Abstract
An imbalance in the angiogenesis axis during pregnancy manifests as clinical preeclampsia due to endothelial dysfunction. Circulating sFLT-1 (soluble fms-like tyrosine kinase 1) increases and PlGF (placental growth factor) reduces prior to and during disease. We investigated the clinical and biochemical effects of replenishing the reduced circulating PlGF with recombinant human PlGF (rhPlGF) and thus restoring the angiogenic balance.
Hypertensive proteinuria was induced in a non-human primate (Papio hamadryas) by uterine artery ligation at 136 days gestation (of an 182 day pregnancy). Two weeks after uteroplacental ischemia (UPI), rhPlGF (rhPlGF, n=3) or normal saline (control, n=4) was administered by subcutaneous injection (100μg/kg/day) for 5 days. Blood pressure (BP) was monitored by intra-arterial radiotelemetry, sFLT-1 and PlGF by ELISA. UPI resulted in experimental preeclampsia evidenced by increased BP, proteinuria and endotheliosis on renal biopsy and elevated sFLT-1. PlGF significantly reduced after UPI. rhPlGF reduced SBP in the treated group (-5.2mmHg+0.8mmHg;from 132.6+6.6mmHg to 124.1+7.6mmHg) compared to an increase in SBP in controls (6.5mmHg+3mmHg; from 131.3+1.5mmHg to 138.6+1.5mmHg). Proteinuria reduced in the treated group (-72.7±55.7mg/mmol) but increased in the control group. Circulating sFLT-1 was not affected by the administration of PlGF, however a reduction in placental sFLT-1 mRNA expression was demonstrated. There was no significant difference in the weights or lengths of the neonates in the rhPlGF or control group, however, this study was not designed to assess fetal safety or outcomes.
Increasing circulating PlGF by the administration of rhPlGF improves clinical parameters in a primate animal model of experimental preeclampsia.
Keywords: hypertension, preeclampsia/pregnancy, animal model, placental growth factor
Introduction
Preeclampsia is a pregnancy related disorder resultant, in part, from the imbalance in the angiogenesis axis1. It has been shown that prior to the disease being clinically apparent there is a reduction in the circulating PlGF and an increase in the circulating sFLT-12. The reduction in PlGF has been noted as early as the first trimester in those women who will go on to develop preeclampsia subsequently in pregnancy3, 4. The reduced PlGF has been used in multifactorial assessments in predicting preeclampsia5. Preeclampsia occurs in approximately 2-5% of pregnancies6, 7 and the clinical manifestations are resultant from endothelial dysfunction and include proteinuria, hypertension and fetal growth restriction amongst other signs and symptoms. Treatment thus far is expectant management, essentially prolongation of pregnancy by controlling blood pressure whilst balancing the risks of worsening endothelial dysfunction to mother and baby compared to the benefits of continued intra-uterine growth8. Ultimately, disease resolution requires the removal of the placenta- although many methods and agents have been evaluated as potential therapies9, 10
Several agents have been tested to reverse the angiogenic imbalance in preclinical or animal models of preeclampsia. VEGF (Vascular endothelial growth factor)11, sildenafil10, statins 12, metformin13 and Kraussianone-214. Most of the agents reduce the available or secreted sFLT-1, however not all has assessed the effect on the PlGF. Statins have been shown to increase PlGF15, 16. Sildenafil has been tested clinically, and despite its biochemical benefits, it has not been shown to improve clinical outcomes17.
Pregnancy and preeclampsia work has been undertaken in rodent18 and sheep19 models, however non-human primate (Papio hamadryas hamadryas) animal models of preeclampsia have many advantages. The placenta is haemomonochorial, implantation is interstitial, the uterine blood flow is antigravity (similarly to humans), typically each pregnancy involves a single fetus and a long gestation as compared to rodents (normal baboon gestation 182 days)20. The baboon placenta differs from human: trophoblast invasion is more superficial and typically does not involve the myometrial segment of the uterine arteries and multinuclear giant cells are not present21. Preeclampsia does not occur frequently in baboons, although there are case reports describing spontaneous preeclampsia 22. Inducing uteroplacental ischemia (by ligating unilateral, non-dominant uterine artery) during pregnancy results in proteinuric hypertension, renal endotheliosis and an associated rise in sFLT-123, the effect on circulating PlGF has not been previously assessed.
Rising PlGF correlates to improved placental perfusion in human pregnancies24. Although, uteroplacental ischemia has been shown to be pivotal to the development of preeclampsia25, the change in PlGF concentrations resultant from placental ischemia has not been investigated. Using a non-human primate model of uteroplacental ischemia23, we investigated the effect of the ischemia on PlGF concentrations. Furthermore, since PlGF does not bind VEGF receptor 2, it may have fewer side-effects than VEGF, such as excess vascular permeability and oedema. We hypothesised that by infusing PlGF, which binds and inactivates sFLT-1, there would be an improvement in clinical signs of an established preeclampsia syndrome (animal model of experimental preeclampsia) including blood pressure and proteinuria, as well as biochemical changes, circulating sFLT-1 and PlGF.
Methods
Animals
Female pregnant baboons (Papio hamadryas, n=7) from the National Baboon Colony, New South Wales, Australia were provided food and water ad libitum. At the beginning of the protocol the animals were 130(±1.8) days gestation of a normally 182 day gestation. Animals were anesthetised using ketamine infusion (0.2mg/kg/min) with a premedication of metoclopramide (5mg intramuscularly as well as clonazepam (intravenously at 0.01mg/kg for seizure prophylaxis). All animals received procedural antibiotics benzylpenicillin and gentamicin intravenously at the time of surgery. They also received buprenorphine (0.02mg/kg, intramuscularly) for analgesia, before, immediately after, 8 and 16 hrs after the procedure. Pain was scored and further analgesia could be administered but was not required. This experiment was approved by the Sydney Local Health District Animal Welfare Committee.
All animals (Papio hamadryas) were acclimatised for several days, a radiotelemeter was surgically inserted into a branch of the femoral artery and passed into the aorta to the level of the renal arteries, to monitor intra-arterial blood pressure (PA-D70, DataSci, Minnesota, USA) as has been described previously23. In brief, blood pressure change is expressed as the absolute change (mmHg) compared to baseline (blood pressure taken for 48hrs prior to the UPI). Drift in blood pressure measurement was measured and readings adjusted appropriately to allow for the degradation of the readings due to battery depletion over the length of the protocol that has been well described previously26. Blood pressure whilst the animals were resting quietly but awake (awake BP) was assessed between 1600-2000 hours and blood pressure during sleep (sleep BP) was measured between 2200 and 0300 hours. The readings were censored for activity.
After 1 week recovery, uteroplacental ischemia (UPI) was induced experimentally as described previously23. Briefly, animals underwent non-dominant unilateral uterine artery complete ligation through a midline abdominal transperitoneal incision. The iliac vessels were visualised and the uterine artery identified at its origin from the internal iliac artery. The uterine artery was irrigated with 1% lignocaine solution to reduce arterial spasm and then ligated with 4.0 silk sutures. Complete ligation was verified by performing repeat uterine artery duplex ultrasonography after the procedure that demonstrated no-flow in the artery where previously flow had been demonstrated. The peritoneum, muscle and skin were closed in layers.
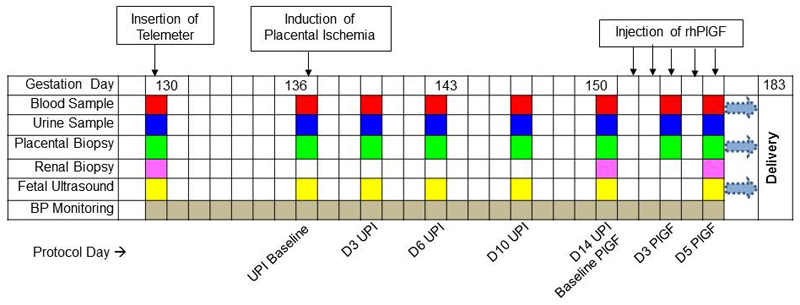
Animals were then observed for two weeks. Blood, urine and chorionic villous samples (CVS) were collected on Day 3, 6, 10 and 14 of UPI. Blood pressure was monitored continuously by telemetry. After two weeks, rhPlGF (recombinant human placental growth factor; R & D, Minneapolis USA) reconstituted in 0.9% normal saline and administered to 3 animals subcutaneously at a dose of 100μg/kg/day (similarly to Suzuki et al)27 as a single injection for 5 days administered between 0600 and 0700 each day. The control group (n=4) received an equal volume vehicle, 10mls 0.9% normal saline. Blood, urine and CVS samples were taken at day 3 and day 5 of rhPlGF (or vehicle) administration. A timeline (Figure 1) demonstrates when the procedures were performed.
Figure 1:
Timeline demonstrating the gestation at which procedures were performed. The timing of blood samples (red box), urine samples (blue box), placental biopsies (green box), renal biopsies (pink box) and fetal ultrasounds (yellow box) are demonstrated. D= day, rhPlGF = recombinant human placental growth factor.
Specimen Collection
Blood was taken from the cubital vein and centrifuged for 10mins at 3000rpm. The plasma was aliquoted (250uL aliquots) and stored at -80C for future analysis of sFLT-1 and PlGF by ELISA. The peripheral blood mononuclear cells (PBMC) were isolated from blood using standard methods28 for PCR of sFLT-1 and PlGF. Catheterised spot urinary protein excretion was adjusted for creatinine excretion and expressed as mg/mmol. Placental chorionic villous samples were performed trans-abdominally as described previously28 and underwent PCR for sFLT-1 and PlGF. Term placentas could not be collected as the animals ingest these immediately.
Renal Biopsy
Percutaneous renal biopsies were performed sequentially in each animal resulting in three biopsies each animal. The baseline biopsy was performed immediately after the insertion of the telemeter. The second biopsy was performed two weeks after UPI. The last biopsy after the 5 days of rhPlGF or 0.9% normal saline injections. Each time the procedure was identical. The animals were placed in the right or left recumbent position on a soft wedge and a 3-4cm sub-costal skin incision anterior to the erector spinae muscle was made. Blunt dissection was performed till the renal capsule kidney was identified without disrupting the peritoneum. A small wedge biopsy was taken from the lower pole (0.5cm × 0.5cm× 0.5cm), the specimen divided and portions immediately placed in 2% glutaraledyhyde for electron microscopy or 10% formalin for later paraffin block embedment or snap frozen in liquid nitrogen. Gelfoam (Upjohn, WA, Australia) was placed in the renal defect to ensure adequate haemostasis and then closure in layers.
ELISA
Baboon plasma sFLT-1 and PlGF and urinary cGMP (cyclic guanosinemonophosphate) was measured by a commercially available human ELISA (R&D, Minneapolis, USA) in duplicate. The CV (coefficient of variation) for all assays was less than 5% and the lower limit of detection for the sFLT-1 ELISA was 20pg/ml, PlGF ELISA was 2pg/mL and for the cGMP was 2pmol/ml. Human sFLT-1 ELISA measures total sFLT-1 and human PlGF ELISA kit measures free PlGF concentrations as described elsewhere29.
Quantitative PCR
RNA was extracted from isolated PBMC and chorionic villous tissue using a commercially available kit (NucleoSpin RNAII; Macherey-Nagel) which has been described previously 30. One microgram of RNA was reverse transcribed to cDNA using Superscript III RNase Reverse Transcriptase (Invitrogen). qPCR was performed by the comparative threshold cycle method and normalised to β2microglobulin expression. The primers used for sFLT-1, PlGF and β2microglobulin have been described 30, 31. RNA expression is expressed as the percentage change compared to baseline (pre- UPI) samples.
Statistical analysis
Statistical analysis was performed (using SPSS™v22.0) with GLM (general linear model) repeated measures, Students t-test and a bonferroni correction was applied where applicable to allow for adjustment of multiple comparisons. Data are expressed as mean±SEM. Significance set at p<0.05.
Results
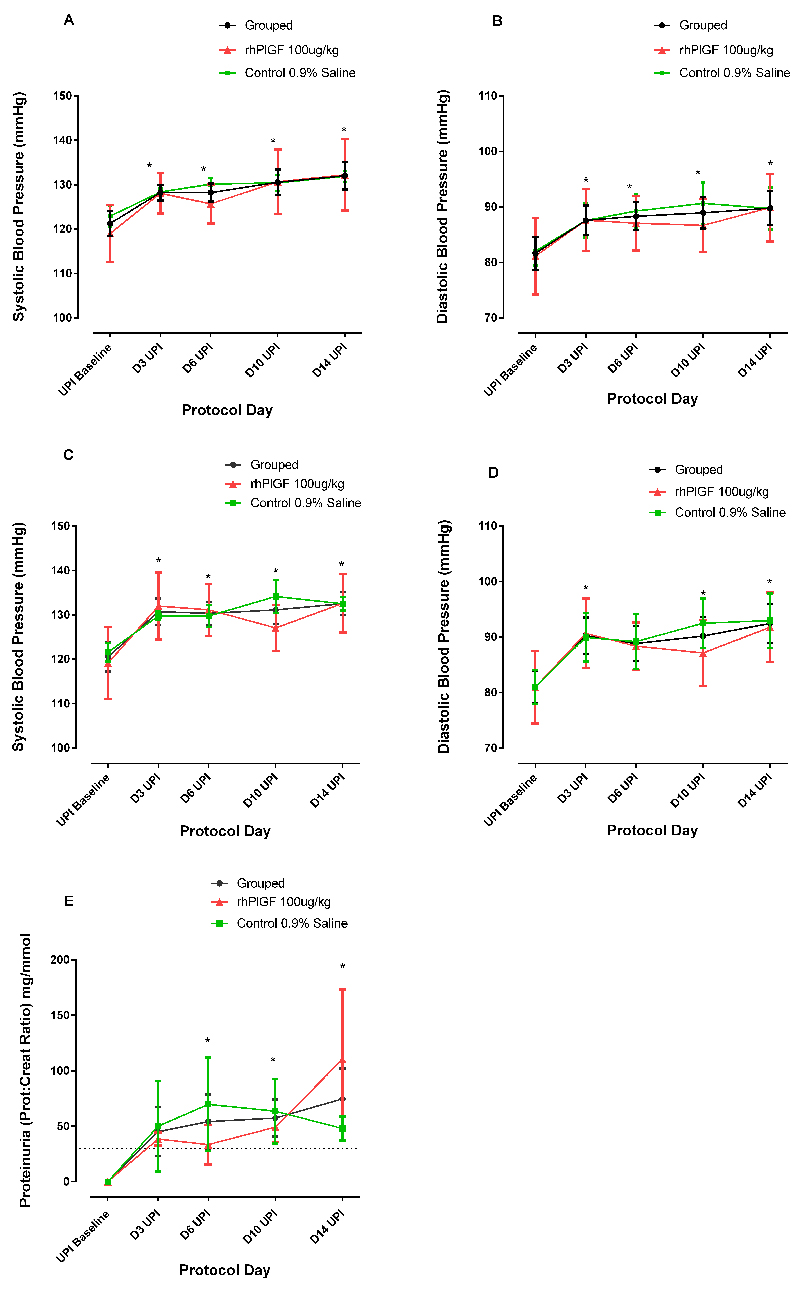
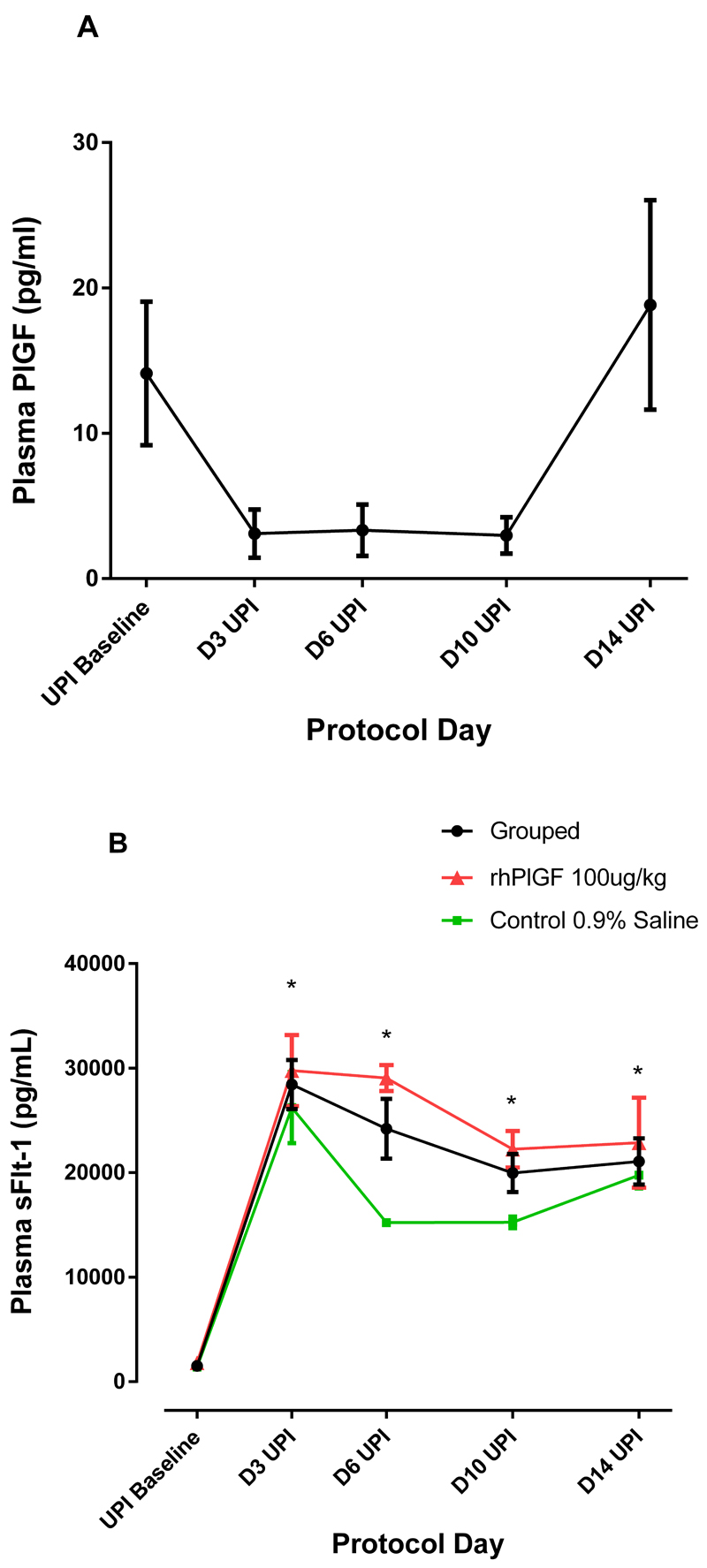
UPI resulted in a significant elevation in blood pressure (p<0.001) which was evident within 3 days of the UPI, as has been demonstrated previously23. There was no significant difference in baseline maternal weight (rhPlGF group: 14.9±1.0kg and control group: 13.9±0.2kg), blood pressure, proteinuria, sFLT-1 or PlGF concentrations between the rhPlGF and control groups. After the UPI, there was a significant rise in blood pressure from a baseline of 120±3.9/80±3.1mmHg (awake) and 121±3.3/81±3.4mmHg (sleep) to a blood pressure of 132±3.0/91±3.6mmHg (awake) and 132±3.7/89±3.4mmHg (sleep) after 14 days of UPI. The changes in blood pressure were evident awake and asleep (Figure 2a-d). Proteinuria (was not present at baseline (spot urinary protein:creatinine concentration ratio <5mg/mmol), but significantly (p<0.05) increased as a result of the UPI from Day 6 UPI onwards (Day 14 UPI spot urinary protein: creatinine concentration ratio 57.4±16.7mg/mmol: Figure 2e). There was a significant change in plasma PlGF after the induction of uteroplacental ischemia (p=0.025, GLM repeated measures) (Figure 3a). There was no significant difference between the two groups. Taken together, the PlGF levels were notably lower after 3 days of UPI compared to baseline (Baseline: 14.1±4.9pg/mL; Day 3: 3.1±1.7 pg/mL; p<0.05). The PlGF levels increased after day 10 UPI back to baseline levels without intervention other than sampling as described above.
Figure 2:
Effects of Uteroplacental ischemia (UPI) on blood pressure a) systolic (SBP) and b) diastolic (DBP) blood pressure (mmHg) during sleep and c) systolic and d) diastolic blood pressure during awake periods and e) proteinuria (expressed as the spot urinary protein: urinary creatinine ratio) on protocol days 3, 6, 10 and 14 after uteroplacental ischemia. Data for the control (n=4, green line), treated (n=3, red line) and total group (n=7, black line) is demonstrated on each graph. * indicates a significant difference of the grouped result compared to baseline blood pressure p<0.001.
Figure 3:
Effects of UPI on a) Plasma PlGF Grouped data (n=7) plasma PlGF at protocol days 3, 6, 10 and 14 after UPI. There is a significant change over time (p=0.025). b) Plasma total sFLT-1 after UPI at protocol days 3, 6, 10 and 14 after UPI for the control (n=4, green line), treated (n=3, red line) and total group (n=7, black line) is demonstrated. * indicates a significant difference of the grouped result compared to baseline sFLT-1 concentration p<0.001.
Uteroplacental ischemia resulted in a significant elevation in total plasma sFLT-1 concentration (n=7, all animals grouped) (Baseline: 1 508±250 pg/mL and Day 14 UPI: 21 077±2 209 pg/mL; p<0.0001) that remained elevated for the duration of UPI. At baseline and after 14 days UPI, there was no significant difference between the rhPlGF and control groups (Day 14 UPI:22 860±4 312.5 pg/mL and 19 294±1 617 pg/mL respectively; p=0.48)(Figure 3b). The urinary cGMP did not change from baseline either with an adjustment for urinary creatinine concentration (Baseline ratio: 78.9+9.7nmol/mmol and Day 14 UPI: 85.6+24.7nmol/mmol; p=0.80) or without a urinary creatinine concentration adjustment (p=0.25).
Administration of rhPLGF
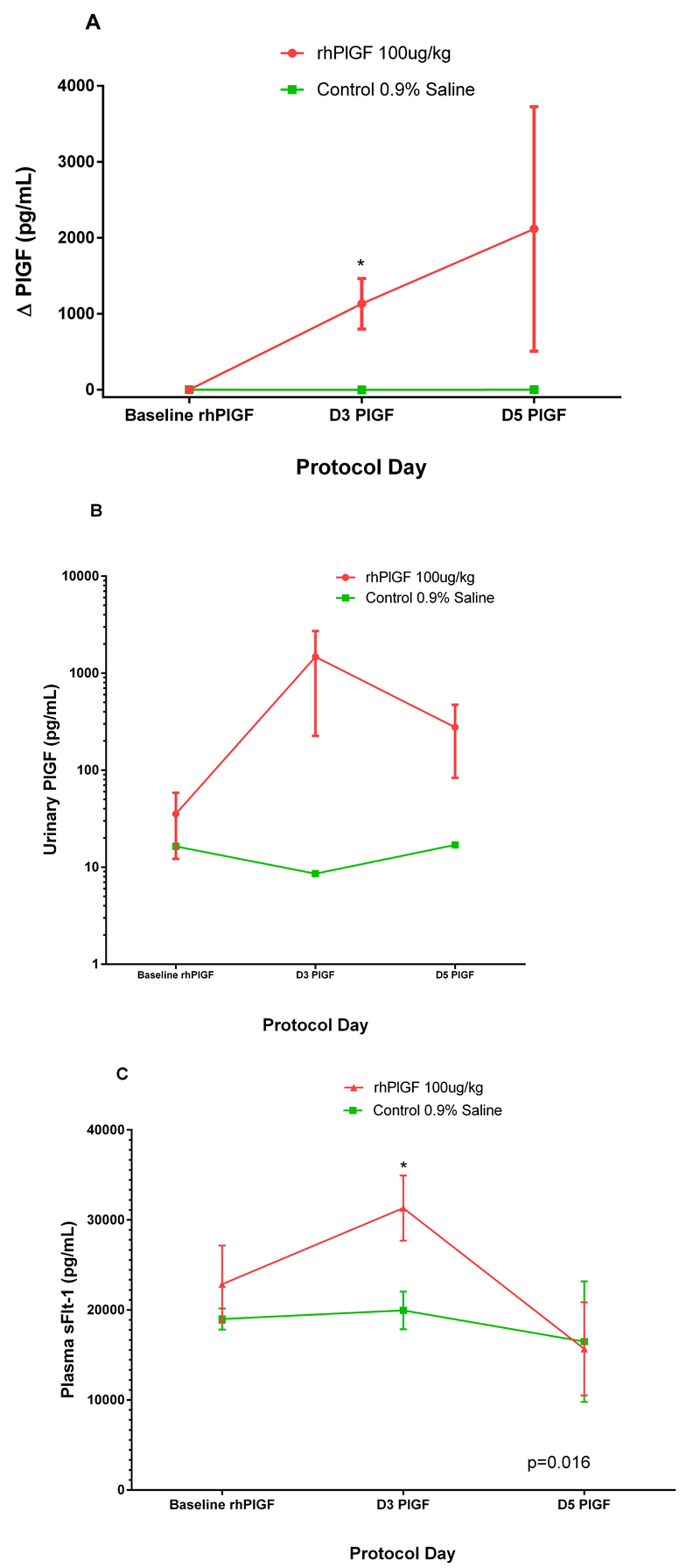
The administration of the PlGF resulted in a rise in plasma PlGF levels in the rhPlGF group. The change in plasma PlGF in the rhPlGF and the control group was 2 116.6±1,610 pg/mL and -0.3±2.6 pg/ml respectively after 5 days of subcutaneous injection. The difference between the two groups was statistically significant at day 3 of injection (Figure 4a; p=0.027). The urinary PlGF (either adjusted or not adjusted for the urinary creatinine excretion) did not differ statistically significantly between the groups after the administration of the rhPlGF (p=0.076), however there was a trend to higher levels in the rhPlGF group (p=0.06) (Figure 4b). The urinary concentrations of PlGF were markedly lower than the contemporaneous circulating PlGF concentrations. Although the concentrations in the circulation remained elevated till Day 5 of injection, the urinary PlGF had reduced at Day 5, suggesting a significant proportion was bound and still circulating. There was no significant change in urinary cGMP after the administration of rhPlGF (p=0.29).
Figure 4:
Changes in PlGF and sFLT-1 after rhPlGF. a) plasma PlGF concentrations (pg/mL) in control (n=4,green line) and treated (n=3, red line) groups after the administration of rhPlGF 100μg/kg or 0.9% saline subcutaneously in equal volumes respectively. *The rhPlGF concentrations at Day 3 of injection are significantly higher in the rhPlGF group compared to the control group (p=0.027). b) urinary PlGF PlGF concentrations (pg/mL) in control (n=4,green line) and treated (n=3, red line) groups after the administration of rhPlGF 100μg/kg or 0.9% saline subcutaneously in equal volumes respectively. c) Changes in plasma sFLT-1 concentrations (pg/mL) in control (n=4, green line) and treated (n=3, red line) groups after the administration of rhPlGF 100μg/kg or 0.9% saline subcutaneously in equal volumes respectively. *The total sFLT-1 concentrations at Day 3 of injection are significantly higher in the rhPlGF group compared to the control group (p=0.016).
The total sFLT-1 concentrations increased significantly more in the rhPlGF group at day 3 of injection compared to the control group (sFLT-1 rhPlGF: baseline 22 860±4,312 and day 3 33 133±3,624 pg/mL Control: 18 997±1 181 and day 3 19 965±2 112 pg/mL: p=0.016 GLM repeated measures, Bonferroni correction repeated measures)(Figure 4c). Although there was a reduction in sFLT-1 concentration after day 3 in the control group, this was not statistically significant.
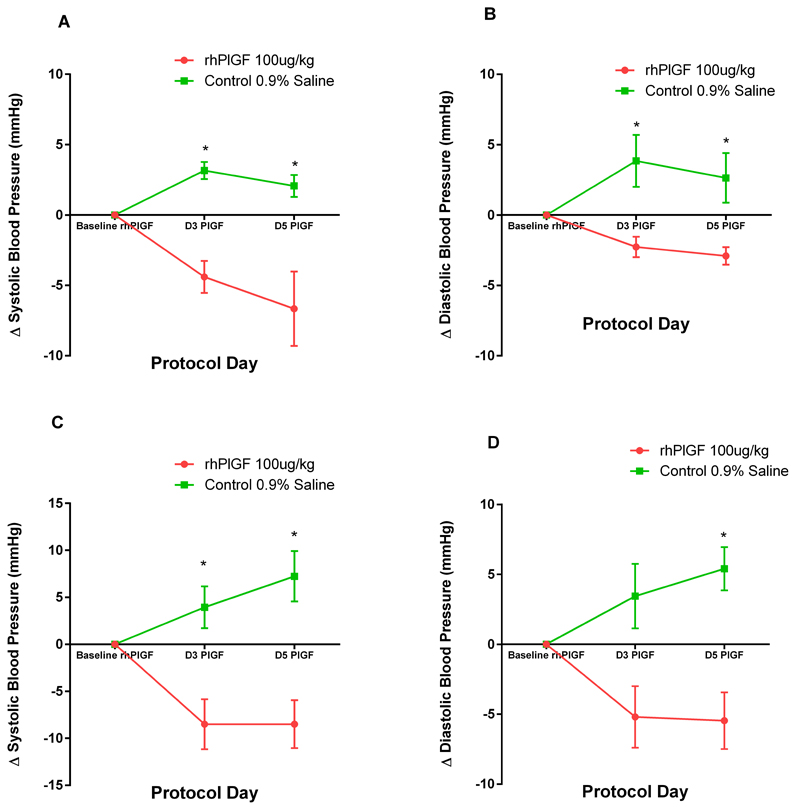
The administration of recombinant human PlGF for 5 days resulted in a reduction in the blood pressure in the rhPlGF group compared to the control group (Figure 5a-d). The reduction in blood pressure was evident after 3 days of rhPlGF injections. There was a reduction in systolic and diastolic blood pressure both whilst awake and during sleep in the rhPlGF compared to an elevation of the BP in the control group (SBP awake : p=0.015, DBP awake : p=0.026, SBP sleep : p=0.025 and DBP sleep : p=0.012). Whilst awake, the SBP and DBP in the rhPlGF and the control groups altered by SBP: -8.5±2.6mmHg and 7.2±2.7mmHg respectively and DBP:-5.5±2.0mmHg and 5.4±1.6mmHg respectively.
Figure 5:
Change in a) systolic blood pressure b) diastolic blood pressure whilst asleep and c) systolic blood pressure and d) diastolic blood pressure awake (mmHg) after 3 and 5 days of rhPlGF 100μg/kg or control (0.9% normal saline) injections. The change in blood pressure in the control group (n=4, green line) and treated group (n=3, red line) is compared to the blood pressure after 14 days of UPI (Baseline PlGF). * indicates a significant difference compared to baseline PlGF, p<0.05.
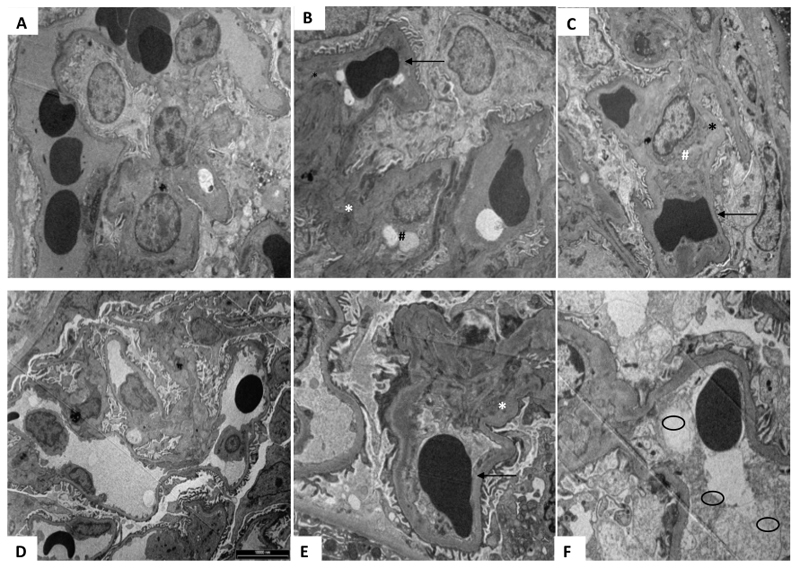
There was a significant reduction in proteinuria with the administration of rhPlGF (p=0.036). The change in proteinuria in the rhPlGF and control group was -72.7±55.7mg/mmol and 16.7±4.8mg/mmol respectively at Day 5 of rhPlGF compared to day 14 of UPI (Figure 6).Renal biopsies, after UPI, demonstrated changes consistent with human-like preeclampsia. These findings were not clearly evident on the light microscopy. However, on electron microscopy there is apparent endotheliosis, subendothelial fibrin deposition as well as fibrin and fibrinoid deposition in the mesangium. These changes were evident in all animals. Qualitatively, there was an improvement in the changes in the rhPlGF group (Figure 7). The control group (Figure 7a-c) renal biopsy electron microscopy did not appreciably change compared to the biopsy undertaken at the end of the UPI. There were still significant changes consistent with human-like preeclampsia. The changes were most marked in the animals given rhPlGF were there was a reduction in fibrin deposition as well as a reduction in subendothelial deposits (figure 7d-f).
Figure 6:
Change in proteinuria in spot urinary protein : creatinine ratio (mg/mmol)) after 3 and 5 days of rhPlGF 100μg/kg (n=3, red line) or control (0.9% normal saline: n=4, green line) injections. The change in proteinuria is compared to the concentration after 14 days of UPI (Baseline PlGF). * indicates a significant difference compared baseline PlGF, p<0.05.
Figure 7:
Electron microscopy of renal biopsies undertaken during the protocol at baseline (A + D), Day 14 of UPI (B+E) and after 5 days (C +F) of rhPlGF or control respectively. (A-C) Demonstrates the changes over time in a control animal. At baseline (A) there are no significant changes noted, importantly the vascular endothelium and vessels appear normal. After 14 days of UPI (B) there are changes consistent human- like preeclampsia such as subendothelial deposits (*), endothelial cells cytoplasmic vacuoles (#) and reduction in capillary lumen demonstrated by a compressed red blood cell (→). After 5 days of 0.9% normal saline (Control group), there is no resolution of the endothelial and other changes noted previously. (D-F) Demonstrates the changes over time in a treated animal (rhPlGF). At baseline (D) there are no significant changes noted, importantly the vascular endothelium and vessels appear normal and endothelial cells are present and appear normal. After 14 days of UPI (E) there are changes consistent with human- like preeclampsia such as subendothelial deposits (*) and reduction in capillary lumen demonstrated by compressed red blood cell (→). After 5 days of rhPlGF, there is a marked improvement in the changes noted. The capillary lumen is improved (○), although not restored, and there is a substantial reduction in subendothelial deposits.
rhPlGF administration resulted in a reduction in the sFLT-1 mRNA expressed in the placenta as measured by serial CVS sampling compared to the control group (p=0.021; Figure 8). The animals given rhPlGF demonstrated a reduction in the placental mRNA expression over time. This was in contrast to the Control group, where the mRNA expression increased after 3 days of rhPlGF. There was no significant change in the placental expression of PlGF. The expression of PlGF and sFLT-1 did not vary in isolated PBMCs as measured by real time PCR.
Figure 8:
Changes in chorionic villous sampling expression of sFLT-1 mRNA after 3 days of rhPlGF 100μg/kg (red bars) or control (0.9% normal saline, green bars) injections. There was a significant difference in the alteration of sFLT-1 mRNA expressed in the placenta (p<0.05).
All animals delivered at term. The neonates appeared normal on physical examination. There were no significant differences in the weights of the offspring of the two groups (rhPlGF group: 643±14g and control group: 650±25g).
Discussion
This study has demonstrated that the induction of uteroplacental ischemia results in a reduction in circulating PlGF. This supports one of the aetiological theories of preeclampsia – that reduced placental perfusion and consequentially, placental hypoxia or ischemia has a role in the clinical manifestation of the disease32. Furthermore, this correlates with indirect evidence that women with reduced PlGF in early pregnancy have an increased risk of placental dysfunction (either as preeclampsia or growth restriction) later in pregnancy5, 33. These women have also been noted to develop smaller placentae with evidence of insufficient perfusion34. The circulating PlGF in the current study increased at day 14 having reached a nadir at day 6 of ischemia. We postulate the PlGF increase was secondary to partial reperfusion of the ischemic portion of the placenta. Improved placental perfusion, although indirectly, has been shown to be associated with increased circulating PlGF – in humans, an increase in PlGF has been shown to correlate with improved uterine perfusion as measured by uterine Doppler35. Conversely, women with demonstrated histopathological evidence of placental hypoperfusion had reduced PlGF compared to women with no placental hypoperfusion demonstrable36, 37. Gilbert et al have shown a similar magnitude reduction in PlGF in a rat RUPP (reduced uterine placenta perfusion) model38.
The administration of exogenous PlGF in an experimental model of preeclampsia reduced the blood pressure, proteinuria and sFLT-1 placental mRNA expression. The circulating PlGF, as would be expected increased after the administration of PlGF, however the total sFLT-1 also increased. The increase in sFlt-1 may have been due to release of pre-formed sFLT-1 from tissues, as demonstrated by Zhao et al39. The reduction in placental mRNA sFLT-1 may thus represent a feedback reduction secondary to the increased circulating levels of sFLT-1. Despite the increase in total sFLT-1 there was a reduction in BP during this time. The increase in sFLT-1 we suggest would be bound to the increased available PlGF, thus unable to exert its anti-angiogenic effect. There are other instances where the circulating sFLT-1 increases without a concomitant elevation in BP, such as with administration of heparin40. So, the elevation in blood pressure in this model although concomitant with an increase in sFLT-1 was corrected by the addition of PlGF which did not significantly alter circulating sFLT-1 but which may have induced the change via a tissue effect not reflected in serum concentrations
The therapeutic benefits of exogenous PlGF demonstrated, reduction in blood pressure and reduction in proteinuria, may be due to other factors as well. The rhPlGF, via activation of FLT-1 and sFLT-1 to a lesser extent, is potentiating the angiogenic response to VEGF41. PlGF has also been shown to behave in an autocrine manner with VEGF as well as to behave independently in activating endothelial cells 42. Furthermore, in hypoxic conditions, such as in the placenta, the formation of VEGF/PlGF heterodimers are up regulated43. All of these are means by which PlGF could have a positive pro-angiogenic effect, other than binding to the free sFLT-1.
The Papio hamadryas PlGF sequence is not yet fully elucidated. However, we have sequenced part of the PlGF molecule (GenBank JX240432.1) This portion of the baboon was 97% homologous with the human PlGF (Genbank: BC 007789.2) mRNA sequence as assessed by BLAST alignment44. Although there is a species difference, given the homology in the mRNA molecules, we would expect the human PlGF to have significant activity with baboon sFLT-1.
Suzuki et al administered a similar dose of PlGF to a mouse adenoviral vector model of preeclampsia and demonstrated the improvement in blood pressure but no change in proteinuria. They gave PlGF for 2 days, intraperitoneally27. Kumasawa et al, increased the concentrations of PlGF in a lentiviral model of preeclampsia indirectly by administering pravastatin but did not demonstrate a change in blood pressure or proteinuria when administration was commenced late in pregnancy (embryonic day 16.5)15. If the pravastatin administration was commenced early in pregnancy (embryonic day 7.5) there was a significant reduction in proteinuria and blood pressure at E16.5. However, with early administration of pravastatin, the magnitude increase in circulating PlGF at E16.5 was more modest than that seen in the current study (60.6 to 116.6pg/mL). When the PlGF concentration was increased to a greater extent (using lentiviral transfection of the blastocyst) Kumasawa did demonstrate a reduction in blood pressure, proteinuria and improved glomerular endotheliosis in the mouse renal biopsies. Finally, Spradley et al administered human recombinant PlGF to a rat UPI model and showed a reduction in blood pressure but proteinuria was not assessed45. In each case the sFLT-1 concentrations fell, which was in contrast to the current study- however in both of these studies – the production of sFLT-1 was constitutive and may not be subject to the same autoregulatory mechanisms as that which occurs as a result of an acute ischemic injury. The administration of PlGF in our study may have resulted in the release of pre-formed membrane bound sFLT-1, which has been described previously with proteases39.
Although there is a wide range of what is considered normal circulating PlGF in uncomplicated pregnancy2, 46, 47, the levels of PlGF after administration of rhPlGF in the current study were at the upper extreme of physiological levels. Others have demonstrated PlGF concentrations at the lower end of the uncomplicated human pregnancy range in the setting of lentivirus and adenoviral induced experimental preeclampsia 15, 27. This may have a role in the differing sFLT-1 levels seen after the increase in PlGF. Furthermore, the circulating sFLT-1 also differed between studies prior to the increase in PlGF. The adenoviral model developed levels of sFLT-1 that were physiologically very high (113ng/ml) and higher than the lentiviral model (5.84ng/ml) whose levels were comparatively at the lower end of the range for women with preeclampsia. The sFLT-1 levels in our model were between the two: at the end 14 days of UPI (22ng/ml).
Changes in the urinary cGMP excretion in women with preeclampsia compared to controls have been inconsistent. No change in cGMP either with UPI or with the administration of rhPlGF was demonstrated. Conrad et al and others found no significant change48, 49, yet Baksu et al found a reduction in urinary cGMP and other metabolites of nitric oxide in women with preeclampsia compared to controls50. In UPI rat models George et al have shown a reduction in renal medullary cGMP expression10 however urinary excretion of cGMP was not assayed. Although we did not find a difference in the cGMP, the concentrations were similar to those published in humans eg Conrad et al found women with preeclampsia to have urinary cGMP concentrations of 1.2nmol/mg which approximates the concentration at Day 14 UPI (0.98nmol/mg). The concentrations may not differ either because cGMP in the urine does not actually change – which is in contrast to changes in cGMP seen in vascular smooth muscles51, 52 or the syndrome produced by UPI is not severe enough to detect a change in cGMP in the urine.
The teratogenicity of any agent given during pregnancy both with regards to birth anomalies and future fetal metabolic or physiologic consequences must be considered. Although our study was not powered to assess the teratogenicity of PlGF- no birth anomalies were noted at birth. Excessive PlGF has been shown to increase survival gene expression and inhibit apoptosis53. When PlGF is constitutively expressed (in transgenic mice that overexpress PlGF) there is evidence of induced vascularisation starting from fetal development and continuing into adulthood. This is associated with enhanced vessel permeability54. Circulating PlGF levels were higher in the transgenic mice both in the fetal period and in adulthood. Interestingly Odorisio et al did show that the over expression of PlGF was associated with up regulation of FLT-1 and FLK-1, although the levels of sFLT-1 were not investigated54.
Perspectives
Clinical features typical of human-like preeclampsia, that is, proteinuric hypertension and associated renal histological changes, can be reduced without terminating a pregnancy in primates. Placental growth factor is demonstrated to be part of the physiological sequence controlling blood pressure and proteinuria as a consequence of placental ischemia. It may well be that if endogenous ischemia is further proven as a mechanism for preeclampsia, that targeting effector proteins such as PLGF and correcting the angiogenic imbalance, may limit the blood pressure changes and the target end organ effects, such as those seen in the kidney.
Novelty and Significance.
What is new?
This study shows that induced placental ischemia reduces circulating PlGF, already demonstrated in humans but presumed to be placental ischemia in origin. In a non-human primate animal model of preeclampsia, infusing a pro-angiogenic molecule, such as PlGF, improves hypertension, proteinuria and renal biopsy proven endotheliosis.
What is relevant?
Placental ischemia causes endothelial dysfunction as evidenced by hypertension and proteinuria. PlGF can improve these clinical signs by improving the endothelial dysfunction.
Summary
Reversing the angiogenic imbalance in preeclampsia by increasing PlGF concentrations, improves the endothelial dysfunction as evidenced by reductions in blood pressure and proteinuria.
Acknowledgments
Funding Sources: NHMRC Project Grant (APP1025258), Australian Postgraduate Award (KY), Howard Hughes (SAK)
Footnotes
Conflicts of Interest/ Disclosures
Ravi Thadhani is co-inventor of patents related to diagnostics in the prediction of preeclampsia that have been out-licensed to diagnostic companies and has financial interest in Aggamin LLC. Ravi Thadhani also reports serving as a consultant to Roche diagnostics. S. Ananth Karumanchi is co-inventor of multiple patents related to the use of angiogenic proteins for the diagnosis and therapy of preeclampsia. These patents have been licensed to multiple companies. S. Ananth Karumanchi also reports serving as a consultant to Roche Diagnostics, Siemens and Thermofisher and has financial interest in Aggamin LLC.
References
- 1.Redman CW, Sargent IL, Staff AC. Ifpa senior award lecture: Making sense of pre-eclampsia - two placental causes of preeclampsia? Placenta. 2014;35(Suppl):S20–25. doi: 10.1016/j.placenta.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 3.Savvidou MD, Akolekar R, Zaragoza E, Poon LC, Nicolaides KH. First trimester urinary placental growth factor and development of pre-eclampsia. Bjog. 2009;116:643–647. doi: 10.1111/j.1471-0528.2008.02074.x. [DOI] [PubMed] [Google Scholar]
- 4.Thadhani R, Mutter WP, Wolf M, Levine RJ, Taylor RN, Sukhatme VP, Ecker J, Karumanchi SA. First trimester placental growth factor and soluble fms-like tyrosine kinase 1 and risk for preeclampsia. J Clin Endocrinol Metab. 2004;89:770–775. doi: 10.1210/jc.2003-031244. [DOI] [PubMed] [Google Scholar]
- 5.Akolekar R, Syngelaki A, Poon L, Wright D, Nicolaides KH. Competing risks model in early screening for preeclampsia by biophysical and biochemical markers. Fetal Diagn Ther. 2013;33:8–15. doi: 10.1159/000341264. [DOI] [PubMed] [Google Scholar]
- 6.Roberts CL, Ford JB, Algert CS, Antonsen S, Chalmers J, Cnattingius S, Gokhale M, Kotelchuck M, Melve KK, Langridge A, Morris C, et al. Population-based trends in pregnancy hypertension and pre-eclampsia: An international comparative study. BMJ Open. 2011;1:e000101. doi: 10.1136/bmjopen-2011-000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abalos E, Cuesta C, Carroli G, Qureshi Z, Widmer M, Vogel JP, Souza JP. Pre-eclampsia, eclampsia and adverse maternal and perinatal outcomes: A secondary analysis of the world health organization multicountry survey on maternal and newborn health. Bjog. 2014;121(Suppl 1):14–24. doi: 10.1111/1471-0528.12629. [DOI] [PubMed] [Google Scholar]
- 8.Lowe SA, Brown MA, Dekker GA, Gatt S, McLintock CK, McMahon LP, Mangos G, Moore MP, Muller P, Paech M, Walters B. Guidelines for the management of hypertensive disorders of pregnancy 2008. Aust N Z J Obstet Gynaecol. 2009;49:242–246. doi: 10.1111/j.1479-828X.2009.01003.x. [DOI] [PubMed] [Google Scholar]
- 9.Thadhani R, Kisner T, Hagmann H, Bossung V, Noack S, Schaarschmidt W, Jank A, Kribs A, Cornely OA, Kreyssig C, Hemphill L, et al. Pilot study of extracorporeal removal of soluble fms-like tyrosine kinase 1 in preeclampsia. Circulation. 2011;124:940–950. doi: 10.1161/CIRCULATIONAHA.111.034793. [DOI] [PubMed] [Google Scholar]
- 10.George EM, Palei AC, Dent EA, Granger JP. Sildenafil attenuates placental ischemia-induced hypertension. Am J Physiol Regul Integr Comp Physiol. 2013;305:R397–403. doi: 10.1152/ajpregu.00216.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilbert JS, Verzwyvelt J, Colson D, Arany M, Karumanchi SA, Granger JP. Recombinant vascular endothelial growth factor 121 infusion lowers blood pressure and improves renal function in rats with placentalischemia-induced hypertension. Hypertension. 2010;55:380–385. doi: 10.1161/HYPERTENSIONAHA.109.141937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bauer AJ, Banek CT, Needham K, Gillham H, Capoccia S, Regal JF, Gilbert JS. Pravastatin attenuates hypertension, oxidative stress, and angiogenic imbalance in rat model of placental ischemia-induced hypertension. Hypertension. 2013;61:1103–1110. doi: 10.1161/HYPERTENSIONAHA.111.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brownfoot FC, Hastie R, Hannan NJ, Cannon P, Tuohey L, Parry LJ, Senadheera S, Illanes SE, Kaitu'u-Lino TJ, Tong S. Metformin as a prevention and treatment for preeclampsia: Effects on soluble fms-like tyrosine kinase 1 (sflt-1) and soluble endoglin secretion, and endothelial dysfunction. Am J Obstet Gynecol. 2016;214:e1–5. doi: 10.1016/j.ajog.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 14.Ramesar SV, Drewes SE, Gathiram P, Moodley J, Mackraj I. The effect of kraussianone-2 (kr2), a natural pyrano-isoflavone from eriosema kraussianum, in an l-name- induced pre-eclamptic rat model. Phytother Res. 2012;26:1375–1380. doi: 10.1002/ptr.3697. [DOI] [PubMed] [Google Scholar]
- 15.Kumasawa K, Ikawa M, Kidoya H, Hasuwa H, Saito-Fujita T, Morioka Y, Takakura N, Kimura T, Okabe M. Pravastatin induces placental growth factor (pgf) and ameliorates preeclampsia in a mouse model. Proc Natl Acad Sci U S A. 2011;108:1451–1455. doi: 10.1073/pnas.1011293108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saad AF, Kechichian T, Yin H, Sbrana E, Longo M, Wen M, Tamayo E, Hankins GD, Saade GR, Costantine MM. Effects of pravastatin on angiogenic and placental hypoxic imbalance in a mouse model of preeclampsia. Reprod Sci. 2014;21:138–145. doi: 10.1177/1933719113492207. [DOI] [PubMed] [Google Scholar]
- 17.Samangaya RA, Mires G, Shennan A, Skillern L, Howe D, McLeod A, Baker PN. A randomised, double-blinded, placebo-controlled study of the phosphodiesterase type 5 inhibitor sildenafil for the treatment of preeclampsia. Hypertens Pregnancy. 2009;28:369–382. doi: 10.3109/10641950802601278. [DOI] [PubMed] [Google Scholar]
- 18.LaMarca BB, Bennett WA, Alexander BT, Cockrell K, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: Role of tumor necrosis factor-alpha. Hypertension. 2005;46:1022–1025. doi: 10.1161/01.HYP.0000175476.26719.36. [DOI] [PubMed] [Google Scholar]
- 19.Leffler CW, Hessler JR, Green RS, Fletcher AM. Effects of sodium chloride on pregnant sheep with reduced uteroplacental perfusion pressure. Hypertension. 1986;8:62–65. doi: 10.1161/01.hyp.8.1.62. [DOI] [PubMed] [Google Scholar]
- 20.Carter AM. Animal models of human placentation--a review. Placenta. 2007;28(Suppl A):S41–47. doi: 10.1016/j.placenta.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Pijnenborg R, D'Hooghe T, Vercruysse L, Bambra C. Evaluation of trophoblast invasion in placental bed biopsies of the baboon, with immunohistochemical localisation of cytokeratin, fibronectin, and laminin. J Med Primatol. 1996;25:272–281. doi: 10.1111/j.1600-0684.1996.tb00210.x. [DOI] [PubMed] [Google Scholar]
- 22.Ramsay MM, Tame JD, Winter JA, Carbone LG, Schlafer DH, Nathanielsz PW. Proteinuric hypertension in a pregnant baboon: Was this pre-eclampsia? J Med Primatol. 1997;26:207–212. doi: 10.1111/j.1600-0684.1997.tb00054.x. [DOI] [PubMed] [Google Scholar]
- 23.Makris A, Thornton C, Thompson J, Thomson S, Martin R, Ogle R, Waugh R, McKenzie P, Kirwan P, Hennessy A. Uteroplacental ischemia results in proteinuric hypertension and elevated sflt-1. Kidney Int. 2007;71:977–984. doi: 10.1038/sj.ki.5002175. [DOI] [PubMed] [Google Scholar]
- 24.Welch PC, Amankwah KS, Miller P, McAsey ME, Torry DS. Correlations of placental perfusion and plgf protein expression in early human pregnancy. Am J Obstet Gynecol. 2006;194:1625–1629. doi: 10.1016/j.ajog.2006.01.012. discussion 1629–1631. [DOI] [PubMed] [Google Scholar]
- 25.Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of preeclampsia: Linking placental ischemia/hypoxia with microvascular dysfunction. Microcirculation. 2002;9:147–160. doi: 10.1038/sj.mn.7800137. [DOI] [PubMed] [Google Scholar]
- 26.Van Vliet BN, Chafe LL, Antic V, Schnyder-Candrian S, Montani JP. Direct and indirect methods used to study arterial blood pressure. Journal of pharmacological and toxicological methods. 2000;44:361–373. doi: 10.1016/s1056-8719(00)00126-x. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki H, Ohkuchi A, Matsubara S, Takei Y, Murakami M, Shibuya M, Suzuki M, Sato Y. Effect of recombinant placental growth factor 2 on hypertension induced by full-length mouse soluble fms-like tyrosine kinase 1 adenoviral vector in pregnant mice. Hypertension. 2009;54:1129–1135. doi: 10.1161/HYPERTENSIONAHA.109.134668. [DOI] [PubMed] [Google Scholar]
- 28.Orange S, Rasko JE, Thompson JF, Vaughan J, Olive E, Pedler M, Horvath JS, Hennessy A. Interleukin-10 regulates arterial pressure in early primate pregnancy. Cytokine. 2005;29:176–185. doi: 10.1016/j.cyto.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 29.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, et al. Excess placental soluble fms-like tyrosine kinase 1 (sflt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Makris A, Xu B, Yu B, Thornton C, Hennessy A. Placental deficiency of interleukin-10 (il-10) in preeclampsia and its relationship to an il10 promoter polymorphism. Placenta. 2006;27:445–451. doi: 10.1016/j.placenta.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Nagamatsu T, Fujii T, Kusumi M, Zou L, Yamashita T, Osuga Y, Momoeda M, Kozuma S, Taketani Y. Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: An implication for the placental vascular development and the pathophysiology of preeclampsia. Endocrinology. 2004;145:4838–4845. doi: 10.1210/en.2004-0533. [DOI] [PubMed] [Google Scholar]
- 32.Roberts JM. Pathophysiology of ischemic placental disease. Semin Perinatol. 2014;38:139–145. doi: 10.1053/j.semperi.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vatten LJ, Asvold BO, Eskild A. Angiogenic factors in maternal circulation and preeclampsia with or without fetal growth restriction. Acta Obstet Gynecol Scand. 2012;91:1388–1394. doi: 10.1111/j.1600-0412.2012.01516.x. [DOI] [PubMed] [Google Scholar]
- 34.Roberts DJ, Post MD. The placenta in pre-eclampsia and intrauterine growth restriction. J Clin Pathol. 2008;61:1254–1260. doi: 10.1136/jcp.2008.055236. [DOI] [PubMed] [Google Scholar]
- 35.Muller PR, James AH, Murtha AP, Yonish B, Jamison MG, Dekker G. Circulating angiogenic factors and abnormal uterine artery doppler velocimetry in the second trimester. Hypertens Pregnancy. 2006;25:183–192. doi: 10.1080/10641950600912968. [DOI] [PubMed] [Google Scholar]
- 36.Ogge G, Chaiworapongsa T, Romero R, Hussein Y, Kusanovic JP, Yeo L, Kim CJ, Hassan SS. Placental lesions associated with maternal underperfusion are more frequent in early-onset than in late-onset preeclampsia. J Perinat Med. 2011;39:641–652. doi: 10.1515/JPM.2011.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baltajian K, Hecht JL, Wenger JB, Salahuddin S, Verlohren S, Perschel FH, Zsengeller ZK, Thadhani R, Karumanchi SA, Rana S. Placental lesions of vascular insufficiency are associated with anti-angiogenic state in women with preeclampsia. Hypertens Pregnancy. 2014:1–13. doi: 10.3109/10641955.2014.926914. [DOI] [PubMed] [Google Scholar]
- 38.Gilbert JS, Babcock SA, Granger JP. Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression. Hypertension. 2007;50:1142–1147. doi: 10.1161/HYPERTENSIONAHA.107.096594. [DOI] [PubMed] [Google Scholar]
- 39.Zhao S, Gu Y, Fan R, Groome LJ, Cooper D, Wang Y. Proteases and sflt-1 release in the human placenta. Placenta. 2010;31:512–518. doi: 10.1016/j.placenta.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hagmann H, Bossung V, Belaidi AA, Fridman A, Karumanchi SA, Thadhani R, Schermer B, Mallmann P, Schwarz G, Benzing T, Brinkkoetter PT. Low-molecular weight heparin increases circulating sflt-1 levels and enhances urinary elimination. PLoS One. 2014;9:e85258. doi: 10.1371/journal.pone.0085258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, Wu Y, Bono F, Devy L, Beck H, Scholz D, et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7:575–583. doi: 10.1038/87904. [DOI] [PubMed] [Google Scholar]
- 42.Migdal M, Huppertz B, Tessler S, Comforti A, Shibuya M, Reich R, Baumann H, Neufeld G. Neuropilin-1 is a placenta growth factor-2 receptor. J Biol Chem. 1998;273:22272–22278. doi: 10.1074/jbc.273.35.22272. [DOI] [PubMed] [Google Scholar]
- 43.Cao Y, Chen H, Zhou L, Chiang MK, Anand-Apte B, Weatherbee JA, Wang Y, Fang F, Flanagan JG, Tsang ML. Heterodimers of placenta growth factor/vascular endothelial growth factor. Endothelial activity, tumor cell expression, and high affinity binding to flk-1/kdr. J Biol Chem. 1996;271:3154–3162. doi: 10.1074/jbc.271.6.3154. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Z, Schwartz S, Wagner L, Miller W. A greedy algorithm for aligning DNA sequences. Journal of computational biology: a journal of computational molecular cell biology. 2000;7:203–214. doi: 10.1089/10665270050081478. [DOI] [PubMed] [Google Scholar]
- 45.Spradley FT, Tan AY, Joo WS, Daniels G, Kussie P, Karumanchi SA, Granger JP. Placental growth factor administration abolishes placental ischemia-induced hypertension. Hypertension. 2016 doi: 10.1161/HYPERTENSIONAHA.115.06783. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, Kusanovic JP, Gotsch F, Erez O, Mazaki-Tovi S, Gomez R, et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med. 2008;21:9–23. doi: 10.1080/14767050701830480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohkuchi A, Hirashima C, Matsubara S, Takahashi K, Matsuda Y, Suzuki M. Threshold of soluble fms-like tyrosine kinase 1/placental growth factor ratio for the imminent onset of preeclampsia. Hypertension. 2011;58:859–866. doi: 10.1161/HYPERTENSIONAHA.111.174417. [DOI] [PubMed] [Google Scholar]
- 48.Conrad KP, Kerchner LJ, Mosher MD. Plasma and 24-h no(x) and cgmp during normal pregnancy and preeclampsia in women on a reduced no(x) diet. Am J Physiol. 1999;277:F48–57. doi: 10.1152/ajprenal.1999.277.1.F48. [DOI] [PubMed] [Google Scholar]
- 49.Schiessl B, Strasburger C, Bidlingmaier M, Mylonas I, Jeschke U, Kainer F, Friese K. Plasma- and urine concentrations of nitrite/nitrate and cyclic guanosinemonophosphate in intrauterine growth restricted and preeclamptic pregnancies. Arch Gynecol Obstet. 2006;274:150–154. doi: 10.1007/s00404-006-0149-8. [DOI] [PubMed] [Google Scholar]
- 50.Baksu B, Davas I, Baksu A, Akyol A, Gulbaba G. Plasma nitric oxide, endothelin-1 and urinary nitric oxide and cyclic guanosine monophosphate levels in hypertensive pregnant women. Int J Gynaecol Obstet. 2005;90:112–117. doi: 10.1016/j.ijgo.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 51.Crews JK, Herrington JN, Granger JP, Khalil RA. Decreased endothelium-dependent vascular relaxation during reduction of uterine perfusion pressure in pregnant rat. Hypertension. 2000;35:367–372. doi: 10.1161/01.hyp.35.1.367. [DOI] [PubMed] [Google Scholar]
- 52.Davis JR, Giardina JB, Green GM, Alexander BT, Granger JP, Khalil RA. Reduced endothelial no-cgmp vascular relaxation pathway during tnf-alpha-induced hypertension in pregnant rats. Am J Physiol Regul Integr Comp Physiol. 2002;282:R390–399. doi: 10.1152/ajpregu.00270.2001. [DOI] [PubMed] [Google Scholar]
- 53.Adini A, Kornaga T, Firoozbakht F, Benjamin LE. Placental growth factor is a survival factor for tumor endothelial cells and macrophages. Cancer Res. 2002;62:2749–2752. [PubMed] [Google Scholar]
- 54.Odorisio T, Schietroma C, Zaccaria ML, Cianfarani F, Tiveron C, Tatangelo L, Failla CM, Zambruno G. Mice overexpressing placenta growth factor exhibit increased vascularization and vessel permeability. J Cell Sci. 2002;115:2559–2567. doi: 10.1242/jcs.115.12.2559. [DOI] [PubMed] [Google Scholar]