mediating belatacept resistance. Importantly, this phenotype is present in the peripheral blood of patients awaiting transplant and assessing the prevalence of CD57+PD1− CD4 T cells prior to transplantation may identify patients not suited for belatacept-based therapy.
Supplementary Material
Table S1: This table illustrates patient demographics, cause of end stage renal disease (ESRD), the type and duration of pre-transplant dialysis received, EBV and CMV serology, percentage of CD57+PD1− CD4 T cells, whether they received a kidney from a deceased or living donor, whether patients received belatacept- or tacrolimus-based immunosuppression following transplantation and whether they experienced a rejection or stable outcome. In this sample set, there was no significant correlation between percentage of CD57+PD1− CD4 T cells and other patient demographics. HTN = hypertension, FSGS = focal segmental glomerulosclerosis, DMII = type 2 diabetes mellitus, PKD = polycystic kidney disease, HD = hemodialysis, PD = peritoneal dialysis, EBV = Epstein-Barr virus, CMV = Cytomegalovirus, DCD = deceased donor, LRD = living related donor, LUD = living unrelated donor, n/a = not available.
Figure S1: There was no artificial segregation of CD57+PD1− CD4 T cells (p<0.74, left) or CD57+PD1− CD8 T cells (p<0.11, right) prior to transplantation in patients that went on to receive belatacept compared to patients that went on to receive tacrolimus.
Figure S2: Gating strategy for cytokine staining represented in Figure 7C and 7D. All gates are made on unstimulated controls groups and the same gates are then applied to cells following stimulation. The first gate is made on lymphocytes, followed by single cells based on FSC-A by FSC-H. Then live CD3 T cells are gated on and segregated into CD4 or CD8 T cells. CD4 T cells were then segregated based on on either CD57+PD1− or CD28-PD1− populations (outlined by black boxes). Indicated cells were then interrogated for their ability to produce cytokines IFNg and TNFa following stimulation. All gates are established on non-stimulated controls. This gating strategy is one representative flow plot from a stimulated patient sample.
Acknowledgments
The authors wish to acknowledge the Emory Transplant Center Biorepository for their help with sample acquisition. This research was supported by a grant from Bristol-Myers Squibb and the National Institute of Allergy and Infectious Disease R01 A1097423 (ADK).
Abbreviations
- ACR
acute cellular rejection
- BRR
belatacept-resistant rejection
- CNIs
calcineurin inhibitors
- CoB
costimulation blockade
- CoBRR
costimulation blockade-resistant rejection
- IPA
ingenuity pathway analysis
- PBMCs
peripheral blood mononuclear cells
- PMA
phorbol 12-myristate 13-acetate
Footnotes
Disclaimer
The research was carried out at the National Institute for Health Research (NIHR)/Wellcome Trust Birmingham Clinical Research Facility. The views expressed are those of the authors and not necessarily those of the National Health Service, the NIHR, or the Department of Health.
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. RT is employed by Bristol-Myers Squibb. The other authors have no conflicts of interest to disclose.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Rooney CM, Smith CA, Ng CY, Loftin SK, Sixbey JW, Gan Y, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92(5):1549–55. [PubMed] [Google Scholar]
- 2.Heslop HE, Slobod KS, Pule MA, Hale GA, Rousseau A, Smith CA, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115(5):925–35. doi: 10.1182/blood-2009-08-239186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisher RA. Cytomegalovirus infection and disease in the new era of immunosuppression following solid organ transplantation. Transplant infectious disease : an official journal of the Transplantation Society. 2009;11(3):195–202. doi: 10.1111/j.1399-3062.2009.00372.x. [DOI] [PubMed] [Google Scholar]
- 4.Xu H, Perez SD, Cheeseman J, Mehta AK, Kirk AD. The allo- and viral-specific immunosuppressive effect of belatacept, but not tacrolimus, attenuates with progressive T cell maturation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014;14(2):319–32. doi: 10.1111/ajt.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Randhawa PS, Shapiro R, Jordan ML, Starzl TE, Demetris AJ. The histopathological changes associated with allograft rejection and drug toxicity in renal transplant recipients maintained on FK506. Clinical significance and comparison with cyclosporine. The American journal of surgical pathology. 1993;17(1):60–8. doi: 10.1097/00000478-199301000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clinical journal of the American Society of Nephrology : CJASN. 2009;4(2):481–508. doi: 10.2215/CJN.04800908. [DOI] [PubMed] [Google Scholar]
- 7.Larsen CP, Knechtle SJ, Adams A, Pearson T, Kirk AD. A new look at blockade of T-cell costimulation: a therapeutic strategy for long-term maintenance immunosuppression. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2006;6(5 Pt 1):876–83. doi: 10.1111/j.1600-6143.2006.01259.x. [DOI] [PubMed] [Google Scholar]
- 8.Vincenti F, Charpentier B, Vanrenterghem Y, Rostaing L, Bresnahan B, Darji P, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study) American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10(3):535–46. doi: 10.1111/j.1600-6143.2009.03005.x. [DOI] [PubMed] [Google Scholar]
- 9.Vincenti F, Larsen CP, Alberu J, Bresnahan B, Garcia VD, Kothari J, et al. Three-year outcomes from BENEFIT, a randomized, active-controlled, parallel-group study in adult kidney transplant recipients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12(1):210–7. doi: 10.1111/j.1600-6143.2011.03785.x. [DOI] [PubMed] [Google Scholar]
- 10.Rostaing L, Vincenti F, Grinyo J, Rice KM, Bresnahan B, Steinberg S, et al. Long-term belatacept exposure maintains efficacy and safety at 5 years: results from the long-term extension of the BENEFIT study. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13(11):2875–83. doi: 10.1111/ajt.12460. [DOI] [PubMed] [Google Scholar]
- 11.O'Callaghan N, Dhillon V, Thomas P, Fenech M. A quantitative real-time PCR method for absolute telomere length. BioTechniques. 2008;44(6):807–9. doi: 10.2144/000112761. [DOI] [PubMed] [Google Scholar]
- 12.Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic acids research. 2009;37(3):e21. doi: 10.1093/nar/gkn1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamann D, Baars PA, Rep MH, Hooibrink B, Kerkhof-Garde SR, Klein MR, et al. Phenotypic and functional separation of memory and effector human CD8+ T cells. The Journal of experimental medicine. 1997;186(9):1407–18. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Byrne JA, Butler JL, Cooper MD. Differential activation requirements for virgin and memory T cells. Journal of immunology (Baltimore, Md : 1950) 1988;141(10):3249–57. [PubMed] [Google Scholar]
- 15.Weaver TA, Charafeddine AH, Agarwal A, Turner AP, Russell M, Leopardi FV, et al. Alefacept promotes co-stimulation blockade based allograft survival in nonhuman primates. Nature medicine. 2009;15(7):746–9. doi: 10.1038/nm.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lo DJ, Weaver TA, Stempora L, Mehta AK, Ford ML, Larsen CP, et al. Selective targeting of human alloresponsive CD8+ effector memory T cells based on CD2 expression. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11(1):22–33. doi: 10.1111/j.1600-6143.2010.03317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitchens WH, Haridas D, Wagener ME, Song M, Ford ML. Combined costimulatory and leukocyte functional antigen-1 blockade prevents transplant rejection mediated by heterologous immune memory alloresponses. Transplantation. 2012;93(10):997–1005. doi: 10.1097/TP.0b013e31824e75d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wherry EJ. T cell exhaustion. Nature immunology. 2011;12(6):492–9. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 19.Jin HT, Ahmed R, Okazaki T. Role of PD-1 in regulating T-cell immunity. Current topics in microbiology and immunology. 2011;350:17–37. doi: 10.1007/82_2010_116. [DOI] [PubMed] [Google Scholar]
- 20.Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101(7):2711–20. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 21.Papagno L, Spina CA, Marchant A, Salio M, Rufer N, Little S, et al. Immune activation and CD8+ T-cell differentiation towards senescence in HIV-1 infection. PLoS biology. 2004;2(2):E20. doi: 10.1371/journal.pbio.0020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morita I, Kizuka Y, Kakuda S, Oka S. Expression and function of the HNK-1 carbohydrate. Journal of biochemistry. 2008;143(6):719–24. doi: 10.1093/jb/mvm221. [DOI] [PubMed] [Google Scholar]
- 23.Palmer BE, Blyveis N, Fontenot AP, Wilson CC. Functional and phenotypic characterization of CD57+CD4+ T cells and their association with HIV-1-induced T cell dysfunction. Journal of immunology (Baltimore, Md : 1950) 2005;175(12):8415–23. doi: 10.4049/jimmunol.175.12.8415. [DOI] [PubMed] [Google Scholar]
- 24.Strioga M, Pasukoniene V, Characiejus D. CD8+ CD28− and CD8+ CD57+ T cells and their role in health and disease. Immunology. 2011;134(1):17–32. doi: 10.1111/j.1365-2567.2011.03470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Focosi D, Bestagno M, Burrone O, Petrini M. CD57+ T lymphocytes and functional immune deficiency. Journal of leukocyte biology. 2010;87(1):107–16. doi: 10.1189/jlb.0809566. [DOI] [PubMed] [Google Scholar]
- 26.Pooley KA, Sandhu MS, Tyrer J, Shah M, Driver KE, Luben RN, et al. Telomere length in prospective and retrospective cancer case-control studies. Cancer research. 2010;70(8):3170–6. doi: 10.1158/0008-5472.CAN-09-4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. Journal of cellular physiology. 2000;182(3):311–22. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 28.Badell IR, Russell MC, Thompson PW, Turner AP, Weaver TA, Robertson JM, et al. LFA-1-specific therapy prolongs allograft survival in rhesus macaques. The Journal of clinical investigation. 2010;120(12):4520–31. doi: 10.1172/JCI43895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Traitanon O, Gorbachev A, Bechtel JJ, Keslar KS, Baldwin WM, 3rd, Poggio ED, et al. IL-15 induces alloreactive CD28(−) memory CD8 T cell proliferation and CTLA4-Ig resistant memory CD8 T cell activation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014;14(6):1277–89. doi: 10.1111/ajt.12719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mou D, Espinosa JE, Stempora L, Iwakoshi NN, Kirk AD. Viral-induced CD28 loss evokes costimulation independent alloimmunity. The Journal of surgical research. 2015;196(2):241–6. doi: 10.1016/j.jss.2015.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sayegh MH, Turka LA. The role of T-cell costimulatory activation pathways in transplant rejection. The New England journal of medicine. 1998;338(25):1813–21. doi: 10.1056/NEJM199806183382506. [DOI] [PubMed] [Google Scholar]
- 32.Ford ML, Adams AB, Pearson TC. Targeting co-stimulatory pathways: transplantation and autoimmunity. Nature reviews Nephrology. 2014;10(1):14–24. doi: 10.1038/nrneph.2013.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gourley TS, Wherry EJ, Masopust D, Ahmed R. Generation and maintenance of immunological memory. Seminars in immunology. 2004;16(5):323–33. doi: 10.1016/j.smim.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science (New York, NY) 1996;272(5258):54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 35.Fernandez S, French MA, Price P. Immunosenescent CD57+CD4+ T-cells accumulate and contribute to interferon-gamma responses in HIV patients responding stably to ART. Disease markers. 2011;31(6):337–42. doi: 10.3233/DMA-2011-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Legendre CM, Guttmann RD, Hou SK, Jean R. Two-color immunofluorescence and flow cytometry analysis of lymphocytes in long-term renal allotransplant recipients: identification of a major Leu-7+/Leu-3+ subpopulation. Journal of immunology (Baltimore, Md : 1950) 1985;135(2):1061–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: This table illustrates patient demographics, cause of end stage renal disease (ESRD), the type and duration of pre-transplant dialysis received, EBV and CMV serology, percentage of CD57+PD1− CD4 T cells, whether they received a kidney from a deceased or living donor, whether patients received belatacept- or tacrolimus-based immunosuppression following transplantation and whether they experienced a rejection or stable outcome. In this sample set, there was no significant correlation between percentage of CD57+PD1− CD4 T cells and other patient demographics. HTN = hypertension, FSGS = focal segmental glomerulosclerosis, DMII = type 2 diabetes mellitus, PKD = polycystic kidney disease, HD = hemodialysis, PD = peritoneal dialysis, EBV = Epstein-Barr virus, CMV = Cytomegalovirus, DCD = deceased donor, LRD = living related donor, LUD = living unrelated donor, n/a = not available.
Figure S1: There was no artificial segregation of CD57+PD1− CD4 T cells (p<0.74, left) or CD57+PD1− CD8 T cells (p<0.11, right) prior to transplantation in patients that went on to receive belatacept compared to patients that went on to receive tacrolimus.
Figure S2: Gating strategy for cytokine staining represented in Figure 7C and 7D. All gates are made on unstimulated controls groups and the same gates are then applied to cells following stimulation. The first gate is made on lymphocytes, followed by single cells based on FSC-A by FSC-H. Then live CD3 T cells are gated on and segregated into CD4 or CD8 T cells. CD4 T cells were then segregated based on on either CD57+PD1− or CD28-PD1− populations (outlined by black boxes). Indicated cells were then interrogated for their ability to produce cytokines IFNg and TNFa following stimulation. All gates are established on non-stimulated controls. This gating strategy is one representative flow plot from a stimulated patient sample.