Abstract
BACKGROUND
It is widely believed that reduced cardiac index (CI) is a significant contributor to renal dysfunction in patients with heart failure (HF). However, recent data have challenged this paradigm.
OBJECTIVES
We sought to determine the relationship between CI and renal function in a multicenter population of HF patients undergoing pulmonary artery catheterization (PAC).
METHODS
Patients undergoing PAC in either the randomized or registry portions of the ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) trial were included (n = 575). We evaluated associations between CI and renal function across multiple subgroups and assessed for nonlinear, threshold, and longitudinal relationships.
RESULTS
There was a weak but statistically significant inverse correlation between CI and estimated glomerular filtration rate (eGFR), such that higher CI was paradoxically associated with worse eGFR (r = −0.12; p = 0.02). CI was not associated with blood urea nitrogen (BUN) or the BUN to creatinine ratio. Similarly, no significant associations were observed between CI and better renal function across multiple subgroups defined by indications for PAC or hemodynamic, laboratory, or demographic parameters. A nonlinear or threshold effect could not be identified. In patients with serial assessments of renal function and CI, we were unable to find within-subject associations between change in CI and eGFR using linear mixed modeling. Neither CI nor change in CI was lower in patients developing worsening renal function (p ≥ 0.28).
CONCLUSIONS
These results reinforce evidence that reduced CI is not the primary driver for renal dysfunction in patients hospitalized for HF, irrespective of the degree of CI impairment or patient subgroup analyzed.
Keywords: blood urea nitrogen, cardiorenal, creatinine, pulmonary artery catheterization, renal function
Central Illustration: CI and Renal Dysfunction in HF

There exists a wide belief that reduced cardiac index (CI) contributes to renal dysfunction in patients with heart failure (HF). However, this was not supported when evaluating the association between CI and renal function as measured by estimated glomerular filtration rate (eGFR), blood urea nitrogen (BUN), creatinine, and BUN-to-creatinine ratio in HF patients undergoing pulmonary artery catheterization. Whiskers represent 1 SD and CI is grouped as top, middle, and bottom third of values in the population.
Patients with chronic heart failure (HF) often have concomitant renal dysfunction and the interdependence of the heart and kidney in this setting has been a topic of extensive study (1,2). Traditionally, the pathophysiological paradigm has held that reduction in cardiac output leads to decreased renal perfusion and serves as a primary driver for renal dysfunction in HF. This notion of a linear relationship between cardiac output and renal function has recently been challenged by several studies demonstrating a lack of correlation or, in some cases, paradoxical correlations between cardiac output and renal function (3–7).
There were important limitations to many of these analyses. The majority were single-center, retrospective studies with the only multicenter experience coming from the ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) trial, a randomized trial of pulmonary artery catheterization (PAC) to guide therapy in patients hospitalized with advanced heart failure (8). However, the ESCAPE trial included only patients for whom equipoise regarding the necessity of invasive hemodynamic guidance existed. As a result, it excluded patients where PAC was felt to be clinically indicated for diagnosis or management, potentially missing important associations in “sicker” patients. Additionally, prior studies have not tested for nonlinear or threshold effects or examined subgroups of patients in which reduced cardiac output may be an important driver of renal dysfunction.
As such, the overall goal of the current study was to provide a comprehensive analysis of the association between CI and renal function in both the ESCAPE trial and ESCAPE registry, datasets encompassing a broad spectrum of disease severity inclusive of patients receiving clinically indicated catheterizations. Using these datasets, we sought to exhaustively evaluate the CI-renal function association looking at nonlinear, threshold, between-patient, within-patient, and multiple subgroup analyses.
METHODS
Our cohort consisted of patients either randomized to the PAC arm of the ESCAPE trial or enrolled in the PAC registry of the ESCAPE trial. Patients were eligible for enrollment into the PAC registry if they were under the care of an HF specialist at 1 of the 26 sites of the ESCAPE trial and were 16 years of age or older undergoing PAC as part of their HF management strategy during hospitalization (9–11). Patients with HF could be enrolled in the PAC registry when they failed to meet the ESCAPE trial eligibility criteria or the investigator perceived that PAC was necessary for management, either for diagnosis or treatment of hemodynamic derangements, and, thus the patient was not a candidate for randomization. Inclusion in the current analysis required data available on cardiac output and renal function upon enrollment. Of the 439 patients in the PAC registry, 7 patients were excluded from the current analysis due to use of renal replacement therapy; of the remaining 432 patients, 383 had data available on renal function and CI.
Since only cross-sectional data were available in the ESCAPE PAC registry, we also analyzed patients in the PAC arm of the ESCAPE trial to test for a longitudinal relationship between CI and renal function. Methods and results of the ESCAPE trial have been previously published (12). Briefly, this was a randomized, multicenter trial comparing PAC-guided therapy to clinical assessment in hospitalized patients with acute decompensated HF. Inclusion criteria included an ejection fraction ≤30%, systolic blood pressure ≤125 mm Hg, and at least 1 sign and 1 symptom of congestion; exclusion criteria included admission creatinine >3.5 mg/dl, use of milrinone before randomization, or use of dopamine or dobutamine >3 μg/kg/min. Of the 433 patients in the ESCAPE trial, 217 were treated in the PAC arm; of these patients, 192 had baseline renal function and hemodynamic data available. CI was assessed at up to 4 time points: baseline (on day of PAC), the day following baseline, the day on which the patient was determined to have “optimal” status by the treating physician, and the last hemodynamic measurement before pulmonary artery catheter removal. Serum creatinine measurements were recorded on admission, hospital days 3, 5, and 7, and at discharge.
Inclusion in the current analysis required at least 1 concomitant pair of CI and serum creatinine measurements. In the ESCAPE trial, the first set of lab values were obtained on the day of randomization and patients were not randomized until they were ready to undergo pulmonary artery catheter placement. In the PAC registry, lab values were obtained 0 to 24 h before PAC, with initial hemodynamics recorded at this time.
Renal function was queried using 4 different metrics: estimated glomerular filtration rate (eGFR), blood urea nitrogen (BUN), serum creatinine, and the blood urea nitrogen to creatinine ratio (BUN/Cr ratio) (13,14). The chronic kidney disease epidemiology collaboration (CKD-EPI) equation was used to calculate eGFR for both cohorts (13). The correlation between CI and eGFR was assessed in the combined cohort using all available pairs of eGFR and CI within each patient, subject to the constraint that the renal function measurement was required to have occurred before the measurement of CI (CI “carried forward,” producing 1,340 data pairs).
The relationship between metrics of decongestion and CI was evaluated in the PAC arm of the ESCAPE trial. Diuretic efficiency was measured in fluid output per milligram of loop diuretic received (ml of net fluid output/40 mg of furosemide equivalents) (15). Changes in hemoglobin were evaluated from baseline to discharge.
STATISTICAL ANALYSIS
The primary goal of this analysis was to comprehensively evaluate the association between CI and renal function in patients with HF. All values are mean ± SD or median with interquartile range for continuous data, and percentage for categorical data. The Mann-Whitney U test was used to compare continuous variables across groups. The chi-square test or Fisher exact test was used to evaluate associations between categorical variables. Spearman’s rank-order correlation was used to evaluate correlations between continuous variables.
A log transformation was applied to eGFR in parametric models due to slight positive skew, in order to ensure that any deviation from normality in this measurement did not result in type II errors. Multivariable linear regression analysis was used to estimate the independent association between CI and the natural log of eGFR. Candidate covariates were hemodynamic parameters that demonstrated a univariate association with eGFR with a p value < 0.2. To test for nonlinear relationships between CI and renal function, linear regression of eGFR on CI was compared to: 1) linear regression of eGFR on 3- and 4-knot cubic spline-smoothed CI; and 2) a fractional polynomial model in CI for the linear regression of eGFR on CI. The differences between each of these models and the standard linear regression were assessed using the likelihood ratio test and partial F-test, respectively.
In the PAC arm of the ESCAPE trial, the longitudinal relationship between CI and the natural log of eGFR was assessed on a subject-specific level using linear mixed effects models. The natural log of baseline eGFR was included as a fixed effect and random effects were evaluated at the level of the patient. Covariance structures were compared using the Akaike and Bayesian information criteria; a model with unstructured covariance provided the best fit.
Statistical analysis was performed with IBM SPSS Statistics version 21 (IBM Corp., Armonk, New York) and Stata version 13.1 (StataCorp, College Station, Texas) and statistical significance was defined as 2-tailed p < 0.05.
RESULTS
OVERALL STUDY POPULATION
Our combined study population included both the PAC arm of the ESCAPE trial and the PAC registry and comprised a total of 575 patients with data on baseline renal function and hemodynamics. Of these patients, 192 were participants in the ESCAPE trial and the remaining 383 were enrolled in the PAC registry. Baseline characteristics of the population are presented in Table 1. Briefly, the cohort consisted predominantly of white males with a median age of 60 years and New York Heart Association (NYHA) class III or IV HF. The mean ejection fraction was 23 ± 12% with 8% of patients having a preserved ejection fraction (>40%). The median eGFR was 52.3 ml/min/1.73 m2 and 24% of our population had an eGFR <30 ml/min/1.73 m2. On average, PAC registry patients had higher ejection fraction and CI as well as worse renal function. Compared to patients in the PAC arm of the ESCAPE trial, registry patients were less likely to have NYHA class IV HF symptoms and more likely to be receiving inotropes (Table 1).
TABLE 1.
Baseline Characteristics
| Overall Cohort N = 575 |
ESCAPE Trial n = 192 |
PAC Registry n = 383 |
|
|---|---|---|---|
| Demographics | |||
| Age, yrs | 58.9 ± 14.2* | 56.8 ± 13.4 | 59.9 ± 14.5 |
| White | 75.1* | 59.9 | 82.8 |
| Male | 71.5 | 75.0 | 69.7 |
| Medical history | |||
| Ischemic etiology of HF | 55.1 | 55.2 | 55.1 |
| NYHA class III | 32.6* | 10.4 | 50.6 |
| NYHA class IV | 67.4* | 89.6 | 49.4 |
| Last EF | 22.6 ± 12.0* | 19.4 ± 6.9 | 24.4 ± 13.8 |
| EF >40% eGFR <30 |
8.1* | 1.1 | 11.9 |
| ml/min/1.73 m2 | 23.3* | 10.4 | 29.8 |
| Diabetes mellitus | 34.4 | 33.3 | 35.0 |
| Hypertension | 44.0 | 47.9 | 42.0 |
| Baseline hemodynamics | |||
| SBP, mm Hg | 105.5 ± 19.9* | 110.0 ± 17.6 | 103.3 ± 20.6 |
| SVR, dynes-sec/cm5 | 1,356.1 ± 696.4* | 1,460.1 ± 814.4 | 1,300.5 ± 618.2 |
| RAP, mm Hg | 14.0 ± 8.6 | 13.6 ± 9.8 | 14.2 ± 7.8 |
| PCWP, mm Hg | 24.1 ± 8.8 | 24.5 ± 9.0 | 23.8 ± 8.7 |
| PA systolic pressure, mm Hg | 54.6 ± 15.1 | 55.0 ± 14.3 | 54.4 ± 15.5 |
| CI, l/min/m2 | 2.3 ± 2.1* | 2.0 ± 0.6 | 2.5 ± 2.5 |
| CO, l/min | 4.7 ± 5.0* | 3.9 ± 1.4 | 5.1 ± 6.1 |
| Medications pre-PAC placement | |||
| ACE inhibitors | 57.4* | 79.2 | 46.5 |
| Inotropes | 27.2* | 11.8 | 34.7 |
| Beta-blocker | 58.8* | 65.1 | 55.6 |
| Loop diuretic | 77.8* | 72.0 | 80.7 |
| Laboratory values | |||
| eGFR, ml/min/1.73 m2 | 52.4 ± 28.3* | 58.5 ± 26.6 | 49.4 ± 28.7 |
| Serum creatinine, mg/dl | 1.8 ± 1.1* | 1.5 ± 0.6 | 1.9 ± 1.3 |
| BUN, mg/dl | 39.3 ± 27.7* | 35.3 ± 22.3 | 41.3 ± 29.9 |
| Serum sodium, meq/l | 135.6 ± 5.0* | 136.4 ± 4.5 | 135.2 ± 5.2 |
Values are mean ± SD or %.
p<0.05 for difference between the PAC arm of the ESCAPE Trial and the PAC Registry.
ACE = angiotensin-converting enzyme; BUN = blood urea nitrogen; HF = heart failure; CI = cardiac index; CO = cardiac output; EF = ejection fraction; eGFR = estimated glomerular filtration rate; NYHA = New York Heart Association; PA = pulmonary artery; PAC = pulmonary artery catheter; PCWP = pulmonary capillary wedge pressure; RAP = right atrial pressure; SBP = systolic blood pressure; SVR = systemic vascular resistance.
Overall, an inverse correlation was observed between baseline CI and baseline eGFR, such that higher CI was paradoxically associated with lower eGFR (Table 2); however, the effect size of this correlation was very small. Similarly, there was a small-magnitude positive correlation between baseline CI and baseline serum creatinine. There was no detectable correlation between baseline CI and BUN or the BUN/Cr ratio. Despite the noted differences in cohort baseline characteristics, the study in which patients were enrolled (PAC registry versus the ESCAPE trial) did not significantly modify the effect of CI on eGFR, BUN, creatinine, or BUN/Cr ratio (p interaction all >0.3).
TABLE 2.
Correlations between CI and Selected Renal Metrics in Multiple Subgroups
| eGFR | BUN | Creatinine | BUN/Cr | |
|---|---|---|---|---|
| Total study population (N = 575) | −0.12* | 0.07 | 0.10* | −0.003 |
| ESCAPE trial (n = 192) | −0.04 | −0.05 | 0.05 | −0.09 |
| PAC registry (n = 383) | −0.12* | 0.10 | 0.09 | 0.04 |
| Medical history | ||||
| NYHA class IV (n = 289) | −0.09 | 0.05 | 0.08 | −0.02 |
| NYHA class III (n = 140) | −0.14 | 0.08 | 0.13 | −0.04 |
| Ejection fraction ≤40% (n = 487) | −0.09* | 0.02 | 0.08 | −0.04 |
| Ejection fraction >40% (n = 43) | −0.17 | 0.31* | 0.18 | 0.33* |
| Diabetes | ||||
| Present (n = 198) | −0.20* | 0.20* | 0.20* | 0.04 |
| Absent (n = 377) | −0.06 | −0.02 | 0.02 | 0.05 |
| Hypertension | ||||
| Present (n = 253) | −0.18* | 0.14* | 0.18* | 0.02 |
| Absent (n = 322) | −0.07 | 0.01 | 0.03 | −0.02 |
| Baseline PAC hemodynamics | ||||
| Systolic blood pressure | ||||
| <100 mm Hg (n = 237) | −0.07 | −0.01 | 0.05 | −0.08 |
| ≥100 mm Hg (n = 333) | −0.17* | 0.14* | 0.14* | 0.06 |
| CI | ||||
| Highest tertile: CI ≥2.3 l/min/m2 (n = 208) | −0.05 | 0.05 | 0.04 | 0.02 |
| Middle tertile: CI 1.8–2.28 l/min/m2 (n = 188) | −0.24* | 0.10 | 0.23* | −0.10 |
| Lowest tertile: CI <1.8 l/min/m2 (n = 179) | −0.05 | 0.01 | −0.02 | 0.06 |
| SVR | ||||
| >1,235 dynes-sec/cm5 (n = 271) | 0.01 | −0.08 | −0.08 | −0.06 |
| ≤1,235 dynes-sec/cm5 (n = 271) | −0.05 | 0.003 | 0.04 | −0.06 |
| RAP | ||||
| >13 mm Hg (n = 253) | −0.18* | 0.16* | 0.16* | 0.03 |
| ≤13 mm Hg (n = 295) | −0.12* | 0.06 | 0.09 | 0.03 |
| PCWP | ||||
| >24 mm Hg (n = 261) | −0.10 | −0.001 | 0.06 | −0.08 |
| ≤24 mm Hg (n = 287) | −0.13* | 0.13* | 0.13* | 0.06 |
| Laboratory values | ||||
| BUN/Cr >20 (n = 301) | −0.18* | 0.18* | 0.16* | 0.04 |
| BUN ≥31 mg/dl (n = 297) | −0.17* | 0.18* | 0.14* | 0.04 |
| eGFR <60 ml/min/1.73 m2 (n = 379) | −0.11* | 0.10 | 0.09 | 0.05 |
| eGFR <30 ml/min/1.73 m2 (n = 134) | −0.06 | 0.12 | 0.08 | 0.06 |
| Medications | ||||
| ACE inhibitors | ||||
| Present (n = 330) | −0.09 | −0.002 | 0.07 | −0.06 |
| Absent (n = 245) | −0.06 | 0.05 | 0.05 | −0.05 |
| Inotropes | ||||
| Present (n = 155) | −0.14 | 0.24* | 0.15 | 0.11 |
| Absent (n = 414) | −0.08 | −0.01 | 0.05 | −0.05 |
| Beta-blockers | ||||
| Present (n = 338) | −0.14* | 0.03 | 0.11 | −0.05 |
| Absent (n = 237) | −0.10 | 0.12 | 0.08 | 0.05 |
| Loop diuretics | ||||
| Present (n = 445) | −0.13* | 0.12* | 0.11* | 0.07 |
| Absent (n = 127) | −0.07 | −0.09 | 0.04 | −0.21* |
p < 0.05.
BUN/Cr = blood urea nitrogen to creatinine ratio; other abbreviations as in Table 1.
No statistically significant association between CI and eGFR was observed in the analysis of all CI-eGFR pairs with CI “carried forward” (1,340 data pairs; r = −0.05; p = 0.06). A nonlinear relationship between CI and renal function was also not detectable (Figure 1). Specifically, the use of a 3-knot cubic spline, a 4-knot cubic spline, and a fractional polynomial could not meaningfully provide a better fit to the data than a linear regression (Figure 1). Furthermore, no threshold effects were observed; the correlation between CI and eGFR was either nonexistent or inverse in the highest, middle, and lowest tertiles of CI (Table 2). Notably, there was little difference in the renal metrics of patients with severely depressed CI (<1.8 l/min/m2) compared to patients with a CI ≥2.3 l/min/m2 (Central Illustration).
Figure 1. eGFR and CI in the Overall Population.

In this scatterplot of all available pairs of cardiac index (CI) and estimated glomerular filtration rate (eGFR) per patient, no association between CI and eGFR was observed. Compared with linear regression, neither 3-knot spline (p = 0.06), 4-knot spline (p = 0.08), nor fractional polynomial (p = 0.06) demonstrated a better fit to the data.
PATIENT SUBGROUPS AND INDICATIONS FOR RHC
Patients with NYHA class III or IV HF also failed to demonstrate any statistically significant correlations between CI and renal function (Table 2). In subgroups of patients with or without preserved ejection fraction, diabetes, or hypertension, a positive relationship with CI was not identifiable across various metrics of renal function (Table 2). Similarly, patient subgroups defined by use of medications such as loop diuretics, beta-blockers, or angiotensin-converting enzyme inhibitors did not demonstrate any positive associations between CI and renal function (Table 2).
There were no correlations between CI and improvement in metrics of renal function in patients with a BUN/Cr ratio >20, eGFR <60 ml/min/1.73 m2, or BUN greater than the median, or in subgroups defined by right atrial pressure, pulmonary capillary wedge pressure, and systemic vascular resistance above or below the median value (Table 2).
Indications for right heart catheterization (RHC), such as suspected cardiogenic shock, inability to wean inotropes, hypotension, and diagnostic uncertainty as to hemodynamic status, did not significantly influence the lack of association between a lower CI and worse renal function (Table 3). These results applied to eGFR, BUN, creatinine, and the BUN/Cr ratio. Notably, patients who underwent RHC to further work up “progressive oliguric renal insufficiency” had a moderate-strength, statistically significant inverse relationship between eGFR and CI, where a lower CI was associated with higher eGFR (r = −0.43; p = 0.01).
TABLE 3.
Correlations between CI and Selected Renal Metrics*
| eGFR | BUN | Creatinine | BUN/Cr | |
|---|---|---|---|---|
|
|
||||
| PAC registry population (N=383) | −0.12* | 0.1 | 0.09 | 0.04 |
| Indications for PAC placement | ||||
| Cardiogenic shock (n = 69) | 0.04 | −0.24* | −0.06 | −0.21 |
| Continued inotrope requirements (n = 46) | −0.23 | 0.36* | 0.23 | 0.22 |
| Hypotension requiring intervention (n = 55) | 0.03 | 0.06 | −0.1 | 0.17 |
| Progressive oliguric renal insufficiency (n = 32) | −0.43* | 0.31 | 0.46* | −0.01 |
| Specific referral for hemodynamic monitoring (n = 18) | −0.29 | 0.37 | 0.21 | −0.29 |
| Diagnostic uncertainty of hemodynamic profile (n = 163) | −0.15 | 0.16* | 0.11 | 0.10 |
In subgroups of patients defined by indication for PAC insertion in the PAC registry.
Abbreviations as in Table 1.
LONGITUDINAL ANALYSIS OF RENAL METRICS AND CI
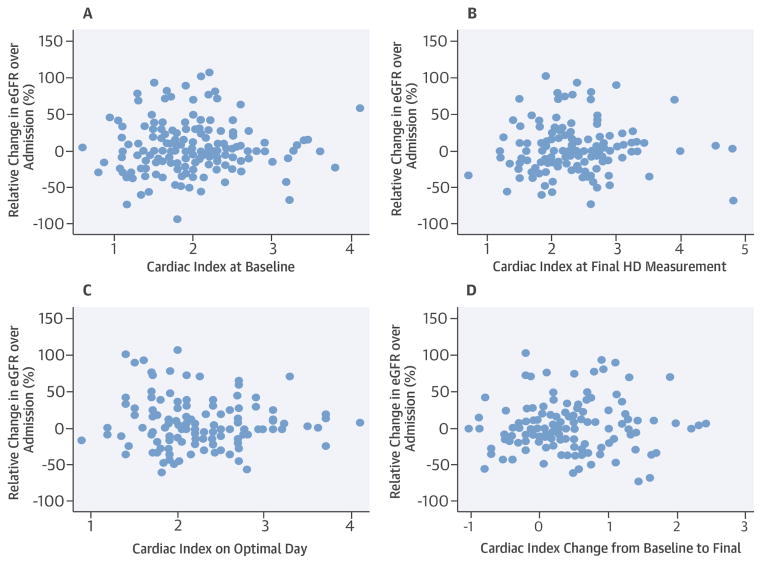
Longitudinal changes were evaluated in the PAC arm of the ESCAPE trial. Changes in renal function were associated with neither individual measurements of CI nor changes in CI (Figure 2). A linear mixed effects model showed no statistically significant effect of CI on the natural log of eGFR on a longitudinal basis (beta = −0.026; p = 0.37).
Figure 2. Relative Change in eGFR against CI Measures.
Scatterplots of relative change are seen compared to baseline cardiac index (A), last measured CI (B), CI on physician-determined optimal day (C), and change in CI (D) in the ESCAPE trial. p≥0.31 for all associations. ESCAPE = Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness; other abbreviations as in Figure 1.
Worsening renal function (WRF), as defined by either a 0.3 mg/dl increase in serum creatinine or a 20% decrease in eGFR compared to baseline at any time point during the hospitalization (days 3, 5, 7, or discharge), occurred in 70 patients or 34% of the randomized ESCAPE trial population. Static measurements of CI and changes in CI did not differ significantly between patients with and without WRF (Table 4).
TABLE 4.
Relationship between Static Measurements and Changes in CI and WRF
| Cardiac Index | p Value | ||
|---|---|---|---|
| Patients with WRF* | Patients without WRF* | ||
| Static measurement of CI | |||
| Baseline | 1.9 ± 0.7 | 2.0 ± 0.6 | 0.43 |
| Day 2 | 2.2 ± 0.7 | 2.1 ± 0.6 | 0.53 |
| At PAC removal | 2.3 ± 0.7 | 2.4 ± 0.6 | 0.28 |
| Change in CI | |||
| Baseline to day 2 | 0.4 ± 0.7 | 0.4 ± 0.7 | 0.51 |
| Baseline to PAC removal | 0.2 ± 0.6 | 0.1 ± 0.6 | 0.91 |
Defined by a 0.3 mg/dl increase in serum creatinine or a 20% decrease in eGFR compared to baseline at 3 days, 5 days, 7 days or discharge.
WRF = worsening renal function; other abbreviations as in Table 1.
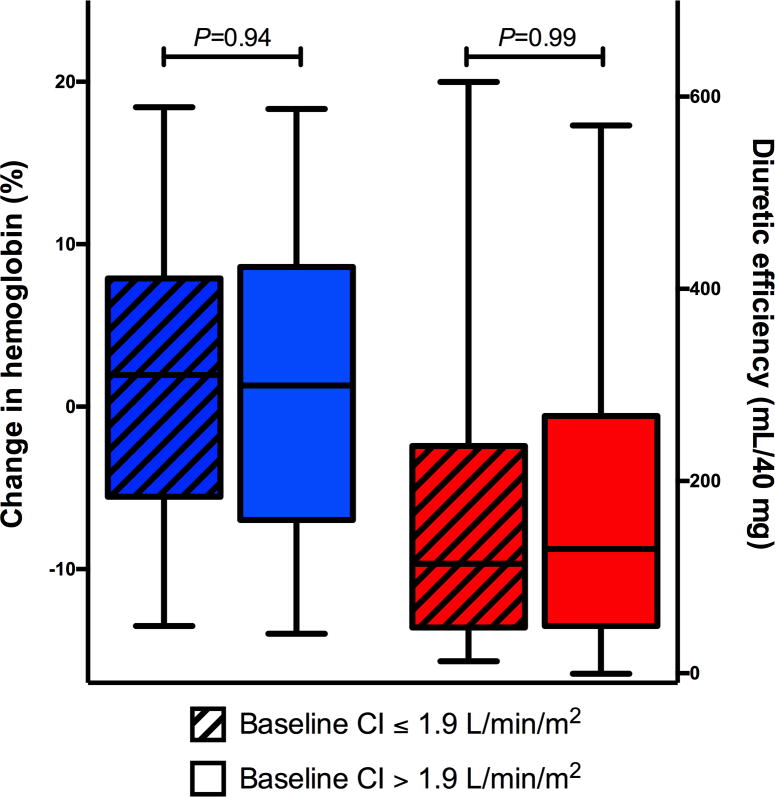
Associations between markers of decongestion and CI were evaluated in the PAC arm of the ESCAPE trial (Figure 3). There was no significant association between relative change in hemoglobin and baseline CI (p = 0.45). Similarly, no significant association was observed between diuretic efficiency and baseline CI (p = 0.59).
Figure 3. Relationship between CI and Markers of Decongestion.
There was no significant association between relative change in hemoglobin and baseline CI (median value: 1.9 l/min/m2; p = 0.45) or between diuretic efficiency and baseline CI (p = 0.59). Abbreviations as in Figure 1.
HEMODYNAMIC PREDICTORS OF eGFR
Several additional hemodynamic parameters were associated with the natural log of eGFR on univariable analysis, namely right atrial pressure (r = −0.18; p < 0.001), pulmonary artery systolic pressure (r = −0.11; p=0.01), CI (r =−0.12; p = 0.005), and systemic vascular resistance (r = 0.19; p < 0.001). On multivariable analysis, right atrial pressure (beta = −0.01; p < 0.001) and CI (beta = −0.10; p = 0.02) both remained significantly and inversely associated with the natural log of eGFR, such that higher CI or right atrial pressure was associated with lower eGFR. Importantly, the adjusted R2 of the multivariable model was only 0.08, indicating that traditional hemodynamic parameters explain a very small proportion of the variability in eGFR in this cohort.
DISCUSSION
The primary finding of this study is the lack of statistically significant associations supporting the hypothesis that low cardiac output is an important driver for renal dysfunction in patients with HF. Where statistically significant associations were found between metrics of renal function and CI, they were generally of very minimal magnitude and, if anything, suggested a weak, paradoxical inverse association between renal function and cardiac output. The overall lack of a positive association between cardiac output and renal function was not due to threshold effects and, despite exhaustive query, there were no patient subgroups where we could identify an important positive influence of CI on renal function. Additional analyses for nonlinear effects also failed to find significant positive associations between CI and renal function. These findings provide further evidence that reduced CI is not the primary driver of renal dysfunction in patients hospitalized for HF.
While no study has previously sought to conduct an in-depth investigation of the relationship between cardiac output and renal function, several studies have reported findings consistent with the current results. In the majority of cases no association was found, with 1 study actually reporting a highly statistically significant increase in the odds for WRF with higher CI (3). Furthermore, we have previously reported that the baseline, discharge, or change in CI during the treatment of acute decompensated HF cannot even differentiate between patients who experience improvement versus worsening in serum creatinine (16). In a randomized trial of decompensated HF patients, randomization to placebo versus high-dose milrinone (which presumably improved cardiac output in these patients) did not lead to a significant change in serum creatinine from admission to discharge between groups (17,18).
With respect to static eGFR, several generally retrospective single-center studies have reported a null association between baseline eGFR and CI (3,6,7,19). There has been 1 analysis of the multicenter ESCAPE trial demonstrating no association between CI and baseline eGFR (8). However, the aim of the prior analysis was to examine whether PAC-guided therapy led to improvement in cardiorenal indices and, as such, it did not exhaustively query the relationship between CI and eGFR on a longitudinal basis using sensitive statistical methodology such as the mixed effects modeling used in the current manuscript. It also lacked evaluation for subgroup, threshold, nonlinear, or within-patient effects. The current analysis adds significantly to the existing literature, as it: 1) draws from a prospective multicenter trial and registry of clinically-indicated RHCs, thus spanning a large spectrum of disease severity; 2) evaluates for nonlinearity and threshold effects in the CI-renal function relationship; 3) explores multiple subgroups, including the indication for RHC; 4) examines the relationship between CI and renal function on a longitudinal basis; 5) evaluates other metrics of renal function such as BUN and the BUN/Cr ratio; and 6) is the first manuscript solely dedicated to addressing this question. Although the current observations are not without limitation, they do provide strong support for the concept that reduced CI is not the major determinant of renal dysfunction in decompensated HF.
The notion that decreases in CI drive renal dysfunction in HF is still pervasive in clinical medicine (20). This is most likely because the concept is intuitively appealing. Since the glomerular filtration rate is the product of filtration fraction and renal plasma flow, it makes sense that any factor that globally worsens perfusion will also worsen glomerular filtration (21). However, this conceptual framework is likely oversimplified, since the kidney cannot directly sense or respond to the amount of blood being pumped into the ascending aorta by the heart (i.e., cardiac output). The level of autoregulation in the kidney is remarkable, and through both tubulo-glomerular feedback and myogenic autoregulatory pathways, renal perfusion and filtration can be held constant over a wide range of conditions (1,21).
While the kidney cannot directly sense cardiac output, this should not be true of many of the other hemodynamic and neurohormonal changes that are common in HF. For example, low systemic blood pressure, venous congestion, high pressure in the abdominal cavity, and neurohormonal activation would all have their effects directly transmitted to the kidney, potentially negatively impacting renal function. To that end, it has now been described in several populations that a drop in systolic blood pressure may in fact be the hemodynamic parameter most strongly associated with worsening of renal function, significantly outperforming and independent of change in CI (6,22,23). Furthermore, multiple studies have demonstrated a relationship between venous congestion or intra-abdominal pressure and static or dynamic renal function (3,4,8,24,25). Lastly, the negative cardiorenal effects of neurohomonal activation have been described and it has been demonstrated that plasma renin activity actually correlates significantly better with measured GFR than does left ventricular ejection fraction (21,26). These and other yet-to-be-described factors that directly influence renal function likely explain how the obligatory indirect effects of cardiac output play a minor role; such considerations also help explain the observed lack of association between decongestion and CI. Thus, with careful consideration of the relevant physiology, additional perspective is gained on the seemingly counterintuitive finding that cardiac output and renal function are not strongly related in HF.
While the current study does not directly address a therapeutic approach to HF patients with renal dysfunction, it does provide some information that can help guide care. The current practice of RHC for the workup of potentially “low output” renal dysfunction and/or starting patients on inotropes to improve renal function is based largely on the concept that cardiac output is an important driver of renal dysfunction. The current data argued the opposite. Notably, though we cannot exclude the possibility that this correlation was found due to chance among multiple tests, an inverse correlation of moderate strength between CI and eGFR in the subgroup of patients who underwent RHC specifically for “progressive oliguric renal insufficiency.” As such, while these data do not provide a specific recommendation for how we should treat patients with HF and renal dysfunction, they should motivate us to entertain alternative diagnostic and therapeutic approaches prior to instituting these interventions that carry significant risk.
STUDY LIMITATIONS
Our study has several limitations. First, the primary metric of renal function was estimated using serum creatinine obtained at the local hospital laboratory at a single time point. In addition to the inherent limitations in estimating glomerular filtration rate using serum creatinine, these were largely decompensated HF patients and so the assumption of steady-state creatinine kinetics may not have always been met. Furthermore, the eGFR in any given patient with HF may be influenced by a multitude of conditions that are not mutually exclusive. As a result, different degrees of intrinsic kidney disease across patients could have masked some of the relationship between true HF-induced renal dysfunction and cardiac output. Additionally, although combining the ESCAPE trial population with the PAC registry allowed us to query the CI-renal function relationship with more sensitive methodology, it also introduced heterogeneity in the study population that should be considered when interpreting the overall cohort results. Finally, the markers of renal function utilized in the current study (serum creatinine and BUN) are parameters that largely reflect glomerular filtration and may not be influenced by many of the other dimensions of renal function, such as tubular function and injury. As such, future research will be needed to determine the influence of cardiac output on other parameter of renal function such as tubular injury.
CONCLUSIONS
In a multicenter population of decompensated HF patients undergoing clinically indicated PAC, we were unable to detect a relationship between low cardiac output and renal dysfunction. This lack of association persisted across multiple patient subgroups, different metrics of renal function, and the spectrum of cardiac indices. Overall these results argue that low cardiac output is not the predominant driver for renal dysfunction in patients presenting with decompensated heart failure.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE
The severity of renal dysfunction in hospitalized patients with heart failure does not correlate with reduced cardiac index.
TRANSLATIONAL OUTLOOK
Additional research is necessary to elucidate the factors responsible for the renal impairment that commonly occurs in patients with heart failure.
Acknowledgments
The results presented in this paper have not been published previously in whole or part, except in abstract format. A preliminary version of the abstract was published in the Journal of the American College of Cardiology. The authors acknowledge support from grants K23DK097201 (FPW), K23HL114868, (JT), L30HL115790 (JT), and K24DK090203 (CP). This manuscript was prepared using ESCAPE Research Materials obtained from the NHLBI Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of the ESCAPE study investigators or the NHLBI.
ABBREVIATIONS AND ACRONYMS
- BUN
blood urea nitrogen
- CI
cardiac index
- eGFR
estimated glomerular filtration rate
- HF
heart failure
- NYHA
New York Heart Association
- PAC
pulmonary artery catheterization
- RHC
right heart catheterization
Footnotes
Disclosures: The authors declare that they have no relevant financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Damman K, Testani JM. The kidney in heart failure: an update. Eur Heart J. 2015;36:1437–44. doi: 10.1093/eurheartj/ehv010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Damman K, Valente MA, Voors AA, O’Connor CM, van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J. 2014;35:455–69. doi: 10.1093/eurheartj/eht386. [DOI] [PubMed] [Google Scholar]
- 3.Mullens W, Abrahams Z, Francis GS, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53:589–96. doi: 10.1016/j.jacc.2008.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mullens W, Abrahams Z, Skouri HN, et al. Elevated intra-abdominal pressure in acute decompensated heart failure: a potential contributor to worsening renal function? J Am Coll Cardiol. 2008;51:300–6. doi: 10.1016/j.jacc.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 5.Guglin M, Rivero A, Matar F, Garcia M. Renal Dysfunction in Heart Failure Is Due to Congestion but Not Low Output. Clin Cardiol. 2011;34:113–6. doi: 10.1002/clc.20831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dupont M, Mullens W, Finucan M, Taylor DO, Starling RC, Tang WH. Determinants of dynamic changes in serum creatinine in acute decompensated heart failure: the importance of blood pressure reduction during treatment. Eur J Heart Fail. 2013;15:433–40. doi: 10.1093/eurjhf/hfs209. [DOI] [PubMed] [Google Scholar]
- 7.Damman K, Navis G, Smilde TD, et al. Decreased cardiac output, venous congestion and the association with renal impairment in patients with cardiac dysfunction. Eur J Heart Fail. 2007;9:872–8. doi: 10.1016/j.ejheart.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Nohria A, Hasselblad V, Stebbins A, et al. Cardiorenal interactions: insights from the ESCAPE trial. J Am Coll Cardiol. 2008;51:1268–74. doi: 10.1016/j.jacc.2007.08.072. [DOI] [PubMed] [Google Scholar]
- 9.Shah MR, O’Connor CM, Sopko G, Hasselblad V, Califf RM, Stevenson LW. Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE): design and rationale. Am Heart J. 2001;141:528–35. doi: 10.1067/mhj.2001.113995. [DOI] [PubMed] [Google Scholar]
- 10.Allen LA, Rogers JG, Warnica JW, et al. High mortality without ESCAPE: the registry of heart failure patients receiving pulmonary artery catheters without randomization. J Card Fail. 2008;14:661–9. doi: 10.1016/j.cardfail.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindenfeld J, Schrier RW. Blood urea nitrogen a marker for adverse effects of loop diuretics? J Am Coll Cardiol. 2011;58:383–5. doi: 10.1016/j.jacc.2011.01.054. [DOI] [PubMed] [Google Scholar]
- 12.Binanay C, Califf RM, Hasselblad V, et al. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA. 2005;294:1625–33. doi: 10.1001/jama.294.13.1625. [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevens LA, Schmid CH, Zhang YL, et al. Development and validation of GFR-estimating equations using diabetes, transplant and weight. Nephrol Dial Transplant. 2010;25:449–57. doi: 10.1093/ndt/gfp510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Testani JM, Brisco MA, Turner JM, et al. Loop diuretic efficiency: a metric of diuretic responsiveness with prognostic importance in acute decompensated heart failure. Circ Heart Fail. 2014;7:261–70. doi: 10.1161/CIRCHEARTFAILURE.113.000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Testani JM, McCauley BD, Kimmel SE, Shannon RP. Characteristics of patients with improvement or worsening in renal function during treatment of acute decompensated heart failure. Am J Cardiol. 2010;106:1763–9. doi: 10.1016/j.amjcard.2010.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuffe MS, Califf RM, Adams KF, Jr, et al. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: A randomized controlled trial. JAMA. 2002;287:1541–7. doi: 10.1001/jama.287.12.1541. [DOI] [PubMed] [Google Scholar]
- 18.Klein L, Massie BM, Leimberger JD, et al. Admission or changes in renal function during hospitalization for worsening heart failure predict postdischarge survival: results from the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) Circ Heart Fail. 2008;1:25–33. doi: 10.1161/CIRCHEARTFAILURE.107.746933. [DOI] [PubMed] [Google Scholar]
- 19.Weinfeld MS, Chertow GM, Stevenson LW. Aggravated renal dysfunction during intensive therapy for advanced chronic heart failure. Am Heart J. 1999;138:285–90. doi: 10.1016/s0002-8703(99)70113-4. [DOI] [PubMed] [Google Scholar]
- 20.Martin PY, Schrier RW. Sodium and water retention in heart failure: pathogenesis and treatment. Kidney Int Suppl. 1997;59:S57–61. [PubMed] [Google Scholar]
- 21.Slotki I, Skorecki K. Disorders of sodium balance. In: Skorecki K, Chertow GM, Marsden PA, Taal MW, Yu ASL, editors. Brenner and Rector’s The Kidney. Philadelphia, PA: Elsevier; 2016. pp. 390–459. [Google Scholar]
- 22.Testani JM, Coca SG, McCauley BD, Shannon RP, Kimmel SE. Impact of changes in blood pressure during the treatment of acute decompensated heart failure on renal and clinical outcomes. Eur J Heart Fail. 2011;13:877–84. doi: 10.1093/eurjhf/hfr070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voors AA, Davison BA, Felker GM, et al. Early drop in systolic blood pressure and worsening renal function in acute heart failure: renal results of Pre-RELAX-AHF. Eur J Heart Fail. 2011;13:961–7. doi: 10.1093/eurjhf/hfr060. [DOI] [PubMed] [Google Scholar]
- 24.Testani JM, Khera AV, St John Sutton MG, et al. Effect of right ventricular function and venous congestion on cardiorenal interactions during the treatment of decompensated heart failure. Am J Cardiol. 2010;105:511–6. doi: 10.1016/j.amjcard.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Damman K, van Deursen VM, Navis G, et al. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53:582–8. doi: 10.1016/j.jacc.2008.08.080. [DOI] [PubMed] [Google Scholar]
- 26.Smilde TD, Damman K, van der Harst P, et al. Differential associations between renal function and “modifiable” risk factors in patients with chronic heart failure. Clin Res Cardiol. 2009;98:121–9. doi: 10.1007/s00392-008-0732-z. [DOI] [PubMed] [Google Scholar]