SUMMARY
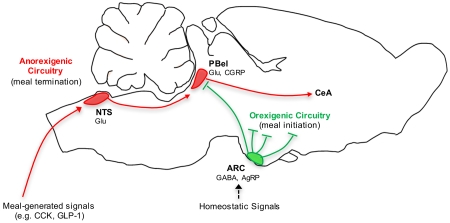
The lateral parabrachial nucleus is a conduit for visceral signals that cause anorexia. We previously identified a subset of neurons located in the external lateral parabrachial nucleus (PBel) that express calcitonin gene-related peptide (CGRP) and inhibit feeding when activated by illness mimetics. We report here that in otherwise normal mice, functional inactivation of CGRP neurons markedly increases meal size, with meal frequency being reduced in a compensatory manner, and renders mice insensitive to the anorexic effects of meal-related satiety peptides. Furthermore, CGRP neurons are directly innervated by orexigenic hypothalamic AgRP neurons, and photostimulation of AgRP fibers supplying the PBel delays satiation by inhibiting CGRP neurons, thereby contributing to AgRP-driven hyperphagia. By establishing a role for CGRP neurons in the control of meal termination and as a downstream mediator of feeding elicited by AgRP neurons, these findings identify a node in which hunger and satiety circuits interact to control feeding behavior.
INTRODUCTION
In most mammals, food is consumed in discrete meals, the size of which is determined by mechanical and chemical signals generated by the gut in response to food ingestion (Ritter, 2004). Many of these “satiety signals”, including the hormones cholecystokinin (CCK) and glucagon-like peptide 1 (GLP-1), act upon vagal sensory fibers that in turn synapse onto and activate caudal hindbrain neurons in the nucleus of the solitary tract (NTS) and area postrema (Abbott et al., 2005; Edwards et al., 1986; Kanoski et al., 2011; Smith et al., 1981; Yuan and Barber, 1993). While some NTS and area postrema neurons project directly to the forebrain, the parabrachial nucleus in rodents (Herbert et al., 1990; Ricardo and Koh, 1978) as well as primates (Beckstead et al., 1980) is a key relay for visceral signals from the caudal hindbrain to forebrain areas associated with appetite control.
Although disruption of this ascending visceral pathway can increase the amount of food consumed during individual meals by delaying meal termination, overall intake does not typically increase. Rather, the resultant increase of meal size is offset by a proportionate decrease in meal frequency (Schwartz et al., 1999), reflecting the integration of meal size within a larger system for energy homeostasis. Specifically, hypothalamic neurons, such as those that synthesize agouti-related peptide (AgRP) and proopiomelanocortin (Morton et al., 2014), are hypothesized to maintain energy homeostasis by concurrently modulating neural pathways that control meal initiation and termination (i.e. orexigenic and anorexigenic pathways). Altogether, this system enables the amount of meal-to-meal food consumption to be adjusted as needed to maintain stable energy reserves.
Contained within the ascending visceral pathway are neurons situated in the lateral parabrachial nucleus that receive excitatory input from the caudal hindbrain (Herbert et al., 1990; Jhamandas and Harris, 1992; Wu et al., 2012) and hence are activated by meal-related satiety signals (Li and Rowland, 1994; Rowland et al., 1997; Wu et al., 2014). Furthermore, parenchymal injections of feeding-related hormones and peptides into the lateral parabrachial nucleus alter feeding, via changes in meal size, suggesting that this structure may integrate meal-related satiety signals with other modulators of feeding behavior (Alhadeff et al., 2015; Alhadeff et al., 2014b). Nonetheless, the identity of lateral parabrachial neurons that control food intake is unknown.
Within the external lateral subdivision of the parabrachial nucleus (PBel) there exists a population of neurons that express calcitonin gene-related peptide (CGRP); their activation can cause profound anorexia in mice, whereas their inhibition ameliorates anorexia induced by visceral malaise or AgRP neuron ablation (Carter et al., 2013). Further establishing their role in pathological anorexia is the observation that CGRPPBel neuronal activation can induce a conditioned taste aversion (Carter et al., 2015). Although CGRPPBel neurons are also activated by meal-related satiety signals (Carter et al., 2013), their contribution to physiological satiation remains unresolved. The current work was undertaken to investigate whether CGRPPBel neurons participate in the control of food intake under physiological conditions, and if this effect involves an interaction between CGRPPBel neurons and hypothalamic neurons that contribute to long-term energy homeostasis. Based on the hypothesis that CGRPPBel neurons are inhibited by orexigenic AgRP neurons (Carter et al., 2013; Wu et al., 2009; Wu et al., 2012), we postulated that AgRP neuron activation increases food intake in part by inhibiting appetite-suppressing CGRPPBel neurons. To examine these hypotheses, we used Cre-dependent viruses in transgenic mice to selectively activate or inactivate CGRPPBel neurons and/or hypothalamic AgRP neurons. Our findings collectively establish that CGRPPBel neurons are critical components of a physiologically relevant circuit controlling satiety and meal size and place these neurons within the context of a larger system controlling energy homeostasis.
RESULTS
CGRPPBel Neurons Control Meal Termination
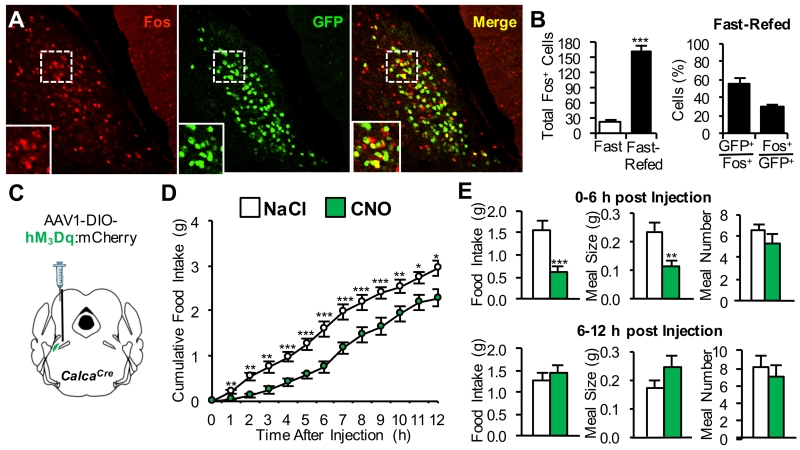
Based on evidence that CGRPPBel neurons are activated by systemic administration of meal-associated gut peptides amylin and CCK (Carter et al., 2013), we predicted that endogenous signals generated during a meal would similarly activate these neurons. Consistent with this hypothesis, Fos expression (a marker of neuronal activation) was elevated in the lateral parabrachial nucleus of mice subjected to a fasting-refeeding protocol, including in the PBel (Figure S1A). Over half of the Fos expression within the PBel following post-fast refeeding was found in green fluorescent protein (GFP)-labeled CGRPPBel neurons (Figures 1A and 1B).
Figure 1. CGRPPBel Neurons are Activated by a Meal and Control Meal Termination.
(A) Representative histological images showing Fos immunoreactivity (red) in GFP-labeled CGRPPBel neurons (green) after fast-refeeding.
(B) Quantification of Fos immunoreactivity in the PBel after fast-refeeding (n = 6 per group).
(C) Unilateral delivery of AAV carrying Cre-dependent hM3Dq:mCherry into the PBel of CalcaCre mice.
(D and E) Cumulative food intake (D) and meal pattern analysis (E) following saline or CNO (1 mg/kg, i.p.) administration immediately prior to onset of dark cycle in non-food-deprived mice (n = 8). All data shown are means ± s.e.m. * P < 0.05; ** P < 0.01; *** P < 0.001. Statistical analysis was performed with an unpaired student’s t-test (B) and two-way ANOVA followed by Bonferroni’s post-hoc test (D-E). See also Figure S1.
If CGRPPBel neurons play a physiological role in satiation, CGRPPBel neuron activation should selectively reduce the size of individual meals, whereas inactivation of these neurons should have the opposite effect. Neuron-specific activation of CGRPPBel neurons was achieved by stereotaxic delivery of adeno-associated virus (AAV) expressing the Cre-dependent hM3Dq:mCherry transgene (AAV1-DIO-hM3Dq:mCherry) into the PBel of mice with Cre recombinase targeted to the CGRP-encoding gene (CalcaCre) (Figure 1C). Food intake was reduced following intraperitoneal (i.p.) injection of clozapine-N-oxide (CNO) to activate hM3Dq in CGRPPBel neurons compared to saline control injection (Figure 1D), and this effect was mediated by reduction of meal size with no change in meal frequency (Figure 1E). Because the reduction of food intake elicited by CGRPPBel activation in this cohort of mice was less robust and of shorter duration (~6 h) than was described in our previous report (Carter et al., 2013), we examined whether this difference may have been due to differences in virus titer. In support of this hypothesis, we found that in a separate group of mice injected with a higher titer of AAV1-DIO-hM3Dq:mCherry, CGRPPBel activation more potently reduced food intake such that a single CNO injection nearly halted food intake for 6 h and continued to decrease meal size (but not frequency) 6-12 h after injection (Figure S1B and S1C). Hence, CGRPPBel activation reduces food intake by engaging a neural circuit that controls meal termination, although with sufficient activation, meal initiation is blunted as well.
CGRPPBel Neuron Inactivation Disrupts Normal Control of Meal Size
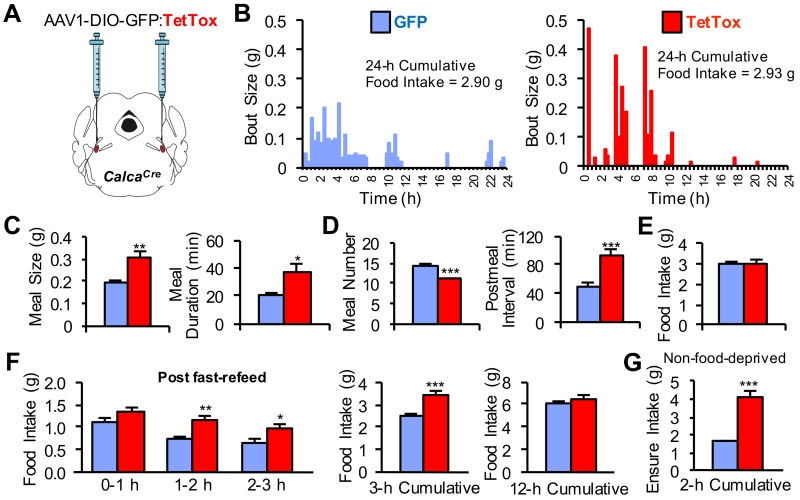
To test the hypothesis that CGRPPBel neurons participate in the physiological control of meal size, we employed a loss-of-function strategy (Figure 2A). CGRPPBel neurons were permanently inactivated following stereotaxic injection of AAV expressing Cre-dependent tetanus toxin light chain (AAV1-DIO-GFP:TetTox) (Han et al., 2015; Kim et al., 2009). Permanent inactivation of CGRPPBel neurons did not affect long-term standard chow intake or body weight (Figure S2A and S2B) consistent with results obtained when these neurons were transiently inhibited with hM4Di (Carter et al., 2013). However, TetTox inactivation of CGRPPBel neurons markedly increased the size of individual feeding bouts during ad libitum food access (standard chow diet) compared to control mice injected with virus encoding only GFP (Figure 2B). Group-wide analysis of meal parameters revealed that TetTox-expressing mice consumed larger meals of longer duration (Figure 2C), but compensated by eating meals less frequently (Figure 2D) such that cumulative intake did not differ from control mice (Figure 2E). Similarly, CGRPPBel inactivation with TetTox significantly increased food intake during the first few hours of refeeding following a fast, but did not affect longer-term food intake (12-h cumulative) compared to control mice (Figure 2F).
Figure 2. Functional Inactivation of CGRPPBel Neurons Disrupts Control of Meal Size.
(A) Bilateral delivery of AAV carrying Cre-dependent GFP:TetTox into the PBel of CalcaCre mice.
(B) Representative bout-size recordings from individual TetTox and GFP control mice during a 24-h recording period.
(C-E) Group-wide, meal-pattern analysis (n = 9 per group) was obtained from bout size recordings using set meal parameters. TetTox inactivation of CGRPPBel neurons increased the size and duration of meals (C) but was accompanied by a decrease in meal frequency (D) such that overall food intake was unaltered (E).
(F) Food intake measured following a 24-h food deprivation (n = 11 per group).
(G) Intake of a palatable liquid diet (Ensure) from non-food-deprived mice given 2 h ad libitum access during the light cycle (n = 11 per group). All data shown are means ± s.e.m. * P < 0.05; ** P < 0.01; *** P < 0.001. Statistical analysis was performed with an unpaired student’s t-test (C-D and G) and two-way ANOVA followed by Bonferroni’s post-hoc test (F). See also Figure S2.
To investigate whether CGRPPBel neuron activation also limits the intake of palatable foods, sated mice were given access to a palatable diet during the light-cycle. We found that CGRPPBel-inactivated mice consumed nearly 3-fold more food than control mice during a 2-h test period (Figure 2G). This result prompted us to examine whether CGRPPBel inactivation exacerbates hyperphagia and weight-gain in mice with ad libitum access to palatable food. However, as with standard chow diet, we found that CGRPPBel inactivation did not affect long-term intake of a palatable high-fat diet compared to mice with intact CGRPPBel neurons and hence did not predispose them to become more obese than normal mice (Figure S2C and S2D). Thus, our observations indicate that CGRPPBel neurons transduce short-term satiety signals generated during food ingestion, but are not necessary for long-term regulation of food intake.
CGRPPBel Neuron Activation Mediates the Anorexic Effect of Meal-related Satiety Signals
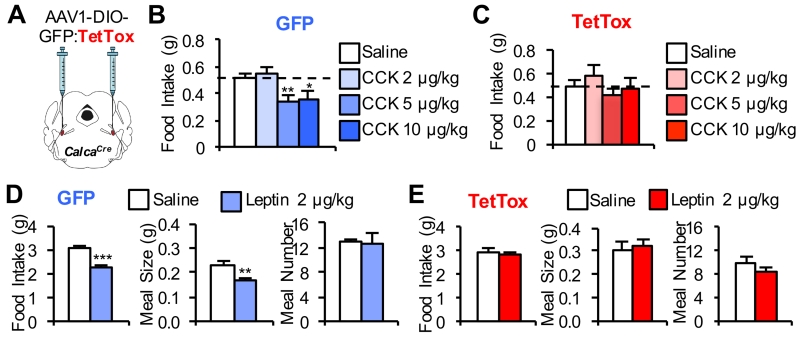
We previously examined the effect of CGRPPBel neuron inhibition on CCK-induced anorexia using a chemogenetic approach, but found that technique to be insufficiently robust to attenuate the anorexic effect of CCK (Carter et al., 2013). Because TetTox completely silences transduced CGRPPBel neurons (Han et al., 2015), whereas chemogenetic inhibition does not, we tested whether food intake is reduced following systemic CCK administration in TetTox-expressing mice. Unlike GFP controls, we found that TetTox-expressing mice did not reduce food intake in response to 5 or 10 μg/kg doses of CCK (Figures 3B and 3C). CGRPPBel neuron activation is therefore required for the effect of CCK, a well-established physiological mediator of satiety (Smith, 2006), to reduce food intake.
Figure 3. CGRPPBel Neuronal Activation is Required for CCK and Leptin-induced Anorexia.
(A) Bilateral delivery of AAV carrying Cre-dependent GFP:TetTox into the PBel of CalcaCre mice.
(B) CCK administration (i.p.) decreases 2-h cumulative food intake in non-food-deprived GFP control mice (n = 8) injected immediately before the dark cycle.
(C) TetTox inactivation of CGRPPBel neurons abolishes CCK-induced anorexia (n = 10).
(D) Leptin administration (i.p.) decreases 12-h cumulative food intake by decreasing meal size in non-food-deprived GFP control mice (n = 6) injected immediately before the dark cycle.
(E) Leptin-induced decrease of 12-h cumulative food intake and reduction of meal size is absent in TetTox mice (n = 9). All data shown are means ± s.e.m. * P < 0.05; ** P < 0.01; *** P < 0.001. Statistical analysis was performed with a one-way ANOVA followed by Tukey’s post-hoc test (B-C) and paired student’s t-test (D-E).
The adipocyte hormone leptin reduces food intake in part by increasing sensitivity to meal-related satiety signals such as CCK and thereby reducing meal size (Barrachina et al., 1997; Morton et al., 2005; Peters et al., 2006). Moreover, acute anorexia induced by leptin administration is mediated entirely by reduced meal size (Flynn et al., 1998; Zheng et al., 2010). We therefore predicted that inactivation CGRPPBel neurons would blunt leptin-induced anorexia. Consistent with this expectation, food intake was not reduced in TetTox-expressing mice following a single systemic leptin injection (Figure 3E), whereas control mice ate less after leptin treatment (Figure 3D). Moreover, the acute inhibitory effect of leptin on food intake in normal mice was the result, as expected, of reduced meal size with no change of meal frequency (Figure 3D). By comparison, TetTox-expressing mice were insensitive to the meal size-reducing effect of leptin (Figure 3E).
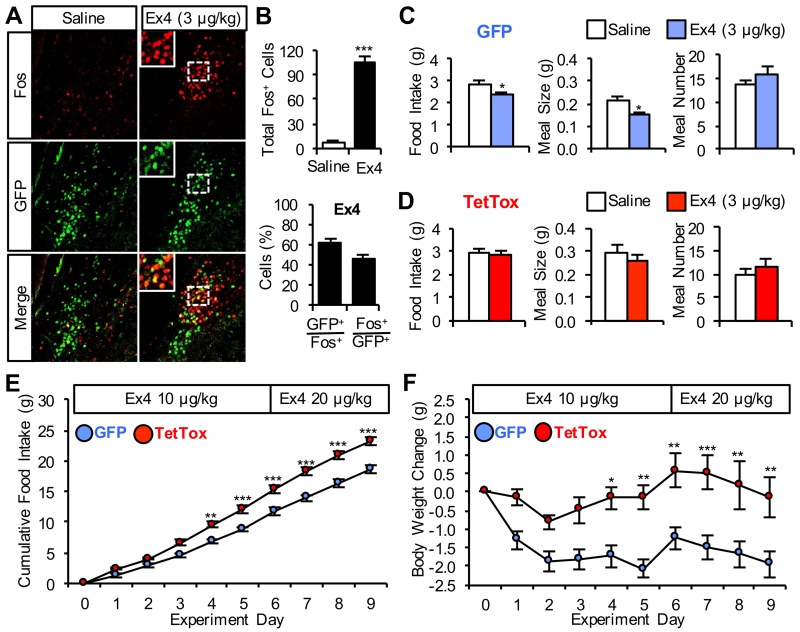
GLP-1 is another anorexic gut hormone that plays a physiological role in satiety perception (Williams et al., 2009). GLP-1 activates the vagus (Gaisano et al., 2010) and is also synthesized by meal-activated NTS neurons (Hayes et al., 2009; Hisadome et al., 2011; Vrang et al., 2003), some of which project to the parabrachial nucleus (Alhadeff et al., 2014a; Richard et al., 2014). Moreover, systemic administration of the potent GLP1-receptor agonist, Exendin-4 (Ex4), requires activation of neurons in the parabrachial nucleus to reduce food intake (Swick et al., 2015). We found that systemic Ex4 administration increases Fos immunoreactivity in GFP-labeled CGRPPBel neurons (Figures 4A and 4B). Moreover, TetTox-expressing mice did not reduce 12-h food intake following administration of an Ex4 dose that was sufficient to reduce 12-h food intake in control mice (Figures 4C and 4D). Higher doses of Ex4 did reduce food intake in TetTox-expressing mice, although the anorexia was blunted compared to controls (Figure S3A and S3B).
Figure 4. CGRPPBel Neuronal Activation Contributes to Anorexia Induced by a GLP-1 Receptor Agonist.
(A) Representative histological images showing Fos immunoreactivity (red) in GFP-labeled CGRPPBel neurons (green) following saline or Ex4 administration.
(B) Quantification of Fos immunoreactivity in the PBel after saline (n = 3) or Ex4 (n = 4) administration.
(C) Ex4 injection (i.p.) decreases 12-h cumulative food intake by decreasing meal size in non-food-deprived GFP control mice (n = 8) administered immediately before the dark cycle.
(D) Ex-induced decrease of 12-h cumulative food intake and reduction of meal size is absent in TetTox mice (n = 8).
(E and F) Twice daily i.p. administration of Ex4 to diet-induced obese mice with access to high-fat diet (TetTox, n = 6; GFP, n = 8). Ex4-induced anorexia (E) and body weight-loss (F) was attenuated in TetTox mice. All data shown are means ± s.e.m. * P < 0.05; ** P < 0.01; *** P < 0.001. Statistical analysis was performed with an unpaired student’s t-test (B), paired student’s t-test (C-D), and two-way ANOVA followed by Bonferroni’s post-hoc test (E-F). See also Figures S3.
Because GLP-1 receptor agonists have been clinically approved for weight loss in humans (Gautron et al., 2015), we examined the effect of chronic Ex4 administration on body weight in lean and obese mice. In control mice maintained on a standard chow diet, single daily injections of Ex4 (10 μg/kg) were sufficient to decrease body weight, an effect that was prevented in TetTox-expressing mice (Figure S3C). In diet-induced obese mice, twice-daily injections of 10 μg/kg and eventually 20 μg/kg of Ex4 were required to sustain anorexia in control mice because they quickly developed tachyphylaxis (Figure S3D and S3E). Nonetheless, CGRPPBel neuron inactivation significantly blunted anorexia and weight-loss in obese mice (Figure 4E and 4F). Collectively, this series of experiments indicates that CGRPPBel neuron activation significantly contributes to the anorexic effect of well-established satiety factors that control meal size.
Identification of CGRPPBel Neuron Projections Involved in Satiety
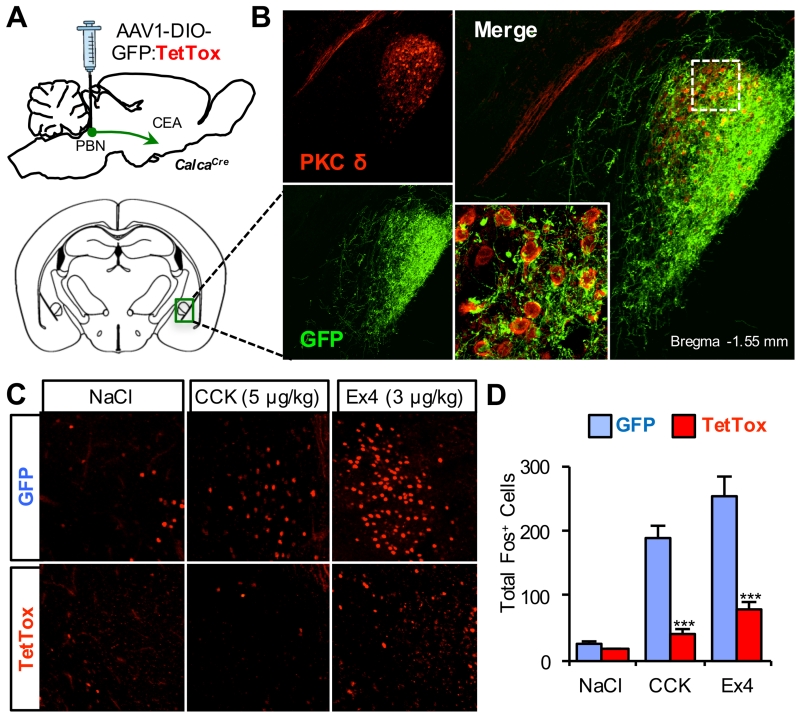
CGRPPBel neurons project their axons to the central nucleus of the amygdala (CeA) and the bed nucleus of the stria terminalis (BNST), and activation of CGRPPBel neuron axon terminals in the CeA has been shown to produce anorexia (Carter et al., 2013). Based on a recent report showing that PKC-δ expressing neurons in the CeA (PKC-δCeA) contribute to the anorexic effect of various agents, including CCK (Cai et al., 2014), we examined whether these neurons are downstream targets of the anorexic effect of CGRPPBel activation. Using virally introduced GFP, double-labeled immunohistochemistry revealed that CGRPPBel axons form putative synapses onto most PKC-δCeA neurons (Figure 5B). Additionally, CCK- and Ex4-induced Fos in the CeA was abolished following CGRPPBel neuron inactivation with TetTox (Figures 5C and 5D), but not in upstream NTS neurons (Figure S4). Given that the majority of CCK-induced Fos occurs in PKC-δCeA neurons (Cai et al., 2014), and that some PKC-δCeA neurons express the CGRP receptor (Han et al., 2015), these data support the hypothesis that PKC-δCeA neurons in the CEA are a downstream target of CGRPPBel-induced anorexia.
Figure 5. Activation of CeA Following Gut Peptide Administration Requires CGRPPBel Neuronal Activation.
(A) Bilateral delivery of AAV carrying Cre-dependent GFP:TetTox into the PBel of CalcaCre mice.
(B) Representative histological images showing overlap of PKC-δ immunoreactivity (red) and GFP-labeled CGRPPBel axon projections (green) in the CeA.
(C and D) Representative histological images (C) and quantification of Fos immunoreactivity (D) in the CeA following saline, CCK, or Ex4 administration to GFP and TetTox mice (n = 4-6 per treatment). All data shown are means ± s.e.m. *** P < 0.001. Statistical analysis was performed with a one-way ANOVA followed by Tukey’s post-hoc test (D). See also Figure S4.
Functional Role of an AgRP Projection to PBel
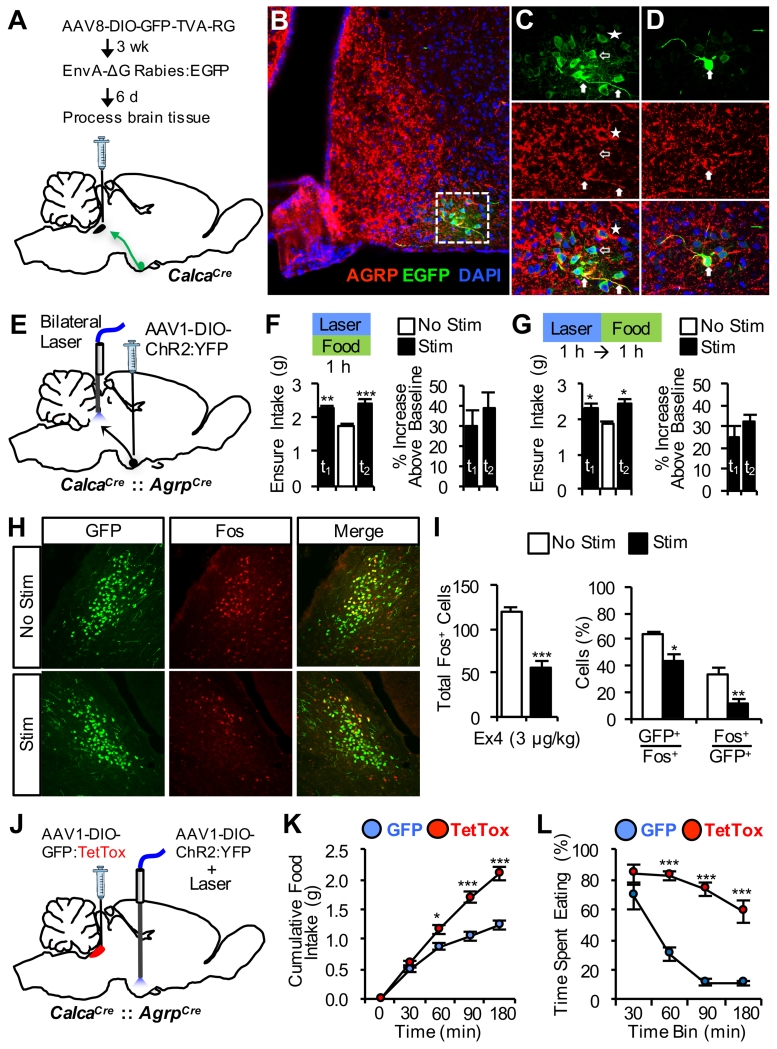
Based on evidence that AgRP neurons provide inhibitory input to the PBel (Carter et al., 2013; Wu et al., 2009), we postulated that the AgRP→PBel pathway plays a physiological role in delaying meal termination and thereby contributes to the orexigenic effect of AgRP neuron activation. To determine whether CGRPPBel neurons receive direct input from AgRP neurons, we performed Cre-dependent, rabies virus-based monosynaptic tracing (Wall et al., 2010; Wickersham et al., 2007) from CGRPPBel neurons (Figure 6A). Using this strategy, we identified upstream, GFP-labeled neurons in the arcuate hypothalamus, most of which were double-labeled with an AgRP antibody (Figures 6B-D and S5). We also observed that some GFP-labeled neurons did not overlap with AgRP labeling. Although it is possible that our AgRP staining did not label all AgRP neurons, it is also possible that CGRPPBel neurons receive monosynaptic input from one or more unidentified subsets of arcuate neurons additional to AgRP neurons. Additional studies are needed to characterize the proportion of CGRPPBel neurons innervated by AgRP neurons.
Figure 6. CGRPPBel Neurons Limit Hyperphagic Response to AgRP Neuron Activation.
(A) Procedure for unilateral delivery of viruses into the PBel of CalcaCre mice allowing for retrograde monosynaptic rabies tracing from CGRPPBel neurons.
(B) Representative confocal image of the arcuate hypothalamus showing overlap of upstream EGFP-labeled neurons (green) and AgRP immunoreactivity (red).
(C and D) High-magnification optical sections of (B) demonstrating examples of EGFP and AgRP double-labeling of soma and fibers (filled arrows), single-labeled EGFP neurons (hollow arrows), and single-labeled EGFP neurons in close apposition to AgRP labeling (star).
(E) Bilateral delivery of AAV carrying Cre-dependent ChR2:YFP into the arcuate hypothalamus of CalcaCre::AgrpCre mice with fiber-optic cannulae bilaterally implanted over the PBel.
(F and G) Concurrent palatable food access and AgRP→PBel photostimulation (F) or AgRP→PBel photostimulation for 1 h immediately prior to palatable food access (G) increases food intake compared to experimental trials without photostimulation (n = 5). Photostimulation trial 1, t1; trial 2, t2.
(H and I) Representative histological images (H) and quantification (I) of Ex4-induced Fos immunoreactivity in GFP-labeled CGRPPBel neurons ipsilateral and contralateral to unilateral AgRP→PBel photostimulation.
(J) Bilateral delivery of AAV carrying Cre-dependent GFP:TetTox into the PBel and bilateral delivery of AAV carrying Cre-dependent ChR2:YFP into the arcuate hypothalamus of CalcaCre::AgrpCre mice with a fiber-optic cannula implanted over the arcuate nucleus.
(K and L) Cumulative food intake (K) and time spent eating (L) following photostimulation of AgRP soma for 2 h in TetTox mice compared to GFP control mice (n = 4 per group). All data shown are means ± s.e.m. * P < 0.05; ** P < 0.01; *** P < 0.001. Statistical analysis was performed with a one-way ANOVA followed by Dunnett’s post-hoc test (F-G), unpaired student’s t-test (I), and two-way ANOVA followed by Bonferroni’s post-hoc test (K-L). See also Figure S5, S6, and Video S1.
To examine a functional interaction between AgRP and CGRPPBel neurons, we generated CalcaCre::AgrpCre double-transgenic mice and transduced AgRP neurons with AAV expressing Cre-dependent channelrhodopsin-2 (AAV1-DIO-ChR2:YFP); fiber-optic cannulae were bilaterally implanted over the PBel (Figure 6E). In agreement with previous reports (Atasoy et al., 2012; Betley et al., 2013), photostimulation of AgRP→PBel axonal projections did not stimulate standard chow intake in sated mice during the light cycle (No Stim, 0.01 ± 0.02 g, Stim, 0.02 ± 0.02 g; mean ± s.e.m.). However, because CGRPPBel inactivation affects food intake by delaying meal termination, we postulated that the feeding effect of AgRP→PBel activation would be detectable in mice already engaged in a meal. We took advantage of the fact that non-food-deprived mice consume food during the light cycle when given access to a palatable diet (Ensure) and found that photostimulation of AgRP→PBel under these conditions increased food intake (Figure 6F). Interestingly, palatable food intake was increased even when AgRP→PBel photostimulation was terminated prior to food access (Figure 6G). Thus, the feeding effect induced by activating AgRP projections to the PBel persists beyond the period of active stimulation. This observation offers a potential explanation for the recent, paradoxical observations that although AgRP neuron activation clearly elicits hyperphagia (Aponte et al., 2011), sensory detection of food inhibits most AgRP neurons prior to food consumption (Betley et al., 2015; Chen et al., 2015).
As a first step to investigate whether hyperphagia elicited by AgRP neuron activation involves inhibition of CGRP neurons, we asked whether AgRP→PBel photostimulation blunts Fos expression in the PBel following Ex4 administration (previously shown to increase Fos in CGRP neurons) (Figure 6H). Consistent with this hypothesis, transduction of AgRP neurons with AAV1-DIO-ChR2:YFP revealed axonal fiber projections near GFP-labeled CGRP neurons (Figure S6), and photostimulation of these fibers attenuated Ex4-induced Fos expression in CGRP neurons (Figures 6G).
Although photostimulation of AgRP neurons potently increases food intake, this increase has its limits and meals are eventually terminated (Aponte et al., 2011). Perhaps anorexigenic signals generated during a meal eventually override AgRP-mediated inhibition of CGRPPBel neurons and thereby result in satiation and meal termination. Accordingly, we postulated that photostimulation of AgRP neurons in conjunction with CGRPPBel inactivation with TetTox (Figure 6J) would result in a failure to terminate meals and consequently enhance the hyperphagic response. Indeed, the ability to terminate feeding during photostimulation of AgRP soma was severely compromised in TetTox-expressing mice relative to controls with intact CGRPPBel neuron function (Video S1). Consequently, both total food intake (Figure 6K) and time spent eating (Figure 6L) were robustly increased during AgRP neuron photostimulation compared to mice with intact CGRPPBel neurons. These data collectively support a model in which AgRP neuron activation increases feeding by activating downstream appetitive pathways while simultaneously inhibiting anorexic signals transduced by CGRPPBel neurons, so as to delay satiety onset.
DISCUSSION
Using a tetanus toxin-based strategy to chronically inactivate a defined neuronal population, we demonstrate that inactivation of CGRPPBel neurons markedly delays satiation while also rendering mice insensitive to satiety signals. We further provide evidence that hypothalamic AgRP neurons stimulate feeding in part by inhibiting CGRPPBel neurons and that inactivation of CGRPPBel neurons greatly enhances feeding elicited by photostimulation of AgRP neurons. These findings implicate CGRPPBel neurons in the physiological mechanism underlying satiation and demonstrate that meal-size control exists within a larger system responsible for long-term energy homeostasis.
Based on previous observations that photostimulation of CGRPPBel neurons both potently reduces food intake and supports the formation of a conditioned taste aversion, a role for these neurons in pathophysiological anorexia, rather than in the physiological control of normal food intake or satiation, has been suggested (Carter et al., 2015; Carter et al., 2013; Garfield et al., 2015; Garfield and Lowell, 2013). By comparison, melanocortin-4 receptor-expressing neurons in the paraventricular hypothalamus (MC4RPVH) have been identified as candidate mediators of food intake inhibition under physiological conditions, in part because they lie downstream of hypothalamic AgRP neurons (Garfield et al., 2015). Consistent with this view, activation of MC4RPVH neurons decreases food intake without inducing an aversive response (Garfield et al., 2015), whereas activation of CGRP neurons is likely to be aversive (although this endpoint has not been tested in the same manner for both neuronal subsets). These observations led to the view that CGRPPBel neuron activation participates solely or primarily in pathophysiological anorexia, whereas MC4RPVH activation mediates physiological satiety (Garfield et al., 2015; Garfield and Lowell, 2013). Our finding that CGRPPBel neurons play a physiological role in controlling meal size forces a reconsideration of this perspective.
Specifically, our findings support an alternative model in which contributions made by MC4RPVH and CGRPPBel neurons to both meal termination and AgRP-driven food intake involve complementary and non-overlapping mechanisms. This model is based upon evidence that satiation following a meal is associated with both positive valence and a feeling of “fullness” – independent processes involving separate neural substrates. For example, administration of nutrients directly into the circulation of food-deprived animals is reinforcing (Oliveira-Maia et al., 2011), despite bypassing gut satiety signals that contribute to satiation. Such observations suggest that animals consume food (in part) to remove aversive hunger signals, and that removal of hunger signals (via nutrient replenishment) reduces appetite. This mechanism of appetite regulation is further supported by evidence that photostimulation of AgRP neurons is aversive (Betley et al., 2015). As MC4RPVH neurons lie downstream of and are inhibited by AgRP neurons, their activation (owing in part to silencing of AgRP neurons as a meal progresses) may contribute to positive affect associated with food consumption. Consistent with this view, MC4RPVH photostimulation is reinforcing only in a food-deprived state when AgRP neurons are active (Garfield et al., 2015).
On the other hand, the feeling of “fullness”, which involves gastric distention and the action of gut-derived satiety peptides, is a negative feedback signal that diminishes the reinforcing effect of food consumption and becomes aversive with supraphysiological stimulation (Bardos, 1989; Gyetvai and Bardos, 1999; Miller, 1954; Perez et al., 1998; Weller and Blass, 1990). Moreover, activation of the ascending visceral pathway that gives rise to the perception of “fullness” is not necessary for the reinforcing quality of food consumption (Lucas and Sclafani, 1996; Perez et al., 1998; Sclafani and Lucas, 1996). Given that CGRPPBel neurons are activated by and contribute to the satiating effect of gut hormones, we postulate that CGRPPBel neuron activation gives rise to the post-meal perception of “fullness” that contributes importantly to meal termination.
This model provides a useful context within which to consider several key observations. First, because sated animals will consume a palatable diet despite the absence of a hunger-driven signal, satiation observed under these conditions cannot be explained by loss of a hunger signal, but nevertheless involves CGRPPBel activation. Second, we observed that increased meal size following CGRPPBel neuron inactivation was offset by a decrease of meal frequency, such that overall food intake remained unchanged. We speculate that the effect of CGRPPBel inactivation to increase food consumption during individual meals delays the onset of hunger signals, thereby reducing meal frequency. This observation supports the hypothesis that energy homeostasis entails mechanisms whereby meal-to-meal intake is regulated over time so as to match energy intake to expenditure and preserve stable body fat mass (Morton et al., 2014).
Consistent with this view is our finding that activation of AgRP→PBel projections increases food intake by delaying satiation. This mechanism of blunting the neuronal response to meal-related satiety signals is likely distinct from that engaged by activation of AgRP projections to MC4RPVH neurons, which initiates feeding (Garfield et al., 2015). We postulate that AgRP-neuron activation robustly increases food intake because it engages these two distinct but complementary mechanisms, a hypothesis that is supported by the fact that food deprivation and hunger-related signals increase the activity of virtually all AgRP neurons (Betley et al., 2013; Betley et al., 2015). In this context, a seemingly paradoxical finding is that sensory detection of food inhibits most AgRP neurons prior to the onset of feeding (Betley et al., 2015; Chen et al., 2015). How can AgRP neuron activation play a physiological role to drive feeding if the neurons become inactive before feeding commences? One possibility is that the behavioral consequences of AgRP neuron activation persist after the activity of these neurons ceases. Consistent with this view, we report that activation of AgRP→PBel projections prior to food access is sufficient to delay satiation; thus, this pathway does not need to be concurrently active during food ingestion to increase food consumption during a meal. Whether other downstream AgRP neuron targets function in a similar fashion, and whether “priming” downstream targets prior to food access involves fast-acting neurotransmitters and/or slow-acting peptides, are questions that merit further investigation.
Additional studies are needed to investigate how CGRPPBel neuron activation contributes to both physiological satiation and illness-associated anorexia. One possibility is that both effects are mediated by a single set of CGRPPBel neurons, with the difference representing changes in stimulus strength. Thus, whereas modest activation of CGRPPBel neurons might contribute to satiation encountered during a typical meal, prolonged or more robust CGRPPBel activation could become aversive – just as the feeling of “fullness” can transition to an uncomfortable feeling after gorging. Alternatively, CGRPPBel neurons involved in the physiological control of meal size may be distinct from those involved in illness and formation of a conditioned taste aversion. Consistent with the latter view, we noticed that a meal or moderate dose of a GLP-1 receptor agonist increases Fos in a dorsal cluster of CGRPPBel neurons (Figures 1A, S1A, and 4A), while visceral illness mimetics appear to increase Fos in both dorsal and ventral populations of CGRPPBel neurons (Carter et al., 2013).
These considerations, however, do not exclude the alternative hypothesis that activation of CGRPPBel neurons is aversive regardless of stimulus strength. Just as NTS neuronal subtypes are differentially activated depending on the meal size (Kreisler et al., 2014), the parabrachial nucleus might similarly transduce this information such that non-CGRP neurons are activated by smaller meals, while CGRP neurons are preferentially activated during larger meals and extreme satiety. Furthermore, it is well-established that the PBel is activated by diverse noxious signals, including uncomfortable gastric distention (Bernard et al., 1994), hypoxia (Teppema et al., 1997) and somatic pain (Bernard and Besson, 1990). We recently demonstrated that CGRPPBel neurons are activated by spinally-mediated noxious stimuli, and that their activation is necessary for ‘freezing responses’ to foot shock (Han et al., 2015). These observations suggest that CGRPPBel neurons might be part of a generalized “alarm” system that elicits appropriate behavioral and motivational responses to harmful stimuli, including decreased appetite during bouts of prolonged feeding. Therefore, it is conceivable that mice inappropriately consume larger meals in the absence of CGRPPBel neurons because they lack this negative-feedback signal.
In conclusion, we report that CGRPPBel neurons play a key role in the physiological system that transduces information from endogenous satiety signals into the decision to terminate a meal. Our findings shed light on how these neurons interact with other neuronal systems involved in food intake control, such as hypothalamic AgRP neurons. This information may ultimately inform our understanding of the pathogenesis and treatment of eating disorders, including anorexia and malaise associated with cancer and other chronic disease states (Morton et al., 2014). As new roles are identified for discrete neuronal subsets and their associated neural circuits, we anticipate that a more comprehensive understanding will emerge of how discrete brain areas (e.g., NTS, PBN, hypothalamus and amygdala) interact in the control of food intake and energy homeostasis.
EXPERIMENTAL PROCEDURES
Mice
CalcaCre:GFP and AgrpCre:GFP mice (C57Bl/6 background) were generated and maintained as previously described (Carter et al., 2013; Sanz et al., 2015). Following stereotaxic surgery, mice were singly housed with ad libitum access to standard chow diet (LabDiet 5053) in temperature- and humidity-controlled facilities with 12-h light/dark cycles. Heterozygous male CalcaCre:GFP/+ and AgrpCre:GFP/+ mice were used for behavioral experiments whereas both male and female mice were used for immunohistochemical Fos studies. All animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Washington.
Virus Production and Stereotaxic Surgeries
Cre-dependent AAV1-DIO-hM3Dq:mCherry, AAV1-DIO-GFP, AAV1-DIO-GFP:TetTox, and AAV1-DIO-ChR2:YFP viral vectors were produced by transfecting HEK cells and then purifying cell extracts by pelleting through sucrose and by CsCl-gradient ultracentrifugation. Final pellets were suspended in 0.1 M PBS at an approximate titer of 2 × 109 viral genomes per microliter (unless stated otherwise) measured with a fluorometer (Hoefer, DQ300). Stereotaxic injection of virus into the PBel of CalcaCre mice was done as described (Carter et al., 2013). Virus (0.5 μl per side) was injected bilaterally (TetTox studies) or unilaterally (hM3Dq-mCherry and monosynaptic retrograde tracing studies) into the PBel using the following coordinates: antero-posterior (AP), −5.10 mm; medio-lateral (ML), ± 1.30; dorso-ventral (DV), 3.25 mm. The following coordinates were used to stereotaxically inject virus into the arcuate nucleus of AgrpCre mice (bilaterally, 0.5 μl per side): AP, −1.25 mm; ML, ± 0.35 mm; DV, −5.80 mm. For optogenetic control of AgRP neurons, a single fiber-optic cannula was implanted above the arcuate nucleus (AP, −1.25 mm; ML, 0.00 mm; DV, −5.50 mm) or bilaterally over the PBel (AP, −4.8 mm; ML, ± 1.40 mm; DV, −2.80 mm). Mice were given at least 3 weeks of post-surgery recovery.
Pharmacology
CNO (1 mg/kg, Sigma), CCK-8s (2-10 μg/kg, Bachem), recombinant murine leptin (2 μg/kg, PeproTech), and Ex4 (3-30 μg/kg, American Peptide) were dissolved in sterile 0.9% saline. All drugs were administered i.p. (10 μl per gram body weight).
Meal Pattern Analysis and Food Monitoring Experiments
Meal-patterning analysis
Mice were habituated to food monitoring home cages (BioDAQ, v. 2.2) for at least 10 days before experimental manipulation. Feeding records were analyzed using BioDAQ Viewer (software v. 2.2.01). A feeding bout was defined as a meal if ≥ 0.06 g of food was ingested and if it was separated from another meal by ≥ 5 min.
Mice had ad libitum access to water during all food-monitoring experiments. All pharmacological behavioral experiments were conducted in non-food-deprived mice injected with drug or control saline injection ~20 min before onset of the dark cycle. A cross-over, within-subjects experimental design was used for pharmacological experiments (except for during chronic Ex4 administration) with at least five days elapsing between experimental trials. Detailed experimental procedures are described in the Supplemental Information.
Photostimulation
A blue light laser (473 nm, LaserGlow) was used to deliver light pulses to the brain through fiber-optic cables (200-μm diameter, Doric Lenses) firmly attached to implanted fiber-optic cannula with zirconia sleeves (Doric Lenses). The frequency and pulse width of the laser light was programmed using a waveform generator (Agilent Technologies, catalog #33220A). The light power exiting the fiber tip (4.8 mW) was estimated to correspond to 3.52 mW mm−2 at the arcuate or PBel based on an online light transmission calculator for brain tissue (http://web.stanford.edu/group/dlab/cgi-bin/graph/chart.php). Based on an established photostimulation protocol for activating AgRP neurons and their fiber projections (Aponte et al., 2011; Atasoy et al., 2012), all of our experiments used the following settings: 10-ms pulses, 20 pulses for 1 s on / 3 s off. During habituation sessions (2 h daily for 5 days), mice were attached to fiber-optic cables but did not receive photostimulation or access to food. All photostimulation experiments were conducted 3 h into the light cycle in non-food-deprived mice. Detailed experimental procedures are described in the Supplemental Information.
Immunohistochemistry and Fos Studies
Fos, PKC-δ, and GFP immunolabeling were performed as described (Han et al., 2015). For studies involving Fos immunolabeling, mice were anaesthetized approximately 90 min after experimental manipulation and intracardially perfused with 0.1 M PBS followed by 4% paraformaldehyde. Detailed experimental procedures are described in the Supplemental Information.
Statistics
All data were analyzed using Prism 5.0 (GraphPad Software) as described in the text and Supplementary Statistical Analysis.
Supplementary Material
Highlights.
Parabrachial CGRP neurons contribute to physiological control of appetite
Inactivation of CGRP neurons decreases perception of meal-induced satiety signals
Hunger-activated AgRP neurons are connected to and inhibit CGRP neurons
CGRP neurons constrict feeding elicited by AgRP neuron activation
ACKNOWLEDEMENTS
We thank Megan Chiang for maintaining the mouse colony. The research was supported by grants from the National Institutes of Health R01-DA24908 (RDP) and DK090320, DK101997, DK083042 and CA184630-01 (MWS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
C.A.C., M.W.S., and R.D.P. conceived and designed the study. C.A.C. performed and analyzed histological and behavioral experiments and A.J.B. performed some histological and behavioral experiments. R.D.P. provided equipment and reagents. C.A.C., M.W.S., and R.D.P. wrote the manuscript.
REFERENCES
- Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JR, Ghatei MA, Bloom SR. The inhibitory effects of peripheral administration of peptide YY(3-36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res. 2005;1044:127–131. doi: 10.1016/j.brainres.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Alhadeff AL, Baird JP, Swick JC, Hayes MR, Grill HJ. Glucagon-like Peptide-1 receptor signaling in the lateral parabrachial nucleus contributes to the control of food intake and motivation to feed. Neuropsychopharmacology. 2014a;39:2233–2243. doi: 10.1038/npp.2014.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhadeff AL, Golub D, Hayes MR, Grill HJ. Peptide YY signaling in the lateral parabrachial nucleus increases food intake through the Y1 receptor. Am. J. Physiol.-Endoc. M. 2015;309:E759–E766. doi: 10.1152/ajpendo.00346.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhadeff AL, Hayes MR, Grill HJ. Leptin receptor signaling in the lateral parabrachial nucleus contributes to the control of food intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014b;307:R1338–1344. doi: 10.1152/ajpregu.00329.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat. Neurosci. 2011;14:351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172–177. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardos G. Behavioral consequences of intestinal distention: aversivity and discomfort. Physiol. Behav. 1989;45:79–85. doi: 10.1016/0031-9384(89)90168-6. [DOI] [PubMed] [Google Scholar]
- Barrachina MD, Martinez V, Wang L, Wei JY, Tache Y. Synergistic interaction between leptin and cholecystokinin to reduce short-term food intake in lean mice. Proc. Natl. Acad. Sci. U.S.A. 1997;94:10455–10460. doi: 10.1073/pnas.94.19.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead RM, Morse JR, Norgren R. The nucleus of the solitary tract in the monkey: projections to the thalamus and brain stem nuclei. J. Comp. Neurol. 1980;190:259–282. doi: 10.1002/cne.901900205. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Besson JM. The spino(trigemino)pontoamygdaloid pathway: electrophysiological evidence for an involvement in pain processes. J. Neurophysiol. 1990;63:473–490. doi: 10.1152/jn.1990.63.3.473. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Huang GF, Besson JM. The parabrachial area: electrophysiological evidence for an involvement in visceral nociceptive processes. J. Neurophysiol. 1994;71:1646–1660. doi: 10.1152/jn.1994.71.5.1646. [DOI] [PubMed] [Google Scholar]
- Betley JN, Cao ZF, Ritola KD, Sternson SM. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell. 2013;155:1337–1350. doi: 10.1016/j.cell.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betley JN, Xu S, Cao ZF, Gong R, Magnus CJ, Yu Y, Sternson SM. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature. 2015;521:180–185. doi: 10.1038/nature14416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Haubensak W, Anthony TE, Anderson DJ. Central amygdala PKC-delta(+) neurons mediate the influence of multiple anorexigenic signals. Nat. Neurosci. 2014;17:1240–1248. doi: 10.1038/nn.3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter ME, Han S, Palmiter RD. Parabrachial calcitonin gene-related peptide neurons mediate conditioned taste aversion. J. Neurosci. 2015;35:4582–4586. doi: 10.1523/JNEUROSCI.3729-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter ME, Soden ME, Zweifel LS, Palmiter RD. Genetic identification of a neural circuit that suppresses appetite. Nature. 2013;503:111–114. doi: 10.1038/nature12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Lin YC, Kuo TW, Knight ZA. Sensory detection of food rapidly modulates arcuate feeding circuits. Cell. 2015;160:829–841. doi: 10.1016/j.cell.2015.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards GL, Ladenheim EE, Ritter RC. Dorsomedial hindbrain participation in cholecystokinin-induced satiety. Am. J. Physiol. 1986;251:R971–977. doi: 10.1152/ajpregu.1986.251.5.R971. [DOI] [PubMed] [Google Scholar]
- Flynn MC, Scott TR, Pritchard TC, Plata-Salaman CR. Mode of action of OB protein (leptin) on feeding. Am. J. Physiol. 1998;275:R174–179. doi: 10.1152/ajpregu.1998.275.1.R174. [DOI] [PubMed] [Google Scholar]
- Gaisano GG, Park SJ, Daly DM, Beyak MJ. Glucagon-like peptide-1 inhibits voltage-gated potassium currents in mouse nodose ganglion neurons. Neurogastroenterol. Motil. 2010;22:470–479. e111. doi: 10.1111/j.1365-2982.2009.01430.x. [DOI] [PubMed] [Google Scholar]
- Garfield AS, Li C, Madara JC, Shah BP, Webber E, Steger JS, Campbell JN, Gavrilova O, Lee CE, Olson DP, et al. A neural basis for melanocortin-4 receptor-regulated appetite. Nat. Neurosci. 2015;18:863–871. doi: 10.1038/nn.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfield Alastair S., Lowell Bradford B. Was It Something I Ate? Cell Metabolism. 2013;18:769–770. doi: 10.1016/j.cmet.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautron L, Elmquist JK, Williams KW. Neural control of energy balance: translating circuits to therapies. Cell. 2015;161:133–145. doi: 10.1016/j.cell.2015.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyetvai B, Bardos G. Modulation of taste reactivity by intestinal distension in rats. Physiol. Behav. 1999;66:529–535. doi: 10.1016/s0031-9384(98)00319-9. [DOI] [PubMed] [Google Scholar]
- Han S, Soleiman MT, Soden ME, Zweifel LS, Palmiter RD. Elucidating an Affective Pain Circuit that Creates a Threat Memory. Cell. 2015;162:363–374. doi: 10.1016/j.cell.2015.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MR, Bradley L, Grill HJ. Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology. 2009;150:2654–2659. doi: 10.1210/en.2008-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J. Comp. Neurol. 1990;293:540–580. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- Hisadome K, Reimann F, Gribble FM, Trapp S. CCK stimulation of GLP-1 neurons involves alpha1-adrenoceptor-mediated increase in glutamatergic synaptic inputs. Diabetes. 2011;60:2701–2709. doi: 10.2337/db11-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhamandas JH, Harris KH. Excitatory Amino-Acids May Mediate Nucleus-Tractus-Solitarius Input to Rat Parabrachial Neurons. American Journal of Physiology. 1992;263:R324–R330. doi: 10.1152/ajpregu.1992.263.2.R324. [DOI] [PubMed] [Google Scholar]
- Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR. Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology. 2011;152:3103–3112. doi: 10.1210/en.2011-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JC, Cook MN, Carey MR, Shen C, Regehr WG, Dymecki SM. Linking genetically defined neurons to behavior through a broadly applicable silencing allele. Neuron. 2009;63:305–315. doi: 10.1016/j.neuron.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisler AD, Davis EA, Rinaman L. Differential activation of chemically identified neurons in the caudal nucleus of the solitary tract in non-entrained rats after intake of satiating vs. non-satiating meals. Physiol. Behav. 2014;136:47–54. doi: 10.1016/j.physbeh.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li BH, Rowland NE. Cholecystokinin- and dexfenfluramine-induced anorexia compared using devazepide and c-fos expression in the rat brain. Regul. Pept. 1994;50:223–233. doi: 10.1016/0167-0115(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Lucas F, Sclafani A. Capsaicin attenuates feeding suppression but not reinforcement by intestinal nutrients. Am. J. Physiol. 1996;270:R1059–1064. doi: 10.1152/ajpregu.1996.270.5.R1059. [DOI] [PubMed] [Google Scholar]
- Miller N. Is distention of the stomach via balloon rewarding or punishing? The American Psychologist. 1954;99:430–431. [Google Scholar]
- Morton GJ, Blevins JE, Williams DL, Niswender KD, Gelling RW, Rhodes CJ, Baskin DG, Schwartz MW. Leptin action in the forebrain regulates the hindbrain response to satiety signals. J. Clin. Invest. 2005;115:703–710. doi: 10.1172/JCI200522081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton GJ, Meek TH, Schwartz MW. Neurobiology of food intake in health and disease. Nat. Rev. Neurosci. 2014;15:367–378. doi: 10.1038/nrn3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira-Maia AJ, Roberts CD, Walker QD, Luo B, Kuhn C, Simon SA, Nicolelis MA. Intravascular food reward. PloS One. 2011;6:e24992. doi: 10.1371/journal.pone.0024992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez C, Lucas F, Sclafani A. Devazepide, a CCK(A) antagonist, attenuates the satiating but not the preference conditioning effects of intestinal carbohydrate infusions in rats. Pharmacol. Biochem. Behav. 1998;59:451–457. doi: 10.1016/s0091-3057(97)00439-5. [DOI] [PubMed] [Google Scholar]
- Peters JH, Ritter RC, Simasko SM. Leptin and CCK selectively activate vagal afferent neurons innervating the stomach and duodenum. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:R1544–1549. doi: 10.1152/ajpregu.00811.2005. [DOI] [PubMed] [Google Scholar]
- Ricardo JA, Koh ET. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res. 1978;153:1–26. doi: 10.1016/0006-8993(78)91125-3. [DOI] [PubMed] [Google Scholar]
- Richard JE, Farkas I, Anesten F, Anderberg RH, Dickson SL, Gribble FM, Reimann F, Jansson JO, Liposits Z, Skibicka KP. GLP-1 receptor stimulation of the lateral parabrachial nucleus reduces food intake: neuroanatomical, electrophysiological, and behavioral evidence. Endocrinology. 2014;155:4356–4367. doi: 10.1210/en.2014-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter RC. Gastrointestinal mechanisms of satiation for food. Physiol. Behav. 2004;81:249–273. doi: 10.1016/j.physbeh.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Rowland NE, Crews EC, Gentry RM. Comparison of Fos induced in rat brain by GLP-1 and amylin. Regul. Pept. 1997;71:171–174. doi: 10.1016/s0167-0115(97)01034-3. [DOI] [PubMed] [Google Scholar]
- Sanz E, Quintana A, Deem JD, Steiner RA, Palmiter RD, McKnight GS. Fertility-regulating Kiss1 neurons arise from hypothalamic POMC-expressing progenitors. J. Neurosci. 2015;35:5549–5556. doi: 10.1523/JNEUROSCI.3614-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz GJ, Salorio CF, Skoglund C, Moran TH. Gut vagal afferent lesions increase meal size but do not block gastric preload-induced feeding suppression. Am. J. Physiol. 1999;276:R1623–1629. doi: 10.1152/ajpregu.1999.276.6.R1623. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Lucas F. Abdominal vagotomy does not block carbohydrate-conditioned flavor preferences in rats. Physiol. Behav. 1996;60:447–453. doi: 10.1016/s0031-9384(96)80018-7. [DOI] [PubMed] [Google Scholar]
- Smith GP. Cholecystokinin and treatment of meal size: proof of principle. Obesity. 2006;14(Suppl 4):168S–170S. doi: 10.1038/oby.2006.300. [DOI] [PubMed] [Google Scholar]
- Smith GP, Jerome C, Cushin BJ, Eterno R, Simansky KJ. Abdominal vagotomy blocks the satiety effect of cholecystokinin in the rat. Science. 1981;213:1036–1037. doi: 10.1126/science.7268408. [DOI] [PubMed] [Google Scholar]
- Swick JC, Alhadeff AL, Grill HJ, Urrea P, Lee SM, Roh H, Baird JP. Parabrachial Nucleus Contributions to Glucagon-Like Peptide-1 Receptor Agonist-Induced Hypophagia. Neuropsychopharmacology. 2015;40:2001–2014. doi: 10.1038/npp.2015.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teppema LJ, Veening JG, Kranenburg A, Dahan A, Berkenbosch A, Olievier C. Expression of c-fos in the rat brainstem after exposure to hypoxia and to normoxic and hyperoxic hypercapnia. J. Comp. Neurol. 1997;388:169–190. doi: 10.1002/(sici)1096-9861(19971117)388:2<169::aid-cne1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Vrang N, Phifer CB, Corkern MM, Berthoud HR. Gastric distension induces c-Fos in medullary GLP-1/2-containing neurons. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285:R470–478. doi: 10.1152/ajpregu.00732.2002. [DOI] [PubMed] [Google Scholar]
- Wall NR, Wickersham IR, Cetin A, De La Parra M, Callaway EM. Monosynaptic circuit tracing in vivo through Cre-dependent targeting and complementation of modified rabies virus. Proc. Natl. Acad. Sci. U.S.A. 2010;107:21848–21853. doi: 10.1073/pnas.1011756107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller A, Blass EM. Cholecystokinin conditioning in rats: ontogenetic determinants. Behav. Neurosci. 1990;104:199–206. doi: 10.1037//0735-7044.104.1.199. [DOI] [PubMed] [Google Scholar]
- Wickersham IR, Lyon DC, Barnard RJO, Mori T, Finke S, Conzelmann KK, Young JAT, Callaway EM. Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron. 2007;53:639–647. doi: 10.1016/j.neuron.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DL, Baskin DG, Schwartz MW. Evidence that Intestinal Glucagon-Like Peptide-1 Plays a Physiological Role in Satiety. Endocrinology. 2009;150:1680–1687. doi: 10.1210/en.2008-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Boyle MP, Palmiter RD. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 2009;137:1225–1234. doi: 10.1016/j.cell.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Clark MS, Palmiter RD. Deciphering a neuronal circuit that mediates appetite. Nature. 2012;483:594–597. doi: 10.1038/nature10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Lemus MB, Stark R, Bayliss JA, Reichenbach A, Lockie SH, Andrews ZB. The temporal pattern of cfos activation in hypothalamic, cortical, and brainstem nuclei in response to fasting and refeeding in male mice. Endocrinology. 2014;155:840–853. doi: 10.1210/en.2013-1831. [DOI] [PubMed] [Google Scholar]
- Yuan CS, Barber WD. Area postrema: gastric vagal input from proximal stomach and interactions with nucleus tractus solitarius in cat. Brain. Res. Bull. 1993;30:119–125. doi: 10.1016/0361-9230(93)90047-f. [DOI] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, Rhodes CJ, Louis GW, Skibicka KP, Grill HJ, Myers MG, Jr., Berthoud HR. A potential role for hypothalamomedullary POMC projections in leptin-induced suppression of food intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;298:R720–728. doi: 10.1152/ajpregu.00619.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.