Abstract
Mini-Abstract
A decade from publication, approximately one in ten surgical devices described in the literature made the leap from the laboratory to a first-in-human study. Clinical involvement was a significant predictor of translation; devices developed with clinical collaboration were over six times more likely to be translated than those without.
Structured Abstract
Objective
To determine the rate and extent of translation of innovative surgical devices from the laboratory to first-in-human studies, and to evaluate the factors influencing such translation.
Summary Background Data
Innovative surgical devices have preceded many of the major advances in surgical practice. However, the process by which devices arising from academia find their way to translation remains poorly understood.
Methods
All biomedical engineering journals, and the five basic science journals with the highest impact factor, were searched between January 1993 and January 2000 using the Boolean search term “surgery OR surgeon OR surgical”. Articles were included if they described the development of a new device and a surgical application was described. A recursive search of all citations to the article was performed using the Web of Science (Thompson-Reuters, New York, USA) to identify any associated first-in-human studies published by January 2015. Kaplan-Meier curves were constructed for the time first-in-human studies. Factors influencing translation were evaluated using Log Rank and Cox proportional hazards models.
Results
8,297 articles were screened, and 205 publications describing unique devices identified. The probability of a first-in-human at 10 years was 9.8%. Clinical involvement was a significant predictor of a first-in-human study (p = 0.02); devices developed with early clinical collaboration were over six times more likely to be translated than those without (RR 6.5 [95% CI 0.9 - 48]).
Conclusions
These findings support initiatives to increase clinical translation through improved interactions between basic, translational, and clinical researchers.
Keywords: Surgery, Innovation, Technology, Diffusion of Innovations
Introduction
The development, evaluation, and adoption of innovative surgical devices are essential to the advancement of clinical practice.1 Despite the enormous importance of these devices to human health, the process by which biomedical innovations arising from academia find their way to translation remains poorly understood.2 Any earnest attempt to foster a more nourishing environment for translational research should be predicated on a better appreciation of this process.
The translation of an innovative surgical device has been described as a continuum of activities, punctuated by several well-defined chasms: (1) the development of the device culminating in a first-in-human study, (2) the evaluation of the device in clinical trials resulting in a license for use, and (3) the adoption of the device by surgeons.3
Previous studies on the translation of biomedical innovation generally report a long lag between innovation and translation – approximately 17 years – and suggest that industry collaboration is the most important predictor of translation.4, 5 However, these studies largely focus on drug rather than device innovation, and on their evaluation and adoption rather than their development. To address this shortfall, we explored the process by which surgical devices described in the biomedical literature make the leap to first-in-human studies.
A multitude of innovative surgical devices arise from academia
We used the ISI Web of Knowledge Journal Citation Report (Thompson-Reuters, New York, USA) to identify all biomedical biomedical engineering journals, and the five basic science journals with the highest impact factor. These journals were then searched on the NCBI PubMed (NCBI, Maryland, USA) and IEEE Xplore (IEEE, New York, USA) databases between January 1993 and January 2000 using the Boolean search term “surgery OR surgical OR surgeon” to capture publications describing innovative surgical devices.
Devices were defined according to the US Food and Drug Administration as “…products which do not achieve their primary intended purposes through chemical actions within or on the body of man or other animals and which are not dependent upon being metabolised for the achievement of any of their primary intended purposes”. When multiple publications were found that described the same surgical device, the earliest publication was used for subsequent analysis.
In all, 8,297 article titles and abstracts were screened, of which 205 described innovative surgical devices and were included. The original articles were most commonly published in ASAIO (57/205; 27.8%) and Artificial Organs (55/205; 26.8%), and the majority of the corresponding authors were found in the USA (59/205; 28.8%) and Japan (56/205; 27.3%). A multitude of devices were observed, but most were implants (149/205; 72.7%) and constructed for a specific disease or application (179/205; 87.3%).
Few devices make the leap from the laboratory to a first-in-human study
We then determined the rate and extent to which innovative surgical devices made the leap from the laboratory to a first-in-human study. A publication was considered to describe the translation of a particular device if it was clearly referenced in the manuscript and an uninterrupted citation chain to the original article could be identified.
For each innovative surgical device, we searched through all citations to the corresponding article published before January 2015 using the Web of Science (Thompson-Reuters, New York, USA). All citations to an article were sorted according to their date of publication (oldest first) and screened to find the first clinical publication using the device. If no clinical publications were found, citations were then screened to identify articles by any of the original authors describing subsequent development of the device and, if so, the process was repeated.
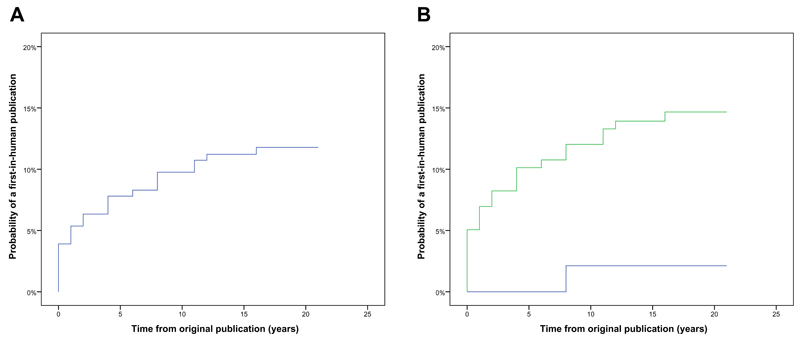
Overall, 24/205 (11.7%) of innovative surgical devices were associated with a first-in-human study. Kaplan-Meier curves were constructed, and the probability of a device resulting in a first-in-human study at 5, 10, and 20 years was 7.8%, 9.8%, and 11.8% respectively (Figure 1).
Figure 1.
Kaplan-Meier graphs illustrating (a) the overall probability of a first-in-human publication over time, and (b) the Log Rank (Mantel-Cox) probability of a first-in-human publication over time stratified according whether there was clinical involvement (green line) or not (blue line).
Contopoulos-Ionnidis et al evaluated the translation of promising basic science research but, unlike the present study, they focused on drug rather than device innovation, and included work that had already been used in humans.5 They concluded that even the most promising basic science research, published in journals with the highest impact factors, was rarely translated; 5.0% of innovations were licensed, and 1.0% were widely adopted. In the present study, the leap from initial device description to first-in-human study represented a major barrier.
Clinical collaboration is key to nourishing the translational research environment
Finally, we evaluated the factors influencing translation (clinical involvement or not; industry involvement or not; instrument or implant; single disease or broader disease category) using Log Rank (Mantel-Cox) and Cox proportional hazards models. The extent of clinical involvement was a significant predictor of a first-in-human study (p = 0.02) (Figure 1). Devices developed with early clinical collaboration were over six times more likely to be translated than those without (RR 6.5 [95% CI 0.9 - 48]). Other variables, including the extent of industry involvement, were not significantly associated with translation (p > 0.1).
In recent years, there have been several initiatives to increase the translation of innovative surgical devices.6, 7 This study is the first to provide quantitative evidence to support the idea that clinical collaboration is associated with more rapid and extensive translation. Interestingly, and in contrast to previous studies, industry collaboration was not associated with increased translation.5 We speculate that the reason for this disparity lies in the varying role of clinical and industry collaboration through the continuum of translation. Early translation may be more reliant on clinicians to drive first-in-human and early clinical trials, while later translation may be more reliant on industry to navigate the complex and costly licensing pathway, and market devices to the wider clinical community.
In summary, improved interactions between basic, translational and clinical researchers may facilitate the translation of innovative surgical devices from the laboratory to the operating room. In the words of Henry Ford: Coming together is a beginning; keeping together is progress; working together is success.
Footnotes
Declaration of competing interests:
H.J. Marcus is supported by an Imperial College Wellcome Trust Clinical Fellowship, and C.J. Payne is supported by a Wates Foundation Fellowship.
Author contributions:
HJM and CJP were involved in the study conception, acquisition of data, analysis of data, and drafting the manuscript. AHH, GG, and KL were involved in the study conception, acquisition of data, analysis of data, and critical revision of the manuscript. DN and GZY were involved in the study conception and critical revision of the manuscript.
References
- 1.Sagar SP, Law PW, Shaul RZ, et al. Hey, I Just Did a New Operation! Introducing Innovative Procedures and Devices Within an Academic Health Center. Ann Surg. 2015;261:30–31. doi: 10.1097/SLA.0000000000000748. [DOI] [PubMed] [Google Scholar]
- 2.Volk HD, Stevens MM, Mooney DJ, et al. Key elements for nourishing the translational research environment. Sci Transl Med. 2015;7:282. doi: 10.1126/scitranslmed.aaa2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drolet BC, Lorenzi NM. Translational research: understanding the continuum from bench to bedside. Transl Res. 2011;157:1–5. doi: 10.1016/j.trsl.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Morris ZS, Wooding S, Grant J. The answer is 17 years, what is the question: understanding time lags in translational research. J R Soc Med. 2011;104:510–20. doi: 10.1258/jrsm.2011.110180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Contopoulos-Ioannidis DG, Ntzani E, Ioannidis JP. Translation of highly promising basic science research into clinical applications. Am J Med. 2003;114:477–84. doi: 10.1016/s0002-9343(03)00013-5. [DOI] [PubMed] [Google Scholar]
- 6.Lost in clinical translation. Nat Med. 2004;10:879. doi: 10.1038/nm0904-879. [DOI] [PubMed] [Google Scholar]
- 7.McMurry-Heath M, Hamburg MA. Creating a space for innovative device development. Sci Transl Med. 2012;4:163fs43. doi: 10.1126/scitranslmed.3005269. [DOI] [PubMed] [Google Scholar]