Abstract
The hypothalamo-pituitary-adrenocortical (HPA axis) is required for stress adaptation. Activation of the HPA axis causes secretion of glucocorticoids, which act on multiple organ systems to redirect energy resources to meet real or anticipated demand. The HPA stress response is driven primarily by neural mechanisms, invoking corticotrophin releasing hormone (CRH) release from hypothalamic paraventricular nucleus (PVN) neurons. Pathways activating CRH release are stressor dependent: reactive responses to homeostatic disruption frequently involve direct noradrenergic or peptidergic drive of PVN neurons by sensory relays, whereas anticipatory responses use oligosynaptic pathways originating in upstream limbic structures. Anticipatory responses are driven largely by disinhibition, mediated by trans-synaptic silencing of tonic PVN inhibition via GABAergic neurons in the amygdala. Stress responses are inhibited by negative feedback mechanisms, whereby glucocorticoids act to diminish drive (brainstem), promote trans-synaptic inhibition by limbic structures (e.g, hippocampus). Glucocorticoids also act at the PVN to rapidly inhibit CRH neuronal activity via membrane glucocorticoid receptors. Chronic stress-induced activation of the HPA axis takes many forms (chronic basal hypersecretion, sensitized stress responses, even adrenal exhaustion), with manifestation dependent upon factors such as stressor chronicity, intensity, frequency and modality. Neural mechanisms driving chronic stress responses can be distinct from those controlling acute reactions, including recruitment of novel limbic, hypothalamic and brainstem circuits. Importantly, an individual’s response to acute or chronic stress is determined by numerous factors, including genetics, early life experience, environmental conditions, sex and age. The context in which stressors occur will determine whether an individual’s acute or chronic stress responses are adaptive or maladaptive (pathological).
Introduction
Survival is a fundamental priority of all organisms in an ever-changing environmental context. Survival frequently hinges on the ability to adapt to various homeostatic challenges. Over the course of evolution, multiple and overlapping mechanisms are in place to deal with both acute and prolonged threats. The so-called ‘stress response’ represents an integrated reaction to stressors, broadly defined as real or perceived threats to homeostasis or well-being. Activation of the hypothalamo-pituitary-adrenocortical (HPA) axis represents a primary hormonal response to homeostatic challenge. In general, some sort of HPA axis change is engendered by all varieties of stressor, and is a hallmark of the physiological reaction to stress. Through the release of glucocorticoids, the HPA axis mobilizes energy reserves to insure that the organism has the resources needed to meet a very real physical insult (a ‘reactive’ response), or prepare for a predicted insult (an ‘anticipatory’ response). Proper control of the stress response is of critical importance, as inappropriate or prolonged HPA axis activation is energetically costly and is linked with numerous physiological and psychological disease states
Glucocorticoids serve to mobilize the appropriate energy needed for the context at hand (116). In brain and pituitary, glucocorticoids signal through at least two receptor subtypes, the mineralocorticoid receptor (MR) and the glucocorticoid receptor (GR). The GR and MR are both ligand-gated transcription factors that alter expression of a large arsenal of genes (32). The MR, which has a higher glucocorticoid binding affinity than GR, regulates basal circadian and ultradian rhythms and is important in dictating HPA axis activity with respect to time of day. In the kidney and other tissues, the MR largely senses aldosterone, due to inactivation of corticosterone (or cortisol) by high levels of 11-β hydroxysteroid dehydrogenase (11-βHSD1) (144). However, in other tissues, including brain, βHSD1 acts in the opposite direction (as a reductase) in brain, and may indeed amplify glucocorticoid action under some conditions (145). Higher levels of glucocorticoids (including those seen following stress) activate the lower-affinity GR, which promotes expression of a wide variety of genes and is thought to mediate glucocorticoid effects on mobilization of energy stores (liver, fat and muscle), inflammation and neural function (among others) (32).
Control of glucocorticoid release is mediated by glucocorticoid feedback at varying levels of the HPA axis, serving to limit prolonged exposure to catabolic actions of glucocorticoids (117). This is of utmost importance, as excess glucocorticoid exposure can lead to pathological outcomes in multiple bodily compartments. Given that stress levels of glucocorticoids are read primarily by the GR, this receptor is generally assumed to subserve the bulk of feedback regulation (117).
This review will focus on regulation of acute and chronic HPA axis responses to stress. At this juncture, it is important to consider several aspects of stress reactivity that should be considered in the context of this summary.
First, it is important to note that the HPA axis is but one of many endogenous stress-reactive systems. For example, stress exposure engages the autonomic nervous system, stimulating sympathoadrenomedullary drive resulting in a widespread response that can optimize survival in the face of challenge (increased heart rate, increased blood pressure, increased hepatic glucose production, etc). Moreover, there are numerous other systems that have temporal links to stressful stimuli, including prolactin release (109), release of gonadal steroid hormones (8) and circulating IL6 levels (87), among others. Consequently, while of substantial importance, HPA axis activation is one among many bodily reactions to stress.
Second, the stress response is not necessarily the primary function of the HPA axis. Glucocorticoids are critical for energy mobilization and distribution in multiple organ systems, and are needed to assure energy availability even in the absence of stress. For example, following acute or chronic exposure to glucocorticoids (e.g., under conditions of chronic stress) promote hepatic glycogenolysis and lipolysis, releasing stored energy. In addition, there is a marked diurnal rhythm of HPA axis activation that occurs in most species, with peak levels corresponding to the waking phase (92). It is thought that diurnal glucocorticoids play an important part in regulating energy homeostasis in order to conduct of daily activities (29, 97, 98). Stress responses may be considered a ‘special case’ of HPA axis drive, boosting energy when it is needed for adaptive responses.
Finally, the literature summarized will concentrate largely on rodent studies, which have yielded a considerable proportion of our mechanistic understanding of HPA axis function. While the focus is on rodents, it is important to note that the organization of the HPA axis is highly conserved throughout mammalian phylogeny. Although some species differences in response characteristics exist, it is safe to say the most of the fundamental aspects of HPA axis mechanics will be true across the phylogenic trajectory from rodents to humans.
In the interest of limiting references (as well as expedience), we have cited our own work in numerous places where multiple labs have contributed. We would encourage readers to query additional sources to obtain a broader view of the literature (see Further Reading List).
Role of the H(ypothalamus), P(ituitary), and A(drenal) in Generation of Stress Responses
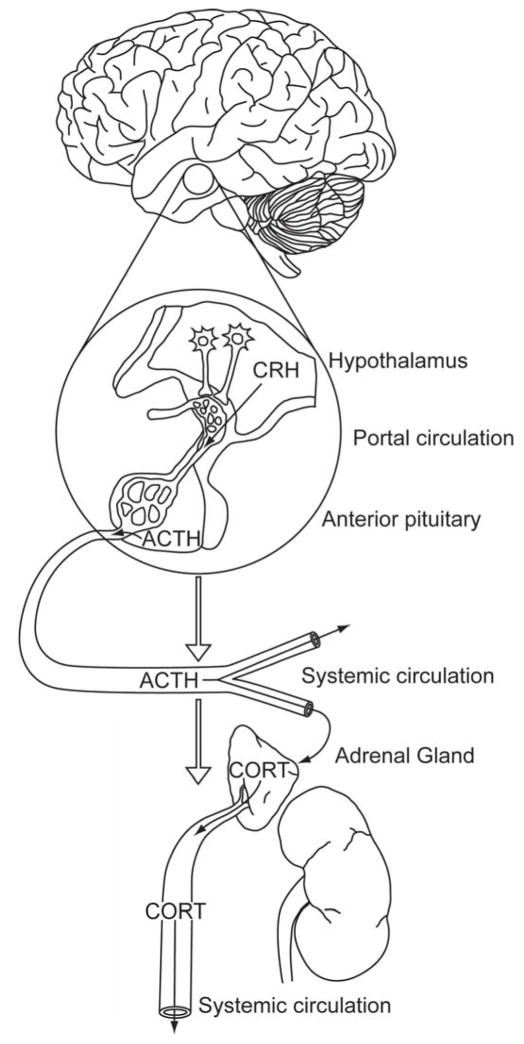
It is important to consider the HPA axis response to stress in terms of interaction between three distinct organs, each of which has its own intrinsic control points for regulation of the ultimate endpoint, glucocorticoids (Figure 1). Understanding of ‘H’ of the HPA axis was spurred by the seminal contributions of Wylie Vale and colleagues (e.g., (156), who identified CRH (corticotropin-releasing hormone) (then named corticotropin releasing factor (CRF)) as the critical hypothalamic secretagogue for adrenocorticotrophic hormone (ACTH). We have come to understand that discrete populations of CRH neurons located in the dorsomedial parvocellular (mp) division of the paraventricular nucleus (PVN) are the prime-movers of the stress response (7). These neurons project to the median eminence, where CRH as well as other peptides (e.g., vasopressin (AVP)) are released into the hypophysial portal plexus of veins. These peptides then travel to the anterior pituitary and bind to cognate receptors on corticotropes, causing release of ACTH.
Figure 1.
Organization of the hypothalamo-pituitary-adrenocortical (HPA) axis. HPA axis stress responses are initiated by corticotropin releasing hormone (CRH) neurons in the paraventricular nucleus of the hypothalamus (PVN). Stressors cause release of CRH into the hypophysial portal vessels, which transport peptide to the anterior pituitary to enable access to corticotrophs. Stimulated corticotrophs then release adrenocorticotrophic hormone (ACTH) into the systemic circulation, whereby it promotes synthesis and secretion of glucocorticoids (cortisol in some species (e.g., man), corticosterone in others (e.g., rats, mice)) at the adrenal cortex. Glucocorticoids are then secreted into the systemic circulation and can access cognate receptors in virtually every organ system, including the brain. Reproduced from (117), with permission.
It is worth commenting on the decision to use ‘CRH’ rather than ‘CRF’ in this review. Use of ‘CRH’ vs. ‘CRF’ is rather contentious in the field. Individuals with an endocrine perspective generally use ‘CRH’, since typically, ‘releasing factors’ are re-named as ‘hormones’ when their definitive peptide, gene and protein structures are identified. Use of ‘CRF’ stems from the multiplicity of peptide action, extending beyond its endocrine function. In the authors’ opinion, both are equally correct. As historically our group has used the ‘endocrine’ definition, and this review has a primary endocrine focus, we have elected to use ‘CRH’ here.
At the corticotrope, CRH binds to G-protein coupled receptors (corticotropin releasing hormone R1 receptors (CRHR1) and activates adenylate cyclase, leading to the release of adrenocorticotropic hormone (ACTH) (2). Genetic studies in crh knockout mice confirm that CRH is required for both basal and stress-induced ACTH release (114). Notably, AVP is co-released from CRH nerve terminals at the median eminence and works in conjunction with CRH to enhance the response during stress (60). Vasopressin works through AVP1B receptors to activate protein kinase C, which complements the actions of CRH on ACTH release but is not sufficient to drive significant ACTH on its own (at least at physiological levels) in most organisms (see below) (3).
Binding of CRH also enhances transcription of the proopiomelanocortin (POMC) gene, which encodes ACTH. Activation of POMC transcription and subsequent release of ACTH are contingent upon the type of stressor that causes the initial stress response (2). Finite control of the transcription of POMC can be credited to both regulation and specificity in transcription factors. CRH promotes transcription of POMC via a cAMP/protein kinase A dependent mechanism (89). Once synthesized, POMC is cleaved by prohormone convertase 1 into ACTH and β-lipotropin (12). ACTH is then packaged into vesicles and released via the regulated exocytosis pathway into the systemic circulation.
At the adrenal cortex, ACTH acts on melanocortin 2 receptors (MC2R) in the zona fasciculata (64). Binding of ACTH to MC2R produces adenylate cyclase-dependent increases in intracellular cAMP levels, which causes a rapid increase in cholesterol biosynthesis (149). Cholesterol is the precursor for most steroids including mineralocorticoids and glucocorticoids.. Cholesterol is transported from cellular stores to the outer mitochondrial membrane, where steroidogenic acute regulatory (StAR) protein facilitates the movement of cholesterol into the inner mitochondrial membrane at locations where the two membranes are in close proximity, referred to as contact sites (65). Due to StAR being produced de novo after trophic hormone stimulation of the cell, this StAR mediated transport of cholesterol becomes the rate limiting step of glucocorticoid production (24). ACTH also increases the level of mRNA that encodes for enzymes important for the steroidogenic pathway, P-450scc, P-450I7a, P-45011/3, P-450C21, and adrenodoxin (149). Once synthesized, glucocorticoids are released into the circulation and travel to various areas of the periphery and also back to the central nervous system.
It is important to consider that the actual biology of the HPA axis is more nuanced than usually presented. For example, large lesions of the PVN do not completely block ACTH release in response to stress, suggesting that PVN neurosecretory neurons may not represent the sole regulator of HPA axis activation (105). In addition, work in crh knockout mice indicates that females can mount a weak corticosterone response to stress, suggesting an alternative source of drive (86). Finally, the supremacy of CRH as the primary driver of ACTH release at the pituitary may not be true of all species, e.g., in vitro study suggest that AVP acts as a stronger stimulus for pituitary ACTH secretion in sheep corticotropes (43).
Intrinsic regulatory properties are evident at the level of the adrenal as well. Increasing numbers of studies are demonstrating marked dissociation between ACTH and glucocorticoid release in numerous species (17). In rodents, there is evidence for sympathetic potentiation of ACTH-induced corticosterone release mediated by the splanchnic nerves. Sympathetic modulation of ACTH efficacy is evident during the active phase of the circadian cycle and following acute stress exposure (88, 161). Adrenal sensitivity is also enhanced after chronic stress exposure, suggesting increased sympathetic drive (162). In man, surgical stress can result in long-term cortisol secretion in the absence of a corresponding increase in ACTH, which is accompanied by marked elevations in circulating cytokines (59). Cytokines are known to directly enhance release of adrenal glucocorticoids, providing an alternative pathway to directly drive the adrenal cortex (13, 17).
Adrenal glucocorticoid release is pulsatile in nature, marked by an ultradian release pattern (100). Pulsatility occurs under basal conditions and is maintained in the face of acute or chronic stress. Indeed, it appears that the magnitude of a stress response can depend on where the stressor occurs with respect to an ultradian pulse: stressors imposed on the rising phase of a pulse produce greater glucocorticoid responses than those occurring during a falling phase (100). Pulsatility is an extremely important component of HPA function, as disruption of pulses (e.g., constant glucocorticoid availability) disrupts GR signaling (135).
Dynamics of the HPA Axis ‘Stress Response’
Temporal activation of the HPA axis depends on stressor duration and modality. Acute stress efficiently drives HPA stress response, and feedback mechanisms effectively terminate the response after the stressor subsides. A typical stress response pattern is illustrated in Figure 2. Generally, the HPA response begins with a pulse of ACTH, beginning within minutes and lasting a relatively short period of time, with duration dependent on stimulus duration, intensity and feedback. The glucocorticoid response lags in time (due to the necessity for de novo glucocorticoid production at the adrenal) and lasts substantially longer, with the time course depending on a combination of active (feedback signaling) and passive (glucocorticoid degradation) processes. Below, we discuss factors influencing the temporal dynamics of the HPA axis stress response, and the implications of response timing on glucocorticoid signaling properties and consequent cellular and systemic outcomes.
Figure 2.
Temporal dynamics of HPA axis stress responses. In response to stress, ACTH is released within minutes of stimulation. The extent of ACTH release is limited by rapid, non-genomic fast feedback mechanisms (usually peaking within 15 min of stressor onset)(see text). Due to the time needed for ACTH to access the adrenal cortex and promote glucocorticoid synthesis and release, there is a substantial delay between time-to-peak for corticosterone relative to ACTH (usually within 30–60 minutes). In addition to shutdown by fast feedback inhibition of ACTH release, the time course of the corticosterone response (usually on the order of 2 hours) can be modulated by delayed glucocorticoid feedback as well as factors controlling glucocorticoid metabolism. The timing of both ACTH and corticosterone responses are dependent on stressor modality and intensity. Modified from (68).
The ‘rising’ phase: neural activation of the HPA axis stress response at the PVN
Direct drive of the HPA axis stress response is largely neuronal in origin, initiated via excitatory neurotransmission at the level of the PVN CRH neuron. As a general rule, neurons that provide direct drive to the HPA axis come from brain sites in receipt of information of relevance to homeostatic need (Figure 3).
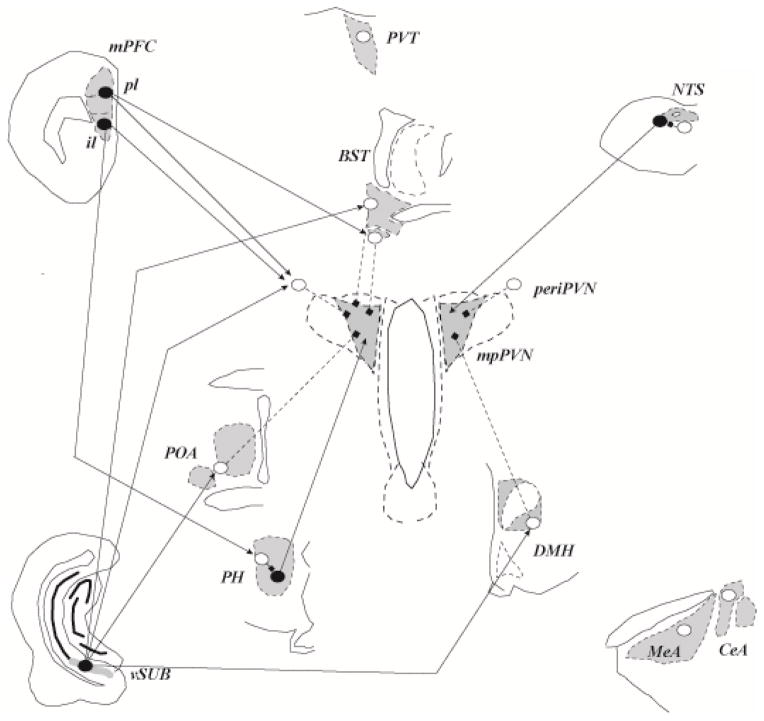
Figure 3.
Neural mechanisms of acute stress excitation. Data suggest corticotropin releasing hormone neurons in the medial dorsal paraventricular nucleus (mpPVN) can be driven by neurons communicating homeostatic challenge, including the nucleus of the solitary tract (NTS), among others. The PVN also has numerous connections with hypothalamic nuclei and subcortical telencephalic structures, including excitatory (posterior hypothalamus (PH), ventrolateral region of the bed nucleus of the stria terminalis (BST)) and inhibitory (medial preoptic nucleus (mPOA), dorsomedial nucleus (DMH), periPVN, and posterior BST) inputs. Inhibitory input to the PVN provides a substantial inhibitory tone, which can be disrupted by inhibition from upstream sites such as the medial and central amygdaloid nuclei (MeA, CeA), providing a mechanism for transsynaptic disinhibition from the limbic forebrain. There is also some evidence suggesting that some cortical regions, such as the infralimbic region (il) of the medial prefrontal cortex, may also provide transsynaptic excitation, perhaps via relays in the brainstem. There is less evidence for excitatory input from other forebrain stress circuits, such as the ventral subiculum (vSUB), prelimbic division of the mPFC or paraventricular thalamus. Input from limbic regions may also access the PVN by interaction with local interneurons in the PVN surround (periPVN). Open circles: inhibitory (e.g., GABAergic) neurons; closed circles: excitatory (e.g., glutamatergic) neurons; squares: inhibitory input; arrowheads: excitatory inputs. Adapted from (79), with permission.
Brainstem noradrenergic neurons (mainly from the nucleus of the solitary tract (NTS)) are required for normal activation of the HPA axis to a variety of stressors, with a bias toward generation of reactive responses of relevance to homeostasis (e.g., following hypovolemia, inflammatory challenges or painful stimuli). These neurons are in receipt of visceral afferents from the vagus and sympathetic nervous system as well as local inflammatory signals, and provide for a rapid response to immediate physiological perturbations (see (74, 163)). The A2 noradrenergic and C2 adrenergic cell groups in the NTS preferentially innervate the CRH-producing subdivision of the PVN, commensurate with a strong role in dictating HPA axis activation (27, 28).
The PVN is a target for numerous stress-excitatory neuropeptidergic neurons, including angiotensin II (subfornical organ), glucagon-like peptide 1 (from the NTS), alpha-MSH (from the arcuate nucleus) and neuropeptide Y (multiple sources), to name a few (75). Peptidergic innervation largely emanates from viscerosensory relays and may in part underlie HPA axis activation in response to fluid/electrolyte balance (subfornical inputs), cardiovascular challenge (NTS), visceral illness (NTS) and metabolic imbalance (arcuate nucleus). In addition, PVN neurons produce numerous neuropeptides that may contribute to activation by local paracrine actions, either by recurrent collaterals or dendritic release (154). It is important to note that neuropeptides are often co-localized with so-called ‘classical’ neurotransmitters (such as norepinephrine, glutamate and GABA). Unlike classical transmitters, neuropeptides, packaged in dense core vesicles require bursting or high frequency activity, but provide a longer, often farther reaching response (see (10)). Neuropeptides almost always coexist with classical neurotransmitters (82), although in many neurons in the hypothalamus (PVN), neuropeptides often colocalize with other neuropeptides such as vasopressin and oxytocin (112). Neuropeptide actions can oppose or synergize with those of co-localized classical neurotransmitters. As it is generally assumed that the threshold for neuropeptide release is greater than that of classical neurotransmitters, neuropeptide signaling may serve to modify the overall impact of classical neurotransmitters on excitation of CRH neurons (a putative ‘neural’ feedback mechanism of sorts).
Glutamate provides synaptic drive to CRH neurons. Given the large number of glutamatergic inputs to the PVN, the precise source of glutamate input to CRH neurons is not completely known: potential sources include the hypothalamus (e.g., dorsomedial, lateral and posterior hypothalamic nuclei) and NTS (164, 178). Recent studies suggest that PVN neurosecretory neurons also release glutamate (83), and thus local release may also account for some excitatory drive.
Serotoninergic neurons of the Raphe nuclei send projections to both the PVN proper and the PVN surround. There is evidence to suggest that serotonin can increase HPA axis activation, although interpretation of the site of action is complicated by this complex innervation pattern (74).
Cytokines and other inflammatory factors (e.g., nitric oxide) also precipitate HPA axis responses at the level of the PVN. While cytokines can signal via ascending afferents via regions such as the NTS, there is some evidence to suggest that cytokines can promote prostaglandin synthesis and release from endothelial cells in the densely vascularized PVN (132). Prostaglandins may then promote PVN activation via EP3 receptors or mobilization of nitric oxide (132).
Recent studies suggest that GABA, usually considered the definitive inhibitory neurotransmitter, may also contribute to HPA axis excitation under conditions of chronic stress. Chronic stress appears to down regulate expression of KCC2, a protein required for maintenance of the cellular chloride gradient. Breakdown of the chloride gradient reverses the direction of ion flow through chloride channels, thus allowing GABA to depolarize, rather than hyperpolarize neurons (81).
The ‘rising’ phase: transsynaptic activation of the HPA axis
The cell groups noted above provide monosynaptic input to the PVN and are thus positioned to directly activate CRH neurons to mount reactive responses to homeostatic challenges. However, generation of anticipatory responses involves synthesis of multi-modal cues that require higher processing of sensory information. A large body of data suggests that anticipatory responses are mediated by descending information from limbic structures, including the ventral hippocampus/subiculum, infralimbic and prelimbic cortices, and amygdala (medial, central and basolateral nuclei). These upstream structures do not project directly to the PVN; rather, descending information is relayed to CRH neurons by intermediary neurons in the regions such as the bed nucleus of the stria terminalis (BST) and various hypothalamic nuclei, including the medial preoptic, dorsomedial and posterior hypothalamic nuclei, as well as neurons in the immediate vicinity of the PVN (periPVN) (see (74, 163)) (Figure 3).
The vast majority of limbic efferents targets GABAergic neurons in intervening nuclei that connect with the PVN. Given that PVN CRH neurons are under robust tonic inhibition (mediated by dense (inhibitory) GABAergic inputs), it is likely that HPA activation is subserved by disinhibition, caused by inhibition of GABAergic input to the PVN. Amygdalar activation of the HPA axis is likely to work by this mechanism, as the vast majority of principle amygdalar output (e.g., from the central and medial nuclei) is GABAergic, and projects to GABAergic, PVN-projecting neurons in the BST and hypothalamus (74, 163).
While a strong case can be made for an ‘amygdalar disinhibitory’ mechanism for generation of psychogenic stress responses, trans-synaptic excitation is also plausible. Descending prefrontal cortical and hippocampal projections are overwhelming glutamatergic in phenotype, requiring drive of the PVN to require intervening excitatory neurons. While hippocampal/cortical-PVN circuits are generally thought to inhibit the HPA axis (see below), there are some exceptions. For example, prefrontal cortex control of the HPA axis appears complicated, with data suggesting that the infralimbic (but not prelimbic) subregion may drive HPA axis responses to stress (127), perhaps in a lateralized fashion (157). Moreover, the infralimbic and prelimbic cortices have opposing roles in regulation of fear-related behaviors (122) and perhaps cardiovascular stress responses (158). Thus, it is possible that different populations of prefrontal neurons may contribute to activation vs. inhibition of stress responses. Of note, the infralimbic cortex projects directly to NTS (143), which sends excitatory norepinephrine, glutamate and GLP-1 projections to the PVN and would be a possible connection conferring trans-synaptic activation of PVN neurons.
The ‘falling’ phase: Inhibition of the stress response at the PVN
Limiting HPA axis activation is extremely critical for management of glucocorticoid responses. The ‘falling’ phase is marked by a rapid shut-off of ACTH release, followed by a more gradual return to baseline glucocorticoid secretory activity (Figure 2). A wealth of research suggests that both components are mediated in part by negative feedback inhibition, likely by way of glucocorticoid receptors (GRs) (78).
Rapid ‘off’ signals are mediated by several different mechanisms:
Glucocorticoid fast feedback mechanisms provide a means for rapid shut-off of the HPA axis at the level of CRH neurons. In the PVN, binding of glucocorticoids to its receptor (GR) cause rapid synthesis and release of endocannabinoids (ECs). The released ECs bind CB1 receptors on presynaptic terminals, inhibiting glutamate release and thereby reducing drive to CRH neurons (both in vitro and in vivo) (37, 42).
-
GABAergic inputs play a major role in limiting HPA axis responses to stress. Numerous hypothalamic nuclei send GABAergic projections to PVN CRH neurons, including the medial preoptic nucleus, dorsomedial hypothalamus, and lateral hypothalamic (Figure 4). In addition, the CRH-containing region of the PVN receives GABAergic input from the BST and zona incerta (74). There are also substantial populations of GABAergic neurons in the subparaventricular nucleus and the region immediately superior to the PVN, forming a GABAergic ‘halo’ around the nucleus. These regions may project into the PVN and likely form a local circuit network communicating PVN inhibition (72).
In general, GABAergic afferents emanate from regions that are well positioned to relay information from forebrain stress-regulatory regions involved in anticipatory stress responses (see below). This property suggests that GABAergic neurons may serve as a ‘gate keeper’ for PVN activation by descending corticolimbic structures.
The PVN is also innervated by neuropeptidergic terminals that can inhibit CRH release and/or HPA axis activation. Some candidates include enkephalin, somatostatin, and melanin concentrating hormone, to name a few (75). Once again, neuropeptides are often (though not always) co-localized with classical transmitters, and may serve the role of augmenting or diminishing the impact of the primary signaling molecule.
Activation of PVN neurons may be controlled by paracrine actions within the nucleus proper. As noted above, there is evidence for local release of endocannabinoids in response to stress, which presumably could act in glucocorticoid-independent fashion to inhibit CRH neurons. Local effects may involve non-neuronal elements; for example, emerging data suggest that local astrocytes may also play a role in EC signaling (38).
Figure 4.
Neural mechanisms of acute stress inhibition. As noted, the PVN receives substantial inhibitory input from hypothalamic (mPOA, DMH, periPVN) and medial forebrain (BST) structures. The regions receive excitatory inputs from forebrain structures such as the IL, PL and vSUB, which are thought to mediate trans-synaptic inhibition of HPA axis stress responses. Upstream limbic pathways may also limit drive of the mpPVN by way of local inhibition of HPA axis excitatory circuits, e.g., the NTS and/or PH. See Figure 3 legend for abbreviations. Adapted from (79), with permission.
Eventual post-stress return to baseline likely involves both passive (steroid ‘clearance’) and active processes (feedback). The active, feedback component involves relatively slow, transcription-independent mechanisms (termed ‘intermediate feedback’ by Dallman and colleagues (93)) and even slower transcriptional mechanisms (‘delayed feedback’) (93). These processes are mediated at least in part by GR (78). The PVN expresses abundant GR, which appears to mediate ‘fast’ (i.e., non-genomic) feedback on CRH neurons, leading to inhibition of the HPA axis stress responses (118, 142, 153). However, deletion of the PVN GR does not affect HPA axis responses to chronic stress exposure, and thus intermediate and delayed feedback may be mediated by GR present in neurons upstream of CRH neurons (153).
The ‘falling’ phase: transsynaptic inhibition of the HPA axis
Both the hippocampus and prefrontal cortex are implicated in trans-synaptic inhibition of CRH neurons (Figure 4). Both regions send glutamatergic projections to GABAergic PVN-projecting neurons in regions such as the BST (74, 163)). Indeed, select neurons in the BST receive inputs from both the prefrontal cortex and ventral subiculum, suggesting these two structures may work independently or in tandem to inhibit PVN activation (128). The hippocampus and prefrontal cortex express GR and MR in high abundance (69), positioning them to participate in feedback regulation of the HPA axis.
The hippocampus is generally divided into a dorsal and ventral component. The dorsal hippocampus primarily mediates cognitive functions, while the ventral hippocampus is important for stress regulation (44). Further, the ventral subiculum provides the stress-regulatory output from the hippocampus (26). Lesions of the ventral hippocampus generally produce prolonged anticipatory (but not reactive) responses, rather than affecting peak response magnitude. Prolonged stress responses in hippocampal (ventral subiculum) lesion rats are accompanied by enhanced depletion of CRH immunoreactivity over time, suggesting that lesions prolong central HPA axis activation, consistent with an effect on central ‘intermediate’ feedback signaling (73). Deletions of GR in the corticolimbic forebrain (including the hippocampus) result in prolonged HPA axis responses, similar to what is observed after hippocampal lesions (19, 54).
Lesions of ventromedial prefrontal cortex (encompassing parts of both infralimbic (IL) and prelimbic (PL) cortices) can exacerbate responses to psychogenic stressors (48, 125), and stimulation of the (prelimbic) prefrontal cortex reduces the magnitude of psychogenic glucocorticoid responses(90), consistent with a role in stress inhibition. Inhibition of HPA axis responses appear to be mediated by glucocorticoid feedback effects at the level of the PFC. Implants of corticosterone into the prefrontal cortex dampen stress responses (40), and local knockdown of GR in either the PL or IL produces exaggerated glucocorticoid responses to stress (111). As prefrontal manipulations affect the magnitude as well as duration of stress responses, prefrontal GRs appear to be involved in both rapid and intermediate feedback responses.
Trans-synaptic inhibition of the HPA axis may also be accomplished by GR binding to primary PVN afferents. Recent data suggests that blockade of GR in the NTS enhances glucocorticoid release and PVN Fos expression following acute psychogenic stress (57), indicating that glucocorticoid feedback may be mediated in part at the level of hindbrain PVN-projecting neurons.
Brain glucocorticoid feedback processes
As noted above, GR-mediated inhibition occurs at numerous CNS nodes, including rapid effects at the PVN and trans-synaptic modulation by way of the hippocampus, prefrontal cortex and hindbrain. Thus, feedback regulation should be considered a distributed process, with the end result at the PVN being a weighted summation of the contribution of multiple signaling pathways.
Glucocorticoids may also provide positive feedback in some brain structures, particularly under conditions of chronic drive (see below). For example, stress and high glucocorticoid levels increase expression of CRH in the central amygdaloid nucleus (106, 108), which is predicted to enhance CeA output and increase HPA axis reactivity.
It is important to appreciate that corticosterone (rats and mice) and cortisol (primates) in isolation cannot be assumed to reflect the HPA axis stress response. For example, decreases in adrenal sensitivity can mask activation of the hypothalamic and pituitary components of the axis, causing underestimation of the stress response. In the vast majority of cases, total corticosterone/cortisol in measured in plasma, reporting both free and CBG-bound glucocorticoid levels. Consequently, increases in CBG would reduce effective glucocorticoid concentrations, causing overestimation of the stress response. Finally, the dynamic aspect of the HPA stress response needs to be considered in the interpretation of data. Treatments that change the time course of the HPA response may affect the interpretation of any single time point: for example, hippocampal lesions impair shut-off of the HPA axis, effects that would be missed if sampling only an early post-stress corticosterone time point. The best practice for measurement of the HPA axis stress would be repeated measures analysis of both ACTH and glucocorticoids, enabling a broader view to the total hormonal response.
Stressor specificity: modalities and pathways
Characteristics of individual stressors by and large determine the magnitude and duration of HPA axis stress responses. Stressor intensity is a major factor in determining the overall trajectory of the HPA axis response: high intensity, long duration challenges (e.g., inflammatory stimuli) typically cause prolonged responses commensurate with the need to limit immune responses. For example, a single injection of lipopolysaccharide, an endotoxin, or interleukin 1 beta, a cytokine, cause prolonged and long-lasting ACTH (peak elevation at 1–2 hours, 4 hours until return to basal levels) and corticosterone (peak elevation at 1 hour, sustained for 6 hours) (141, 160). Shorter responses are typically observed following exposure to psychological stressor, for example, a strange or threatening environment or situation. Restraint, a commonly used stressor in rodents, produces an early peak of ACTH (typically 15–20 after initiation of stress; peak corticosterone responses typically occur between 30–60 min, and terminate within 2–3 hours after onset (examples: (22, 167)(rat);(153)(mouse). Importantly, in the case of restraint recovery begins while the stressor is still present (i.e., before they are released from the retrainer), suggesting the initial response to the stressor is waning even before the animal is removed from the stressful situation. Stressors of a presumptive ‘lesser’ severity (5 min exposure to a novel open field) produce lower peak HPA activation, corresponding roughly with the time-course seen following restraint (example: compare profiles in (54)).
As noted above, the mechanisms that underlie activation and inhibition of the HPA axis also differ between stressors. In general, stressors that signal threats to homeostasis (e.g., systemic inflammation, blood loss, hypoxia, fluid and electrolyte balance, hypoglycemia) are communicated directly to PVN CRH neurons, generally by monosynaptic relays from sensory organs (74). For example, information on blood volume or oxygenation is communicated via baro- and chemoreceptors to the NTS (1), which can then send direct noradrenergic projections to the PVN insuring a rapid HPA axis response (see (74, 163)). Information on fluid balance is likely relayed through excitatory projections from sensory circumventricular organs to the PVN (46). Information on energy balance appears to access the PVN via projections from the arcuate nuclei, which process circulating signals relevant to metabolic status (e.g., insulin, leptin) (9).
In contrast, responses that are elicited by psychological stimuli typically require relays linking one or more limbic structures with the PVN via intermediary nuclei, which are largely inhibitory in nature (see above). The limbic structures synthesize polymodal sensory information regarding the stimuli presented with mnemonic information regarding potential threat, resulting in generation of a response in anticipation of a plausible threat to health or well-being (74). Recent data suggest that inputs from multiple limbic regions converge on regions sending direct projections to the PVN (128), suggesting a mechanism whereby information from stress-excitatory and stress-inhibitory are synthesized to ‘optimize’ the net stress response (74, 163). It is important to note that different types of psychological stimuli may recruit the various limbic stress-regulatory pathways to different degrees.
The distinction between homeostatic and psychological stressors has been noted by numerous investigators, and has received a number of different designations: physical vs. psychological (or emotional) (31, 85); interoceptive vs. exteroceptive (140); systemic vs. processive (71); and more recently, reactive vs. anticipatory (74). The reactive vs. anticipatory designation that we favor stems from the observation that responses to homeostatic challenge occur by virtue of sensory events signaling physiological imbalance, and thus are in reaction to these conditions. The glucocorticoid response is recruited to meet this perceived need. In contrast, polysensory stimuli coming from the external environment (e.g., presence of a threatening conspecific or predator) are not accompanied by a physiological challenge, but may predict one (on the basis of mnemonic information or instinctual predispositions). Consequently, the response is initiated in anticipation of an adverse outcome. Glucocorticoids are released to enable the organism to meet a perceived challenge, in the event that additional resources (e.g., enhanced plasma glucose) would be needed to insure survival.
The overall temporal course does not appear to depend on modality. For example, in mice comparable periods of restraint and hypoxia produce corticosterone responses of comparable peak and duration (54), despite falling into the ‘anticipatory’ and ‘reactive’ categories, respectively. This observation suggests it is the net signal to the PVN that determines HPA axis drive, rather than the nature of the signal.
Factors modulating glucocorticoid response to stress
Neural and hormonal mechanisms discussed above are all subject to modification by genetic and experiential factors, changing the temporal dynamics of stress responses. Some factors that shape the stress response include:
Sex
Perhaps the most pronounced physiological modulators of the HPA axis are gonadal steroids. There is a substantial sex difference in both magnitude and duration of the stress responses, shaped by male (testosterone) and female (estradiol (E2), progesterone) sex hormones. In general, testosterone inhibits stress reactivity (see (171)). On the other hand, estradiol appears to enhance HPA axis responses. In rat studies, ovariectomized females or female in diestrus (period of low E2 secretion) exhibit low resting glucocorticoid secretion and crisp on-off responses to stressors, similar to those observed in males. Animals in estrus (high E2) and proestrus (high E2, high progesterone) have elevated basal corticosterone, enhanced peak corticosterone release, and delayed return to baseline (20, 49, 172). Importantly, an important site of E2 action appears to be at the ‘A’ of the HPA axis, whereby E2 enhances adrenal sensitivity to ACTH (50).
For the most part, the data summarized in the above sections are from male subjects. This is a critical consideration, as it is inappropriate to assume that regulatory processes (or even circuits) are common to males and females. Indeed, recent evidence indicates that female mice with corticolimbic deletion of the GR do not hypersecrete glucocorticoids in response to psychogenic stress (in contrast to male littermates), suggesting that fundamentally different feedback mechanisms may exist in the two sexes (151). Moreover, the energetic needs of males and females are different, which may account for the differing shapes of HPA axis responses. This may be particularly apparent during the receptive phase of the estrus cycle, where the physiological agenda of the female may shift to prepare for the possibility of fertilization and/or possible ‘stress’ associated with mating behavior.
The sexual dimorphism in HPA axis activity is also consistently observed in humans (168). Women show greater variability in stress-induced HPA axis activity than men (175). Indeed, circulating cortisol levels change across the menstrual cycle, with low levels associated with high estrogen states (94). A recent study demonstrated that women during the high estrogen state have lower activation of limbic regions associated with stress activation as measured by MRI following exposure to a psychosocial stressor (6). These and other studies provide evidence for the important modulatory role of gonadal steroids, particularly estradiol in the activation of the neuroendocrine stress response (91, 133). Because women during the reproductive age are twice as likely than man to develop stress-related psychopathologies including depression and PTSD, cyclic fluctuations of estradiol may contribute to the increased vulnerability of women (175).
Development and Aging
The HPA axis stress response varies widely with age. For example, rodents are born with the ability to mount HPA axis responses to stressors, but this ability is substantially impaired over the first and second week of life (the so-called ‘stress hyporesponsive period’(SHRP)) (see (139)). This is thought to be a process connected with final maturation of the adrenal that correlate to changes in adrenal sensitivity to ACTH as well as potential changes in CBG present in young rodents (e.g., corticosterone clearance in decreased and CBG is elevated during the SHRP period) (142).
Over the peri-pubertal period, HPA axis stress responses are exaggerated relative to adults, both in terms of ACTH and glucocorticoid release (134). Pronounced glucocorticoid release during this period suggests either enhanced central drive or a lag in the development of feedback pathways or mechanisms. As these responses occur in the absence of pathology, it is evident that these dynamics changes are critical for normal developmental progressions. Indeed, ‘forcing’ HPA drive during the SHRP with powerful immune stressors (34, 55) may be associated with hormonal dysregulation and behavioral stress hyper-reactivity in later life (see (173)).
Recent evidence from humans suggests that SHRP may also occur in infancy extending into childhood (63). Although cortisol stress responses are observed in newborn babies, this sensitivity is diminished by the first year of life. The rodent literature suggests that the SHRP is disrupted following maternal separation, suggesting that optimal maternal care is necessary for the maintenance of the SHRP. Although not as well studied, in humans maintenance of the SHRP may also depend on parental care and social stimulation (63), suggesting that the absence of these factors may disrupt the SHRP and disrupt the normal development of the stress response (104).
Development of the HPA axis can be influenced by maternal interactions. Prolonged maternal deprivation or an impoverished maternal environment produce disorganized maternal behavior that results in an over-reactive HPA axis in offspring (see (96)). Conversely, brief maternal separation increases attentiveness to pups and results in reduced stress reactivity in offspring later in life. Finally, pups of attentive dams (in terms of frequent licking and grooming) are less responsive to stressors in adulthood (103). The latter effect can be attributed to an epigenetic modification in the GR gene that results in elevated expression in the hippocampus (174), which may result in tighter feedback regulation of stress responses.
While the development tuning of the HPA axis stress response is very real, one should interpret the significance of these lasting changes with caution. The glucocorticoid response to stress is important for survival, and needs to be sufficient to allow the organism to meet real or perceived challenges. Clearly, an overactive HPA axis places the organism at the mercy of catabolic actions of glucocorticoids, which could damage key physiological systems (as well as neurons). For example, glucocorticoids exert pronounced catabolic effects on hippocampal neurons and, consequently, glucocorticoid concentration correlates with neuronal density in the hippocampus across the lifespan. Specifically, glucocorticoid-induced neuronal catabolism produces vulnerability to metabolic insults, an effect mitigated by supplementation with energy substrates (e.g. glucose, mannose, etc.) (137). However, an insufficient glucocorticoid response under conditions of pronounced stress render the individual ill-prepared to meet a life-threatening challenge. For example, in humans, a low GR (cortisol) response to stress predicts enhanced susceptibility to development of PTSD (126).
A sizable literature indicates that aging increases basal HPA axis function and stress responsiveness (see (136)). Age-related enhancement of glucocorticoid secretion is thought to be the result of loss of glucocorticoid negative feedback, primarily through loss of GR in hippocampal circuits (138). (138). In humans, aging is associated with increased cortisol levels (123, 146), increased cortisol production (123), and increased cortisol responses to cognitive challenge (146). This aging-induced increase in cortisol occurs in both males (11) and females (115), but may be more robust in females (146, 176). Aging increases total cortisol exposure by impairing HPA feedback inhibition, as the elderly have delayed or blunted ACTH inhibition responses to cortisol infusion (18, 129, 176, 177). These higher cortisol levels are also associated with increased cognitive decline in elderly humans (99).
As one may expect with aging, there are marked individual differences in age effects on the HPA axis: marked HPA axis hyperactivity is observed in some individuals, whereas others preserve normal HPA axis function throughout life. Importantly, HPA axis hyperactivity is correlated with negative outcomes and longevity (e.g., memory deficits occur primarily in aged rats showing hippocampal GR loss (84), and the HPA axis hyperactivity seen in aged F344 rats (24 months) is not observed in the more long-lived F344/Brown-Norway strain (36 months)) (147)). Overall, the data suggest that appropriate control of HPA axis function is associated with successful aging.
One cannot discuss the stress and aging connection without acknowledging that the reproducibility of aging effects across laboratories has been problematic. Replication issues include differences in HPA regulatory patterns (e.g., unchanged vs. increased basal glucocorticoid release; increased, unchanged or decreased PVN CRH mRNA expression; enhanced vs. reduced adrenal sensitivity to ACTH) (e.g., compare (23, 67, 77, 136)). These discrepancies may be linked to differences in the strains employed, or perhaps to the life history of the animals themselves. Aging tends to increase the variability in HPA axis reactivity. Genetics and life history (e.g., mothering and perinatal environment (see above), cumulative stress exposure) may predispose dysregulation of the HPA axis or protect against it, depending on the challenges placed before the aged individual.
Corticosteroid Binding Proteins
In blood, corticosteroids are extensively bound by plasma proteins, including a specific binding molecule, corticosteroid binding globulin (CBG) (approximately 80% under basal secretory conditions), as well as albumins (15%). Thus, only about 5% of hormone is readily available for receptor action under resting conditions (113). However, plasma binding protein capacity becomes saturated in the low physiological range, and is not able to buffer the impact of stress-related glucocorticoid secretion on receptor systems (113). Nonetheless, the presence of CBG causes most standard assay methods (i.e, those that do not separate free corticosterone from bound) to vastly overestimate the amount of corticosteroids capable of binding receptors in the low- to mid-physiological range of steroids.
Generally, plasma CBG secretion does not show a substantial diurnal rhythm and is not altered in the time frame of an acute stress response, leading most to discount an active role in shaping dynamic responses to HPA axis stimulation (113). However, there are numerous conditions where alterations in baseline secretion of CBG may be a factor. The most obvious is in the context of sex differences, given that levels of CBG in females exceed those in males (159), at least during periods of elevated estrogens. Increased circulating CBG may compensate (at least in part) for higher basal glucocorticoid release in females. In addition, CBG is negatively regulated by corticosteroids (45), and as a consequence is reduced under conditions of chronic stress (155). Thus, it is probable that CBG (as well as plasma albumins) may play a role in adjustment of HPA axis stress responses under various conditions, and perhaps increase the impact of glucocorticoids during periods of elevated secretory activity.
Recent studies suggest that in addition to binding circulating steroids, CBG may also play an active role in glucocorticoid signaling, either by modulating steroid delivery or taking an active role in signaling at the membrane (see (113)). In combination with its impact on glucocorticoid binding, these data suggest that CBG needs to be considered in discussion of HPA axis activity and stress responses.
Chronic Stress
The sections above largely deal with responses of the HPA axis to novel challenges. However, stress is often repeated or prolonged, causing chronic central drive to neurons controlling the stress response. Chronic stress exposure causes marked changes in both baseline HPA function and responsiveness to stress that are long-lasting in nature and invoke different regulatory mechanisms (see (70, 162). Moreover, the overall impact of chronic stress on the organism depends on the severity of the stressor or stressors, their modality, and the extent to which the organism can predict or cope with the challenge.
It is important to consider chronic stress to be a cumulative process. An HPA axis response occurs to each individual stressor over the course of repeated or prolonged exposure, and consequently the cumulative glucocortiocoid burden is increased. This is evident from somatic changes in HPA responsive organs. The adrenal frequently (but not always) increases in size, a product of repeated exposure to ACTH and perhaps increased sympathetic drive (see (162)). Moreover, the adrenal becomes more sensitive to ACTH, meaning that glucocorticoid responses to a given stressor will be amplified (see (162)). In addition, glucocorticoid-sensitive immune organs (e.g., thymus) will undergo cell death and involution, and consequently give evidence of a history of increased glucocorticoid release (70)). In addition, chronic stress regimens can result in a frank increase in baseline glucocorticoid release, usually observed during the nadir of the circadian rhythm (70, 162). The mechanism for this increase may be related to loss of glucocorticoid feedback control of the HPA axis, which could be associated with decreased GR in regions such as the hippocampus and prefrontal cortex (as well as the PVN). The presence or degree of baseline hypersecretion (and indeed, GR down-regulation) varies greatly across stress regimens and between strains of rat or mouse (e.g., see (61, 70, 80).
Habituation to repeated stress
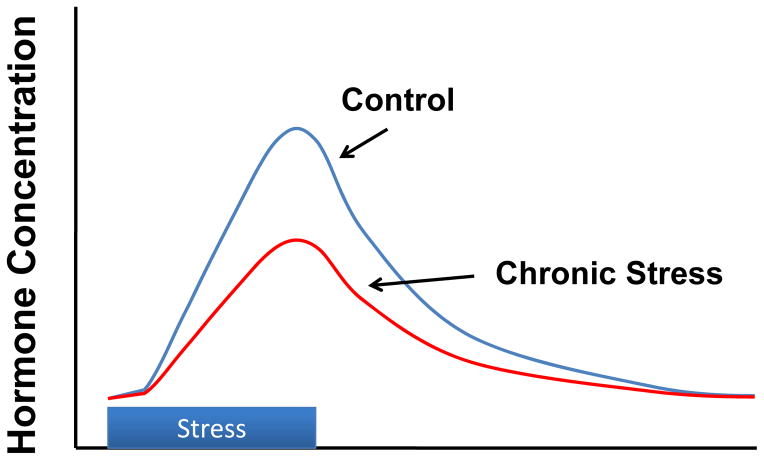
Repeated exposure to the same stressor (a so-called ‘homotypic’ stressor) can result in habituation of the HPA axis response, characterized by a decreasing glucocorticoid response over time (see (36)(Figure 5). Importantly, while the response decreases with subsequent experiences, it does not go away: the organism is still undergoing repeated stress. Research into mechanism suggests that the decrement in HPA drive requires the MR, as blockade impairs the ability to habituate (25). Habituation appears to be mediated at least in part by the paraventricular thalamic nucleus (PVT): this region undergoes selective activation upon presentation of a familiar stressor, and lesion or inactivation of this area blocks the habituation process (15).
Figure 5.
Habituation of glucocorticoid stress responses following chronic stress, often observed after repeated or predictable stressor exposure. Modified from (68)
Importantly, some degree of habituation is evident following exposure to varying (heterotypic) stressors as well. For example, animals typically show a marked weight loss and hypophagia in the first few days of a chronic variable stress regimen (as part of the integrated bodily response to stress), but return to normal eating patterns over the ensuing time period (indicative of some degree of stress resilience) (51). While the HPA axis endpoints following a non-habituating stress regimen (e.g., chronic variable stress) are typically more pronounced than those seen following repeated homotypic stressor exposure, it is incorrect to assume that the individual is not ‘adapting’ to the regimen.
Not all homotypic stressors cause response habituation. Responses to more ‘severe’ stressors (e.g., repeated predator exposure (47)) are maintained over time (47), perhaps due to the higher perceived ‘cost’ required to adapt to the particular situational demands.
Facilitation of Stress Responses
Despite being exposed to repeated stress, the HPA axis stress response to a new stressor is either maintained or increased, indicating the existence of mechanisms that drive the HPA axis despite the increased feedback signal generated by episodic or cumulative increases in glucocorticoid exposure (4). Exposure to a prior stressor facilitates the glucocorticoid response to a second stressor, resulting in a faster onset of glucocorticoid release and higher peak glucocorticoid levels to the successive stimulus (33, 102). This partly depends on stressor stimulus intensity. Two successive small challenges to the HPA axis result in an attenuation of the adrenocortical response to the second stimulus, due to feedback inhibition. However, if either of the stimuli are sufficiently intense, facilitation will override the feedback inhibition (56). Importantly, potentiation of HPA responses to repeated stressors appear to mediated at least in part by post-stress increases in ACTH release or enhanced adrenal sensitivity (101, 102, 130, 162).
Facilitation occurs following habituating and non-habituating regimens (Figure 6)(see data in (15, 165)), indicating it is a general reaction to repeated stress exposure. Facilitation provides a mechanism for the organism to respond to new stimuli despite a history of stress exposure, either familiar or variant.
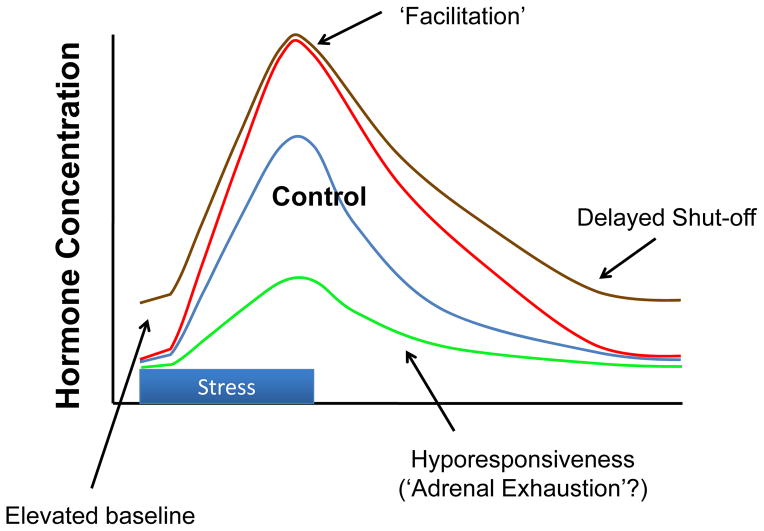
Figure 6.
Potential glucocorticoid profiles seen following non-habituating chronic stressors (e.g., seen after chronic unpredictable stress, chronic social stress or severe stress regimens). Depending on both the regimen and the individual, chronic stress profiles may be manifest as increased basal glucocorticoid secretion (usually at the time of the circadian nadir); delayed shut-off of the stress response (due to reduced feedback efficacy); facilitated or sensitized responses to novel stressors; or in extreme cases, hyporesponsiveness driven by adrenal exhaustion. Modified from (68).
The mechanism underlying facilitation involves circuits connecting with the PVT (14), further suggesting an important role for this region in integrating stress ‘chronicity’. As was the case for habituation, blockade of PVT signaling (lesions) prohibits stress facilitation (15). The PVT has rich connections with the circadian timing system (suprachiasmatic nucleus), medial prefrontal cortex and basolateral amygdala, suggesting that it may integrate mnemonic as well as temporal information regarding stressor exposure.
Modality and Severity
An in-depth discussion of the merits and shortcomings of experimental chronic stress regimens would require a review of its own, and is beyond the scope of the current discussion. Nonetheless, it is important to consider attributes that are common or variant across stress regimens, in order to sort properties that drive the overall impact of chronic stress and/or glucocorticoids on the organism.
Above, we highlighted HPA axis changes consequent to chronic stress. As noted above, genetics and environment determine which form an HPA axis response will be manifest. Nonetheless, one should take care not to define ‘chronic stress’ by the HPA axis response, as numerous other systems participate in physiological adaptation (e.g., autonomic nervous system, immune system, brain behavioral prioritization, etc).
In general, the more ‘severe’ the stress regimen, the more pronounced the HPA axis phenotype (119). For example, a study comparing stress regimens in rats show marked habituation with repeated exposure to a mild stressor (mild footshock), whereas adaptation is less pronounced following immobilization (124). A recent study in our group demonstrated that CVS causes increased basal corticosterone secretion, whereas repeated restraint performed in a parallel cohort does not (58). Examination of two different ‘intensities’ of repeated restraint stress (0.5 h vs. 3 h per day) reveal intensity-related increases in BST and CeA CRH mRNA expression, along with increases in PVN AVP mRNA expression only in the 3 h/day restraint group (62). Stress regimens that expose mice to repeated social defeat and social instability can cross the line from enhanced adrenal reactivity to adrenal exhaustion, a condition associated with substantial physical morbidity (131)(Figure 6). Nonetheless, one should take care in defining ‘severity’, as the response to chronic stress is dependent on the animal’s reaction, and avoid preconceived notions regarding what would constitute ‘severity’ or ‘intensity’ to the organism.
Recently, the field has leaned toward use of chronic stress regimens that have putative ‘ethological relevance’ for the subjects. The argument for ethological approaches stems from the assumption that exposure to ‘stressors’ that are not within the animal’s natural repertiore of experience are less relevant to understanding stress processing, and thus less applicable to extrapolation to the human condition. While this logic can be argued, the methods chosen (including chronic social defeat; chronic predator or predator odor exposure; chronic social stress) elicit robust HPA responses and can be readily employed to study mechanisms of stress control.
One important product of social stress models is the ability to sort stress-susceptible from stress-resistant individuals. For instance, social defeat stress involves daily exposure of rats or mice to a larger conspecific in the latter’s home cage. The larger individual usually defends its home cage ‘territory’ by initiating aggressive interactions with the ‘intruder’ animal. Repeated defeat can produce groups of animals that will largely avoid interaction with the resident (variably referred to as ‘passive copers’ or ‘stress susceptible’) and groups that will re-engage the resident (variably referred to as ‘active copers’ or ‘stress resistant’) (35, 95)). Animals that actively re-engage the resident have lower corticosterone responses following defeat and reduced PVN CRH expression relative to passive copers, suggesting that reduced HPA function may either contribute to stress resistance or be a consequence of it.
Social stress can also occur in a colony environment, where male rats are housed in the presence of females in a large enclosure (‘visible burrow’) (16). In this environment, one rat generally becomes dominant, engaging in aggressive interactions with the other colony dwellers (who become ‘submissive’). The dominants and submissives show marked phenotypic differences to living in the colony environment: the submissives lose considerable weight and exhibit a pattern of defensive wounds. The pattern of HPA activity also differs considerably: the dominants respond to a novel restraint challenge in similar fashion to control rats (paired housed), along with an increase in PVN CRH expression. In contrast, two patterns of HPA axis disturbance become evident in submissives: one subgroup (stress-responsive) exhibits a corticosterone and CRH profile similar to that of the dominant, along with a down-regulation of hippocampal GR expression not observed in the latter (5). In contrast, the second (stress non-responsive) mounts a severely blunted HPA axis response, along with a marked diminution of PVN CRH mRNA expression. The ‘non-responsive’ phenotype is associated with normal expression of GR in hippocampus and reduced CBG levels, suggesting an enhanced resting feedback signal (16). While impossible to determine whether these differences are ‘state’ or ‘trait’, reduced HPA responsiveness and loss of CRH expression is consistent with an enhanced feedback signal over time, either via adrenal drive or enhanced central GR signaling.
Neurocircuit Mechanisms of Chronic Stress Adaptation or Maladaptation
Since chronic stress and disease states such as PTSD and depression are linked, it is important to elucidate neurocircuit mechanisms underlying physiological and behavioral changes seen under conditions of prolonged stress exposure. As noted above, the PVT seems to be very important in registering chronicity of stressors, having an important role in both stress habituation and sensitization. However, it is important to also consider several other nodes that show differential involvement in acute vs. chronic stress (see Figure 7).
Figure 7.
Neural mechanisms controlling chronic stress regulation of the HPA axis. Pathways responsible for drive of the HPA axis under chronic stress are not as well understood as those mediating acute response. There is strong evidence that the PVT, which is not involved in acute stress excitation or inhibition, is required for both stress habituation and stress facilitation, suggesting a role in communicating stress chronicity. Importantly, the PVT has extensive reciprocal projections to the IL, PL and vSUB, as well as projections to the area of the BST. Neuronal activation studies indicate the existence of a small network of structures that are differentially activated by chronic unpredictable stress (relative to restraint), including the IL, PL, PH and NTS. Importantly, the PH and NTS are both connected with the IL, and both mediate acute stress excitation, suggesting a possible integrated circuit mediating chronic stress drive. Finally, chronic stress increases tone of CRH-expressing stress circuitry, suggesting that CRH systems may be recruited by chronic stress and participate in HPA axis hyperdrive. See Figure 3 legend for abbreviations. Adapted from (79), with permission.
Human imaging studies link the subgenual anterior cingulate cortex (sgACC, area 25) with both depression and PTSD (41, 110, 148). The rodent homologue of human sgACC is the IL (121), which is intimately involved in regulation of HPA axis and behavioral responses to stress in rodents (111). The IL (as well as PL) is disproportionately activated by chronic unpredictable stress exposure (CVS) relative to an habituating chronic restraint stress procedure as assessed by expression of fosB, a marker of chronic stress activation, suggesting this structure may be differentially recruited under chronic stress conditions (53). Other stress-regulatory regions, such as the hippocampus, basolateral amygdala, central amygdaloid nucleus and PVT, do not show CVS-induced activation in excess of that seen following chronic restraint, suggesting that IL and PL hyperactivation by unpredictable stress is unique among upstream limbic regulatory sites (53). Interestingly, the only other brain regions exhibiting selective recruitment by CVS are the posterior hypothalamic region and the nucleus of the solitary tract, both of which are targeted by the IL (53). Moreover, while the PVT was not differentially activated, it has heavy reciprocal connections with both IL and PL (170), and is thus positioned to relay information on stress chronicity to and from the cortex. Consequently, it is possible that IL (and perhaps PL) play an important role in regulating the physiologic and behavioral impact of chronic stress.
There is also a considerable literature linking central CRH pathways with chronic stress integration. In particular, CRH expression is markedly enhanced in the CeA as well as the BST following exposure to high levels of glucocorticoids (106, 107) and in some (e.g., chronic social subordination, chronic pain) (5, 166)) but not all (e.g., CVS, predator) (47, 165) chronic stress paradigms. As both the CeA and BST project strongly to behavioral, neuroendocrine and autonomic effectors circuits, up-regulation of CRH predicts enhanced capacity for stress excitation, and is purported to reflect a ‘chronic stress-recruited’ pathway (30).
In addition to involvement in CRH signaling, the BST also shows marked regulatory heterogeneity following acute vs. chronic stress. The anterior BST appears to be required for acute stress excitation, in that damage to this structure reduces ACTH and corticosterone responses to stress. This contrasts with the posterior BST, which is required for stress inhibition (22). When animals are exposed to a chronic stress regimen (CVS), the role of the anterior BST appears to be reversed, as lesions potentiate the impact of a novel stressor on HPA axis activation (21). These data suggest that chronic stress may invoke functional changes in existing stress regulatory circuits, essentially using the same pathways to different ends.
Finally, it is important to note that in many cases, regions regulating acute stress responses are not necessarily involved in regulating chronic reactivity. For example, lesions of noradrenergic pathways or the medial amygdaloid nucleus result in blunted acute stress responses, but do not block thymic atrophy, adrenal hypertrophy or HPA axis corticosterone secretion following chronic stress (52, 152)). Conversely, circuit manipulations that potentiate acute HPA axis reactivity (e.g., ventral subiculum lesions) (76) do not exacerbate HPA axis responses to chronic stress. Thus, chronic stress-induced HPA axis drive is likely to use circuitry that is distinct from that employed under acute stimulation conditions.
Perspective
The ability of the HPA axis to change dynamically with respect to experience is critical for coping with adversity. As noted above, genes, environmental circumstances and gene x environment interactions can affect response patterns. It is critical to note that in all of these cases, adaptations accomplished by the HPA axis should not be considered pathological. For example, under conditions of chronic stress, it is important to engage the system so as to maintain responsiveness even in the face of glucocorticoid feedback: hence, the sensitized adrenal and the enhanced neural response to novel threats. Changes caused by adversity in early development may well be a mechanism to prepare the individual for a ‘cruel world’, assuring that appropriate responses can be mounted when faced with stressors later in life. The latter effect is consistent with the ‘match-mismatch’ hypothesis regarding developmental programming of stress reactivity: whereas early stress may prepare the organism for coping with stress later in life, an ‘unstressed’ early life may make individual more prone to mounting inappropriate stress responses (120).
The key factor in determining adaptation vs. pathology is likely to be context. There are conditions where robust responses are needed; if they are not mounted, the organism will have suboptimal coping capacity (see Figure 8). For example, low HPA axis activity is thought to be a trait variable affecting susceptibility to post-traumatic stress disorder. Conversely, mounting inappropriate HPA axis responses when they are not needed may be equally problematic, as increased glucocorticoid ‘burden’ may contribute to physical pathologies and perhaps major depressive illness. Thus a healthy HPA axis may be linked to moderation: the axis should be able to be engaged in an appropriate context, and disengaged quickly when threats have passed.
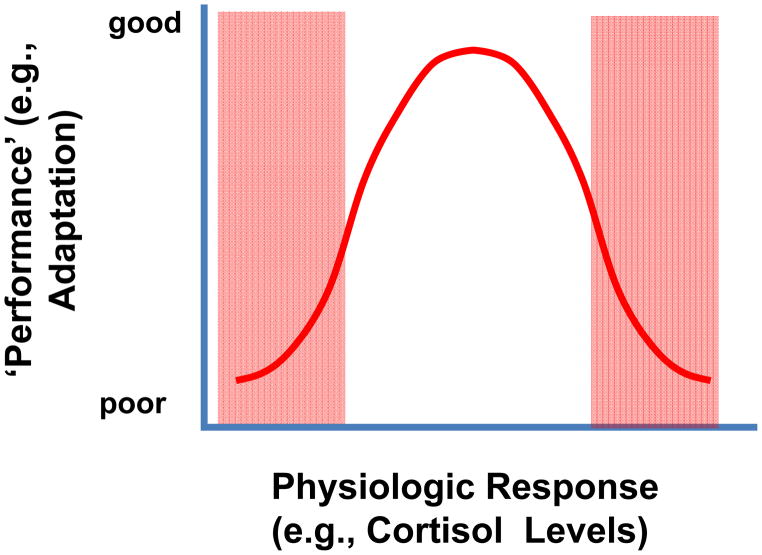
Figure 8.
Inverted U-shaped relationship between increasing levels of glucocorticoids (arrow) and ‘systems performance’ (e.g., spatial memory). Optimal systems performance is generally observed at intermediate levels of glucocorticoid availability, consistent with the need for glucocorticoids to supply an appropriate context for adaptation. Performance is generally degraded if glucocorticoid secretion is insufficient or hyperresponsive. See deKloet (1998) (32) for discussion. Modified from (68).
Glucocortiocoids also have profound context- specific effects on cognition that are of relevance to neuropsychiatric disorders linked to stress, including PTSD and depression. For instance, hydrocortisone, a synthetic glucocorticoid, impairs memory when given immediately prior to a memory encoding task, but enhances it when given over 2 hours prior to the task (169). This experiment highlights the dual ability of glucocorticoids to both impair and enhance cognitive function, depending on the context. Hydrocortisone given to patients immediately following a traumatic experience has also been shown to diminish PTSD-like symptomology in patients (66, 116, 179). Thus, exploiting the timing of glucocorticoid action using hydrocortisone or cortisol in conjunction with traumatic exposure, has been proposed as a potential way to reduce PTSD in humans (66). Prolonged glucocorticoid exposure is also thought to increase perserveration in humans and in rodents, which is relevant to depression, a disorder characterized in part by constant negative rumination (39, 150). This switch to habitual responding can be adaptive when responding to the stimulus repeatedly, limiting energy expenditure and cognitive appraisal. However, when behavioral flexibility is required, this leaves the habitually responding individual at a disadvantage and may be considered pathological. Thus, glucocorticoids affect ‘systems performance’ (e.g. memory, behavioral flexibility, decision-making, etc) in a context-specific manner that can be either adaptive or pathological depending on the circumstances.
Conclusion
The HPA axis stress response is one of many bodily systems that is designed to help the organism cope with adversity. The importance of this system is underscored by its conservation across species and maintenance of dynamic responsiveness across the life span. An array of intrinsic regulatory processes governs the activity of hypothalamic CRH neurons, anterior pituitary corticotropes, and steroidogenesis in the adrenal cortex. Further, organismal regulation of the HPA axis can be mediated by mechanisms as diverse as plasma binding proteins, sex steroids, and the autonomic nervous system. Importantly, previous stress history interacts with current environmental demands to regulate HPA axis activity. Critical components of this interaction are the divergent and interconnected forebrain limbic sites that provide substantial regulation of HPA axis stress responding. The dynamics of the HPA axis require a tightly-controlled balance of excitation and inhibition for appropriate stress responding and adaptation to environmental demand. Understanding the mechanisms mediating the beneficial and (potentially) deleterious aspects of glucocorticoid action will certainly advance our understanding of health and disease.
References
- 1.Accorsi-Mendonca D, Machado BH. Synaptic transmission of baro- and chemoreceptors afferents in the NTS second order neurons. Auton Neurosci. 2013;175:3–8. doi: 10.1016/j.autneu.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Aguilera G. Regulation of pituitary ACTH secretion during chronic stress. Front Neuroendocrinol. 1994;15:321–350. doi: 10.1006/frne.1994.1013. [DOI] [PubMed] [Google Scholar]
- 3.Aguilera G, Rabadan-Diehl C. Vasopressinergic regulation of the hypothalamic-pituitary-adrenal axis: implications for stress adaptation. Regul Pept. 2000;96:23–29. doi: 10.1016/s0167-0115(00)00196-8. [DOI] [PubMed] [Google Scholar]
- 4.Akana SF, Dallman MF, Bradbury MJ, Scribner KA, Strack AM, Walker CD. Feedback and facilitation in the adrenocortical system: unmasking facilitation by partial inhibition of the glucocorticoid response to prior stress. Endocrinology. 1992;131:57–68. doi: 10.1210/endo.131.1.1319329. [DOI] [PubMed] [Google Scholar]
- 5.Albeck DS, McKittrick CR, Blanchard DC, Blanchard RJ, Nikulina J, McEwen BS, Sakai RR. Chronic social stress alters levels of corticotropin-releasing factor and arginine vasopressin mRNA in rat brain. J Neurosci. 1997;17:4895–4903. doi: 10.1523/JNEUROSCI.17-12-04895.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albert K, Pruessner J, Newhouse P. Estradiol levels modulate brain activity and negative responses to psychosocial stress across the menstrual cycle. Psychoneuroendocrinology. 2015;59:14–24. doi: 10.1016/j.psyneuen.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antoni FA. Hypothalamic control of adrenocorticotropin secretion: Advances since the discovery of 41-residue corticotropin-releasing factor. Endocrine Rev. 1986;7:351–378. doi: 10.1210/edrv-7-4-351. [DOI] [PubMed] [Google Scholar]
- 8.Armario A, Castellanos JM. Effect of acute and chronic stress on testosterone secretion in male rats. J Endocrinol Invest. 1984;7:659–661. doi: 10.1007/BF03349502. [DOI] [PubMed] [Google Scholar]
- 9.Baskin DG, Figlewicz Lattemann D, Seeley RJ, Woods SC, Porte D, Jr, Schwartz MW. Insulin and leptin: dual adiposity signals to the brain for the regulation of food intake and body weight. Brain Res. 1999;848:114–123. doi: 10.1016/s0006-8993(99)01974-5. [DOI] [PubMed] [Google Scholar]
- 10.Bean AJ, Roth RH. Extracellular dopamine and neurotensin in rat prefrontal cortex in vivo: effects of median forebrain bundle stimulation frequency, stimulation pattern, and dopamine autoreceptors. J Neurosci. 1991;11:2694–2702. doi: 10.1523/JNEUROSCI.11-09-02694.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belanger A, Candas B, Dupont A, Cusan L, Diamond P, Gomez JL, Labrie F. Changes in serum concentrations of conjugated and unconjugated steroids in 40- to 80-year-old men. J Clin Endocrinol Metab. 1994;79:1086–1090. doi: 10.1210/jcem.79.4.7962278. [DOI] [PubMed] [Google Scholar]
- 12.Benjannet S, Rondeau N, Day R, Chretien M, Seidah NG. PC1 and PC2 are proprotein convertases capable of cleaving proopiomelanocortin at distinct pairs of basic residues. Proc Natl Acad Sci U S A. 1991;88:3564–3568. doi: 10.1073/pnas.88.9.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bethin KE, Vogt SK, Muglia LJ. Interleukin-6 is an essential, corticotropin-releasing hormone-independent stimulator of the adrenal axis during immune system activation. Proc Natl Acad Sci U S A. 2000;97:9317–9322. doi: 10.1073/pnas.97.16.9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary- adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84:1025–1039. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- 15.Bhatnagar S, Huber R, Nowak N, Trotter P. Lesions of the posterior paraventricular thalamus block habituation of hypothalamic-pituitary-adrenal responses to repeated restraint. J Neuroendocrinol. 2002;14:403–410. doi: 10.1046/j.0007-1331.2002.00792.x. [DOI] [PubMed] [Google Scholar]
- 16.Blanchard DC, Sakai RR, McEwen B, Weiss SM, Blanchard RJ. Subordination stress: behavioral, brain, and neuroendocrine correlates. Behav Brain Res. 1993;58:113–121. doi: 10.1016/0166-4328(93)90096-9. [DOI] [PubMed] [Google Scholar]
- 17.Bornstein SR, Engeland WC, Ehrhart-Bornstein M, Herman JP. Dissociation of ACTH and glucocorticoids. Trends Endocrinol Metab. 2008;19:175–180. doi: 10.1016/j.tem.2008.01.009. Highlights work demonstrating regulation of corticosteroid secretion at the level of the adrenal. [DOI] [PubMed] [Google Scholar]
- 18.Boscaro M, Paoletta A, Scarpa E, Barzon L, Fusaro P, Fallo F, Sonino N. Age-related changes in glucocorticoid fast feedback inhibition of adrenocorticotropin in man. J Clin Endocrinol Metab. 1998;83:1380–1383. doi: 10.1210/jcem.83.4.4745. [DOI] [PubMed] [Google Scholar]
- 19.Boyle MP, Kolber BJ, Vogt SK, Wozniak DF, Muglia LJ. Forebrain glucocorticoid receptors modulate anxiety-associated locomotor activation and adrenal responsiveness. J Neurosci. 2006;26:1971–1978. doi: 10.1523/JNEUROSCI.2173-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carey MP, Deterd CH, de Koning J, Helmerhorst F, de Kloet ER. The influence of ovarian steroids on hypothalamic-pituitary-adrenal regulation in the female rat. J Endocrinol. 1995;144:311–321. doi: 10.1677/joe.0.1440311. [DOI] [PubMed] [Google Scholar]
- 21.Choi DC, Evanson NK, Furay AR, Ulrich-Lai YM, Ostrander MM, Herman JP. The anteroventral bed nucleus of the stria terminalis differentially regulates hypothalamic-pituitary-adrenocortical axis responses to acute and chronic stress. Endocrinology. 2008;149:818–826. doi: 10.1210/en.2007-0883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, Herman JP. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputs. J Neurosci. 2007;27:2025–2034. doi: 10.1523/JNEUROSCI.4301-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cizza G, Calogero AE, Brady LS, Bagdy G, Bergamini E, Blackman MR, Chrousos GP, Gold PW. Male Fischer 344/N rats show a progressive central impairment of the hypothalamic-pituitary-adrenal axis with advancing age. Endocrinol. 1994;134:1611–1620. doi: 10.1210/endo.134.4.8137722. [DOI] [PubMed] [Google Scholar]
- 24.Clark BJ, Wells J, King SR, Stocco DM. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR) J Biol Chem. 1994;269:28314–28322. [PubMed] [Google Scholar]
- 25.Cole MA, Kalman BA, Pace TW, Topczewski F, Lowrey MJ, Spencer RL. Selective blockade of the mineralocorticoid receptor impairs hypothalamic-pituitary-adrenal axis expression of habituation. J Neuroendocrinol. 2000;12:1034–1042. doi: 10.1046/j.1365-2826.2000.00555.x. [DOI] [PubMed] [Google Scholar]
- 26.Cullinan WE, Herman JP, Watson SJ. Ventral subicular interaction with the hypothalamic paraventricular nucleus: Evidence for a relay in the bed nucleus of the stria terminalis. J Comp Neurol. 1993;332:1–20. doi: 10.1002/cne.903320102. [DOI] [PubMed] [Google Scholar]
- 27.Cunningham ET, Jr, Sawchenko PE. Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. J Comp Neurol. 1988;274:60–76. doi: 10.1002/cne.902740107. [DOI] [PubMed] [Google Scholar]
- 28.Cunningham ET, Jr, Bohn MC, Sawchenko PE. Organization of adrenergic inputs to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J Comp Neurol. 1990;292:651–667. doi: 10.1002/cne.902920413. [DOI] [PubMed] [Google Scholar]
- 29.Dallman MF, La Fleur SE, Pecoraro NC, Gomez F, Houshyar H, Akana SF. Minireview: Glucocorticoids - Food intake, abdominal obesity, and wealthy nations in 2004. Endocrinology. 2004;145:2633–2638. doi: 10.1210/en.2004-0037. [DOI] [PubMed] [Google Scholar]
- 30.Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, Bell ME, Bhatnagar S, Laugero KD, Manalo S. Chronic stress and obesity: a new view of “comfort food”. Proc Natl Acad Sci U S A. 2003;100:11696–11701. doi: 10.1073/pnas.1934666100. An influential paper positing both the importance of CRH neuron recruitment to chronic stress drive and their regulation by peripheral metabolic signals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dayas CV, Buller KM, Crane JW, Xu Y, Day TA. Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur J Neurosci. 2001;14:1143–1152. doi: 10.1046/j.0953-816x.2001.01733.x. [DOI] [PubMed] [Google Scholar]
- 32.De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. Definitive exposition of the interplay between MR and GR, wedding the ‘inverted U-shaped curve’ to glucocorticoid signaling mechanisms. [DOI] [PubMed] [Google Scholar]
- 33.DeMaria EJ, Lilly MP, Gann DS. Potentiated hormonal responses in a model of traumatic injury. J Surg Res. 1987;43:45–51. doi: 10.1016/0022-4804(87)90045-x. [DOI] [PubMed] [Google Scholar]
- 34.Dent GW, Smith MA, Levine S. The ontogeny of the neuroendocrine response to endotoxin. Brain Res Dev Brain Res. 1999;117:21–29. doi: 10.1016/s0165-3806(99)00091-7. [DOI] [PubMed] [Google Scholar]
- 35.Der-Avakian A, Mazei-Robison MS, Kesby JP, Nestler EJ, Markou A. Enduring deficits in brain reward function after chronic social defeat in rats: susceptibility, resilience, and antidepressant response. Biol Psychiatry. 2014;76:542–549. doi: 10.1016/j.biopsych.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dhabhar FS, McEwen BS, Spencer RL. Adaptation to prolonged or repeated stress--comparison between rat strains showing intrinsic differences in reactivity to acute stress. Neuroendocrinology. 1997;65:360–368. doi: 10.1159/000127196. [DOI] [PubMed] [Google Scholar]
- 37.Di S, Malcher-Lopes R, Halmos KC, Tasker JG. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J Neurosci. 2003;23:4850–4857. doi: 10.1523/JNEUROSCI.23-12-04850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di S, Popescu IR, Tasker JG. Glial control of endocannabinoid heterosynaptic modulation in hypothalamic magnocellular neuroendocrine cells. J Neurosci. 2013;33:18331–18342. doi: 10.1523/JNEUROSCI.2971-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, Costa RM, Sousa N. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325:621–625. doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- 40.Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamo-pituitary-adrenal responses to stress. J Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Drevets WC. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog Brain Res. 2000;126:413–431. doi: 10.1016/S0079-6123(00)26027-5. [DOI] [PubMed] [Google Scholar]
- 42.Evanson NK, Tasker JG, Hill MN, Hillard CJ, Herman JP. Fast Feedback Inhibition of the HPA Axis by Glucocorticoids Is Mediated by Endocannabinoid Signaling. Endocrinology. 2010;151:4811–4819. doi: 10.1210/en.2010-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Familari M, Smith AI, Smith R, Funder JW. Arginine vasopressin is a much more potent stimulus to ACTH release from ovine anterior pituitary cells than ovine corticotropin-releasing factor. 1. In vitro studies. Neuroendocrinology. 1989;50:152–157. doi: 10.1159/000125214. [DOI] [PubMed] [Google Scholar]
- 44.Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feldman D, Mondon CE, Horner JA, Weiser JN. Glucocorticoid and estrogen regulation of corticosteroid-binding globulin production by rat liver. Am J Physiol. 1979;237:E493–499. doi: 10.1152/ajpendo.1979.237.6.E493. [DOI] [PubMed] [Google Scholar]
- 46.Ferguson AV. Angiotensinergic regulation of autonomic and neuroendocrine outputs: critical roles for the subfornical organ and paraventricular nucleus. Neuroendocrinology. 2009;89:370–376. doi: 10.1159/000211202. [DOI] [PubMed] [Google Scholar]
- 47.Figueiredo HF, Bodie BL, Tauchi M, Dolgas CM, Herman JP. Stress integration after acute and chronic predator stress: differential activation of central stress circuitry and sensitization of the hypothalamo-pituitary-adrenocortical axis. Endocrinology. 2003;144:5249–5258. doi: 10.1210/en.2003-0713. [DOI] [PubMed] [Google Scholar]
- 48.Figueiredo HF, Bruestle A, Bodie B, Dolgas CM, Herman JP. The medial prefrontal cortex differentially regulates stress-induced c-fos expression in the forebrain depending on type of stressor. Eur J Neurosci. 2003;18:2357–2364. doi: 10.1046/j.1460-9568.2003.02932.x. [DOI] [PubMed] [Google Scholar]
- 49.Figueiredo HF, Dolgas CM, Herman JP. Stress activation of cortex and hippocampus is modulated by sex and stage of estrus. Endocrinology. 2002;143:2534–2540. doi: 10.1210/endo.143.7.8888. [DOI] [PubMed] [Google Scholar]
- 50.Figueiredo HF, Ulrich-Lai YM, Choi DC, Herman JP. Estrogen potentiates adrenocortical responses to stress in female rats. Am J Physiol Endocrinol Metab. 2007;292:E1173–1182. doi: 10.1152/ajpendo.00102.2006. [DOI] [PubMed] [Google Scholar]
- 51.Flak JN, Jankord R, Solomon MB, Krause EG, Herman JP. Opposing effects of chronic stress and weight restriction on cardiovascular, neuroendocrine and metabolic function. Physiol Behav. 2011;104:228–234. doi: 10.1016/j.physbeh.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flak JN, Myers B, Solomon MB, McKlveen JM, Krause EG, Herman JP. Role of paraventricular nucleus-projecting norepinephrine/epinephrine neurons in acute and chronic stress. Eur J Neurosci. 2014;39:1903–1911. doi: 10.1111/ejn.12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Flak JN, Solomon MB, Jankord R, Krause EG, Herman JP. Identification of chronic stress-activated regions reveals a potential recruited circuit in rat brain. Eur J Neurosci. 2012;36:2547–2555. doi: 10.1111/j.1460-9568.2012.08161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Furay AR, Bruestle AE, Herman JP. The role of the forebrain glucocorticoid receptor in acute and chronic stress. Endocrinology. 2008;149:5482–5490. doi: 10.1210/en.2008-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Furukawa H, del Rey A, Monge-Arditi G, Besedovsky HO. Interleukin-1, but not stress, stimulates glucocorticoid output during early postnatal life in mice. Ann N Y Acad Sci. 1998;840:117–122. doi: 10.1111/j.1749-6632.1998.tb09555.x. [DOI] [PubMed] [Google Scholar]
- 56.Gann DS, Cryer GL, Pirkle JC., Jr Physiological inhibition and facilitation of adrenocortical response to hemorrhage. Am J Physiol. 1977;232:R5–9. doi: 10.1152/ajpregu.1977.232.1.R5. [DOI] [PubMed] [Google Scholar]
- 57.Ghosal S, Bundzikova-Osacka J, Dolgas CM, Myers B, Herman JP. Glucocorticoid receptors in the nucleus of the solitary tract (NTS) decrease endocrine and behavioral stress responses. Psychoneuroendocrinology. 2014;45:142–153. doi: 10.1016/j.psyneuen.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ghosal S, Myers B, Herman JP. Role of central glucagon-like peptide-1 in stress regulation. Physiol Behav. 2013;122:201–207. doi: 10.1016/j.physbeh.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gibbison B, Spiga F, Walker JJ, Russell GM, Stevenson K, Kershaw Y, Zhao Z, Henley D, Angelini GD, Lightman SL. Dynamic Pituitary-Adrenal Interactions in Response to Cardiac Surgery. Crit Care Med. 2014 doi: 10.1097/CCM.0000000000000773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gillies GE, Linton EA, Lowry PJ. Corticotropin-releasing activity of the new CRF is potentiated several times by vasopressin. Nature. 1982;299:355–357. doi: 10.1038/299355a0. [DOI] [PubMed] [Google Scholar]
- 61.Gomez F, Lahmame A, de Kloet ER, Armario A. Hypothalamic-pituitary-adrenal response to chronic stress in five inbred rat strains: differential responses are mainly located at the adrenocortical level. Neuroendocrinology. 1996;63:327–337. doi: 10.1159/000126973. [DOI] [PubMed] [Google Scholar]
- 62.Gray M, Bingham B, Viau V. A comparison of two repeated restraint stress paradigms on hypothalamic-pituitary-adrenal axis habituation, gonadal status and central neuropeptide expression in adult male rats. J Neuroendocrinol. 2010;22:92–101. doi: 10.1111/j.1365-2826.2009.01941.x. [DOI] [PubMed] [Google Scholar]
- 63.Gunnar M, Quevedo K. The neurobiology of stress and development. Annu Rev Psychol. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- 64.Hadley ME, Haskell-Luevano C. The proopiomelanocortin system. Ann N Y Acad Sci. 1999;885:1–21. doi: 10.1111/j.1749-6632.1999.tb08662.x. [DOI] [PubMed] [Google Scholar]
- 65.Hanukoglu I. Steroidogenic enzymes: structure, function, and role in regulation of steroid hormone biosynthesis. J Steroid Biochem Mol Biol. 1992;43:779–804. doi: 10.1016/0960-0760(92)90307-5. [DOI] [PubMed] [Google Scholar]
- 66.Hauer D, Kaufmann I, Strewe C, Briegel I, Campolongo P, Schelling G. The role of glucocorticoids, catecholamines and endocannabinoids in the development of traumatic memories and posttraumatic stress symptoms in survivors of critical illness. Neurobiol Learn Mem. 2014;112:68–74. doi: 10.1016/j.nlm.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 67.Hauger RL, Thrivikraman KV, Plotsky PM. Age-related alterations of hypothalamic-pituitary-adrenal axis function in male Fischer 344 rats. Endocrinology. 1994;134:1528–1536. doi: 10.1210/endo.134.3.8119195. [DOI] [PubMed] [Google Scholar]
- 68.Herman JP. Neural control of chronic stress adaptation. Front Behav Neurosci. 2013;7:61. doi: 10.3389/fnbeh.2013.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Herman JP. Regulation of adrenocorticosteroid receptor mRNA expression in the central nervous system. Cell Mol Neurobiol. 1993;13:349–372. doi: 10.1007/BF00711577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Herman JP, Adams D, Prewitt C. Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology. 1995;61:180–190. doi: 10.1159/000126839. [DOI] [PubMed] [Google Scholar]
- 71.Herman JP, Cullinan WE. Neurocircuitry of stress: Central control of the hypothalamo-pituitary-adrenocortical axis. TINS. 1997;20:78–83. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- 72.Herman JP, Cullinan WE, Ziegler DR, Tasker JG. Role of the paraventricular nucleus microenvironment in stress integration. Eur J Neurosci. 2002;16:381–385. doi: 10.1046/j.1460-9568.2002.02133.x. [DOI] [PubMed] [Google Scholar]
- 73.Herman JP, Dolgas CM, Carlson SL. Ventral subiculum regulates hypothalamo-pituitary-adrenocortical and behavioural responses to cognitive stressors. Neuroscience. 1998;86:449–459. doi: 10.1016/s0306-4522(98)00055-4. [DOI] [PubMed] [Google Scholar]
- 74.Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 75.Herman JP, Figueiredo HF, Mueller NK, Ostrander MM, Zhang R, Tauchi M, Choi DC, Furay AR, Evanson NK, Nelson EB, Ulrich-Lai YM. Neurochemical Systems Regulating the Hypothalamo-Pituitary-Adrenocortical Axis. In: Blaustein J, Lajtha A, editors. Handbook of neurochemistry and molecular neurobiology Behavioral neurochemistry, neuroendocrinology, and molecular neurobiology. New York: Springer; 2007. p. xiv.p. 954. [Google Scholar]
- 76.Herman JP, Flak J, Jankord R. Chronic stress plasticity in the hypothalamic paraventricular nucleus. Prog Brain Res. 2008;170:353–364. doi: 10.1016/S0079-6123(08)00429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Herman JP, Larson BR, Speert DB, Seasholtz AF. Hypothalamo-pituitary-adrenocortical dysregulation in aging F344/Brown-Norway F1 hybrid rats. Neurobiol Aging. 2001;22:323–332. doi: 10.1016/s0197-4580(00)00242-6. [DOI] [PubMed] [Google Scholar]
- 78.Herman JP, McKlveen JM, Solomon MB, Carvalho-Netto E, Myers B. Neural regulation of the stress response: glucocorticoid feedback mechanisms. Braz J Med Biol Res. 2012;45:292–298. doi: 10.1590/S0100-879X2012007500041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 80.Herman JP, Watson SJ, Spencer RL. Defense of adrenocorticosteroid receptor expression in rat hippocampus: effects of stress and strain. Endocrinology. 1999;140:3981–3991. doi: 10.1210/endo.140.9.6962. [DOI] [PubMed] [Google Scholar]
- 81.Hewitt SA, Wamsteeker JI, Kurz EU, Bains JS. Altered chloride homeostasis removes synaptic inhibitory constraint of the stress axis. Nat Neurosci. 2009;12:438–443. doi: 10.1038/nn.2274. [DOI] [PubMed] [Google Scholar]
- 82.Hokfelt T. Neuropeptides in perspective: the last ten years. Neuron. 1991;7:867–879. doi: 10.1016/0896-6273(91)90333-u. [DOI] [PubMed] [Google Scholar]
- 83.Hrabovszky E, Liposits Z. Novel aspects of glutamatergic signalling in the neuroendocrine system. J Neuroendocrinol. 2008;20:743–751. doi: 10.1111/j.1365-2826.2008.01719.x. [DOI] [PubMed] [Google Scholar]
- 84.Issa AM, Rowe W, Gauthier S, Meaney MJ. Hypothalamic-pituitary-adrenal activity in aged, cognitively impaired and cognitively unimpaired rats. J Neurosci. 1990;10:3247–3254. doi: 10.1523/JNEUROSCI.10-10-03247.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jacobson L. Hypothalamic-pituitary-adrenocortical axis regulation. Endocrinol Metab Clin North Am. 2005;34:271–292. vii. doi: 10.1016/j.ecl.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 86.Jacobson L, Muglia LJ, Weninger SC, Pacak K, Majzoub JA. CRH deficiency impairs but does not block pituitary-adrenal responses to diverse stressors. Neuroendocrinology. 2000;71:79–87. doi: 10.1159/000054524. [DOI] [PubMed] [Google Scholar]
- 87.Jankord R, Zhang R, Flak JN, Solomon MB, Albertz J, Herman JP. Stress activation of IL-6 neurons in the hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2010;299:R343–351. doi: 10.1152/ajpregu.00131.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jasper MS, Engeland WC. Splanchnicotomy increases adrenal sensitivity to ACTH in nonstressed rats. Am J Physiol. 1997;273:E363–368. doi: 10.1152/ajpendo.1997.273.2.E363. [DOI] [PubMed] [Google Scholar]
- 89.Jenks BG. Regulation of proopiomelanocortin gene expression: an overview of the signaling cascades, transcription factors, and responsive elements involved. Ann N Y Acad Sci. 2009;1163:17–30. doi: 10.1111/j.1749-6632.2008.03620.x. [DOI] [PubMed] [Google Scholar]
- 90.Jones KR, Myers B, Herman JP. Stimulation of the prelimbic cortex differentially modulates neuroendocrine responses to psychogenic and systemic stressors. Physiol Behav. 2011;104:266–271. doi: 10.1016/j.physbeh.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31:151–178. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 92.Kalsbeek A, van der Spek R, Lei J, Endert E, Buijs RM, Fliers E. Circadian rhythms in the hypothalamo-pituitary-adrenal (HPA) axis. Mol Cell Endocrinol. 2012;349:20–29. doi: 10.1016/j.mce.2011.06.042. [DOI] [PubMed] [Google Scholar]
- 93.Keller-Wood M, Dallman MF. Corticosteroid inhibition of ACTH secretion. Endocrine Rev. 1984;5:1–24. doi: 10.1210/edrv-5-1-1. A still-definitive synopsis of a vast literature on the problem of negative feedback regulation of the HPA axis. [DOI] [PubMed] [Google Scholar]
- 94.Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 95.Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, De Jong IC, Ruis MA, Blokhuis HJ. Coping styles in animals: current status in behavior and stress-physiology. Neurosci Biobehav Rev. 1999;23:925–935. doi: 10.1016/s0149-7634(99)00026-3. [DOI] [PubMed] [Google Scholar]
- 96.Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM. Long-term behavioral and neuroendocrine adaptations to adverse early experience. Prog Brain Res. 2000;122:81–103. doi: 10.1016/s0079-6123(08)62132-9. [DOI] [PubMed] [Google Scholar]
- 97.Le Minh N, Damiola F, Tronche F, Schutz G, Schibler U. Glucocorticoid hormones inhibit food-induced phase-shifting of peripheral circadian oscillators. EMBO J. 2001;20:7128–7136. doi: 10.1093/emboj/20.24.7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Leal AM, Moreira AC. Food and the circadian activity of the hypothalamic-pituitary-adrenal axis. Braz J Med Biol Res. 1997;30:1391–1405. doi: 10.1590/s0100-879x1997001200003. [DOI] [PubMed] [Google Scholar]
- 99.Li G, Cherrier MM, Tsuang DW, Petrie EC, Colasurdo EA, Craft S, Schellenberg GD, Peskind ER, Raskind MA, Wilkinson CW. Salivary cortisol and memory function in human aging. Neurobiol Aging. 2006;27:1705–1714. doi: 10.1016/j.neurobiolaging.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 100.Lightman SL, Wiles CC, Atkinson HC, Henley DE, Russell GM, Leendertz JA, McKenna MA, Spiga F, Wood SA, Conway-Campbell BL. The significance of glucocorticoid pulsatility. Eur J Pharmacol. 2008;583:255–262. doi: 10.1016/j.ejphar.2007.11.073. [DOI] [PubMed] [Google Scholar]
- 101.Lilly MP, Engeland WC, Gann DS. Pituitary-adrenal responses to repeated small hemorrhage in conscious dogs. Am J Physiol. 1986;251:R1200–1207. doi: 10.1152/ajpregu.1986.251.6.R1200. [DOI] [PubMed] [Google Scholar]
- 102.Lilly MP, Engeland WC, Gann DS. Responses of cortisol secretion to repeated hemorrhage in the anesthetized dog. Endocrinology. 1983;112:681–688. doi: 10.1210/endo-112-2-681. [DOI] [PubMed] [Google Scholar]
- 103.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic- pituitary-adrenal responses to stress [see comments] Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 104.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 105.Makara GB, Stark E, Karteszi M, Palkovits M, Rappay G. Effects of paraventricular lesions on stimulated ACTH release and CRF in stalk-median eminence of the rat. Am J Physiol. 1981;240:E441–446. doi: 10.1152/ajpendo.1981.240.4.E441. [DOI] [PubMed] [Google Scholar]
- 106.Makino S, Gold PW, Schulkin J. Corticosterone effects on corticotropin-releasing hormone mRNA in the central nucleus of the amygdala and the parvocellular region of the paraventricular nucleus of the hypothalamus. Brain Res. 1994;640:105–112. doi: 10.1016/0006-8993(94)91862-7. [DOI] [PubMed] [Google Scholar]
- 107.Makino S, Gold PW, Schulkin J. Effects of corticosterone on CRH mRNA and content in the bed nucleus of the stria terminalis; comparison with the effects in the central nucleus of the amygdala and the paraventricular nucleus of the hypothalamus. Brain Res. 1994;657:141–149. doi: 10.1016/0006-8993(94)90961-x. [DOI] [PubMed] [Google Scholar]
- 108.Makino S, Shibasaki T, Yamauchi N, Nishioka T, Mimoto T, Wakabayashi I, Gold PW, Hashimoto K. Psychological stress increased corticotropin-releasing hormone mRNA and content in the central nucleus of the amygdala but not in the hypothalamic paraventricular nucleus in the rat. Brain Res. 1999;850:136–143. doi: 10.1016/s0006-8993(99)02114-9. [DOI] [PubMed] [Google Scholar]
- 109.Marti O, Armario A. Anterior pituitary response to stress: time-related changes and adaptation. Int J Dev Neurosci. 1998;16:241–260. doi: 10.1016/s0736-5748(98)00030-6. [DOI] [PubMed] [Google Scholar]
- 110.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 111.McKlveen JM, Myers B, Flak JN, Bundzikova J, Solomon MB, Seroogy KB, Herman JP. Role of prefrontal cortex glucocorticoid receptors in stress and emotion. Biol Psychiatry. 2013;74:672–679. doi: 10.1016/j.biopsych.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Merighi A. Costorage and coexistence of neuropeptides in the mammalian CNS. Prog Neurobiol. 2002;66:161–190. doi: 10.1016/s0301-0082(01)00031-4. [DOI] [PubMed] [Google Scholar]
- 113.Moisan MP, Minni AM, Dominguez G, Helbling JC, Foury A, Henkous N, Dorey R, Beracochea D. Role of corticosteroid binding globulin in the fast actions of glucocorticoids on the brain. Steroids. 2014;81:109–115. doi: 10.1016/j.steroids.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 114.Muglia LJ, Jacobson L, Weninger SC, Karalis KP, Jeong K, Majzoub JA. The physiology of corticotropin-releasing hormone deficiency in mice. Peptides. 2001;22:725–731. doi: 10.1016/s0196-9781(01)00385-0. [DOI] [PubMed] [Google Scholar]
- 115.Murakami K, Nakagawa T, Shozu M, Uchide K, Koike K, Inoue M. Changes with aging of steroidal levels in the cerebrospinal fluid of women. Maturitas. 1999;33:71–80. doi: 10.1016/s0378-5122(99)00040-7. [DOI] [PubMed] [Google Scholar]
- 116.Myers B, McKlveen JM, Herman JP. Glucocorticoid actions on synapses, circuits, and behavior: implications for the energetics of stress. Front Neuroendocrinol. 2014;35:180–196. doi: 10.1016/j.yfrne.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Myers B, McKlveen JM, Herman JP. Neural Regulation of the Stress Response: The Many Faces of Feedback. Cell Mol Neurobiol. 2012;352:683–596. doi: 10.1007/s10571-012-9801-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nahar J, Haam J, Chen C, Jiang Z, Glatzer NR, Muglia LJ, Dohanich GP, Herman JP, Tasker JG. Rapid Nongenomic Glucocorticoid Actions in Male Mouse Hypothalamic Neuroendocrine Cells Are Dependent on the Nuclear Glucocorticoid Receptor. Endocrinology. 2015;156:2831–2842. doi: 10.1210/en.2015-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Natelson BH, Ottenweller JE, Cook JA, Pitman D, McCarty R, Tapp WN. Effect of stressor intensity on habituation of the adrenocortical stress response. Physiol Behav. 1988;43:41–46. doi: 10.1016/0031-9384(88)90096-0. [DOI] [PubMed] [Google Scholar]
- 120.Nederhof E, Schmidt MV. Mismatch or cumulative stress: toward an integrated hypothesis of programming effects. Physiol Behav. 2012;106:691–700. doi: 10.1016/j.physbeh.2011.12.008. Elegant discussion of the match:mismatch hypothesis of how early life stress affects stress reactivity later in life. [DOI] [PubMed] [Google Scholar]
- 121.Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 122.Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16:279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Purnell JQ, Brandon DD, Isabelle LM, Loriaux DL, Samuels MH. Association of 24-hour cortisol production rates, cortisol-binding globulin, and plasma-free cortisol levels with body composition, leptin levels, and aging in adult men and women. J Clin Endocrinol Metab. 2004;89:281–287. doi: 10.1210/jc.2003-030440. [DOI] [PubMed] [Google Scholar]
- 124.Rabasa C, Munoz-Abellan C, Daviu N, Nadal R, Armario A. Repeated exposure to immobilization or two different footshock intensities reveals differential adaptation of the hypothalamic-pituitary-adrenal axis. Physiol Behav. 2011;103:125–133. doi: 10.1016/j.physbeh.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 125.Radley JJ, Arias CM, Sawchenko PE. Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. J Neurosci. 2006;26:12967–12976. doi: 10.1523/JNEUROSCI.4297-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Radley JJ, Kabbaj M, Jacobson L, Heydendael W, Yehuda R, Herman JP. Stress risk factors and stress-related pathology: neuroplasticity, epigenetics and endophenotypes. Stress. 2011;14:481–497. doi: 10.3109/10253890.2011.604751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Radley JJ, PES . Society for Neuroscience. Washington, DC: 2005. Differential role of the dorsal versus ventral medial prefrontal cortex (mPFC) in paraventircular hypothalamic responses to acute restraint stress.. In. Online. Program No. 637.611. [Google Scholar]
- 128.Radley JJ, Sawchenko PE. A common substrate for prefrontal and hippocampal inhibition of the neuroendocrine stress response. J Neurosci. 2011;31:9683–9695. doi: 10.1523/JNEUROSCI.6040-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Raff H, Raff JL, Duthie EH, Wilson CR, Sasse EA, Rudman I, Mattson D. Elevated salivary cortisol in the evening in healthy elderly men and women: correlation with bone mineral density. J Gerontol A Biol Sci Med Sci. 1999;54:M479–483. doi: 10.1093/gerona/54.9.m479. [DOI] [PubMed] [Google Scholar]
- 130.Raff H, Shinsako J, Dallman MF. Surgery potentiates adrenocortical responses to hypoxia in dogs. Proc Soc Exp Biol Med. 1983;172:400–406. doi: 10.3181/00379727-172-41578. [DOI] [PubMed] [Google Scholar]
- 131.Reber SO, Birkeneder L, Veenema AH, Obermeier F, Falk W, Straub RH, Neumann ID. Adrenal insufficiency and colonic inflammation after a novel chronic psychosocial stress paradigm in mice: implications and mechanisms. Endocrinology. 2007;148:670–682. doi: 10.1210/en.2006-0983. [DOI] [PubMed] [Google Scholar]
- 132.Rivest S. How circulating cytokines trigger the neural circuits that control the hypothalamic-pituitary-adrenal axis. Psychoneuroendocrinology. 2001;26:761–788. doi: 10.1016/s0306-4530(01)00064-6. [DOI] [PubMed] [Google Scholar]
- 133.Roca CA, Schmidt PJ, Deuster PA, Danaceau MA, Altemus M, Putnam K, Chrousos GP, Nieman LK, Rubinow DR. Sex-related differences in stimulated hypothalamic-pituitary-adrenal axis during induced gonadal suppression. J Clin Endocrinol Metab. 2005;90:4224–4231. doi: 10.1210/jc.2004-2525. [DOI] [PubMed] [Google Scholar]
- 134.Romeo RD. Pubertal maturation and programming of hypothalamic-pituitary-adrenal reactivity. Front Neuroendocrinol. 2010;31:232–240. doi: 10.1016/j.yfrne.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 135.Russell GM, Kalafatakis K, Lightman SL. The importance of biological oscillators for HPA activity and tissue glucocorticoid response: Coordinating stress and neurobehavioural adaptation. J Neuroendocrinol. 2014 doi: 10.1111/jne.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sapolsky RM. Do glucocorticoid concentrations rise with age in the rat? Neurobiol Aging. 1991;13:171–174. doi: 10.1016/0197-4580(92)90025-s. [DOI] [PubMed] [Google Scholar]
- 137.Sapolsky RM. Glucocorticoids and Hippocampal Damage. Trends in Neurosciences. 1987;10:346–349. [Google Scholar]
- 138.Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr Rev. 1986;7:284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- 139.Sapolsky RM, Meaney MJ. Maturation of the adrenal stress response: Neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res Rev. 1986;11:65–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- 140.Sawchenko PE, Li HY, Ericsson A. Circuits and mechanisms governing hypothalamic responses to stress: a tale of two paradigms. Prog Brain Res. 2000;122:61–78. doi: 10.1016/s0079-6123(08)62131-7. [DOI] [PubMed] [Google Scholar]
- 141.Schotanus K, Tilders FJ, Berkenbosch F. Human recombinant interleukin-1 receptor antagonist prevents adrenocorticotropin, but not interleukin-6 responses to bacterial endotoxin in rats. Endocrinology. 1993;133:2461–2468. doi: 10.1210/endo.133.6.8243265. [DOI] [PubMed] [Google Scholar]
- 142.Schroeder RJ, Henning SJ. Roles of plasma clearance and corticosteroid-binding globulin in the developmental increase in circulating corticosterone in infant rats. Endocrinology. 1989;124:2612–2618. doi: 10.1210/endo-124-5-2612. [DOI] [PubMed] [Google Scholar]
- 143.Schwaber JS, Kapp BS, Higgins GA, PRR Amygdala and basal forebrain direct connections with the nucleus of the solitary tract and the dorsal motor nucleus. J Neurosci. 1982;2:1424–1438. doi: 10.1523/JNEUROSCI.02-10-01424.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Seckl JR. 11 beta-hydroxysteroid dehydrogenase isoforms and their implications for blood pressure regulation. Eur J Clin Invest. 1993;23:589–601. doi: 10.1111/j.1365-2362.1993.tb00720.x. [DOI] [PubMed] [Google Scholar]
- 145.Seckl JR, Walker BR. Minireview: 11beta-hydroxysteroid dehydrogenase type 1- a tissue-specific amplifier of glucocorticoid action. Endocrinology. 2001;142:1371–1376. doi: 10.1210/endo.142.4.8114. [DOI] [PubMed] [Google Scholar]
- 146.Seeman TE, Singer B, Wilkinson CW, McEwen B. Gender differences in age-related changes in HPA axis reactivity. Psychoneuroendocrinology. 2001;26:225–240. doi: 10.1016/s0306-4530(00)00043-3. [DOI] [PubMed] [Google Scholar]
- 147.Segar TM, Kasckow JW, Welge JA, Herman JP. Heterogeneity of neuroendocrine stress responses in aging rat strains. Physiol Behav. 2009;96:6–11. doi: 10.1016/j.physbeh.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- 149.Simpson ER, Waterman MR. Regulation of the synthesis of steroidogenic enzymes in adrenal cortical cells by ACTH. Annu Rev Physiol. 1988;50:427–440. doi: 10.1146/annurev.ph.50.030188.002235. [DOI] [PubMed] [Google Scholar]
- 150.Soares JM, Sampaio A, Ferreira LM, Santos NC, Marques F, Palha JA, Cerqueira JJ, Sousa N. Stress-induced changes in human decision-making are reversible. Transl Psychiatry. 2012;2:e131. doi: 10.1038/tp.2012.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Solomon MB, Furay AR, Jones K, Packard AE, Packard BA, Wulsin AC, Herman JP. Deletion of forebrain glucocorticoid receptors impairs neuroendocrine stress responses and induces depression-like behavior in males but not females. Neuroscience. 2012;203:135–143. doi: 10.1016/j.neuroscience.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Solomon MB, Jones K, Packard BA, Herman JP. The medial amygdala modulates body weight but not neuroendocrine responses to chronic stress. J Neuroendocrinol. 2010;22:13–23. doi: 10.1111/j.1365-2826.2009.01933.x. [DOI] [PubMed] [Google Scholar]
- 153.Solomon MB, Loftspring M, de Kloet AD, Ghosal S, Jankord R, Flak JN, Wulsin AC, Krause EG, Zhang R, Rice T, McKlveen J, Myers B, Tasker JG, Herman JP. Neuroendocrine Function After Hypothalamic Depletion of Glucocorticoid Receptors in Male and Female Mice. Endocrinology. 2015;156:2843–2853. doi: 10.1210/en.2015-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Son SJ, Filosa JA, Potapenko ES, Biancardi VC, Zheng H, Patel KP, Tobin VA, Ludwig M, Stern JE. Dendritic peptide release mediates interpopulation crosstalk between neurosecretory and preautonomic networks. Neuron. 2013;78:1036–1049. doi: 10.1016/j.neuron.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Spencer RL, Miller AH, Moday H, McEwen BS, Blanchard RJ, Blanchard DC, Sakai RR. Chronic social stress produces reductions in available splenic type II corticosteroid receptor binding and plasma corticosteroid binding globulin levels. Psychoneuroendocrinology. 1996;21:95–109. doi: 10.1016/0306-4530(95)00020-8. [DOI] [PubMed] [Google Scholar]
- 156.Spiess J, Rivier J, Rivier C, Vale W. Primary structure of corticotropin-releasing factor from ovine hypothalamus. Proc Natl Acad Sci U S A. 1981;78:6517–6521. doi: 10.1073/pnas.78.10.6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sullivan RM, Gratton A. Lateralized effects of medial prefrontal cortex lesions on neuroendocrine and autonomic stress responses in rats. J Neurosci. 1999;19:2834–2840. doi: 10.1523/JNEUROSCI.19-07-02834.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tavares RF, Correa FM, Resstel LB. Opposite role of infralimbic and prelimbic cortex in the tachycardiac response evoked by acute restraint stress in rats. J Neurosci Res. 2009;87:2601–2607. doi: 10.1002/jnr.22070. [DOI] [PubMed] [Google Scholar]
- 159.Tinnikov AA. Responses of serum corticosterone and corticosteroid-binding globulin to acute and prolonged stress in the rat. Endocrine. 1999;11:145–150. doi: 10.1385/ENDO:11:2:145. [DOI] [PubMed] [Google Scholar]
- 160.Turnbull AV, Rivier CL. Sprague-Dawley rats obtained from different vendors exhibit distinct adrenocorticotropin responses to inflammatory stimuli. Neuroendocrinology. 1999;70:186–195. doi: 10.1159/000054475. [DOI] [PubMed] [Google Scholar]
- 161.Ulrich-Lai YM, Engeland WC. Adrenal splanchnic innervation modulates adrenal cortical responses to dehydration stress in rats. Neuroendocrinology. 2002;76:79–92. doi: 10.1159/000064426. [DOI] [PubMed] [Google Scholar]
- 162.Ulrich-Lai YM, Figueiredo HF, Ostrander MM, Choi DC, Engeland WC, Herman JP. Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. Am J Physiol Endocrinol Metab. 2006;291:E965–973. doi: 10.1152/ajpendo.00070.2006. [DOI] [PubMed] [Google Scholar]
- 163.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ulrich-Lai YM, Jones KR, Ziegler DR, Cullinan WE, Herman JP. Forebrain origins of glutamatergic innervation to the rat paraventricular nucleus of the hypothalamus: differential inputs to the anterior versus posterior subregions. J Comp Neurol. 2011;519:1301–1319. doi: 10.1002/cne.22571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ulrich-Lai YM, Ostrander MM, Thomas IM, Packard BA, Furay AR, Dolgas CM, Van Hooren DC, Figueiredo HF, Mueller NK, Choi DC, Herman JP. Daily limited access to sweetened drink attenuates hypothalamic-pituitary-adrenocortical axis stress responses. Endocrinology. 2007;148:1823–1834. doi: 10.1210/en.2006-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ulrich-Lai YM, Xie W, Meij JT, Dolgas CM, Yu L, Herman JP. Limbic and HPA axis function in an animal model of chronic neuropathic pain. Physiol Behav. 2006;88:67–76. doi: 10.1016/j.physbeh.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 167.Vahl TP, Ulrich-Lai YM, Ostrander MM, Dolgas CM, Elfers EE, Seeley RJ, D’Alessio DA, Herman JP. Comparative analysis of ACTH and corticosterone sampling methods in rats. Am J Physiol Endocrinol Metab. 2005;289:E823–828. doi: 10.1152/ajpendo.00122.2005. [DOI] [PubMed] [Google Scholar]
- 168.Vamvakopoulos NV. Sexual dimorphism of stress response and immune/inflammatory reaction: the corticotropin releasing hormone perspective. Mediators Inflamm. 1995;4:163–174. doi: 10.1155/S0962935195000275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.van Ast VA, Cornelisse S, Meeter M, Joels M, Kindt M. Time-dependent effects of cortisol on the contextualization of emotional memories. Biol Psychiatry. 2013;74:809–816. doi: 10.1016/j.biopsych.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 170.Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- 171.Viau V, Meaney MJ. The inhibitory effect of testosterone on hypothalamo-pituitary-adrenal responses to stress is mediated by the medial preoptic area. J Neurosci. 1996;16:1866–1876. doi: 10.1523/JNEUROSCI.16-05-01866.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Viau V, Meaney MJ. Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology. 1991;129:2503–2511. doi: 10.1210/endo-129-5-2503. [DOI] [PubMed] [Google Scholar]
- 173.Walker AK, Nakamura T, Byrne RJ, Naicker S, Tynan RJ, Hunter M, Hodgson DM. Neonatal lipopolysaccharide and adult stress exposure predisposes rats to anxiety-like behaviour and blunted corticosterone responses: implications for the double-hit hypothesis. Psychoneuroendocrinology. 2009;34:1515–1525. doi: 10.1016/j.psyneuen.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 174.Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 175.Weiss EL, Longhurst JG, Mazure CM. Childhood sexual abuse as a risk factor for depression in women: psychosocial and neurobiological correlates. Am J Psychiatry. 1999;156:816–828. doi: 10.1176/ajp.156.6.816. [DOI] [PubMed] [Google Scholar]
- 176.Wilkinson CW, Peskind ER, Raskind MA. Decreased hypothalamic-pituitary-adrenal axis sensitivity to cortisol feedback inhibition in human aging. Neuroendocrinology. 1997;65:79–90. doi: 10.1159/000127167. [DOI] [PubMed] [Google Scholar]
- 177.Wilkinson CW, Petrie EC, Murray SR, Colasurdo EA, Raskind MA, Peskind ER. Human glucocorticoid feedback inhibition is reduced in older individuals: evening study. J Clin Endocrinol Metab. 2001;86:545–550. doi: 10.1210/jcem.86.2.7232. [DOI] [PubMed] [Google Scholar]
- 178.Ziegler DR, Edwards MR, Ulrich-Lai YM, Herman JP, Cullinan WE. Brainstem origins of glutamatergic innervation of the rat hypothalamic paraventricular nucleus. J Comp Neurol. 2012;520:2369–2394. doi: 10.1002/cne.23043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zohar J, Yahalom H, Kozlovsky N, Cwikel-Hamzany S, Matar MA, Kaplan Z, Yehuda R, Cohen H. High dose hydrocortisone immediately after trauma may alter the trajectory of PTSD: interplay between clinical and animal studies. Eur Neuropsychopharmacol. 2011;21:796–809. doi: 10.1016/j.euroneuro.2011.06.001. [DOI] [PubMed] [Google Scholar]