Abstract
Inflammation in the central nervous system (CNS) is associated with epilepsy and is characterized by the increased levels of a complex set of soluble molecules and their receptors in epileptogenic foci with profound neuromodulatory effects. These molecules activate receptor-mediated pathways in glia and neurons that contribute to hyperexcitability in neural networks that underlie seizure generation. As a consequence, exciting new opportunities now exist for novel therapies targeting the various components of the immune system and the associated inflammatory mediators, especially the IL-1β system. This review summarizes recent findings that increased our understanding of the role of inflammation in reducing seizure threshold, contributing to seizure generation, and participating in epileptogenesis. We will discuss preclinical studies supporting the hypothesis that pharmacological inhibition of specific proinflammatory signalings may be useful to treat drug-resistant seizures in human epilepsy, and possibly delay or arrest epileptogenesis.
Keywords: Inflammation, IL-1β, TNF-α, IL-6, Reactive astrogliosis
14.1 Introduction
The state-of-the-art knowledge acquired in the last decade of experimental and clinical work indicates that cytokines and related molecules are increased in brain tissue after epileptogenic injuries or during seizures. In the experimental setting, these molecules, endowed with proinflammatory properties, contribute significantly to the generation and maintenance of a hyperexcitable neuronal network, thus decreasing seizure threshold (Fig. 14.1) and making the occurrence of a seizure more likely.
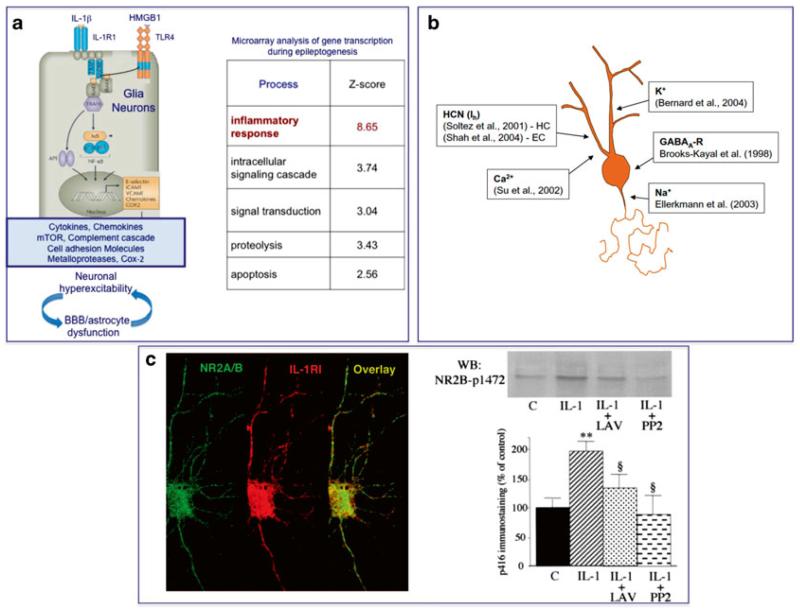
Fig. 14.1. Schematic representation of the pathophysiologic outcomes of innate immunity activation in epilepsy.
Activation of innate immune signaling occurs in epilepsy also in the absence of infection, thus triggering the so-called “sterile” inflammatory cascade (a). Endogenous molecules (damage associated molecular patterns, DAMPs) such as IL-1β and the High Mobility Group Box 1 (HMGB1) protein are released by neurons and glia following epileptogenic inciting events, or during recurrent seizures. The activation of their cognate receptors (IL-1R type 1 and TLR4, respectively) upregulated in astrocytes triggers the NFkB-dependent infammatory genes cascade, thus inducing various molecules with proinflammatory and neuromodulatory properties. The signaling activation in neurons increases excitability by provoking acquired channelopathies involving voltage-gated channels (HCN1) or AMPA and GABA-A receptor complexes (b), as well as by rapid activation of Src kinase inducing the phosphorylation of the NR2B subunit of the NMDA receptor thereby promoting neuronal Ca2+ influx (c). This chain of event contributes to the generation and establishment of an hyperexcitable neuronal network by direct receptor-mediated neuronal effects or indirectly by inducing astrocytes and BBB dysfunctions
A key question that basic science has been addressing is how these proinflammatory molecules affect neuronal and glial functions. Answers to this question will increase our knowledge of the complex mechanistic aspects of hyperexcitability following inflammation and will be instrumental in highlighting novel targets for developing drugs and therapies that raise seizure threshold, prevent seizure generation after an inciting event, and inhibit their recurrence in chronic epilepsy.
14.1.1 Inflammatory Molecules as Neuromodulators
The presence of molecules with proinflammatory properties in brain specimens obtained from patients with epilepsy has been described as “brain inflammation” (Table 14.1). However, there is emerging evidence that these molecules have neuromodulatory functions that activate signaling in neurons and glia that are different from those induced by the same molecules in leukocytes in the frame of a classical inflammatory response to infection. During infection, proinflammatory cytokines and related molecules are released during innate immunity activation by immuno-competent cells following “pathogen associated molecular patterns” (PAMPs) activation of toll-like receptors (TLRs) or nucleotide-binding oligomerization domain (NOD-like) receptors. Cytokine release activates inflammatory programs for pathogen removal and the subsequent induction of homoeostatic tissue repair mechanisms. Notably, in humans affected by various forms of pharmacoresistant epilepsy of differing etiologies (e.g. Rasmussen’s (RE) and limbic encephalitis (LE), malformations of cortical development, and mesial temporal lobe epilepsy (mTLE)) increased inflammatory mediators are measured in epileptogenic foci in the absence of an identifiable active infectious process. However, it is also important to note that CNS infection, which is a common cause of TLE, can also result in a cytokine storm that affects excitability. In this context, evidence of HHV6 infected astrocytes and neurons has been reported in about 2/3 of patients with mTLE [108]. Moreover, recent work has shown the presence of Human Papilloma virus in human focal cortical dysplasia type II which might be responsible for focal epileptogenic malformations during fetal brain development in association with enhanced mTORC1 signaling [18].
Table 14.1.
Inflammatory mediators in human epilepsies and experimental models
| Clinical evidence |
|---|
| Inflammatory mediators are overexpressed in epileptogenic foci in human pharmacoresistant epilepsy of differing etiologies (e.g. RE, LE, MCD, mTLE) |
| Microglia and astrocytes are main sources of inflammatory mediators in brain tissue; neurons and endothelial cells of the blood brain barrier (BBB) also contribute to the generation of brain inflammation |
| Leukocyte extravasation in brain depends on the etiology of epilepsy |
| BBB damage is often detected together with brain inflammation |
| Experimental evidence |
| Recurrent seizures and epileptogenic brain injuries induce inflammatory mediators in astrocytes, microglia, neurons, and microvessels in brain areas involved in seizure onset and generalization |
| This phenomenon is long lasting and may exceed the initial precipitating event by days or weeks depending on the epilepsy model. It is inadequately controlled by anti-inflammatory mechanisms |
| In models of epileptogenesis, inflammation initiates before the development of epilepsy |
| Specific anti-inflammatory treatments reduce acute and chronic seizures and delay their time of onset |
| Transgenic mice with perturbed cytokine signaling show altered seizure susceptibility |
|
Proinflammatory insults decrease seizure threshold (acutely and long-term) |
The so-called sterile inflammation in the brain can be induced when TLRs are activated by endogenous molecules released by injured brain cells, named “danger signals” or “damage-associated molecular patterns” (DAMPs). In particular, the activation of TLR4, which can also be activated by gram-negative bacteria, is induced by the ubiquitous nuclear protein High Mobility Group Box 1 (HMGB1) which is released, upon its cytoplasmatic translocation, by neurons and glial cells. In concert with IL-1β released by glia, thereafter activating IL-1 receptor type 1 (IL-1R1), HMGB1 induces the transcriptional up-regulation of various inflammatory genes, therefore promoting the generation of the brain inflammatory cascade in glia and endothelial cells of the BBB (Fig. 14.1). In the context of malformations of cortical development, the inflammatory cascade is also induced in aberrant neuronal cells [3]. The activation of the IL-1R1/TLR4 signaling in neurons, which overexpress these receptors in pathologic conditions, in concert with pathways induced by other cytokines such as TNF-α, IL-6, the complement system and some prostaglandins, alters neuronal excitability by modifying either glutamate or GABA receptor subunit composition, or trafficking of receptors, or the function of voltage-gated ion channels via rapid onset post-translational mechanisms [118, 123]. Furthermore, initiation of the JAK/STAT and other signaling pathways through these mechanisms can also result in activation of glial cells, inducing a cascade of events that alters their structure and function in a variety of ways that can also contribute to aberrant excitability [99].
In animal models, pharmacological intervention to block or activate specific inflammatory pathways induced in human epilepsy brain specimens has shown that: (i) cytokines such as IL-1β, TNF-α, and IL-6, and danger signals such as HMGB1 and S100β, contribute to seizures in a receptor-dependent manner; (ii) the complement system contributes to seizure generation and cell loss; and (iii) PGE2 contributes to cell loss by activating EP2 receptors in neurons (Table 14.2). This set of evidence is corroborated by the assessment of susceptibility to seizures and cell loss in transgenic mouse models with impaired or overexpressed inflammatory signalings [118].
Table 14.2.
Antagonism of IL-1R1/TLR4 in rodent models of seizures
|
Seizure reduction in rodents exposed to an acute
challenge |
|---|
| Kainic acid (lesional model), bicuculline and febrile seizures (non lesional models) [28, 87, 114, 119] |
| Status epilepticus [24, 64] |
| Electrical rapid kindling [88, 5, 6] |
| Chronic recurrent seizures reduced in |
| mTLE mouse model [66, 67] |
| SWD in GAERS & WAG/Rij (absence seizures models) [1, 49] |
|
Other inflammatory signaling contributing to seizures
are mediated by |
| TNF-α, IL-6, COX-2 & complement system (reviewed in [50, 115, 3]) |
14.1.2 IL-1β, HMGB1 and the NMDA and GABA Receptors
IL-1β and HMGB1 both potentiate NMDA receptor function in cultured hippocampal neurons using post-translational mechanisms mediated by activation of IL-1R1 and TLR4, respectively [8, 53, 121]. In particular, these cytokines enhance NMDA-mediated Ca2+ influx by activating Src kinases-dependent NR2B phosphorylation (Fig. 14.2). This signaling has been demonstrated to underlie the proictogenic and proneurotoxic properties of these cytokines [7, 8, 40, 121].
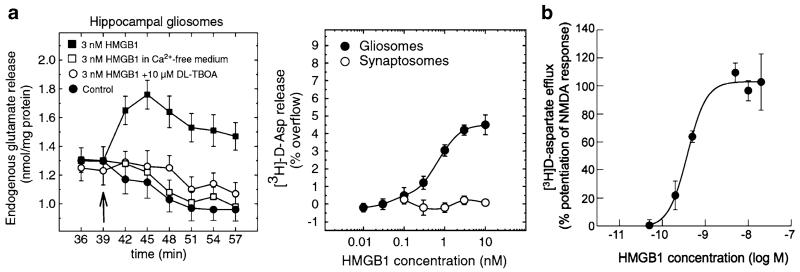
Fig. 14.2. Presynaptic and postsynaptic effects of HMGB1 on glutamatergic transmission.
HMGB1 protein evokes (3H)D-aspartate and glutamate release from re-sealed glial (gliosomes) and neuronal (synaptosomes) subcellular particles isolated from the mouse hippocampus (a). This protein per se augments the calcium-independent neurotransmitter outflow from gliosomes, but not from synaptosomes, in a concentration-dependent manner. This outflow is likely mediated by reversal of glutamate transporter (GLAST) since it is blocked by DL-threo-b-benzyloxyaspartate (TBOA) [81]. HMGB1 augments the NMDA-induced (3 H)D-aspartate calcium-dependent release from synaptosomes (b). This enhancing effect is mediated by increased intracellular calcium via the MK-801 sensitive channel. This HMGB1-NMDA receptor interaction involves the NR2B subunit [80]
This rapid onset (within 2 min) mechanism is reminiscent of that induced by IL-1β in hypothalamic neurons, which underlies the initial rise in body temperature induced by this cytokine [23, 91, 105], and it involves MyD88-dependent and ceramide-mediated activation of Src kinases. IL-1β also down-regulates AMPA receptor expression and their phosphorylation state in a Ca2+ - and NMDA-dependent manner in hippocampal neurons [53]. Recent evidence shows that HMGB1 effects on neuronal excitability may also include a physical, receptor unrelated, interaction with presynaptic NMDA receptors resulting in enhanced Ca2+-dependent glutamate release from presynaptic terminals evoked upon NMDAR stimulation [80]. Notably, HMGB1 per se can also induce glutamate release from hippocampal gliosome preparations implying that this molecule may increase gliotransmission [81]. While the effect of IL-1β and HMGB1 on NMDA-induced Ca2+-influx in neuronal cell soma and dendrites mediates cell loss and increases seizures [7, 8, 121], whether the effect of HMGB1 on presynaptic or glial glutamate release results in pathologic outcomes has not been yet investigated.
Excitatory actions of IL-1β have been reported in hippocampal slices or cultured pyramidal neurons where the cytokine reduces synaptically-mediated GABA inhibition in CA3 hippocampal region via still unidentified kinases [123, 129], and increases CA1 neurons excitability by reducing NMDA-induced outward current. This latter action involves activation of cytoplasmatic P38 MAPK phosphorylating large-conductance Ca2+-dependent K channels [131].
14.1.3 Cytokines, Synaptic Transmission/Plasticity and Seizures
Cytokine receptors are expressed by the same resident CNS cells that express their cognate cytokines, namely neurons, microglia, and astrocytes. Binding of ligands to these receptors set into motion a variety of signaling pathways that activate glial cells and can also lead to enhanced excitability of neurons.
IL-1β
In the hippocampus, IL-1β was reported to induce rapid changes in synaptic transmission, and to inhibit LTP via activation of MAPK and PKC [12, 75, 84, 96]. Fast neuronal actions of IL-1β were described in the preoptic/anterior hypothalamic neurons involving A-type K+ currents and the consequent reduced synaptic release of GABA [105].
TNF-α
Work by Stellwagen et al. demonstrated that TNF-α released by astrocytes binds to the TNF-α receptors (TNFR) on neurons and induces an increase in AMPA-type glutamate receptors and a concomitant decrease of GABAA receptors at synapses [102]. Specifically, TNF-α has been shown to increase trafficking of GluR2-lacking AMPA receptors to synaptic membranes in both hippocampal and motor neurons [11, 55, 56, 102, 103, 126]. In hippocampal neurons, this trafficking has been shown to depend on the PI3K–Akt pathway [102]. GluR2-lacking receptors are permeable to Ca2+ and activation of these receptors could dramatically alter synaptic strengths at these synapses or contribute to excitotoxicity. While TNFR knock out mice do not appear to have impaired long term potentiation (LTP) or long term depression (LTD), synaptic scaling may be modulated by TNF-α [101, 103]. While it is currently unclear what role TNF-α signaling may be playing in receptor trafficking in epilepsy, recent work using the Theiler’s Murine Encephalomyelitis Virus (TMEV) model of TLE has demonstrated that there is over a 120-fold increase in whole brain TNF-α mRNA soon after infection in C57Bl/6 mice [47]. This dramatic increase in TNF-α expression is associated with acute seizures and changes in mEPSC amplitudes and decay times in hippocampal brain slices prepared from animals acutely infected with TMEV [57, 98, 104]. In addition, TNFR1 knockout mice are much less likely to exhibit seizures during the acute infection period. Taken together, the evidence suggests an important role of TNF-α in modulating excitatory circuits and excessive amounts of TNF-α may contribute to seizure activity. Accordingly, a proictogenic role of TNF-α mediated by TNFR1, and an opposite anti-ictogenic role of this cytokine mediated by TNFR2 have been reported in chemoconvulsant models of seizures [7-9, 124]. Molecular and functional interactions between TNFR and the glutamatergic system in the hippocampus appear to be implicated in the effect of this cytokine in seizure susceptibility [8].
In addition to modifying synaptic transmission, TNF-α is also known to stimulate the release of glutamate from microglia [17, 107] and astrocytes [92, 93], and these additional sources of extracellular glutamate likely contribute to excitoxicity in injured brain regions. Activation of TNFR in cultured microglia results in an increased expression of glutaminase, which converts glutamine to glutamate. This excess intracellular glutamate is then released through connexin 36 hemi-channels and can be blocked by the gap junction inhibitor, carbenoxolone [107]. It is thought that this mechanism can contribute to neuronal cell death that often accompanies chronic or prolonged tissue inflammation.
IL-6
Recent work has demonstrated that IL-6, another cytokine that is increased in response to epileptogenic insults, decreases GABA and glycine-mediated inhibitory synaptic currents following bath application to spinal cord slices [46]. Such changes in synaptic neurotransmitter receptor function can result in tipping the balance of excitation and inhibition towards hyperexcitability. Binding of IL-6 to its receptor results in the activation of the JAK/STAT pathway and this pathway is known to regulate the expression of many different receptor gated ion channel subunits [60] and underlies NMDA-dependent LTD in the hippocampus [72]. Therefore, changes in IL-6 expression levels could dramatically influence excitability of neural circuits responsible for seizure generation. Recent work with the TMEV mouse model of TLE, demonstrated that IL-6 mRNA expression increases significantly during the acute infection period and this increase parallels the onset of seizures in this model. Furthermore, IL-6 receptor knockout mice have a reduced incidence of seizures following TMEV infection, suggesting that this cytokine, which is largely expressed in this animal model by infiltrating macrophages, contributes to lowering seizure thresholds [21, 47]. Finally, treatment of TMEV infected mice with either minocycline or wogonin, were both found to dramatically reduce concomitantly the number of infiltrating macrophages in the brain and seizure incidence [21]. These results suggest that IL-6 may be an important regulator, possibly through the JAK/STAT pathway, of synaptic plasticity and seizure activity.
14.1.4 Cytokines and Voltage-Gated Ion Channels
While cytokines have been extensively studied in neuropathic pain and in epilepsy, very few studies have examined the effects of the prominent cytokines on voltage gated ion channels (see [122]). Nevertheless, the limited available literature demonstrates that cytokines can modulate a variety of voltage gated ion channels through multiple mechanisms [95]. For example, TNF-α has been shown to increase expression of TTX resistant sodium channels in isolated dorsal root ganglion cells, increase Ca2+ currents in cultured hippocampal neurons and decrease inwardly rectifying K+ currents in cultured cortical astrocytes [35, 44, 48]. IL-1β has been shown to decrease Ca2+ currents in cultured hippocampal and cortical neurons [83, 84, 132, 133] as well as Na+ and K+ currents in dissociated retinal ganglion cells [26].
The effect of cytokines on ion channel function is an area where clearly further work is necessary so as to inform hypotheses about the full range of activity of cytokines in epilepsy, particularly in view of the plethora of differing effects on neuronal functions that cytokines may have depending on their concentration, timing of tissue exposure, the type of neuronal cells expressing the relevant receptors, and the concomitant presence of other neuromodulatory molecules.
14.1.5 Prostaglandins, Synaptic Plasticity and Seizure Activity
Arachidonic acid (AA) is converted to prostanoids via activity of the enzyme cyclooxygenase (COX). COX-2 is constitutively active at low levels in the hippocampus, its expression rapidly increases as a consequence of neural activity, and is necessary for some forms of synaptic plasticity, such as LTP in the dentate gyrus [42]. Prostaglandin E2 (PGE2), one of the most common of the prostanoids to be formed in the hippocampus, binds to the G-protein coupled EP2 receptor on neurons, activates cAMP and mediates synaptic plasticity via the cAMP-protein kinase A (PKA)-cAMP–responsive element binding protein (CREB) pathway [42, 116]. Following status epilepticus (SE), COX-2 expression is increased in the hippocampus and prostaglandins, including PGE2, are also subsequently increased and hypothesized to be involved in mediating neurodegeneration that occurs in multiple brain regions following SE. This neurotoxic effect may be due to excessive stimulation of EP2 receptors expressed by microglia and the consequent activation of an alternative pathway, the cAMP-Epac signaling pathway promoting upregulation of various inflammatory mediators and oxidative stress [42]. Whereas pharmacological inhibition of COX-2 can be neuroprotective following CNS insults, this approach has not yielded great success in preventing the development of epilepsy following SE although disease-modifying effects have been reported [45, 51, 61, 85]. Depending on the drug used to inhibit COX-2 and the trigger of SE, adverse events have also been described in epileptic rats [39, 85]. Therefore, the search is on for drugs that can selectively interfere with downstream pathways of COX-2 in an effort to mitigate the detrimental inflammatory actions that can occur in the CNS following SE. Recently, Jiang et al. evaluated the ability of a novel small molecule and brain permeable EP2 antagonist, TG6-10-1, to confer neuroprotection and prevent the development of epilepsy in mice treated with pilocarpine [43]. Encouragingly, there was significant neuroprotection and decreased mortality following SE in the treated mice. However, there were no differences with vehicle-treated mice in spontaneous seizure frequency, suggesting that epileptogenesis was not interrupted with this treatment [43]. This suggests that adjunctive therapy with an EP2 antagonist may be important for attaining neuroprotection in patients experiencing SE, but additional approaches will be necessary to prevent the development of epilepsy. In this context, a recent study reported that co-treatment with IL-1 receptor antagonist (IL-1Ra, anakinra) and a COX-2 inhibitor given at the time of SE induction were required to reduce both cell loss and epileptogenesis in rats [52]. Similarly, combined treatment with IL-1Ra and VX-765, an inhibitor of IL-1β biosynthesis, given systemically to rats after 3 h of uninterrupted SE, afforded significant neuroprotection although not inhibiting epilepsy development [74]. This evidence highlights the need of both early intervention and combined anti-inflammatory treatments for optimizing beneficial clinical outcomes.
Another strategy to be investigated is a combination of specific antiinflammatory drugs with classical antiepileptic drugs (AED) targeting complementary mechanisms. Indeed, some AEDs afford neuroprotection or decrease the severity of spontaneous seizures induced in SE models [71].
14.1.6 TLR4 and Neuronal Excitability
Out of 11 members of the TLRs family, TLR4 is the most extensively studied in CNS for its involvement in increasing brain excitability and cell loss, and for reducing neurogenesis.
Rat cortical application of lipopolysaccharide (LPS), a PAMP component of gram-negative bacteria wall and prototypical activator of TLR4, has been reported to rapidly increase the excitability of local neurons as assessed by measuring amplitudes of sensory evoked field potentials following rat forepaw stimulation and spontaneous activity [90]. A ten-fold higher LPS concentration could evoke epileptiform activity which was prevented by pre-application of IL-1Ra, implicating a role of IL-1β released from LPS-activated microglia [90].
We recently discovered that intracerebral LPS application reduces hyperpolarization-activated ion channel (HCN1) protein in hippocampal tissue, an effect associated with a reduction in Ih current as assessed in whole-cell patch recording of CA1 pyramidal neurons. This effect is long-lasting but reversible upon resolution of both microglia activation and induction of proinflammatory cytokines in these cells. The activation of IL-1R1/TLR4 signaling is responsible for this effect since it was precluded in TLR4 or IL-1R1 knock-out mice, and by pharmacological blockade of these receptors with selective antagonists (Bernard et al., 2013, personal communication).
The reported LTP and LTD impairment induced by TLR4 stimulation is compatible with neurological dysfunction and cognitive deficits induced by early life exposure to LPS which are associated with specific and persistent changes in NMDA receptor subunits expression in the cortex and hippocampus, predicting modifications in CNS excitability (for review see [89, 127]).
14.1.7 Inflammation-Induced Functional Changes in Astrocytes
Reactive astrogliosis occurs as a consequence of cytokine activation of the IL-1R/TLR and JAK/STAT pathway and other signaling pathways following CNS insults such as traumatic brain injury (TBI), SE, and infection [99]. Astrogliosis is a graded process and is characterized by hypertrophy of primary processes, dramatic increases in the expression of intermediate filament proteins such as glial fibrillary acidic protein (GFAP), a decrease and cell redistribution in glutamine synthetase [20, 29, 78, 125], an increase in expression of adenosine kinase, and, in some cases, a disruption in domain organization of glial processes [76, 99]. There is also a dramatic increase in gap junction coupling between astrocytes in animal models [106] and resected human tissue [19, 32, 70], and a number of specific subunits of kainate receptors (KAR) were recently found to be expressed in reactive astrocytes following chemoconvulsant-induced SE in rodents [112]. There are, therefore, a multitude of changes in astrocytes following seizure-inducing insults and these changes may have a dramatic impact on the circuit dynamics underlying seizure generation [25, 36].
As astrocytes are intricately involved in regulating neuronal activity at the tri-partite synapse (review [2]), some of the changes in glial function that are observed in rodent models and human epilepsy could easily lead to hyperexcitability in neural circuits and contribute to seizure generation. For example, decreases in the endogenous anticonvulsant adenosine as a consequence of increased expression of adenosine kinase can lead to hyperexcitability and seizure activity [4, 15] and, while early after SE, glutamate uptake by astrocytes seems to be functioning well [106], there are numerous reports of cytokine-mediated decreases in glutamate transporter function in epilepsy and other disorders which could readily lead to excess excitation and cell death in vulnerable neurons [62, 68, 86, 94]. Reactive astrocytes have also been reported to have a decrease in the inward rectifier potassium channel (KIR), namely Kv4.1, a critical ion channel that aids in the buffering of extracellular potassium concentrations, and this altered expression may be mediated by IL-1β [134]. Electrophysiological recordings in acute brain slices obtained from surgical specimens of patients with mTLE, have revealed a reduced KIR conductance in reactive astrocytes [38]. However, we recently demonstrated that KIR mediated currents were not altered in astrocytes during the latent period up to 2 weeks following SE in the KA-treated rat [106], and this is consistent with a recent report demonstrating that initial decreases in Kv4.1 mRNA and protein return to control levels by day 7 after SE [134]. Therefore, reactive astrocyte function may change over time as epilepsy develops.
While many of the observed changes in astrocytes that occur as a consequence of inflammation may actively contribute to network hyperexcitability, other components of reactive astrogliosis, such as increased gap junctional coupling, or increased neurotrophins may be critical compensatory mechanisms following injury, and may act to dampen excitability and protect neurons [36]. Thus, simply blocking the inflammatory response in glial cells may be too global an approach for disease modification during epileptogenesis, while targeting specific processes, such as maintaining KIR function, might prove to be a more useful approach.
14.1.8 Cytokines Effects on BBB: Consequences for Neuronal Excitability
Evidence obtained using in vitro models of the BBB [31, 130] or epilepsy models [58, 77, 111, 116] demonstrated that cytokines and prostaglandins compromise the permeability properties of the BBB, and that such alteration in brain vessels is a common feature of drug-resistant epileptogenic foci in humans and experimental models. In particular, there is evidence of the presence of IL-1β in perivascular glia and astrocytic endfeet impinging on brain vessels in epilepsy tissue where the BBB is altered, as shown by the parenchymal extravasation of serum macromolecules such as albumin and IgG. One mechanism of BBB damage induced by cytokines involves breakdown of tight-junction proteins in brain vessels [58, 59, 69, 73] induced by activation of Src kinases. This evidence highlights that key molecular pathways activated by cytokines in epilepsy result in different outcomes depending on the target cell population (expressing the relevant receptors), i.e. BBB permeability function is compromised in vessels, hyperexcitability is induced in neurons, and astrocyte function is greatly modified.
BBB damage leads to albumin extravasation which induces TGF-β signaling in astrocytes by activating the TGF-β receptor type 2 [33]. This signaling mediates transcriptional up-regulation of IL-1β and other inflammatory genes in astrocytes [16, 34] while glutamate transporter and Kir4.1 channels are down-regulated. These pathologic changes have been shown to establish a hyperexcitable milieu in surrounding neurons due to increased extracellular K+ and glutamate [97] which decreases seizure threshold and may induce per se epileptiform activity [22, 34].
14.1.9 Leukocytes, Autoantibodies and Neuronal Excitability
There is evidence of adaptive immunity activation in rare disorders such as Rasmussen’s encephalitis (RE), viral and limbic encephalitis and neurologic or systemic autoimmune disorders. These conditions are often associated with seizures and epilepsy development. In RE brain tissue, cytotoxic CD8+ T lymphocytes have been demonstrated in close apposition to neurons and astrocytes, then provoking their apoptosis by releasing granzyme B [10, 79]. The presence of these cells, and more in general CD3+ leuckocytes, appears to be much less prominent in more common forms of epilepsy. For example, in focal cortical dysplasia (FCD) type 2, scattered lymphocytes have been described in brain tissue while this phenomenon occurs at a minor extent in FCD type 1, and is almost undetectable in mTLE [41, 65, 110]. Others have detected leukocytes in brain parenchyma surrounding brain vessels also in mTLE [30, 128]. In animal models of epilepsy the role of these cells is still uncertain since they were reported to mediate anti-epileptogenic and neuroprotective effects in KA-treated rats [128] whereas they contribute to the pathology in pilocarpine-treated mice [30]. Notably, in this latter instance the effects of leukocytes may be ascribed to the peculiar mechanisms mediating seizures caused by pilocarpine and which are not shared by other chemoconvulsants [64, 109, 117].
A recent randomized clinical study using tacrolimus, which impedes T cell proliferation and activation, in recent onset RE patients showed delayed deterioration of neurological deficits but the treatment did not ameliorate drug resistant seizures [13]. However, case reports have shown decreased seizure frequency in one RE patient treated with natalizumab, a blocker of T cell entry into the CNS [14] and in a patient with multiple sclerosis and refractory epilepsy [101]. The authors discussed that interpretation of data was limited by an additional coadministration of varying antiepileptic medications.
In limbic encephalitis and autoimmune disorders, circulating autoantibodies against various neuronal proteins have been detected (for review, see [120]). These antibodies recognizing membrane neuronal proteins may have a pathologic role, in addition to their diagnostic value. In particular, antibodies against NR1/NR2 subunits obtained from serum of affected patients can increase extracellular hippocampal glutamate levels when intracerebrally infused in rats. Increased sensitivity to AMPA receptor-mediated neuronal excitability and GABAergic dysfunction have also been reported [63]. Antibodies directed against voltage-gated K+ channel complex increase excitability of hippocampal CA3 pyramidal cells by reducing channel function at mossy fiber-CA3 synapses [54]. AMPA receptor antibodies alter synaptic receptor location and number by reducing those receptors containing the GLUR2 subunit, therefore increasing the relative abundance of Ca2+-permeable receptors [53].
14.2 Conclusions
While understanding of the role of the innate immune system and the associated molecules with inflammatory properties in epilepsy and seizure threshold changes has advanced tremendously over the last decade, there are still a number of questions that yet remain open and require further investigation. For example, it is not yet clear which molecules and inflammatory pathways activated following epileptogenic brain insults will make the most appropriate targets for intervening to prevent seizure occurrence and/or the process of epileptogenesis. The complex network changes that occur in a number of cell types in the CNS, including neurons, microglia and astrocytes, in response to increases in a myriad of neuromodulatory and inflammatory molecules such as IL-1β, TNF-α, IL-6 and interferon-γ to name but a few, are difficult to decipher. Moreover, it has still to be determined which are the master regulators of the inflammatory cascade, and when and how to prevent the induction of brain inflammation or rather promote its resolution by implementing the effects of the endogenous antiinflammatory molecules, which are defective in epilepsy [82, 87].
Nevertheless, the increasing recognition that the innate immune system is tightly coupled to epileptogenesis and seizure threshold changes is encouraging as it opens up many potential novel molecular targets for therapeutics. Most AEDs are mainly antiseizure, symptomatic drugs that target neuronal proteins such as sodium channels or glutamate receptors. Their adverse effects on cognition and induction of sedation, coupled with the knowledge that nearly 30 % of patients with epilepsy do not have their seizures adequately controlled with current AEDs, suggest that targeting the neuromodulatory inflammatory pathways is a promising novel strategy with disease-modifying potential. Considering that prolonged administration in epilepsy is likely to be required, and the constraints imposed by the BBB, both the efficacy and the safety of drugs that preclude or reverse the over-activation of specific innate immune mechanisms should be carefully considered. Importantly, some of these antiinflammatory drugs are already in clinical use showing therapeutic effects in peripheral inflammatory conditions [27, 37, 113]. These drugs might be considered to complement the symptomatic treatment provided by available AEDs for resolving the inflammatory processes in the brain, therefore raising seizure threshold and decreasing the likelihood of seizure recurrence. In this context, a phase 2 clinical study with VX765 has given promising results in adult patients with drug resistant partial onset seizures (http://clinicaltrials.gov/ct2/show/NCT01048255; www.epilepsy.com/files/Pipeline2012/6-7).
Acknowledgements
A. Vezzani is very grateful to Phil Schwarzkroin for the intense and fruitful collaboration during the 8 years of shared editorial work for Epilepsia. During the time A.V. was serving as associated editor for basic science, she could fully appreciate P.S. extensive and deep scientific knowledge, his patience, constructive criticism, support and commitment. She thanks Phil, in particular, for his willingness to share responsibility and decisions as well as complaints and rewards, and for the very educational and formative time.
K.S. Wilcox would like to acknowledge the kind support Phil Schwartzkroin has demonstrated over the years. While she never had the opportunity to work with him, he was always interested in her work, encouraged her to speak out at meetings, to get involved in the American Epilepsy Society, and provided a welcoming environment for someone entering the field of epilepsy. He is a mentor to all.
Other Acknowledgements This work was supported by National Institutes of Health Grants NS 078331 (KSW), NS065434 (KSW), and the Margolis Foundation (KSW) and Ministero della salute Grant N. RF-2009-1506142 (AV).
Contributor Information
Karen S. Wilcox, Department of Pharmacology and Toxicology, University of Utah, Salt Lake City, UT 84108, USA
Annamaria Vezzani, Department of Neuroscience, IRCSS-Istituto di Ricerche Farmacologiche “Mario Negri”, via G. La Masa 19, Milan 20156, Italy, annamaria.vezzani@marionegri.it.
References
- 1.Akin D, Ravizza T, Maroso M, Carcak N, Eryigit T, Vanzulli I, et al. IL-1beta is induced in reactive astrocytes in the somatosensory cortex of rats with genetic absence epilepsy at the onset of spike-and-wave discharges, and contributes to their occurrence. Neurobiol Dis. 2011;44(3):259–269. doi: 10.1016/j.nbd.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 2.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22(5):208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 3.Aronica E, Ravizza T, Zurolo E, Vezzani A. Astrocyte immune responses in epilepsy. Glia. 2012;60(8):1258–1268. doi: 10.1002/glia.22312. [DOI] [PubMed] [Google Scholar]
- 4.Aronica E, Sandau US, Iyer A, Boison D. Glial adenosine kinase – a neuropathological marker of the epileptic brain. Neurochem Int. 2013;63:688–695. doi: 10.1016/j.neuint.2013.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Auvin S, Mazarati A, Shin D, Sankar R. Inflammation enhances epileptogenesis in the developing rat brain. Neurobiol Dis. 2010;40(1):303–310. doi: 10.1016/j.nbd.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Auvin S, Shin D, Mazarati A, Sankar R. Inflammation induced by LPS enhances epileptogenesis in immature rat and may be partially reversed by IL1RA. Epilepsia. 2010;51(Suppl 3):34–38. doi: 10.1111/j.1528-1167.2010.02606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balosso S, Maroso M, Sanchez-Alavez M, Ravizza T, Frasca A, Bartfai T, et al. A novel non-transcriptional pathway mediates the proconvulsive effects of interleukin-1beta. Brain. 2008;131(Pt 12):3256–3265. doi: 10.1093/brain/awn271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balosso S, Ravizza T, Aronica E, Vezzani A. The dual role of TNF-alpha and its receptors in seizures. Exp Neurol. 2013;247C:267–271. doi: 10.1016/j.expneurol.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Balosso S, Ravizza T, Perego C, Peschon J, Campbell IL, De Simoni MG, et al. Tumor necrosis factor-alpha inhibits seizures in mice via p75 receptors. Ann Neurol. 2005;57(6):804–812. doi: 10.1002/ana.20480. [DOI] [PubMed] [Google Scholar]
- 10.Bauer J, Vezzani A, Bien CG. Epileptic encephalitis: the role of the innate and adaptive immune system. Brain Pathol. 2012;22(3):412–421. doi: 10.1111/j.1750-3639.2012.00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, et al. Control of synaptic strength by glial TNFalpha. Science. 2002;295(5563):2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- 12.Bellinger FP, Madamba S, Siggins GR. Interleukin 1 beta inhibits synaptic strength and long-term potentiation in the rat CA1 hippocampus. Brain Res. 1993;628(1-2):227–234. doi: 10.1016/0006-8993(93)90959-q. [DOI] [PubMed] [Google Scholar]
- 13.Bien CG, Tiemeier H, Sassen R, Kuczaty S, Urbach H, von Lehe M, et al. Rasmussen encephalitis: incidence and course under randomized therapy with tacrolimus or intravenous immunoglobulins. Epilepsia. 2013;54(3):543–550. doi: 10.1111/epi.12042. [DOI] [PubMed] [Google Scholar]
- 14.Bittner S, Simon OJ, Gobel K, Bien CG, Meuth SG, Wiendl H. Rasmussen encephalitis treated with natalizumab. Neurology. 2013;81(4):395–397. doi: 10.1212/WNL.0b013e31829c5ceb. [DOI] [PubMed] [Google Scholar]
- 15.Boison D. Adenosine kinase: exploitation for therapeutic gain. Pharmacol Rev. 2013;65(3):906–943. doi: 10.1124/pr.112.006361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cacheaux LP, Ivens S, David Y, Lakhter AJ, Bar-Klein G, Shapira M, et al. Transcriptome profiling reveals TGF-beta signaling involvement in epileptogenesis. J Neurosci. 2009;29(28):8927–8935. doi: 10.1523/JNEUROSCI.0430-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen CJ, Ou YC, Chang CY, Pan HC, Liao SL, Chen SY, et al. Glutamate released by Japanese encephalitis virus-infected microglia involves TNF-alpha signaling and contributes to neuronal death. Glia. 2012a;60(3):487–501. doi: 10.1002/glia.22282. [DOI] [PubMed] [Google Scholar]
- 18.Chen J, Tsai V, Parker WE, Aronica E, Baybis M, Crino PB. Detection of human papillo-mavirus in human focal cortical dysplasia type IIB. Ann Neurol. 2012b;72(6):881–892. doi: 10.1002/ana.23795. [DOI] [PubMed] [Google Scholar]
- 19.Collignon F, Wetjen NM, Cohen-Gadol AA, Cascino GD, Parisi J, Meyer FB, et al. Altered expression of connexin subtypes in mesial temporal lobe epilepsy in humans. J Neurosurg. 2006;105(1):77–87. doi: 10.3171/jns.2006.105.1.77. [DOI] [PubMed] [Google Scholar]
- 20.Coulter DA, Eid T. Astrocytic regulation of glutamate homeostasis in epilepsy. Glia. 2012;60(8):1215–1226. doi: 10.1002/glia.22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cusick MF, Libbey JE, Patel DC, Doty DJ, Fujinami RS. Infiltrating macrophages are key to the development of seizures following virus infection. J Virol. 2013;87(3):1849–1860. doi: 10.1128/JVI.02747-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.David Y, Cacheaux LP, Ivens S, Lapilover E, Heinemann U, Kaufer D, et al. Astrocytic dysfunction in epileptogenesis: consequence of altered potassium and glutamate homeostasis? J Neurosci. 2009;29(34):10588–10599. doi: 10.1523/JNEUROSCI.2323-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis CN, Tabarean I, Gaidarova S, Behrens MM, Bartfai T. IL-1beta induces a MyD88-dependent and ceramide-mediated activation of Src in anterior hypothalamic neurons. J Neurochem. 2006;98(5):1379–1389. doi: 10.1111/j.1471-4159.2006.03951.x. [DOI] [PubMed] [Google Scholar]
- 24.De Simoni MG, Perego C, Ravizza T, Moneta D, Conti M, Marchesi F, et al. Inflammatory cytokines and related genes are induced in the rat hippocampus by limbic status epilepticus. Eur J Neurosci. 2000;12(7):2623–2633. doi: 10.1046/j.1460-9568.2000.00140.x. [DOI] [PubMed] [Google Scholar]
- 25.Devinsky O, Vezzani A, Najjar S, De Lanerolle NC, Rogawski MA. Glia and epilepsy: excitability and inflammation. Trends Neurosci. 2013;36(3):174–184. doi: 10.1016/j.tins.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Diem R, Hobom M, Grotsch P, Kramer B, Bahr M. Interleukin-1 beta protects neurons via the interleukin-1 (IL-1) receptor-mediated Akt pathway and by IL-1 receptor-independent decrease of transmembrane currents in vivo. Mol Cell Neurosci. 2003;22(4):487–500. doi: 10.1016/s1044-7431(02)00042-8. [DOI] [PubMed] [Google Scholar]
- 27.Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11(8):633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dube C, Vezzani A, Behrens M, Bartfai T, Baram TZ. Interleukin-1beta contributes to the generation of experimental febrile seizures. Ann Neurol. 2005;57(1):152–155. doi: 10.1002/ana.20358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eid T, Thomas MJ, Spencer DD, Runden-Pran E, Lai JC, Malthankar GV, et al. Loss of glutamine synthetase in the human epileptogenic hippocampus: possible mechanism for raised extracellular glutamate in mesial temporal lobe epilepsy. Lancet. 2004;363(9402):28–37. doi: 10.1016/s0140-6736(03)15166-5. [DOI] [PubMed] [Google Scholar]
- 30.Fabene PF, Navarro Mora G, Martinello M, Rossi B, Merigo F, Ottoboni L, et al. A role for leukocyte-endothelial adhesion mechanisms in epilepsy. Nat Med. 2008;14(12):1377–1383. doi: 10.1038/nm.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferrari CC, Depino AM, Prada F, Muraro N, Campbell S, Podhajcer O, et al. Reversible demyelination, blood–brain barrier breakdown, and pronounced neutrophil recruitment induced by chronic IL-1 expression in the brain. Am J Pathol. 2004;165(5):1827–1837. doi: 10.1016/S0002-9440(10)63438-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fonseca CG, Green CR, Nicholson LF. Upregulation in astrocytic connexin 43 gap junction levels may exacerbate generalized seizures in mesial temporal lobe epilepsy. Brain Res. 2002;929(1):105–116. doi: 10.1016/s0006-8993(01)03289-9. [DOI] [PubMed] [Google Scholar]
- 33.Friedman A, Kaufer D, Heinemann U. Blood–brain barrier breakdown-inducing astrocytic transformation: novel targets for the prevention of epilepsy. Epilepsy Res. 2009;85(2-3):142–149. doi: 10.1016/j.eplepsyres.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frigerio F, Frasca A, Weissberg I, Parrella S, Friedman A, Vezzani A, et al. Long-lasting pro-ictogenic effects induced in vivo by rat brain exposure to serum albumin in the absence of con-comitant pathology. Epilepsia. 2012;53(11):1887–1897. doi: 10.1111/j.1528-1167.2012.03666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furukawa K, Mattson MP. The transcription factor NF-kappaB mediates increases in calcium currents and decreases in NMDA- and AMPA/kainate-induced currents induced by tumor necrosis factor-alpha in hippocampal neurons. J Neurochem. 1998;70(5):1876–1886. doi: 10.1046/j.1471-4159.1998.70051876.x. [DOI] [PubMed] [Google Scholar]
- 36.Gibbons MB, Smeal RM, Takahashi DK, Vargas JR, Wilcox KS. Contributions of astrocytes to epileptogenesis following status epilepticus: opportunities for preventive therapy? Neurochem Int. 2013;63:660–669. doi: 10.1016/j.neuint.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hennessy EJ, Parker AE, O’Neill LA. Targeting Toll-like receptors: emerging therapeutics? Nat Rev Drug Discov. 2010;9(4):293–307. doi: 10.1038/nrd3203. [DOI] [PubMed] [Google Scholar]
- 38.Hinterkeuser S, Schroder W, Hager G, Seifert G, Blumcke I, Elger CE, et al. Astrocytes in the hippocampus of patients with temporal lobe epilepsy display changes in potassium conductances. Eur J Neurosci. 2000;12(6):2087–2096. doi: 10.1046/j.1460-9568.2000.00104.x. [DOI] [PubMed] [Google Scholar]
- 39.Holtman L, van Vliet EA, Edelbroek PM, Aronica E, Gorter JA. Cox-2 inhibition can lead to adverse effects in a rat model for temporal lobe epilepsy. Epilepsy Res. 2010;91(1):49–56. doi: 10.1016/j.eplepsyres.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 40.Iori V, Maroso M, Rizzi M, Iyer AM, Vertemara R, Carli M, et al. Receptor for advanced glycation endproducts is upregulated in temporal lobe epilepsy and contributes to experimental seizures. Neurobiol Dis. 2013;58:102–114. doi: 10.1016/j.nbd.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 41.Iyer A, Zurolo E, Spliet WG, van Rijen PC, Baayen JC, Gorter JA, et al. Evaluation of the innate and adaptive immunity in type I and type II focal cortical dysplasias. Epilepsia. 2010;51(9):1763–1773. doi: 10.1111/j.1528-1167.2010.02547.x. [DOI] [PubMed] [Google Scholar]
- 42.Jiang J, Dingledine R. Prostaglandin receptor EP2 in the crosshairs of anti-inflammation, anti-cancer, and neuroprotection. Trends Pharmacol Sci. 2013;34(7):413–423. doi: 10.1016/j.tips.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang J, Quan Y, Ganesh T, Pouliot WA, Dudek FE, Dingledine R. Inhibition of the prostaglandin receptor EP2 following status epilepticus reduces delayed mortality and brain inflammation. Proc Natl Acad Sci U S A. 2013;110(9):3591–3596. doi: 10.1073/pnas.1218498110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jin X, Gereau RW. Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. J Neurosci. 2006;26(1):246–255. doi: 10.1523/JNEUROSCI.3858-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jung KH, Chu K, Lee ST, Kim J, Sinn DI, Kim JM, et al. Cyclooxygenase-2 inhibitor, celecoxib, inhibits the altered hippocampal neurogenesis with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Neurobiol Dis. 2006;23(2):237–246. doi: 10.1016/j.nbd.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 46.Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci. 2008;28(20):5189–5194. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kirkman NJ, Libbey JE, Wilcox KS, White HS, Fujinami RS. Innate but not adaptive immune responses contribute to behavioral seizures following viral infection. Epilepsia. 2010;51(3):454–464. doi: 10.1111/j.1528-1167.2009.02390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koller H, Allert N, Oel D, Stoll G, Siebler M. TNF alpha induces a protein kinase C-dependent reduction in astroglial K+ conductance. Neuroreport. 1998;9(7):1375–1378. doi: 10.1097/00001756-199805110-00023. [DOI] [PubMed] [Google Scholar]
- 49.Kovacs Z, Czurko A, Kekesi KA, Juhasz G. Intracerebroventricularly administered lipopoly-saccharide enhances spike-wave discharges in freely moving WAG/Rij rats. Brain Res Bull. 2011;85(6):410–416. doi: 10.1016/j.brainresbull.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 50.Kulkarni SK, Dhir A. Cyclooxygenase in epilepsy: from perception to application. Drugs Today (Barc) 2009;45(2):135–154. doi: 10.1358/dot.2009.45.2.1322481. [DOI] [PubMed] [Google Scholar]
- 51.Kunz T, Oliw EH. The selective cyclooxygenase-2 inhibitor rofecoxib reduces kainate-induced cell death in the rat hippocampus. Eur J Neurosci. 2001;13(3):569–575. doi: 10.1046/j.1460-9568.2001.01420.x. [DOI] [PubMed] [Google Scholar]
- 52.Kwon YS, Pineda E, Auvin S, Shin D, Mazarati A, Sankar R. Neuroprotective and antiepileptogenic effects of combination of anti-inflammatory drugs in the immature brain. J Neuroinflammation. 2013;10:30. doi: 10.1186/1742-2094-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lai AY, Swayze RD, El-Husseini A, Song C. Interleukin-1 beta modulates AMPA receptor expression and phosphorylation in hippocampal neurons. J Neuroimmunol. 2006;175(1-2):97–106. doi: 10.1016/j.jneuroim.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 54.Lalic T, Pettingill P, Vincent A, Capogna M. Human limbic encephalitis serum enhances hippocampal mossy fiber-CA3 pyramidal cell synaptic transmission. Epilepsia. 2011;52(1):121–131. doi: 10.1111/j.1528-1167.2010.02756.x. [DOI] [PubMed] [Google Scholar]
- 55.Leonoudakis D, Braithwaite SP, Beattie MS, Beattie EC. TNFalpha-induced AMPA-receptor trafficking in CNS neurons; relevance to excitotoxicity? Neuron Glia Biol. 2004;1(3):263–273. doi: 10.1017/S1740925X05000608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leonoudakis D, Zhao P, Beattie EC. Rapid tumor necrosis factor alpha-induced exocytosis of glutamate receptor 2-lacking AMPA receptors to extrasynaptic plasma membrane potentiates excito-toxicity. J Neurosci. 2008;28(9):2119–2130. doi: 10.1523/JNEUROSCI.5159-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Libbey JE, Kirkman NJ, Smith MC, Tanaka T, Wilcox KS, White HS, et al. Seizures following picornavirus infection. Epilepsia. 2008;49(6):1066–1074. doi: 10.1111/j.1528-1167.2008.01535.x. [DOI] [PubMed] [Google Scholar]
- 58.Librizzi L, Noe F, Vezzani A, de Curtis M, Ravizza T. Seizure-induced brain-borne inflammation sustains seizure recurrence and blood-brain barrier damage. Ann Neurol. 2012;72(1):82–90. doi: 10.1002/ana.23567. [DOI] [PubMed] [Google Scholar]
- 59.Librizzi L, Regondi MC, Pastori C, Frigerio S, Frassoni C, de Curtis M. Expression of adhesion factors induced by epileptiform activity in the endothelium of the isolated guinea pig brain in vitro. Epilepsia. 2007;48(4):743–751. doi: 10.1111/j.1528-1167.2007.01047.x. [DOI] [PubMed] [Google Scholar]
- 60.Lund IV, Hu Y, Raol YH, Benham RS, Faris R, Russek SJ, et al. BDNF selectively regulates GABAA receptor transcription by activation of the JAK/STAT pathway. Sci Signal. 2008;1(41):ra9. doi: 10.1126/scisignal.1162396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma L, Cui XL, Wang Y, Li XW, Yang F, Wei D, et al. Aspirin attenuates spontaneous recurrent seizures and inhibits hippocampal neuronal loss, mossy fiber sprouting and aberrant neurogenesis following pilocarpine-induced status epilepticus in rats. Brain Res. 2012;1469:103–113. doi: 10.1016/j.brainres.2012.05.058. [DOI] [PubMed] [Google Scholar]
- 62.Mandolesi G, Musella A, Gentile A, Grasselli G, Haji N, Sepman H, et al. Interleukin-1beta alters glutamate transmission at purkinje cell synapses in a mouse model of multiple sclerosis. J Neurosci. 2013;33(29):12105–12121. doi: 10.1523/JNEUROSCI.5369-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Manto M, Dalmau J, Didelot A, Rogemond V, Honnorat J. In vivo effects of antibodies from patients with anti-NMDA receptor encephalitis: further evidence of synaptic glutamatergic dysfunction. Orphanet J Rare Dis. 2010;5:31. doi: 10.1186/1750-1172-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marchi N, Fan Q, Ghosh C, Fazio V, Bertolini F, Betto G, et al. Antagonism of peripheral inflammation reduces the severity of status epilepticus. Neurobiol Dis. 2009;33(2):171–181. doi: 10.1016/j.nbd.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marchi N, Teng Q, Ghosh C, Fan Q, Nguyen MT, Desai NK, et al. Blood–brain barrier damage, but not parenchymal white blood cells, is a hallmark of seizure activity. Brain Res. 2010;1353:176–186. doi: 10.1016/j.brainres.2010.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maroso M, Balosso S, Ravizza T, Iori V, Wright CI, French J, et al. Interleukin-1beta biosynthesis inhibition reduces acute seizures and drug resistant chronic epileptic activity in mice. Neurotherapeutics. 2011;8(2):304–315. doi: 10.1007/s13311-011-0039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maroso M, Balosso S, Ravizza T, Liu J, Aronica E, Iyer AM, et al. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med. 2010;16(4):413–419. doi: 10.1038/nm.2127. [DOI] [PubMed] [Google Scholar]
- 68.Mathern GW, Mendoza D, Lozada A, Pretorius JK, Dehnes Y, Danbolt NC, et al. Hippocampal GABA and glutamate transporter immunoreactivity in patients with temporal lobe epilepsy. Neurology. 1999;52(3):453–472. doi: 10.1212/wnl.52.3.453. [DOI] [PubMed] [Google Scholar]
- 69.Morin-Brureau M, Lebrun A, Rousset MC, Fagni L, Bockaert J, de Bock F, et al. Epileptiform activity induces vascular remodeling and zonula occludens 1 downregulation in organotypic hippo-campal cultures: role of VEGF signaling pathways. J Neurosci. 2011;31(29):10677–10688. doi: 10.1523/JNEUROSCI.5692-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Naus CC, Bechberger JF, Caveney S, Wilson JX. Expression of gap junction genes in astrocytes and C6 glioma cells. Neurosci Lett. 1991;126(1):33–36. doi: 10.1016/0304-3940(91)90364-y. [DOI] [PubMed] [Google Scholar]
- 71.Nehlig A. What is animal experimentation telling us about new drug treatments of status epilepticus? Epilepsia. 2007;48(Suppl 8):78–81. doi: 10.1111/j.1528-1167.2007.01358.x. [DOI] [PubMed] [Google Scholar]
- 72.Nicolas CS, Peineau S, Amici M, Csaba Z, Fafouri A, Javalet C, et al. The Jak/STAT pathway is involved in synaptic plasticity. Neuron. 2012;73(2):374–390. doi: 10.1016/j.neuron.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nicoletti JN, Shah SK, McCloskey DP, Goodman JH, Elkady A, Atassi H, et al. Vascular endothelial growth factor is up-regulated after status epilepticus and protects against seizure-induced neuronal loss in hippocampus. Neuroscience. 2008;151(1):232–241. doi: 10.1061/j.neuroscience.2007.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Noe FM, Polascheck N, Frigerio F, Bankstahl M, Ravizza T, Marchini S, et al. Pharmacological blockade of IL-1beta/IL-1 receptor type 1 axis during epileptogenesis provides neuroprotection in two rat models of temporal lobe epilepsy. Neurobiol Dis. 2013;59:183–193. doi: 10.1016/j.nbd.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 75.O’Donnell E, Vereker E, Lynch MA. Age-related impairment in LTP is accompanied by enhanced activity of stress-activated protein kinases: analysis of underlying mechanisms. Eur J Neurosci. 2000;12(1):345–352. doi: 10.1046/j.1460-9568.2000.00900.x. [DOI] [PubMed] [Google Scholar]
- 76.Oberheim NA, Tian GF, Han X, Peng W, Takano T, Ransom B, et al. Loss of astrocytic domain organization in the epileptic brain. J Neurosci. 2008;28(13):3264–3276. doi: 10.1523/JNEUROSCI.4980-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oby E, Janigro D. The blood–brain barrier and epilepsy. Epilepsia. 2006;47(11):1761–1774. doi: 10.1111/j.1528-1167.2006.00817.x. [DOI] [PubMed] [Google Scholar]
- 78.Ortinski PI, Dong J, Mungenast A, Yue C, Takano H, Watson DJ, et al. Selective induction of astrocytic gliosis generates deficits in neuronal inhibition. Nat Neurosci. 2010;13(5):584–591. doi: 10.1038/nn.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pardo CA, Vining EP, Guo L, Skolasky RL, Carson BS, Freeman JM. The pathology of Rasmussen syndrome: stages of cortical involvement and neuropathological studies in 45 hemispherectomies. Epilepsia. 2004;45(5):516–526. doi: 10.1111/j.0013-9580.2004.33103.x. [DOI] [PubMed] [Google Scholar]
- 80.Pedrazzi M, Averna M, Sparatore B, Patrone M, Salamino F, Marcoli M, et al. Potentiation of NMDA receptor-dependent cell responses by extra-cellular high mobility group box 1 protein. PLoS One. 2012;7(8):e44518. doi: 10.1371/journal.pone.0044518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pedrazzi M, Raiteri L, Bonanno G, Patrone M, Ledda S, Passalacqua M, et al. Stimulation of excitatory amino acid release from adult mouse brain glia subcellular particles by high mobility group box 1 protein. J Neurochem. 2006;99(3):827–838. doi: 10.1111/j.1471-4159.2006.04120.x. [DOI] [PubMed] [Google Scholar]
- 82.Pernhorst K, Herms S, Hoffmann P, Cichon S, Schulz H, Sander T, et al. TLR4, ATF-3 and IL8 inflammation mediator expression correlates with seizure frequency in human epileptic brain tissue. Seizure. 2013;22(8):675–678. doi: 10.1016/j.seizure.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 83.Plata-Salaman CR, Ffrench-Mullen JM. Interleukin-1 beta depresses calcium currents in CA1 hippocampal neurons at pathophysiological concentrations. Brain Res Bull. 1992;29(2):221–223. doi: 10.1016/0361-9230(92)90029-w. [DOI] [PubMed] [Google Scholar]
- 84.Plata-Salaman CR, ffrench-Mullen JM. Interleukin-1 beta inhibits Ca2+ channel currents in hippocampal neurons through protein kinase C. Eur J Pharmacol. 1994;266(1):1–10. doi: 10.1016/0922-4106(94)90202-x. [DOI] [PubMed] [Google Scholar]
- 85.Polascheck N, Bankstahl M, Loscher W. The COX-2 inhibitor parecoxib is neuroprotective but not antiepileptogenic in the pilocarpine model of temporal lobe epilepsy. Exp Neurol. 2010;224(1):219–233. doi: 10.1016/j.expneurol.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 86.Proper EA, Hoogland G, Kappen SM, Jansen GH, Rensen MG, Schrama LH, et al. Distribution of glutamate transporters in the hippocampus of patients with pharmaco-resistant temporal lobe epilepsy. Brain. 2002;125(Pt 1):32–43. doi: 10.1093/brain/awf001. [DOI] [PubMed] [Google Scholar]
- 87.Ravizza T, Lucas SM, Balosso S, Bernardino L, Ku G, Noe F, et al. Inactivation of caspase-1 in rodent brain: a novel anticonvulsive strategy. Epilepsia. 2006;47(7):1160–1168. doi: 10.1111/j.1528-1167.2006.00590.x. [DOI] [PubMed] [Google Scholar]
- 88.Ravizza T, Noe F, Zardoni D, Vaghi V, Sifringer M, Vezzani A. Interleukin converting enzyme inhibition impairs kindling epileptogenesis in rats by blocking astrocytic IL-1beta production. Neurobiol Dis. 2008;31(3):327–333. doi: 10.1016/j.nbd.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 89.Riazi K, Galic MA, Pittman QJ. Contributions of peripheral inflammation to seizure susceptibility: cytokines and brain excitability. Epilepsy Res. 2010;89(1):34–42. doi: 10.1016/j.eplepsyres.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 90.Rodgers KM, Hutchinson MR, Northcutt A, Maier SF, Watkins LR, Barth DS. The cortical innate immune response increases local neuronal excitability leading to seizures. Brain. 2009;132(Pt 9):2478–2486. doi: 10.1093/brain/awp177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sanchez-Alavez M, Tabarean IV, Behrens MM, Bartfai T. Ceramide mediates the rapid phase of febrile response to IL-1beta. Proc Natl Acad Sci U S A. 2006;103(8):2904–2908. doi: 10.1073/pnas.0510960103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Santello M, Bezzi P, Volterra A. TNFalpha controls glutamatergic gliotransmission in the hippocampal dentate gyrus. Neuron. 2011;69(5):988–1001. doi: 10.1016/j.neuron.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 93.Santello M, Volterra A. TNFalpha in synaptic function: switching gears. Trends Neurosci. 2012;35(10):638–647. doi: 10.1016/j.tins.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 94.Sarac S, Afzal S, Broholm H, Madsen FF, Ploug T, Laursen H. Excitatory amino acid transporters EAAT-1 and EAAT-2 in temporal lobe and hippocampus in intractable temporal lobe epilepsy. APMIS. 2009;117(4):291–301. doi: 10.1111/j.1600-0463.2009.02443.x. [DOI] [PubMed] [Google Scholar]
- 95.Schafers M, Sorkin L. Effect of cytokines on neuronal excitability. Neurosci Lett. 2008;437(3):188–193. doi: 10.1016/j.neulet.2008.03.052. [DOI] [PubMed] [Google Scholar]
- 96.Schneider H, Pitossi F, Balschun D, Wagner A, del Rey A, Besedovsky HO. A neuromodulatory role of interleukin-1beta in the hippocampus. Proc Natl Acad Sci U S A. 1998;95(13):7778–7783. doi: 10.1073/pnas.95.13.7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Seiffert E, Dreier JP, Ivens S, Bechmann I, Tomkins O, Heinemann U, et al. Lasting blood–brain barrier disruption induces epileptic focus in the rat somatosensory cortex. J Neurosci. 2004;24(36):7829–7836. doi: 10.1523/JNEUROSCI.1751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Smeal RM, Stewart KA, Iacob E, Fujinami RS, White HS, Wilcox KS. The activity within the CA3 excitatory network during Theiler’s virus encephalitis is distinct from that observed during chronic epilepsy. J Neurovirol. 2012;18(1):30–44. doi: 10.1007/s13365-012-0082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32(12):638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sotgiu S, Murrighile MR, Constantin G. Treatment of refractory epilepsy with natalizumab in a patient with multiple sclerosis. Case report BMC Neurol. 2010;10:84. doi: 10.1186/1471-2377-10-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Steinmetz CC, Turrigiano GG. Tumor necrosis factor-alpha signaling maintains the ability of cortical synapses to express synaptic scaling. J Neurosci. 2010;30(44):14685–14690. doi: 10.1523/JNEUROSCI.2210-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stellwagen D, Beattie EC, Seo JY, Malenka RC. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J Neurosci. 2005;25(12):3219–3228. doi: 10.1523/JNEUROSCI.4486-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440(7087):1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 104.Stewart KA, Wilcox KS, Fujinami RS, White HS. Development of postinfection epilepsy after Theiler’s virus infection of C57BL/6 mice. J Neuropathol Exp Neurol. 2010;69(12):1210–1219. doi: 10.1097/NEN.0b013e3181ffc420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tabarean IV, Korn H, Bartfai T. Interleukin-1beta induces hyperpolarization and modulates synaptic inhibition in preoptic and anterior hypothalamic neurons. Neuroscience. 2006;141(4):1685–1695. doi: 10.1016/j.neuroscience.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 106.Takahashi DK, Vargas JR, Wilcox KS. Increased coupling and altered glutamate transport currents in astrocytes following kainic-acid-induced status epilepticus. Neurobiol Dis. 2010;40(3):573–585. doi: 10.1016/j.nbd.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Takeuchi H, Jin S, Wang J, Zhang G, Kawanokuchi J, Kuno R, et al. Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J Biol Chem. 2006;281(30):21362–21368. doi: 10.1074/jbc.M600504200. [DOI] [PubMed] [Google Scholar]
- 108.Theodore WH, Epstein L, Gaillard WD, Shinnar S, Wainwright MS, Jacobson S. Human herpes virus 6B: a possible role in epilepsy? Epilepsia. 2008;49(11):1828–1837. doi: 10.1111/j.1528-1167.2008.01699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Uva L, Librizzi L, Marchi N, Noe F, Bongiovanni R, Vezzani A, et al. Acute induction of epileptiform discharges by pilocarpine in the in vitro isolated guinea-pig brain requires enhancement of blood–brain barrier permeability. Neuroscience. 2008;151(1):303–312. doi: 10.1016/j.neuroscience.2007.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.van Gassen KL, de Wit M, Koerkamp MJ, Rensen MG, van Rijen PC, Holstege FC, et al. Possible role of the innate immunity in temporal lobe epilepsy. Epilepsia. 2008;49(6):1055–1065. doi: 10.1111/j.1528-1167.2007.01470.x. [DOI] [PubMed] [Google Scholar]
- 111.van Vliet EA, Zibell G, Pekcec A, Schlichtiger J, Edelbroek PM, Holtman L, et al. COX-2 inhibition controls P-glycoprotein expression and promotes brain delivery of phenytoin in chronic epileptic rats. Neuropharmacology. 2010;58(2):404–412. doi: 10.1016/j.neuropharm.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 112.Vargas JR, Takahashi DK, Thomson KE, Wilcox KS. The expression of kainate receptor subunits in hippocampal astrocytes after experimentally induced status epilepticus. J Neuropathol Exp Neurol. 2013;72(10):919–932. doi: 10.1097/NEN.0b013e3182a4b266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vezzani A, Balosso S, Maroso M, Zardoni D, Noe F, Ravizza T. ICE/caspase 1 inhibitors and IL-1beta receptor antagonists as potential therapeutics in epilepsy. Curr Opin Investig Drugs. 2010;11(1):43–50. [PubMed] [Google Scholar]
- 114.Vezzani A, Conti M, De Luigi A, Ravizza T, Moneta D, Marchesi F, et al. Interleukin-1beta immunoreactivity and microglia are enhanced in the rat hippocampus by focal kainate application: functional evidence for enhancement of electrographic seizures. J Neurosci. 1999;19(12):5054–5065. doi: 10.1523/JNEUROSCI.19-12-05054.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol. 2011;7(1):31–40. doi: 10.1038/nrneurol.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vezzani A, Friedman A, Dingledine RJ. The role of inflammation in epileptogenesis. Neuropharmacology. 2013;69:16–24. doi: 10.1016/j.neuropharm.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vezzani A, Janigro D. Leukocyte-endothelial adhesion mechanisms in epilepsy: cheers and jeers. Epilepsy Curr. 2009;9(4):118–121. doi: 10.1111/j.1535-7511.2009.01312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vezzani A, Maroso M, Balosso S, Sanchez MA, Bartfai T. IL-1 receptor/Toll-like receptor signaling in infection, inflammation, stress and neurodegeneration couples hyperexcitability and seizures. Brain Behav Immun. 2011;25(7):1281–1289. doi: 10.1016/j.bbi.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 119.Vezzani A, Moneta D, Conti M, Richichi C, Ravizza T, De Luigi A, et al. Powerful anticonvulsant action of IL-1 receptor antagonist on intracerebral injection and astrocytic overexpression in mice. Proc Natl Acad Sci U S A. 2000;97(21):11534–11539. doi: 10.1073/pnas.190206797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vincent A, Irani SR, Lang B. Potentially pathogenic autoantibodies associated with epilepsy and encephalitis in children and adults. Epilepsia. 2011;52(Suppl 8):8–11. doi: 10.1111/j.1528-1167.2011.03224.x. [DOI] [PubMed] [Google Scholar]
- 121.Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, et al. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci. 2003;23(25):8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Viviani B, Gardoni F, Marinovich M. Cytokines and neuronal ion channels in health and disease. Int Rev Neurobiol. 2007;82:247–263. doi: 10.1016/S0074-7742(07)82013-7. [DOI] [PubMed] [Google Scholar]
- 123.Wang S, Cheng Q, Malik S, Yang J. Interleukin-1beta inhibits gamma-aminobutyric acid type A (GABA(A)) receptor current in cultured hippocampal neurons. J Pharmacol Exp Ther. 2000;292(2):497–504. [PubMed] [Google Scholar]
- 124.Weinberg MS, Blake BL, McCown TJ. Opposing actions of hippocampus TNFalpha receptors on limbic seizure susceptibility. Exp Neurol. 2013;247:429–437. doi: 10.1016/j.expneurol.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wilhelmsson U, Bushong EA, Price DL, Smarr BL, Phung V, Terada M, et al. Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proc Natl Acad Sci U S A. 2006;103(46):17513–17518. doi: 10.1073/pnas.0602841103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yin HZ, Hsu CI, Yu S, Rao SD, Sorkin LS, Weiss JH. TNF-alpha triggers rapid membrane insertion of Ca(2+) permeable AMPA receptors into adult motor neurons and enhances their susceptibility to slow excitotoxic injury. Exp Neurol. 2012;238(2):93–102. doi: 10.1016/j.expneurol.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25(2):181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 128.Zattoni M, Mura ML, Deprez F, Schwendener RA, Engelhardt B, Frei K, et al. Brain infiltration of leukocytes contributes to the pathophysiology of temporal lobe epilepsy. J Neurosci. 2011;31(11):4037–4050. doi: 10.1523/JNEUROSCI.6210-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zeise ML, Espinoza J, Morales P, Nalli A. Interleukin-1beta does not increase synaptic inhibition in hippocampal CA3 pyramidal and dentate gyrus granule cells of the rat in vitro. Brain Res. 1997;768(1-2):341–344. doi: 10.1016/s0006-8993(97)00787-7. [DOI] [PubMed] [Google Scholar]
- 130.Zhang J, Takahashi HK, Liu K, Wake H, Liu R, Maruo T, et al. Anti-high mobility group box-1 monoclonal antibody protects the blood–brain barrier from ischemia-induced disruption in rats. Stroke. 2011;42(5):1420–1428. doi: 10.1161/STROKEAHA.110.598334. [DOI] [PubMed] [Google Scholar]
- 131.Zhang R, Sun L, Hayashi Y, Liu X, Koyama S, Wu Z, et al. Acute p38-mediated inhibition of NMDA-induced outward currents in hippocampal CA1 neurons by interleukin-1beta. Neurobiol Dis. 2010;38(1):68–77. doi: 10.1016/j.nbd.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 132.Zhou C, Tai C, Ye HH, Ren X, Chen JG, Wang SQ, et al. Interleukin-1beta downregulates the L-type Ca2+ channel activity by depressing the expression of channel protein in cortical neurons. J Cell Physiol. 2006a;206(3):799–806. doi: 10.1002/jcp.20518. [DOI] [PubMed] [Google Scholar]
- 133.Zhou C, Ye HH, Wang SQ, Chai Z. Interleukin-1beta regulation of N-type Ca2+ channels in cortical neurons. Neurosci Lett. 2006b;403(1-2):181–185. doi: 10.1016/j.neulet.2006.04.043. [DOI] [PubMed] [Google Scholar]
- 134.Zurolo E, de Groot M, Iyer A, Anink J, van Vliet EA, Heimans JJ, et al. Regulation of Kir4.1 expression in astrocytes and astrocytic tumors: a role for interleukin-1 beta. J Neuroinflammation. 2012;9:280. doi: 10.1186/1742-2094-9-280. [DOI] [PMC free article] [PubMed] [Google Scholar]