Abstract
Objective
Metabolic syndrome is associated with insulin resistance and increased future risk of type 2 diabetes. This study investigates the relationship between insulin secretion, insulin resistance and individual metabolic syndrome components in subjects without a prior diagnosis of diabetes.
Research Design and Methods
We assessed insulin secretion during hyperglycemic clamps by infusing dextrose to maintain hyperglycemia (200 mg/dL), followed by L-arginine administration. Studies in 98 individuals (mean age 45.3±1.2 years, 56% female, 22% African-American, 49% with metabolic syndrome) were analyzed. We tested the association between the number of metabolic syndrome components and individual outcome variables using linear mixed-effects models to adjust for potential confounding effects of age, sex, and race.
Results
Insulin sensitivity index was reduced in the presence of 1 or more metabolic syndrome components. Insulin sensitivity was independently associated with age, waist circumference, male gender and decreased HDL cholesterol. The acute insulin response was greater with two or more metabolic syndrome components, and late glucose-stimulated and L-arginine-stimulated insulin responses exhibited a similar trend. In contrast, the disposition index, a measure of beta cell compensation for insulin resistance, was linearly lower with the number of metabolic syndrome components, and was negatively associated with age, Caucasian race, waist circumference, fasting glucose, and decreased HDL cholesterol.
Conclusions
The insulin secretory response in metabolic syndrome is inadequate for the worsening insulin sensitivity, as demonstrated by a decline in disposition index. A dysfunctional insulin secretory response is evident in non-diabetic individuals and worsens with accumulation of metabolic syndrome components.
Keywords: Metabolic syndrome, Insulin sensitivity, Insulin secretion, Disposition Index
1. Introduction
The metabolic syndrome was originally defined as a group of cardiovascular risk factors which cluster together more often than predicted by chance alone. The most commonly applied definition utilizes waist circumference, blood pressure, fasting glucose, triglycerides, and HDL cholesterol [1, 2]. The presence of three or more metabolic syndrome components is associated with impaired insulin sensitivity and a four-fold increased risk of developing type 2 diabetes mellitus (T2DM) [3-5].
Although nearly all patients with T2DM have reduced insulin sensitivity, this alone is not sufficient for development of diabetes, because compensatory insulin secretion can overcome insulin resistance early in the development of disease [6]. Therefore, impaired insulin secretion is essential for development of T2DM [6, 7]. An impaired insulin response to an acute glucose load is the earliest measurable beta cell defect, and is associated with an increased risk for progression to T2DM [8, 9]. A few studies have examined the relationship between metabolic syndrome components and the insulin response during oral glucose tolerance tests [10, 11] and frequently-sampled intravenous glucose tolerance test (FSIGTT) [12], but none have directly assessed insulin secretion using the gold-standard hyperglycemic clamp. Insulin secretion should also be examined in context with insulin sensitivity, to assess whether it is appropriate for the degree of insulin resistance [13]. Thus, the hyperglycemic clamp provides a measure of insulin sensitivity which correlates well with hyperinsulinemic clamp estimates and allows the simultaneous assessment of both measures [14].
The disposition index is the product of insulin sensitivity and the insulin secretory response and can be interpreted as the ability of the beta cell to compensate for insulin resistance [13]. A decreased disposition index is associated with future development of T2DM in populations at high risk [8, 15], although the association between disposition index and individual components of the metabolic syndrome components has not been directly investigated. The metabolic syndrome is often dichotomized based on the presence or absence of three components. We postulated that the cumulative number of components or analysis as continuous measures may better quantify the diabetes risk. Therefore, in the present study, we investigated the association between the number of metabolic syndrome components and predictors of T2DM (insulin sensitivity, insulin secretory response and disposition index) in a cohort of individuals without previously diagnosed T2DM using hyperglycemic clamps.
2. Research design and methods
All studies were approved by the Vanderbilt University Institutional Review Board and conducted in accordance with the Declaration of Helsinki. We recruited subjects 18-60 years of age from the Vanderbilt research participant volunteer registry. Informed consent was obtained and subjects underwent a screening history and physical before study enrollment. Exclusion criteria were a fasting plasma glucose ≥ 7 mmol/L (≥ 126 mg/dL), use of any anti-diabetes medication, ongoing glucocorticoid therapy, eGFR < 60, or any serious medical conditions. Pregnancy was excluded in women of childbearing potential by measurement of urine β-subunit of human chorionic gonadotropin (β-hCG) at screening and on each study day. We analyzed data from protocols which utilized hyperglycemic clamps conducted to investigate insulin secretion, and only studies conducted during normal dietary sodium intake (150-200 mEq Na/day) were used in the present analysis. Race was self-reported as Caucasian, African-American, or other at the time of enrollment.
2.1 Baseline Metabolic Characterization
We classified the metabolic syndrome using the 2004 revision of the National Cholesterol Education Program Adult Treatment Panel III (NCEP/ATP III) criteria [1, 2]. Subjects were considered to have metabolic syndrome if three of any of the following five traits were present: 1) abdominal obesity defined as a waist circumference in men ≥ 102 cm (40 inches) and in women ≥ 88 cm (35 inches), 2) triglycerides ≥ 1.7 mmol/L (≥ 150 mg/dL), 3) high density lipoprotein (HDL) cholesterol < 1.04 mmol/L (< 40 mg/dL) in males and < 1.29 mmol/L (< 50 mg/dL) in females, 4) systolic blood pressure > 130 mmHg or diastolic blood pressure > 85 mmHg, or 5) fasting plasma glucose ≥ 5.6 mmol/L (≥ 100 mg/dL). Waist (horizontal umbilicus) circumference was measured with a spring-loaded tape measure (Gulick II by Country Technology, Gay Mills, WI). Electrolytes and lipid panels were performed in the Vanderbilt clinical laboratory. Blood was collected in EDTA and plasma was separated and stored at −80 °C until assayed. Aprotinin was also added to plasma for measurement of insulin by radioimmunoassay (Millipore, St. Charles, MO). Classification of glycemic status was based on American Diabetes Association criterion with fasting plasma and/or 2-hour plasma glucose when available. Baseline fasting plasma glucose on the day of the hyperglycemic clamps (average of t=−20, −10, and 0) was used for determination of metabolic syndrome for all subjects.
2.2 Hyperglycemic clamps
Anti-hypertensive medications were discontinued in 25 of the 98 subjects for ≥3 weeks prior to study and were monitored to ensure that blood pressure remained <180/110 without hypertensive symptoms. Subjects ingested either their ad lib diet or a sodium controlled diet (160 mmol/d, representing the average regional sodium intake) for ≥5 days prior to the study. Subjects fasted after midnight and clamp studies began at 08:00-09:00 AM on each study day. During hyperglycemic clamp studies, a catheter was inserted in a retrograde hand vein for blood sampling, and the hand was warmed throughout the study for blood arterialization. An antecubital catheter was inserted in the contralateral arm for glucose infusion. To account for pulsatile insulin secretion, baseline glucose and insulin were measured at −20, −10, and −1 minutes before glucose infusion and the average value was used to calculate baseline values (t=0). Blood for plasma glucose was drawn every 5 minutes and immediately centrifuged and analyzed using the glucose oxidase method (YSI 2300 STAT Plus Glucose Analyzer, YSI Life Sciences; Yellow Springs, IL). A standardized priming 20% dextrose (Hospira, Lake Forest, IL) infusion was administered during the first 10 minutes (200 mg/kg body weight), and then infusion rates were adjusted every 5 minutes to maintain plasma glucose at 11.1 mmol/L (200 mg/dL) for 150 minutes, adjusted by the calculations from DeFronzo et al.[14, 16]. At 120 minutes, 5g L-Arginine (R-Gene®10, Pfizer, New York, NY) was administered intravenously over 5 minutes, and blood samples for insulin were collected every 2.5 minutes for 10 minutes, then every 5 minutes thereafter.
Hyperglycemic clamp outcomes included the acute glucose-stimulated insulin response (AUCΔInsulin0-10 = area under insulin curve from 0-10 minutes corrected for mean fasting insulin concentration), insulin sensitivity index (average glucose infusion rate in mg/kg/min divided by average insulin concentration from 90-120 minutes in μU/mL × 100), disposition index (AUCΔInsulin0-10 × insulin sensitivity index), late phase insulin response (ΔInsulin90-120 = average insulin values from time 90-120 minus fasting insulin), L-Arginine-stimulated insulin response (ΔInsulinL-Arginine = peak insulin within 10 minutes minus average insulin from 90-120 minutes), and maximal disposition index (disposition indexL-arginine = insulin sensitivity index × ΔInsulinL-Arginine).
2.3 Statistical Analysis
Study data were collected and managed using REDCap electronic data capture tools hosted at Vanderbilt [17]. Comparisons of baseline values between subjects with and without metabolic syndrome were made using the Wilcoxon rank-sum test. The effect of individual continuous metabolic syndrome components on each outcome measure (i.e., insulin sensitivity index, AUCΔInsulin0-10, disposition index, ΔInsulin90-120, and ΔInsulinL-Arginine) was evaluated using linear mixed-effects models, with compound symmetry correlation structure for repeated measurements with age, gender, race, and the five metabolic syndrome components as fixed effects. The model for AUCΔinsulin0-10 was also adjusted for insulin sensitivity index. All statistical analyses were performed using SPSS® for Windows (version 22, IBM® SPSS®, Chicago, IL) and SAS® release 9 (Cary, North Carolina). A two-tailed P-value <0.05 was considered statistically significant. Data are presented as mean ± SEM unless otherwise indicated. Data in all figures are presented as the least-square estimated marginal means for the multivariable model, calculated at the mean age (44.7 years).
3. Results
Subject Characteristics
Baseline characteristics for the 98 participants stratified by metabolic syndrome components are presented in Table 1. Metabolic syndrome was present in 49% of the cohort, classified by: increased waist circumference (85.7%), decreased HDL (62.2%), elevated fasting plasma glucose (39.8%), elevated blood pressure (34.7%), and elevated plasma triglycerides (22.4%). Oral glucose tolerance test (OGTT) data was available for 73 (74.5%) individuals. While no subjects had a prior diagnosis of diabetes at the time of screening, glucose tolerance based on 2-hr glucose was classified as diabetic in 9 (12.3%), impaired in 14 (19.2%), and normal in 50 (68.5%).
Table 1.
Study participant characteristics
| Total (n=98) | Metabolic Syndrome Absent (n=50) |
Metabolic Syndrome Present (n=48) |
||||||
|---|---|---|---|---|---|---|---|---|
| Number of components | 0 (n=6) | 1 (n=14) | 2 (n=30) | 3 (n=30) | 4 (n=14) | 5 (n=4) | P-Value | |
| Age (years) | 44.7±1.3 | 27.8±2.1 | 34.4±2.8 | 43.7±2.4 | 50.5±1.6 | 51.2±1.8 | 46.7±6.5 | <0.001 |
| Female (%) | 56(57%) | 2(33%) | 7(50%) | 19(63%) | 18(60%) | 8(57%) | 2(50%) | 0.82 |
| Race | 0.22 | |||||||
| Caucasian (%) | 74(76%) | 4(67%) | 12(85.7%) | 20(67%) | 23(77%) | 12(86%) | 3(75%) | |
| African American (%) | 24(24%) | 2(33%) | 2(14.3%) | 10(33%) | 7(23%) | 2(14%) | 1 (25%) | |
| Body Mass Index (kg/m2) | 33.8±0.7 | 25.3±1.3 | 27.7±1.2 | 34.8±1.5 | 35.6±1.0 | 36.9±1.5 | 36.6±2.0 | <0.001 |
| Waist Circumference (cm) | 109.3±1.6 | 86.4±4.4 | 96.0±3.8 | 110.4±2.8 | 113.0±2.1 | 119.2±2.5 | 119.1±3.6 | <0.001 |
| Blood pressure (mmHg) | ||||||||
| Systolic | 124±2 | 113±3 | 111±4 | 120±2 | 129±3 | 136±5 | 144±8 | <0.001 |
| Diastolic | 72±1 | 64±1 | 66±3 | 69±1 | 76±2 | 78±3 | 82±4 | <0.001 |
| Heart Rate (bpm) | 65±1 | 55±4 | 60±3 | 64±2 | 66±2 | 70±4 | 70±4 | 0.007 |
| Total Cholesterol (mM) | 4.56±0.09 | 3.92±0.30 | 4.10±0.16 | 4.66±0.17 | 4.69±0.18 | 4.61±0.25 | 5.02±0.34 | 0.12 |
| Triglycerides (mM) | 1.31±0.11 | 0.51±0.09 | 0.69±0.11 | 1.01±0.07 | 1.41±0.13 | 1.81±0.31 | 4.38±1.10 | <0.001 |
| HDL (mM) | 1.17±0.04 | 1.44±0.10 | 1.58±0.16 | 1.16±0.05 | 1.08±0.04 | 0.96±0.04 | 0.87±0.06 | <0.001 |
| LDL (mM) | 2.81±0.09 | 2.24±0.33 | 2.21±0.16 | 3.04±0.16 | 2.97±0.16 | 2.87±0.24 | 2.5±0.45 | 0.38 |
| Body Fat (%) | 35.0±1.3 | 18.3±2.9 | 23.7±3.9 | 37.0±2.3 | 39.0±1.7 | 40.5±2.8 | 37.9±6.3 | 0.002 |
| Fasting glucose (mM) | 5.6±0.1 | 5.0±0.2 | 5.3±0.1 | 5.3±0.1 | 5.7±0.1 | 6.1±0.3 | 6.5±0.4 | <0.001 |
*P-value indicates comparison between those with versus without metabolic syndrome. HDL, high density lipoprotein cholesterol; LDL, low density lipoprotein cholesterol
3.1 Insulin sensitivity index
Increasing age and each component of the metabolic syndrome negatively correlated with insulin sensitivity when analyzed individually, although this does not adjust for potential confounding factors (Supplemental Table 1). In the adjusted analysis, insulin sensitivity index negatively associated with age and waist circumference and positively with HDL (Table 2). Insulin sensitivity index was not associated with sex, race, systolic blood pressure, triglycerides, or fasting plasma glucose. The largest decline in adjusted mean insulin sensitivity index across metabolic syndrome components occurred from 0 to 2 components, with no further significant difference between 2 and 5 components (Figure 1A).
Table 2.
Multivariable analysis of hyperglycemic clamp outcomes by metabolic syndrome components as continuous variables.
| Insulin Sensitivity Index |
AUCΔInsulin0-10 |
Disposition Index |
||||
|---|---|---|---|---|---|---|
| β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | |
| Age (years) | −0.17 (−0.32,−0.01) | 0.033 | −1.7 (−7.1, 3.7) | 0.54 | −31.4 (−66.6, 3.9) | 0.08 |
| Sex* | 3.1 (−6.5, 0.4) | 0.079 | −97.5 (−220.0, 25.2) | 0.12 | −746.5 (−1546.0, 530) | 0.067 |
| Race† | −0.22 (−3.8, 3.3) | 0.90 | 275.6 (150.4, 400.8) | <10−4 | 1443.2 (589.2, 2297.3) | 0.001 |
| Glucose (mmol/L) | −0.57 (−2.6, 1.5) | 0.58 | −177.8 (−250.3, −105.3) | <10−5 | −910.8 (−1402.3, −419.3) | <0.001 |
| Waist circumference (cm) | −0.28 (−0.40, −0.16) | <0.001 | −1.3 (−6.0, 3.4) | 0.58 | −42.8 (−71.3, −14.3) | 0.004 |
| HDL Cholesterol (mmol/L) | 6.2 (0.9, 11.4) | 0.022 | 20.9 (−168.6, 210.4) | 0.83 | 1187.4 (−65.0, 2439.9) | 0.063 |
| SBP (mmHg) | 0.02 (−0.07, 0.11) | 0.66 | −3.0 (−6.3, 0.3) | 0.07 | −15.1 (−38.3, 8.1) | 0.20 |
| Triglycerides (mmol/L) | −0.69 (−2.3, 0.93) | 0.40 | 16.5 (−40.3, 73.3) | 0.57 | 106.4 (−271.3, 483.9) | 0.58 |
| ISI (mg/kg/min per μU/mL•100) | NA | −13.9 (−21.0, −6.9) | <0.001 | NA | ||
β-coefficient represents change in outcome per unit change in variable.
Female referent
Caucasian referent
Figure 1. Hyperglycemic clamp measures stratified by number of metabolic syndrome components.
A, Insulin sensitivity index (ISI, in mg/kg/min per μU/mL•100). B, Acute insulin response (AUC for insulin during first 10 minutes of hyperglycemic clamp, μU/mL•min). C, Disposition index (product of ISI and acute insulin response), D, Late-phase insulin response (ΔInsulin90-120). E, L-arginine-stimulated insulin response. F, Maximal disposition index calculated using the L-arginine insulin response. Data are presented as least square mean±SEM adjusted for age, sex, and race. *P<0.05 vs 0, †P<0.05 vs 1, and ‡P<0.05 vs 2 components. Two symbols indicate P<0.01 and 3 indicates P<0.001.
3.2 Acute glucose-stimulated insulin response
In univariate analyses, the acute insulin response (AUCΔInsulin0-10) correlated with fasting glucose, with a trend for insulin sensitivity index (Supplemental Table 1). After multivariate adjustment, the acute insulin response to glucose was not significantly altered by the number of metabolic syndrome components (Figure 1B). The acute insulin response was greater in subjects with two metabolic syndrome components, however (Figure 1B). Similar results were obtained when insulin response was analyzed as the maximal increase above baseline or as the baseline-adjusted area-under-the-curve within 10 minutes after glucose administration. When metabolic syndrome components were analyzed as continuous measures, however, the acute insulin response was negatively associated with fasting glucose, and positively associated with African American race (Table 2). Because of the known relationship between insulin secretion and insulin sensitivity in normal individuals [6, 18], we also adjusted for insulin sensitivity index, which negatively associated with the acute insulin response but did not alter the significance of other variables in the model.
3.3 Late-phase glucose-stimulated insulin response
In unadjusted analyses, the late-phase insulin secretion (ΔInsulin90-120) correlated negatively with insulin sensitivity index and positively with waist circumference, triglycerides, and the acute insulin response (Supplemental Table 1). The late-phase insulin response was greater in subjects with 2-4 metabolic syndrome components (Figure 1C). In the multivariable model, InsulinΔ90-120 was negatively associated with fasting glucose and insulin sensitivity index, and tended to be greater in African Americans (Supplemental Table 2).
3.4 L-Arginine stimulated insulin response
The insulin response after L-Arginine administration (ΔInsulinL-Arginine) correlated negatively with HDL and insulin sensitivity index, and positively with triglycerides (Supplemental Table 1). After multivariable adjustment, only HDL cholesterol remained significant (Supplemental Table 2), although a linear increase was apparent with number of metabolic syndrome components, with a significantly greater response in subjects with 4 and tendency to decrease with 5 components (Figure 1D).
3.5 Qualitative insulin secretory responses
Qualitative differences in the time-to-peak insulin concentration also varied by presence of metabolic syndrome. The acute insulin response time-to-peak occurred at a later time point in metabolic syndrome subjects (5.7±0.3 versus 6.4±0.3 minutes for non-metabolic syndrome versus +metabolic syndrome; P=0.04), but there was no significant difference when analyzed by number of metabolic components. In contrast, there was no difference in time-to-peak L-Arginine response (6.1±0.2 versus 6.3±0.2 minutes; P=0.51). A negative value for the acute insulin response rarely occurred (4.6%), but was present only in subjects with ≥2 metabolic syndrome components, and only in subjects with elevated fasting glucose and waist circumference.
3.6 Disposition index
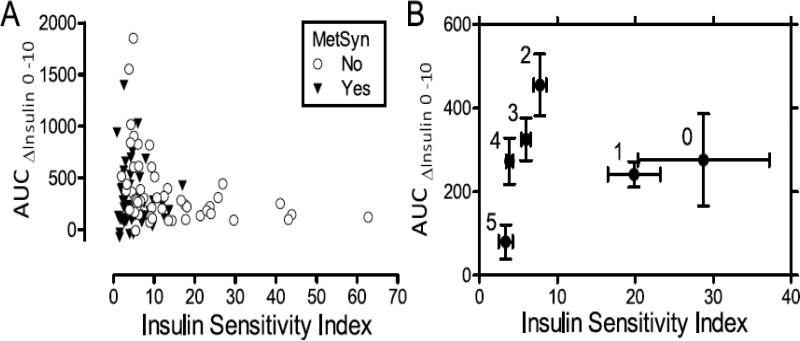
In univariate analysis, the disposition index correlated negatively with age, fasting glucose, waist circumference, SBP, and triglycerides, and positively with HDL (Supplemental Table 1). After multivariate adjustment, disposition index was inversely associated with number of metabolic syndrome components (Figure 1E). Disposition index also associated negatively with waist circumference and fasting plasma glucose and positively with HDL (Table 2). Disposition index was not associated with age, systolic blood pressure, triglycerides, or gender. The maximal disposition index calculated using the L-arginine insulin response appeared qualitatively similar to the disposition index trend, although the variability was greater. The ability of beta cell compensation for insulin resistance can be further visualized by plotting insulin sensitivity index against insulin secretory response (Figure 2A), grouped by presence or absence of metabolic syndrome. The grouped means by number of metabolic syndrome components are presented in Figure 2B, with groups with >3 components trending towards the graph origin.
Figure 2. Relationship between insulin secretion and insulin sensitivity.
A, Individual data points for insulin secretion (AUC for insulin during first 10 minutes of hyperglycemic clamp) plotted again insulin sensitivity index in subjects with and without metabolic syndrome. B, Data are presented as least square mean±SEM adjusted for age, sex, and race for insulin sensitivity index versus insulin secretion. Numbers adjacent to each data point indicate the number of metabolic syndrome components.
3.7 HOMA-2 estimates and hyperglycemic clamp outcomes
The correlation for hyperglycemic clamp and HOMA-2 estimates, based on fasting glucose and insulin, are presented in Supplemental Table 3. The HOMA2 estimate for beta cell function (HOMA2-B), correlated significantly, but weakly with dynamic insulin responses (ΔInsulin0-10, r=0.32, P=0.001; ΔInsulinL-arginine, r=0.19, P=0.047), but correlated more strongly with terminal-clamp insulin (ΔInsulin90-120 r=0.58, p<0.001). In comparison, the HOMA2 estimate of insulin sensitivity (HOMA2-S) correlated well with the insulin sensitivity index during hyperglycemic clamps (r=−0.85, P<10−9) and to the disposition index (r=0.50, P<10−6).
4. Discussion
Presence of the metabolic syndrome is associated with insulin resistance, increased cardiovascular complications [19] and a four-fold increased risk of incident diabetes [3, 4, 20]. The risk of T2DM is highest in subjects with impaired glucose-stimulated insulin secretion, reflecting an inadequate beta cell response for insulin resistance (i.e. reduced disposition index). We found that insulin sensitivity was strongly associated with all individual metabolic syndrome components, whereas the insulin secretory response was not. The disposition index progressively declined according to number of metabolic syndrome components, with a significant difference with as few as two components. Our findings are clinically relevant, because subjects with only two metabolic syndrome components may have an increased risk for diabetes and could benefit from earlier lifestyle interventions.
To our knowledge, the association between individual metabolic syndrome components and insulin secretion has not been investigated using the gold-standard hyperglycemic clamp or by modeling to adjust for potential confounders. Several studies have demonstrated a negative association between metabolic syndrome and either HOMA estimates of beta cell function or OGTT measures of insulin secretion, although these methods may underestimate the degree of impairment [11, 21-23]. These methods have been criticized because the measurements are largely driven by the change in plasma glucose rather than an absolute difference in insulin secretion [24]. The presence of metabolic syndrome has also been associated with impaired FSIGTT-derived measures of insulin secretion in the Insulin Resistance Atherosclerosis (IRAS) multi-ethnic cohort [12], which analyzed individual correlations between components but did not adjust for confounding variables.
The hyperglycemic clamp method further allows the analysis of late-phase insulin secretion and the response to non-glucose stimuli, such as L-Arginine [25]. The L-arginine insulin response was the largest insulin peak observed during the present study. In contrast to the acute glucose-stimulated insulin response, the L-arginine response increased with accumulation of metabolic syndrome components before a decline seemed to appear with 5 components. The L-arginine insulin response is reduced in subjects with established diabetes [26], indicating that this impairment may develop later in the course of disease and could provide a measure of functional beta cell mass. A larger response has been observed in combination with GLP-1 [27].
The increased risk of T2DM due to inadequate beta cell compensation may be represented by the disposition index [15]. This relationship is normally hyperbolic, such that individuals with comparable disposition index fall on the same line, regardless of their degree of insulin resistance. The disposition index is determined by genetic and environmental factors and is inversely associated with T2DM risk [15, 28]. We noted a relationship between insulin sensitivity, insulin secretion, and number of metabolic syndrome components which reflects the increased diabetes risk (Figure 2B). This data is consistent with the previously reported loss of insulin secretion with worsening insulin resistance as subjects progress from normal glucose tolerance to overt T2DM [6, 9].
There was a clear racial contribution to the insulin secretory response, such that the acute- and late-phase insulin secretion and disposition index are notably higher in African American subjects. Similar observations have been reported in prepubertal children and healthy premenopausal women of African American origin [29]. In these studies, the increased insulin secretory response was not explained by diet or body composition differences [30]. African Americans pre-diabetes also have increased acute insulin secretory response and disposition index determined by FSIGTT [31]. The present study expands previous observations by demonstrating that this racial difference persists after adjusting for confounding factors, including components of the metabolic syndrome [12, 32]. Multiple studies have also reported reduced insulin sensitivity in African Americans or black South Africans compared to whites [33-35]. In our study, however, we observed no difference in insulin sensitivity, and the racial differences in insulin secretory response remained significant even after adjusting for insulin sensitivity. Other factors, such as racially-linked genetic variants that affect beta cell function could explain these racial differences [23, 36].
African Americans have a lower incidence of metabolic syndrome, as defined using standard definitions, despite an increased risk of cardiovascular disease and T2DM [37-40]. This observation has been described as the “metabolic syndrome paradox” [41]. Individual components of the metabolic syndrome, particularly low HDL and high triglycerides levels, are rarely abnormal in African Americans explaining this low prevalence [41]. Increased disposition index has been associated with reduced T2DM risk in African Americans [15]. Therefore, our finding of increased disposition index in African Americans was surprising, and raises the question whether increased disposition index is truly protective in this population. Future studies are needed to evaluate and determine how cardiometabolic risk can be adequately measured and identified in this population.
The cross-sectional nature of this study does not allow us to establish causation between accumulation of metabolic syndrome components and the decrease in beta cell function. We excluded subjects with pre-existing or symptomatic T2DM, which limited the range of fasting glycemia and decreased heterogeneity. This more focused population allows us to examine early changes in a cohort with increased diabetes risk, before the occurrence of established T2DM.
The metabolic syndrome contributes to cardiovascular disease and T2DM risk, and our study demonstrates a clear relationship between metabolic syndrome and impaired beta cell compensatory ability. This impairment is evident before the occurrence of overt hyperglycemia and helps explain the increased risk of T2DM in metabolic syndrome. Interventions to improve insulin sensitivity and beta cell function should target patients with as few as two metabolic syndrome components.
Supplementary Material
Acknowledgments
Funding
This work was supported by NIH grants DK081662, DK096994, HL060906, HL103976, and DK20593 (VUMC DRTC Hormone Assay Core), and UL1 RR024975 and UL1 TR000445 from NCATS/NIH (Vanderbilt Institute for Clinical and Translational Research), T32 DK007061, PhRMA Foundation Career Development Award (C.A.S.), Fogarty International Center of the National Institutes of Health Number R25 TW009337, and VA Merit BX000666.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
SS, CR, CS, CY, and JML researched data. SS, CY, and JML wrote the manuscript. CR, CS, and AP edited the manuscript and contributed to discussion. JML is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Disclosure Summary: The authors have nothing to disclose
The authors declare no conflicts of interest.
References
- 1.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM, Brewer HB, Jr., Cleeman JI, Smith SC, Jr., Lenfant C, American Heart A et al. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–8. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 3.Lorenzo C, Okoloise M, Williams K, Stern MP, Haffner SM, San Antonio Heart S. The metabolic syndrome as predictor of type 2 diabetes: the San Antonio heart study. Diabetes Care. 2003;26:3153–9. doi: 10.2337/diacare.26.11.3153. [DOI] [PubMed] [Google Scholar]
- 4.Ford ES, Li C, Sattar N. Metabolic syndrome and incident diabetes: current state of the evidence. Diabetes Care. 2008;31:1898–904. doi: 10.2337/dc08-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson PW, D'Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–72. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- 6.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–6. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 7.Ferrannini E, Mari A. beta-Cell function in type 2 diabetes. Metabolism. 2014;63:1217–27. doi: 10.1016/j.metabol.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Defronzo RA, Tripathy D, Schwenke DC, Banerji M, Bray GA, Buchanan TA, et al. Prediction of diabetes based on baseline metabolic characteristics in individuals at high risk. Diabetes Care. 2013;36:3607–12. doi: 10.2337/dc13-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999;104:787–94. doi: 10.1172/JCI7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nijpels G, van der Wal PS, Bouter LM, Heine RJ. Comparison of three methods for the quantification of beta-cell function and insulin sensitivity. Diabetes Res Clin Pract. 1994;26:189–95. doi: 10.1016/0168-8227(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 11.Malin SK, Finnegan S, Fealy CE, Filion J, Rocco MB, Kirwan JP. beta-Cell dysfunction is associated with metabolic syndrome severity in adults. Metab Syndr Relat Disord. 2014;12:79–85. doi: 10.1089/met.2013.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanley AJ, Wagenknecht LE, D'Agostino RB, Jr., Zinman B, Haffner SM. Identification of subjects with insulin resistance and beta-cell dysfunction using alternative definitions of the metabolic syndrome. Diabetes. 2003;52:2740–7. doi: 10.2337/diabetes.52.11.2740. [DOI] [PubMed] [Google Scholar]
- 13.Bergman RN, Ader M, Huecking K, Van Citters G. Accurate assessment of beta-cell function: the hyperbolic correction. Diabetes. 2002;51(Suppl 1):S212–20. doi: 10.2337/diabetes.51.2007.s212. [DOI] [PubMed] [Google Scholar]
- 14.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–23. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 15.Lorenzo C, Wagenknecht LE, Rewers MJ, Karter AJ, Bergman RN, Hanley AJ, et al. Disposition index, glucose effectiveness, and conversion to type 2 diabetes: the Insulin Resistance Atherosclerosis Study (IRAS). Diabetes Care. 2010;33:2098–103. doi: 10.2337/dc10-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luther JM, Byrne LM, Yu C, Wang TJ, Brown NJ. Dietary sodium restriction decreases insulin secretion without affecting insulin sensitivity in humans. J Clin Endocrinol Metab. 2014;99:E1895–902. doi: 10.1210/jc.2014-2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, et al. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes. 1993;42:1663–72. doi: 10.2337/diab.42.11.1663. [DOI] [PubMed] [Google Scholar]
- 19.Dekker JM, Girman C, Rhodes T, Nijpels G, Stehouwer CD, Bouter LM, et al. Metabolic syndrome and 10-year cardiovascular disease risk in the Hoorn Study. Circulation. 2005;112:666–73. doi: 10.1161/CIRCULATIONAHA.104.516948. [DOI] [PubMed] [Google Scholar]
- 20.Hanley AJ, Karter AJ, Williams K, Festa A, D'Agostino RB, Jr., Wagenknecht LE, et al. Prediction of type 2 diabetes mellitus with alternative definitions of the metabolic syndrome: the Insulin Resistance Atherosclerosis Study. Circulation. 2005;112:3713–21. doi: 10.1161/CIRCULATIONAHA.105.559633. [DOI] [PubMed] [Google Scholar]
- 21.Festa A, Williams K, Hanley AJ, Haffner SM. Beta-cell dysfunction in subjects with impaired glucose tolerance and early type 2 diabetes: comparison of surrogate markers with first-phase insulin secretion from an intravenous glucose tolerance test. Diabetes. 2008;57:1638–44. doi: 10.2337/db07-0954. [DOI] [PubMed] [Google Scholar]
- 22.Eriksson J, Franssila-Kallunki A, Ekstrand A, Saloranta C, Widen E, Schalin C, et al. Early metabolic defects in persons at increased risk for non-insulin-dependent diabetes mellitus. N Engl J Med. 1989;321:337–43. doi: 10.1056/NEJM198908103210601. [DOI] [PubMed] [Google Scholar]
- 23.Pimenta W, Korytkowski M, Mitrakou A, Jenssen T, Yki-Jarvinen H, Evron W, et al. Pancreatic beta-cell dysfunction as the primary genetic lesion in NIDDM. Evidence from studies in normal glucose-tolerant individuals with a first-degree NIDDM relative. JAMA. 1995;273:1855–61. [PubMed] [Google Scholar]
- 24.Reaven GM. Insulin secretory function in type 2 diabetes: Does it matter how you measure it? Journal of diabetes. 2009;1:142–50. doi: 10.1111/j.1753-0407.2009.00016.x. [DOI] [PubMed] [Google Scholar]
- 25.Elahi D. In praise of the hyperglycemic clamp. A method for assessment of beta-cell sensitivity and insulin resistance. Diabetes Care. 1996;19:278–86. doi: 10.2337/diacare.19.3.278. [DOI] [PubMed] [Google Scholar]
- 26.Ward WK, Bolgiano DC, McKnight B, Halter JB, Porte D., Jr. Diminished B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Invest. 1984;74:1318–28. doi: 10.1172/JCI111542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stumvoll M, Fritsche A, Haring HU. Clinical characterization of insulin secretion as the basis for genetic analyses. Diabetes. 2002;51(Suppl 1):S122–9. doi: 10.2337/diabetes.51.2007.s122. [DOI] [PubMed] [Google Scholar]
- 28.Palmer ND, Langefeld CD, Ziegler JT, Hsu F, Haffner SM, Fingerlin T, et al. Candidate loci for insulin sensitivity and disposition index from a genome-wide association analysis of Hispanic participants in the Insulin Resistance Atherosclerosis (IRAS) Family Study. Diabetologia. 2010;53:281–9. doi: 10.1007/s00125-009-1586-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arslanian S. Insulin secretion and sensitivity in healthy African-American vs American white children. Clin Pediatr (Phila) 1998;37:81–8. doi: 10.1177/000992289803700204. [DOI] [PubMed] [Google Scholar]
- 30.Lindquist CH, Gower BA, Goran MI. Role of dietary factors in ethnic differences in early risk of cardiovascular disease and type 2 diabetes. Am J Clin Nutr. 2000;71:725–32. doi: 10.1093/ajcn/71.3.725. [DOI] [PubMed] [Google Scholar]
- 31.Ebenibo S, Edeoga C, Wan J, Dagogo-Jack S. Glucoregulatory function among African Americans and European Americans with normal or pre-diabetic hemoglobin A1c levels. Metabolism. 2014;63:767–72. doi: 10.1016/j.metabol.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rasouli N, Spencer HJ, Rashidi AA, Elbein SC. Impact of family history of diabetes and ethnicity on -cell function in obese, glucose-tolerant individuals. J Clin Endocrinol Metab. 2007;92:4656–63. doi: 10.1210/jc.2007-0919. [DOI] [PubMed] [Google Scholar]
- 33.Ellis AC, Alvarez JA, Granger WM, Ovalle F, Gower BA. Ethnic differences in glucose disposal, hepatic insulin sensitivity, and endogenous glucose production among African American and European American women. Metabolism. 2012;61:634–40. doi: 10.1016/j.metabol.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haffner SM, Howard G, Mayer E, Bergman RN, Savage PJ, Rewers M, et al. Insulin sensitivity and acute insulin response in African-Americans, non-Hispanic whites, and Hispanics with NIDDM: the Insulin Resistance Atherosclerosis Study. Diabetes. 1997;46:63–9. doi: 10.2337/diab.46.1.63. [DOI] [PubMed] [Google Scholar]
- 35.Goedecke JH, Dave JA, Faulenbach MV, Utzschneider KM, Lambert EV, West S, et al. Insulin response in relation to insulin sensitivity: an appropriate beta-cell response in black South African women. Diabetes Care. 2009;32:860–5. doi: 10.2337/dc08-2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.t Hart LM, Simonis-Bik AM, Nijpels G, van Haeften TW, Schafer SA, Houwing- Duistermaat JJ, et al. Combined risk allele score of eight type 2 diabetes genes is associated with reduced first-phase glucose-stimulated insulin secretion during hyperglycemic clamps. Diabetes. 2010;59:287–92. doi: 10.2337/db09-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harris MI, Flegal KM, Cowie CC, Eberhardt MS, Goldstein DE, Little RR, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988-1994. Diabetes Care. 1998;21:518–24. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 38.Brancati FL, Kao WH, Folsom AR, Watson RL, Szklo M. Incident type 2 diabetes mellitus in African American and white adults: the Atherosclerosis Risk in Communities Study. JAMA. 2000;283:2253–9. doi: 10.1001/jama.283.17.2253. [DOI] [PubMed] [Google Scholar]
- 39.Weiss R, Dziura JD, Burgert TS, Taksali SE, Tamborlane WV, Caprio S. Ethnic differences in beta cell adaptation to insulin resistance in obese children and adolescents. Diabetologia. 2006;49:571–9. doi: 10.1007/s00125-005-0109-z. [DOI] [PubMed] [Google Scholar]
- 40.Winkleby MA, Kraemer HC, Ahn DK, Varady AN. Ethnic and socioeconomic differences in cardiovascular disease risk factors: findings for women from the Third National Health and Nutrition Examination Survey, 1988-1994. JAMA. 1998;280:356–62. doi: 10.1001/jama.280.4.356. [DOI] [PubMed] [Google Scholar]
- 41.Fitzpatrick SL, Lai BS, Brancati FL, Golden SH, Hill-Briggs F. Metabolic syndrome risk profiles among African American adolescents: national health and nutrition examination survey, 2003-2010. Diabetes Care. 2013;36:436–42. doi: 10.2337/dc12-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.