Abstract
DREAM (also known as K+ channel interacting protein 3 and calsenilin) is a calcium binding protein and an active modulator of KV4 channels in neuronal cells as well as a novel Ca2+-regulated transcriptional modulator. DREAM has also been associated with the regulation of Alzheimer’s disease through the prevention of presenilin-2 fragmentation. Many interactions of DREAM with its binding partners (Kv4, calmodulin, DNA, and drugs) have been shown to be dependent on calcium. Therefore, understanding the structural changes induced by binding of metals to DREAM is essential for elucidating the mechanism of signal transduction and biological activity of this protein. Here, we show that the fluorescence emission and excitation spectra of the calcium luminescent analogue, Tb3+, are enhanced upon binding to the EF-hands of DREAM due to a mechanism of energy transfer between Trp and Tb3+. We also observe that unlike Tb3+-bound calmodulin, the luminescence lifetime of terbium bound to DREAM decays as a complex multiexponential (τaverage ~ 1.8 ms) that is sensitive to perturbation of the protein structure and drug (NS5806) binding. Using isothermal calorimetry, we have determined that Tb3+ binds to at least three sites with high affinity (Kd = 1.8 μM in the presence of Ca2+) and displaces bound Ca2+ through an entropically driven mechanism (ΔH ~ 12 kcal mol−1, and TΔS ~ 22 kcal mol−1). Furthermore, the hydrophobic probe 1,8-ANS shows that Tb3+, like Ca2+, triggers the exposure of a hydrophobic surface on DREAM, which modulates ligand binding. Analogous to Ca2+ binding, Tb3+ binding also induces the dimerization of DREAM. Secondary structural analyses using far-UV circular dichroism and trapped ion mobility spectrometry–mass spectrometry reveal that replacement of Ca2+ with Tb3+ preserves the folding state with minimal changes to the overall structure of DREAM. These findings pave the way for further investigation of the metal binding properties of DREAM using lanthanides as well as the study of DREAM–protein complexes by lanthanide resonance energy transfer or nuclear magnetic resonance.
Graphical abstract
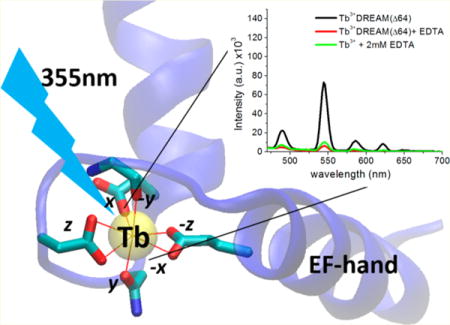
DREAM (downstream regulatory element antagonist modulator), also named KChIP3 and calsenilin, is a 29 kDa multifunctional Ca2+-sensing protein found in different neuronal cell compartments.1 Outside the nucleus, DREAM interacts with presenilin to regulate amyloid precursor protein processing and with potassium channels to regulate their membrane translocation and gating.2,3 Moreover, DREAM represents a new class of Ca2+-sensing protein that can translocate to the nucleus and directly bind DNA.1 In the nucleus, it regulates prodynorphin and c-fos gene expression by binding to the DRE regulatory sequence of those genes.1,4 Association of DREAM with the DRE promoter regions in the absence of calcium leads to inhibition of gene transcription. These genes have been shown to be involved in apoptosis, cell homeostasis, and pain modulation.5,6 The role of DREAM in pain sensing, memory retention, learning, and Alzheimer’s disease highlights the multifunctional properties of this protein.6 As a calcium signal transducer, DREAM does not possess endogenous catalytic activity, and its regulatory effect in biological processes arises from interaction with numerous binding partners. Therefore, understanding how calcium and other metals trigger structural changes in DREAM, and how this protein reorganization controls target recognition, would provide important insight into its mechanism of action.
The three-dimensional structure of Ca2+-bound DREAM has been obtained through nuclear magnetic resonance (NMR) and is presented in Figure 1a.7 DREAM has four EF-hand motifs; EF-hand 3 and EF-hand 4 are able to bind Ca2+, while EF-hand 1 is unable to bind either Mg2+ or Ca2+. The coordination of calcium/magnesium in the EF-hand motif has been widely studied, and it has been shown to form hexa- or heptacoordination with oxygen atoms of proteins to form a pentagonal bipyramidal coordination (Figure 1b,c).8,9 The oxygen-donating amino acids of the calcium selective metal binding loop in EF-hands follow a common organization such that positions 1, 3, and 5 are either an aspartic or an asparagine amino acid while the amino acid at position 12 is a well-conserved glutamic acid (Figure 1b). Modulation of metal affinity and selectivity arises from distinct combinations of negatively charged amino acids at these positions. For instance, EF-hand 2 of DREAM has been proposed to selectively bind Mg2+ due to Glu → Asp mutation at position 12, which eliminates the heptacoordination necessary for strong binding of Ca2+.10,11 Similarly, the presence of a lysine at position 1, proline at position 5, and aspartic acid at position 12 renders EF-hand 1 of DREAM unable to bind most metals.10,12
Figure 1.
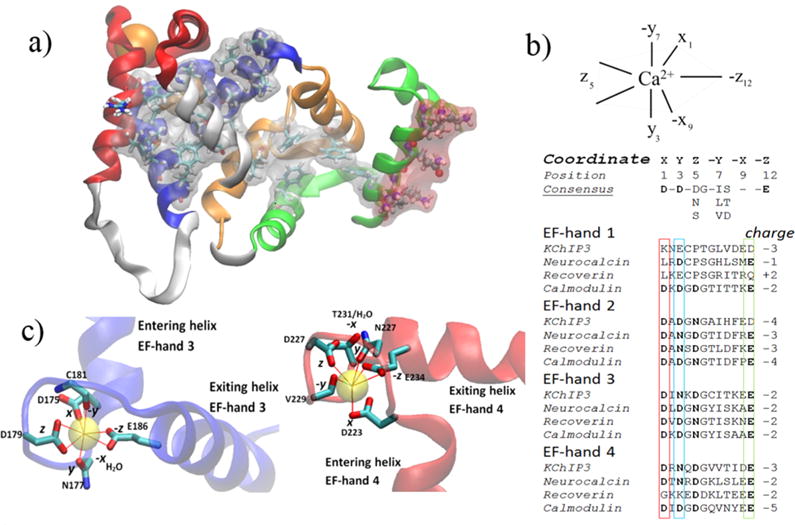
(a) NMR structure of DREAM monomer with highlighted hydrophobic residues (gray mesh) and charged residues (red mesh) (Protein Data Bank entry 2JUL).7 The four EF-hands of DREAM are colored green (EF-hand 1), orange (EF-hand 2), blue (EF-hand 3), and red (EF-hand 4). Calcium is shown as an orange sphere. (b) Coordination geometry of calcium bound to EF-hand 3 (left) and EF-hand 4 (right). Residues involved in coordination of Ca2+ are shown as a licorice model. EF-hand 3 shows a clear pentagonal bipyramidal coordination, whereas EF-hand 4 is distorted. (c) Geometry and consensus sequence of EF-hand binding loops as well as sequence homology between neuronal calcium sensors and calmodulin at the metal binding loops.
Association of calcium at the metal binding loop of the active EF-hand pair at the C-terminus of DREAM induces a structural rearrangement that leads to exposure of a hydrophobic surface as well as changes in oligomerization state.12,13 However, details about the underlying molecular mechanism by which calcium binds and induces structural changes in DREAM are not known. Nonetheless, experiments using NMR to monitor the glycine residues in the EF-hand loops and the associated chemical shift broadening upon metal binding would provide insight into the role of amino acids of DREAM. Of particular interest is the use of lanthanide ions, which have been shown to possess physical properties similar to those of calcium ions and have been widely applied to study the metal binding properties of EF-hands.14,15 Additionally, lanthanide–protein complexes have been shown to undergo magnetic alignment during NMR experiments, which is of great help in elucidating the three-dimensional structure of protein–metal and protein–protein complexes.17 The advantage of employing lanthanides to understand the mechanism of calcium binding is their unique luminescence properties as well as their ability to effectively displace calcium from EF-hand loops. Replacement of Ca2+ with Tb3+ has also been shown to induce structural changes in the EF-hand loops that are highly homologous to those observed upon calcium binding.16 In this report, we implement a combination of fluorescence, luminescence, TIMS–MS, and calorimetric techniques to show that Tb3+ binds at the EF-hands of DREAM and functions as a calcium biomimetic. Moreover, we show that association of Tb3+ at EF-hands 3 and 4 of DREAM leads to a calcium-like conformation with hydrophobic surface exposure, oligomeric transition, and ion-neutral collisional cross section (CCS) similar to those observed for the Ca2+-bound protein. Nonetheless, we observe small deviations in the dynamics of the environment near Trp169 as well as secondary structure organization, indicating that not all aspects of Tb3+ binding are identical to those of Ca2+ binding. Using ITC and the fluorescence properties of Tb3+, we are able to gain insight into the role of Mg2+ and ligand binding to DREAM. Initial results of this study have been previously presented in an abstract form.18
MATERIALS AND METHODS
General
NS5806 {1-[2,4-dibromo-6-(1H-tetrazol-5-yl)-phenyl]-3-(3,5-bis-trifluoromethyl-phenyl)urea, >99% pure} was purchased from Tocris Bioscience, trifluoperazine (TFP) from Sigma-Aldrich, and 1,8-ANS (8-anilino-1-naphthalenesulfonic acid) from Cayman Chemical Co. Concentrated stock solutions were prepared as previously described.19 TbCl3·6H2O was obtained from Sigma-Aldrich and used without further purification. Terbium stocks of ~0.5 M were prepared gravimetrically in decalcified ultrapure 18 MΩ water, and the concentrations of Tb3+ stocks were confirmed by titrations against EDTA standards.
Isolation and Purification of DREAM Constructs
Recombinant mouse DREAM(Δ64), DREAM(Δ160), and DREAM(Y174A) constructs were expressed in Escherichia coli BL21(DE3) cells and purified according to previously published procedures.12,20 Two additional protein constructs with single-amino acid mutations of glutamic acid at position 12 in the loop of EF-hand 3 and EF-hand 4 were obtained and are named DREAM(E186Q) and DREAM(E234Q), respectively. Rat calmodulin was purified as previously described.19,21
Photophysics of the DREAM:Tb3+ Complex
Fluorescence experiments were conducted on a custom PC1-ChronosFD instrument (ISS, Champaign, IL) in steady-state mode for excitation and emission spectra and in frequency domain mode for fluorescence decay measurements. The intrinsic protein fluorescence arising from tyrosine residues in CaM and tryptophan on DREAM was obtained by exciting the sample with 280 ± 2 and 295 ± 2 nm light, respectively. The fluorescence of 1,8-ANS was monitored by exciting the sample with 350 ± 4 nm light, through a vertically oriented polarizer. The sensitized emission spectra of terbium(III)-bound proteins were obtained by exciting the sample at 280 ± 4 nm while collecting the emission through a 400 nm long pass filter to minimize the contribution of the protein intrinsic fluorescence and the second harmonic peaks. Displacement of Ca2+ from DREAM was monitored by adding small aliquots of a 2.0 mM Tb3+ in 20 mM TRIS (pH 7.4) stock solution to 10–20 μM DREAM construct in the same buffer with 5 mM Mg2+ and/or 100 μM Ca2+. The resulting spectra were normalized by dividing the intensity at each wavelength by the background value at 530 nm, and the resulting titration plots were fitted using a noncooperative n-site quadratic equation that assumes a similar affinity for all the sites.20 All spectra were corrected for the PMT wavelength-dependent response as well as the lamp wavelength-dependent changes in intensity. The intrinsic fluorescence lifetime of DREAM was measured by exciting the sample with the modulated light of a 280 nm diode, and the fluorescence collected through a 320 nm long pass filter with 2,5-diphenyloxazole (PPO) in ethanol (τ = 1.40 ns) used a lifetime reference.
Circular dichroism measurements were conducted in a Jasco J-815 CD spectrometer along the 1 mm path of a quartz cuvette (model J-815, Jasco, Easton, MD). Luminescence measurements were conducted on a home-built instrument where the sample was placed in a 2 mm × 10 mm quartz cuvette in a temperature-controlled sample holder (Quantum Northwest, Liberty Lake, WA) and excited along the 10 mm path. The 355 nm line of a Nd:YAG laser (Minilite II Continuum, San Jose, CA) was used to directly excite Tb3+ ions, while the luminescence was measured perpendicularly through a 550 ± 10 nm band-pass filter and detected by a H7360-01 PMT (Hamamatsu). The signal was digitized by a 400 MHz oscilloscope (WaveSurfer 42Xs, Teledyne Lecroy), and the initial 100 μs of each trace were deleted to eliminate the contribution from scattered light and PMT recovery time. The fluorescence modulation-phase plots and luminescence decay traces were fit using Globals for spectroscopy software (LFD, Irvine, CA).
Thermodynamics of the DREAM:Tb3+ Complex
Isothermal calorimetry titrations were employed to determine the thermodynamics of Tb3+ displacement of Ca2+ from DREAM or CaM and were conducted using a VP-ITC isothermal calorimeter (Microcal Inc., Northampton, MA). Protein constructs were dialyzed overnight in 5 mM TRIS (Ph 7.4), 100 mM NaCl, and 100 μM CaCl2 with or without 5 mM MgCl2. The use of 10 mM EDTA during purification of calmodulin required multiple overnight dialysis steps to ensure complete removal of contaminating EDTA. Terbium stock solutions were prepared in ITC dialysate buffer. The reaction cell was loaded with an ~10 μM protein solution, determined spectrophotometrically prior to the ITC experiment, and the concentration of Tb3+ in the syringe (297 μL) was 1.00 mM. Thirty injections of increasing volume were titrated into the protein solution with increasing time intervals between injections. Isotherms were corrected for the heat of dilution of ligand, and all ITC experiments were conducted in triplicate. The recovered thermodynamic parameters were obtained by fitting the isotherms modeled either with an N-set-of-sites model or with a sequential model using the Microcal ITC analysis plug in Origin 7.0.
Trapped Ion Mobility Spectrometry–Mass Spectrometry (TIMS–MS) Studies
Experimental Section
Details regarding the TIMS operation and specifics compared to traditional IMS can be found elsewhere.22–25 Briefly, in TIMS, mobility separation is based on holding the ions stationary using an electric field against a moving gas. The separation in a TIMS device can be described by the center of the mass frame using the same principles as in a conventional IMS drift tube.26 The TIMS analyzer was coupled to a maXis Impact Q-UHR-ToF instrument (Bruker Daltonics Inc., Billerica, MA). Data acquisition was controlled using in-house software, written in National Instruments Lab VIEW (2012, version 12.0f3) and synchronized with the maXis Impact acquisition program. TIMS separation was performed using nitrogen as a bath gas at ~300 K, and typical P1 and P2 values are 1.8 and 0.6 mbar, respectively. The same RF (880 kHz and 200–350 Vpp) was applied to all electrodes, including the entrance funnel, the mobility separating section, and the exit funnel. Protein samples were prepared at 15 μM protein and 15 μM TbCl3·6H2O using HPLC grade solvents from Thermo Fisher Scientific Inc. (Waltham, MA) in 10 mM ammonium acetate under physiological conditions (pH 6.7). A custom-built, nano-electrospray ionization source was coupled to the TIMS–MS analyzer and was used for all analyses. A typical source voltage of 600–1200 V was used, and analyses were performed in positive ion mode.
Theoretical
Theoretical CCS were calculated for the previously reported 2JUL NMR structure of DREAM7 using IMoS (version 1.04b)27–29 with nitrogen as a bath gas at ~300 K. In the IMoS calculations, 100 total rotations were performed using the diffuse hard sphere scattering method with a Maxwell distribution.
RESULTS
The well-known calcium biomimetic behavior of europium-(III), terbium(III), and neodymium(III) and the unique spectroscopic properties of protein:lanthanide complexes have been widely employed to characterize the sequence of metal binding to calcium binding proteins,30–32 to observe protein conformational heterogeneity,33 to determine water coordination of metals bound at the EF-hand motif,34 and as binding assays.35 Therefore, we envisioned that the properties of lanthanides could be employed to obtain information about the biophysical properties of DREAM protein. However, the association of lanthanides with the KChIP subfamily of calcium binding proteins has not been extensively studied. Thus, we first set forth to determine whether Tb3+ can directly associate with the EF-hand of DREAM. This is important, because previous studies of DREAM(Δ64) using mass spectrometry and studies of NCS-1 using sensitized emission have presented contradicting results on whether Tb3+ can bind to neuronal calcium sensors.10,36 Additionally, we are interested in investigating whether Tb3+ binding induces structural changes in DREAM homologous similar to those observed for calcium.
Terbium(III) Binds to DREAM and Is Sensitized by Energy Transfer from W169
Calmodulin and DREAM are well-known to undergo distinct structural changes upon binding of calcium, which are accompanied by changes in fluorescence of tyrosine and tryptophan residues, respectively.1,37 These fluorescence transitions are shown in panels a and b of Figure 2. When calcium binds, the tyrosine fluorescence of CaM increases whereas the tryptophan fluorescence of DREAM decreases, in agreement with previous reports. Of particular interest is the observation that in the presence of Tb3+, at molar ratios of 4:1 for CaM and 2:1 for DREAM, the fluorescence emission is nearly identical to that observed in the presence of saturating calcium (Figure 2a,b). The slightly lower tyrosine fluorescence of CaM in the presence of Tb3+ is likely due to an efficient quenching of Tb3+ by an aromatic amino acid or due to incomplete binding of this ion. We also investigated whether binding of Tb3+ on DREAM leads to the transfer of energy from nearby aromatic residues toward the metal ligand, as previously observed for CaM.32 The presence of the characteristic sharp emission bands in the sensitized emission spectra of the DREAM(Δ64):Tb3+ complex supports the idea that aromatic residues, likely at the C-terminus of DREAM, are able to transfer energy to bound terbium (Figure 2c,d). The sensitized emission intensity of terbium bound to DREAM(Δ64) is approximately half of that observed for CaM, which is likely due to the presence of only two Tb3+ ions bound to EF-hands 3 and 4 of DREAM(Δ64), whereas four Tb3+ ions are bound to CaM.10 Nonetheless, it is also possible that the presence of Tyr100 at position 7 of the EF-hand 3 binding loop and Tyr139 at position 10 of EF-hand 4 on CaM provides a more efficient energy transfer to Tb3+.38
Figure 2.
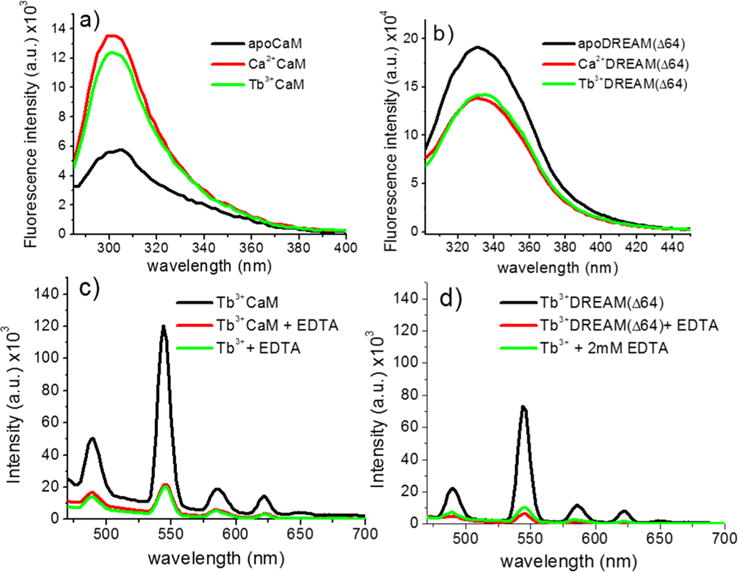
Intrinsic fluorescence changes of 40 μM (a) CaM and (b) DREAM(Δ64) upon binding of 1 mM calcium or 160 μM Tb3+ on CaM or 80 μM Tb3+ for DREAM(Δ64), excited at 280 ± 4 nm. The observed tyrosine fluorescence change upon binding of Tb3+ to CaM shows a small deviation from that observed for Ca2+, likely due to quenching of the fluorescence by energy transfer to Tb3+. The Tb3+-induced transition for DREAM(Δ64) is identical to that observed in the presence of Ca2+. Sensitized emission of (c) 160 μM Tb3+ bound to CaM and (d) 80 μM Tb3+ bound to DREAM(Δ64), excited at 280 nm with and without 2 mM EDTA. The background emission of the Tb3+:EDTA complex is shown as a reference. The observed sensitized emission of Tb3+ shows the characteristic sharp bands at 489, 544 (major), 586, and 622 nm due to the 5D4 → 7F6, 5D4 → 7F5, 5D4 → 7F4, and 5D4 → 7F3 transitions of Tb3+, respectively. The major peak at 545 nm for DREAM(Δ64) is 40% smaller than that observed for CaM. Addition of 2 mM EDTA to either CaM or DREAM(Δ64) resulted in an emission identical to that of Tb3+ in solution.
Detailed analysis of DREAM(Δ64) excitation spectra due to the 5D4 → 7F5 transition at 545 nm shows a broad peak with maxima at 280 and 32 nm fwhm (Figure 3a). The ratio of terbium luminescence intensity upon excitation at 295 and 280 nm is 0.40, which indicates that tyrosine and tryptophan residues are able to transfer energy.39 The excitation spectra of DREAM(Δ160), DREAM(E186Q), and DREAM(E234Q) are identical to those of DREAM(Δ64), with a broader fwhm of 36 nm and a higher 295 nm/280 nm ratio of 0.52 for DREAM(Δ161). Analysis of the C-terminal domain of DREAM shows the presence of three tyrosine residues (Y174, Y195, and Y203); however, only Y174 is within the range of 5–10 Å necessary for an efficient energy transfer to Tb3+ bound at EF-hands 3 and 4 (Figure 3b).40,41 Therefore, to quantify the energy transfer contribution of Y174, we constructed a DREAM(Y174A) mutant and determined the effect of this mutation on the sensitized emission and excitation spectra. This mutant shows an identical circular dichroism transition and amplitude as well as the same Tb3+-induced tryptophan fluorescence change, as the DREAM(Δ64) construct (data not shown). On the other hand, the efficiency of energy transfer in the DREAM(Y174A) mutant is decreased by ~60%, judging from the decreased sensitized emission at 545 nm (Figure 3a). The excitation spectra of this construct also show a 5 nm blue shift to 275 nm with a fwhm of 38 nm and a 295 nm/280 nm ratio of 0.41. The decrease in the sensitized emission and the blue shift of the excitation spectra support the role of Y174 as an energy donor, while the identical 295 nm/280 nm ratio for DREAM(Δ64) and DREAM-(Y174A) is indicative of Tyr → Trp → Tb3+ being the predominant energy transfer pathway. The similar Tb3+ sensitization observed in DREAM(Δ161) and DREAM(Δ64) constructs indicates that aromatic amino acids at the N-terminus do not transfer energy to Tb3+ and that the observed luminescence arises from terbium bound at EF-hands 3 and 4. For comparison, the excitation spectra of CaM are also shown, and a characteristic maximum at 277 nm with fwhm of 24 nm and a 295 nm/280 nm ratio of 0.07 is observed, which is in good agreement with a Tyr → Tb3+ energy transfer. Moreover, the lack of vibronic structures on the excitation spectra on all constructs indicates that phenylalanine residues do not play a major role in energy transfer.
Figure 3.
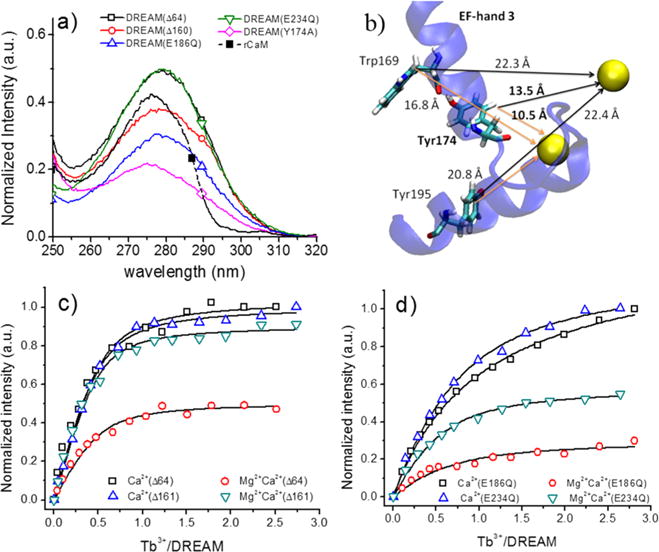
(a) Excitation spectra of DREAM constructs bound to Tb3+ (20 μM protein with 40 μM Tb3+). The spectra were normalized so that the intensity corresponding to backbone to Tb3+ energy transfer at 240 nm is the same for DREAM(Δ64), DREAM(Δ160), and DREAM(Y174A) while the magnitude is half for DREAM(E186Q) and DREAM(E234Q). (b) Calcium/terbium binding sites of EF-hands 3 and 4. Only EF-hand 3 is shown (blue) for the sake of clarity. (c) Titrations of Tb3+ into DREAM(Δ64) and DREAM(Δ160) in 20 mM TRIS (pH 7.4) and 100 μM Ca2+ with and without 5 mM Mg2+. (d) Titration of Tb3+ into DREAM(E186Q) and DREAM(E234Q) under the conditions described for panel c. Solid lines represent the best fit using the quadratic equation for N binding sites.
The shorter distance from Tyr174 to EF-hand 3 (10.5 Å) than to EF-hand 4 (13.5 Å) supports the idea that monitoring the binding of Tb3+ using the sensitized emission may allow us to identify whether aromatic amino acids on DREAM can transfer energy to Tb3+ bound at both EF-hands. To confirm this hypothesis, we employed two DREAM constructs in which glutamic acid at position 12 of the calcium binding loop has been mutated to glutamine, thus inactivating either EF-hand 3 in DREAM(E186Q) or EF-hand 4 in DREAM(E234Q).42 As expected, the excitation spectra of Tb3+ bound at EF-hand 3 in the DREAM(E234Q) mutant shows fluorescence ~39% greater than that of Tb3+ bound at EF-hand 4 in the DREAM(E186Q) mutant (Figure 3a).
Titration of Tb3+ into Ca2+-bound DREAM(Δ64) or DREAM(Δ161) shows that both construct have a similar affinity for Tb3+ in the presence of calcium. The dissociation constant obtained using the quadratic equation shows identical affinity for Tb3+ in the presence of Ca2+ with a Kd of 1.8 ± 0.6 μM for DREAM(Δ64) and a Kd of 1.6 ± 0.2 μM for DREAM(Δ161). Interestingly, in the presence of 5 mM MgCl2, the sensitized emission of Tb3+ is decreased by 53% for DREAM(Δ64) but no significant changes are observed for DREAM(Δ161) (Figure 3c). To better understand the mechanism of binding of Tb3+ to EF-hands 3 and 4 of DREAM, we have also conducted titrations in the presence of Ca2+ or Mg2+ and Ca2+ using the DREAM(E186Q) and DREAM(E234Q) protein mutants (Figure 3d). Displacement of Ca2+ from DREAM(E186Q) by Tb3+ shows a weaker dissociation constant of 11 ± 1 μM compared to that of DREAM(E234Q) (5.9 ± 0.5 μM). However, a decrease in the sensitized emission upon addition of 5 mM MgCl2 similar to that for DREAM(Δ64) was observed, with emission decreases of 55 and 80% for DREAM(E234Q) and DREAM(E186Q), respectively (Figure 3d). Under the conditions used for these titrations (500 μM CaCl2 or 500 μM CaCl2 and 5 mM MgCl2), Tb3+ binds first to the inactivated EF-hand (see ITC experiments below). Therefore, the data shown in Figure 3d highlight the fact that binding of Mg2+ to EF-hand 2 induces structural rearrangements that have a greater impact on EF-hand 3. This is in good agreement with the fact that EF-hand 2 is adjacent to EF-hand 3, and that the exiting helix of EF-hand 2 is in direct contact with the entering helix of EF-hand 3 (Figure 1a). Tryptophan 169 and tyrosine 174, both of which are shown to play a major role in energy transfer, both reside on the entering helix of EF-hand 3. The presence of Mg2+ also enhanced the binding of Tb3+, with dissociation constants of 5.8 ± 1.9 μM for DREAM(E186Q) and 2.7 ± 0.4 μM for DREAM(E234Q). This increase in apparent affinity is likely due to a decrease in the level of nonspecific binding of Tb3+ to secondary sites or to EF-hand 2. Together, these results support a model in which terbium displaces Ca2+ from EF-hand 4 and EF-hand 3 and highlight the role of Mg2+ binding at EF-hand 2 as a structural cofactor in DREAM.
Binding of Terbium(III) to DREAM Leads to a Structural Rearrangement Similar to Those Observed for Ca2+
The terbium(III)-induced tryptophan emission quenching observed in Figure 2b supports the idea that Tb3+ is able to mimic the structural changes induced by binding of Ca2+ to EF-hands 3 and 4. To test whether binding of Tb3+ to DREAM induces a structural transition analogous to that of Ca2+, we monitored the fluorescence and anisotropy decay of Trp169, the fluorescence of the extrinsic hydrophobic probe 1,8-ANS bound at the C-terminus of DREAM(Δ64),13 and changes in secondary structure. Detailed information regarding the environment and dynamics of Trp169 and how they are affected by metal binding can be obtained by measuring the fluorescence and anisotropy decay lifetimes. As previously reported,43 the fluorescence decay of Trp169 on DREAM-(Δ64) was best fitted by a Gaussian-discrete bimodal decay model, whose parameters are listed in Table 1 and shown in Figure 4a. The small discrepancies between our results and those published previously likely arise from the lack of LDAO detergent under our conditions. Nonetheless, we observe that addition of Ca2+ or Tb3+ results in a decrease in the average excited-state lifetime from 4.8 to 3.8 ns, which is due to a decrease in the fractional intensity contribution of the long lifetime and a slightly faster Gaussian decay from 1.8 to 1.5 ns. The lack of significant lifetime quenching of Trp169 by Tb3+ is likely due to the poor efficiency of this energy transfer process. Moreover, anisotropy decay measurements were conducted to identify whether binding of Tb3+ induces dimerization of DREAM as observed for Ca2+. The frequency domain anisotropy decay data were best fitted with a double discrete model, in which the fast Θ1 is associated with fast local fluctuations of tryptophan and the slow Θ2 corresponds to the global rotation of the protein (Table 1). A clear transition from 15 to 29 ns upon binding of Ca2+ and 27 ns in the presence of Tb3+ is observed. These rotational correlation times match well with the values of 14 ns for a monomeric and 28 ns for a dimeric DREAM(Δ64) protein approximated by the Einstein–Stokes equation at 17 °C, η = 0.0100 P, and 0.73 g/mL hydration. Differences in rotational correlation times of the fast local tryptophan motion can be observed between Ca2+- and Tb3+-bound DREAM(Δ64), where a 2-fold faster local motion is measured in the presence of terbium (0.52 ns vs 0.23 ns). In the metal-free DREAM(Δ64), the local flexibility accounts for 35% of the depolarization, while in the Ca2+- and Tb3+-bound DREAM(Δ64) form, this rotation contributes 48 and 58% to depolarization, respectively. Altogether, excited-state and anisotropy decay of Trp169 reveals that even though binding of Tb3+ can induce dimerization of DREAM(Δ64) this metal also induces a more dynamic structure near the Trp169 compared to Ca2+.
Table 1.
Fluorescence and Anisotropy Decay Parameters of DREAM(Δ64) Bound to Ca2+ or Tb3+a
| (f1) (ns) | w1 (ns) | τ2 (f2) (ns) | 〈τ〉 (ns) | Θ1 (f1) (ns) | Θ2 (f2) (ns) | χ2 | |
|---|---|---|---|---|---|---|---|
| apoDREAM(Δ64) | 1.8 (0.39) | 0.69 | 5.7 (0.61) | 4.2 | 0.40 (0.35) | 15 (0.65) | 0.5 |
| Ca2+DREAM(Δ64) | 1.5 (0.48) | 0.60 | 5.9 (0.52) | 3.8 | 0.52 (0.40) | 29 (0.60) | 0.9 |
| Tb3+DREAM(Δ64) | 1.5 (0.47) | 0.64 | 5.8 (0.53) | 3.8 | 0.23 (0.58) | 27 (0.42) | 1.5 |
Values in parentheses represent the fraction intensity and fractional depolarization. τ1 is the mean decay time of the Gaussian distribution with a width of distribution w1. The lifetime of the discrete single-exponential term is denoted as τ2. The average lifetime, 〈τ〉, was calculated using eqs 1 and 2 of the Supporting Information. α1 and α2 are normalized pre-exponential decay fractions and f1 and f2 exponential decay fractions.
Figure 4.
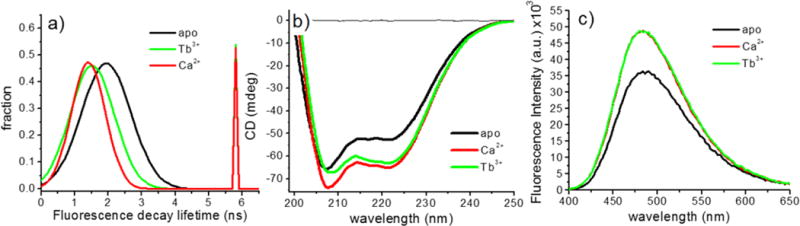
(a) Tryptophan fluorescence lifetime distribution of DREAM(Δ64) in the presence of Ca2+ or Tb3+. Decay data were modeled as a Gaussian distribution and a discrete decay (parameters listed in Table 1). (b) Circular dichroism spectra of 40 μM DREAM(Δ64) in the presence of 1 mM Ca2+, 2 mM EDTA, or 160 μM Tb3+ in 5 mM TRIS (pH 7.4). (c) Binding of Tb3+ or Ca2+ to 10 μM DREAM(Δ64) in the presence of 40 μM 1,8-ANS.
As shown in Figure 4b, the CD spectrum of metal-free DREAM(Δ64) shows a characteristic profile with minima at 222 and 208 nm (the maximum at 190 nm is not shown) in good agreement with an α-helical structure. Upon binding of Ca2+, an ellipticity decrease at 200–225 nm and an increase at 190 nm are observed, which can be explained by an increase in α-helical content and/or rearrangement of the α-helices. On the other hand, in the presence of Tb3+, a CD spectrum intermediate between those measured for apo and Ca2+-bound DREAM is observed. This intermediate structure was also observed when a 2-fold excess of terbium was added to a sample containing 100 μM calcium (not shown). The largest deviation in CD spectra between Ca2+- and Tb3+-bound DREAM(Δ64) is observed near the 208 nm minimum. Thus, the CD data indicate that Tb3+ is able to displace Ca2+ and induce a structural change distinct from those observed for metal-free and Ca2+-bound DREAM(Δ64).
Despite the small differences in the protein dynamics near Trp169, as well as the deviation in secondary structure upon binding of Tb3+, we observed similar binding of 1,8-ANS at the C-terminal hydrophobic cavity of DREAM(Δ64).13 The fluorescence emission of this probe has been extensively shown to be sensitive to the immediate environment, and while the total intensity upon Ca2+ binding is observed to increase, no changes in fluorescence of 1,8-ANS were observed upon displacement of Ca2+ by Tb3+ (Figure 4c). Moreover, frequency domain analysis of the excited state of 1,8-ANS as well as the depolarization time reveals no significant differences between Ca2+- and Tb3+-bound DREAM(Δ64) (data not shown). Overall, the indication is that the hydrophobic cavity exposure induced by calcium is also triggered by terbium.
DREAM Binds Tb3+ through an Entropy-Driven Mechanism
To complement the Tb3+:DREAM binding studies using sensitized emission, we also conducted calorimetric studies in which the heat associated with Tb3+ displacement of Ca2+ bound at the EF-hands of CaM and DREAM is measured. Isothermal calorimetry reveals that displacement of Ca2+ from CaM is endothermic and can be best modeled as a sequential process (Figure 5a). The profile of the ITC isotherm for Tb3+ displacement of Ca2+ bound to CaM is similar to that obtained for binding of calcium to a plant–mammalian CaM chimera,44 albeit with slightly different thermodynamic parameters. The associations of Tb3+ ions with CaM at EF-hands 1–4 have association constants slightly lower than those determined for Ca2+ binding, which is likely due to competition effects. We associate the site with K1 parameters as representing the displacement of Ca2+ by Tb3+, while the two other observed binding processes (K2 and K3) likely arise from a convolution of specific and nonspecific association of Tb3+ ions. The presence of nonspecific binding of Tb3+ ions is supported by the fact that addition of 5 mM Mg2+ results in drastic changes in the thermodynamic parameters associated with K2 and K3. The observed decreases in enthalpy and entropy of ~160 and ~280 kcal mol−1 for K2 and K3, respectively, also support the idea that these supplementary sites are strikingly different and may involve different ligands and/or ion displacement. We associate the thermodynamic values recovered in the presence of Mg2+ with the displacement of Ca2+ specifically bound at the loops of EF-hands 1–4, convoluted with any additional protein rearrangement to accommodate Tb3+. It can be observed that in the presence of Mg2+ there is a 3-fold decrease in affinity for the K1 site, with a minimal change in enthalpy and entropy, whereas sites K2 and K3 show similar affinity, with K2 having an enthalpy and entropy 2-fold larger than those of K3 sites. Altogether, these values can be interpreted as corresponding to Tb3+ displacement of Ca2+ ions from four EF-hands with K1 representing the weakest Ca2+ binding hand and K2 and K3 corresponding to the three remaining EF-hands. Following previous studies in which calmodulin was shown to follow a sequential filling of EF-hands 1 → 2 → 3 and 4 by Tb3+ and Ca2+, we associate the K1 binding site to that of binding of Tb3+ to EF-hand 4.32,45 Interestingly, EF-hand 4 in calmodulin shows the presence of glutamic acid at position 11 near the −z metal coordination (Figure 1), which may explain the smaller enthalpic and entropic contribution upon displacement of Ca2+ by Tb3+. The Tb3+ binding sites corresponding to K2 show ΔH2 and ΔS2 thermodynamic parameters that are ~2-fold larger than those of ΔH3 and ΔS3, which is likely due to two Tb3+ binding sites being reported by the K2 parameters. This allows us to approximate a ΔH value of ~23 kcal mol−1 and a TΔS of ~27 kcal mol−1 for Tb3+ displacement of Ca2+ bound at EF-hands 1–3 and a ΔH of 13 kcal mol−1 and a TΔS of 28 kcal mol−1 for EF-hand 4.
Figure 5.
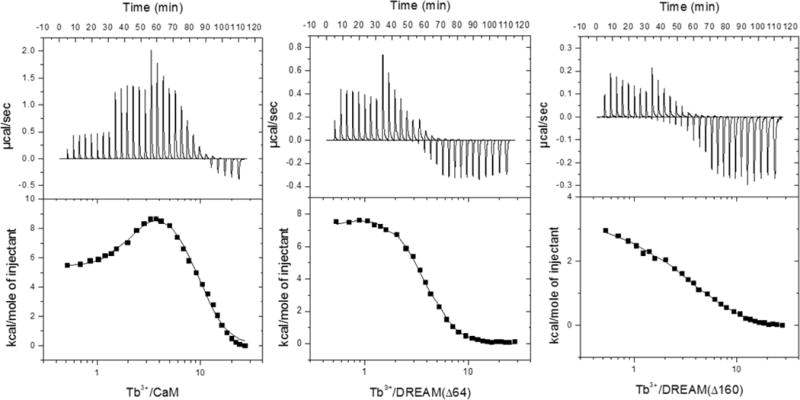
ITC isotherms for Tb3+ displacement of Ca2+ from CaM, DREAM(Δ64), and DREAM(Δ160). The top panels of each profile reflect the thermal power expressed in units of microcalories per second. The bottom panels show integrated reaction heats (ΔH) expressed in units of kilocalories per mole. The solid lines present the best fitting curve with the parameters listed in Table 2.
Furthermore, displacement of Ca2+ bound to DREAM(Δ64) by Tb3+ shows a simpler isotherm (Figure 5b), which is similar to that obtained for displacement of Ca2+ by Tm3+ in the Entamoeba histolytica calcium binding protein.14 Despite the ability to model the isotherm using the simpler one-set-of-sites model, we decided to present the results obtained from a three-site sequential model based on three accounts. First, it is expected that binding of Tb3+ is not associated with a cooperative behavior because the protein undergoes a transition from a calcium-bound structure to a calcium-like structure. Second, replacement of glutamic acid at position 12 of the loop in EF-hand 3 resulted in an isotherm that could not be fitted by a one-set-of-sites model. Lastly, displacement of Ca2+ by Tb3+ in other calcium binding proteins with known calcium cooperativity have been observed to follow a sequential mechanism.14,44 The recovered parameters using the sequential model are listed in Table 2. For the sake of completeness, the parameters recovered with an N-set-of-sites model are presented in Table S1. The data reveal that three Tb3+ ions can bind to DREAM(Δ64), with slightly different affinities, such that K1 > K2 > K3. Interestingly, the associated enthalpy and entropy of sites K1 and K2 are similar to those found for Ca2+ displacement of K1 sites on CaM, while the enthalpy and entropy of the site corresponding to K3 show significantly lower enthalpic and entropic contributions. Titration of Tb3+ in the presence of Ca2+ and 5 mM Mg2+ in DREAM(Δ64) increases ΔΔH3 and TΔΔS3 by ~6 kcal mol−1, while the enthalpy and entropy of sites K1 and K2 remain unchanged. Unlike CaM, in which all four EF-hands are believed to prefer binding to Ca2+ versus Mg2+, EF-hand 2 on DREAM is proposed to bind Mg2+ preferentially.8,10 Therefore, we assign the recovered thermodynamic parameters for sites with K1 and K2 association constants as representing Ca2+ displacement from EF-hand 3 or 4 while those recovered for the site with K3 being representative of binding of Tb3+ to EF-hand 2. The assignment of K3 to EF-hand 2 is also supported by the displacement of Ca2+ by Tb3+ in a DREAM(Δ160) construct lacking the Mg2+ binding EF-hand 2, which shows only two sites with affinities and thermodynamic parameters similar to those of K1 and K2 on DREAM(Δ64). The recovered association constant and thermodynamic parameter for binding of Tb3+ to DREAM-(Δ160) are also independent of Mg2+, in agreement with the sensitized emission titrations. However, despite the similarities, a decrease of ~10 kcal mol−1 in the enthalpy and entropy for the K2 site is observed for DREAM(Δ160) compared to that of DREAM(Δ64). This decrease in energetics could be due to a distinct metal coordination or loss of structural rearrangement at the missing N-terminus. It is tempting to assign the K2 parameters of DREAM(Δ160) as representing binding of Tb3+ to EF-hand 3, due to closer proximity to the now deleted N-terminal domain, but these results do not permit unequivocal assignment. Together, the recovered thermodynamic parameters for DREAM(Δ64) and DREAM(Δ160) show that Tb3+ can efficiently displace Ca2+ from the C-terminal EF-hands 3 and 4, and that deletion of the N-terminal amino acids (1–160) results in modification of the metal binding properties of EF-hands 3 and 4. Thus, we can approximate a ΔH value of ~15 kcal mol−1 and a TΔS of ~22 kcal mol−1 for Tb3+ displacement of Ca2+ bound at EF-hands 3 and 4 of DREAM(Δ64).
Table 2.
ITC Parameters Recovered for Tb3+ Displacement of Ca2+ from EF-Hands of CaM and DREAM Using a Sequential Modela
| K1 × 104 | K2 × 104 | K3 × 104 | ΔH1 | ΔH2 | ΔH3 | TΔS1 | TΔS2 | TΔS3 | |
|---|---|---|---|---|---|---|---|---|---|
| CaM | 29 ± 4.2 | 0.7 ± 0.4 | 5.1 ± 8.4 | 12 ± 5 | 212 ± 38 | −307 ± 127 | 19 ± 3.9 | 217 | −302 |
| CaM with Mg2+ | 8.9 ± 1.8 | 1.9 ± 0.2 | 1.4 ± 0.2 | 13 ± 1.5 | 47 ± 0.6 | 23 ± 7 | 20 ± 1.4 | 52 ± 0.6 | 28 ± 7 |
| DREAM(Δ64) | 22 ± 1.3 | 8.1 ± 1.6 | 2.8 ± 0.2 | 13 ± 9.0 | 18 ± 0.6 | −2.5 ± 2.7 | 20 ± 9.1 | 24 ± 0.4 | 3.6 ± 2.7 |
| DREAM(Δ64) with Mg2+ | 16 ± 2.5 | 11 ± 1.1 | 6.0 ± 0.5 | 12 ± 1.3 | 18 ± 6.0 | 3.4 ± 3.2 | 19 ± 1.3 | 25 ± 5.9 | 9.9 ± 3.1 |
| DREAM (Δ160) | 6.9 ± 3.2 | 8.9 ± 9.2 | 13 ± 1.7 | 8.2 ± 1.3 | 19 ± 1.5 | 15 ± 1.1 | |||
| DREAM (Δ160) with Mg2+ | 6.8 ± 1.4 | 1.8 ± 0.6 | 11 ± 2.2 | 7.7 ± 2.4 | 17 ± 2.1 | 14 ± 2.4 | |||
| DREAM (E186Q) with Mg2+ | 14.0 ± 0.9 | 8.6 ± 1.3 | 8.8 ± 2.2 | 7.5 ± 1.2 | 15 ± 1.7 | 2.2 ± 2.6 | 14 ± 0.8 | 22 ± 1.8 | 9 ± 2.7 |
| DREAM (E234Q) with Mg2+ | 5.7 ± 0.9 | 27 ± 1.5 | 16 ± 6.2 | 11 ± 1.9 | 3.6 ± 2.7 | 4.0 ± 1.3 | 18 ± 1.9 | 9.6 ± 2.5 | 11 ± 0.9 |
All experiments were conducted in triplicate at 25 °C; errors are standard deviations. Enthalpy and entropy changes shown in units of kilocalories per mole; association constants in units of inverse molar.
Additional experiments in which we monitored the thermodynamics of association of Tb3+ with Ca2+-bound DREAM(E186Q) with inactivated EF-hand 3 and DREAM(E234Q) with inactivated EF-hand 4 were also conducted, and the recovered parameters are listed in Table 2. Displacement of Ca2+ by Tb3+ on the DREAM(E186Q) construct is best modeled as a three-site sequential binding process, in which the K1 site has the highest affinity, while ΔH1 and ΔS1 are ~5 kcal mol−1 lower than those obtained for DREAM(Δ64). Tb3+ binding at the site with K2 shows an affinity identical to that observed for K2 on DREAM(Δ64) in the presence of Mg2+, and similar enthalpic and entropic contributions. The third site, K3, shows parameters similar to those recovered for Tb3+ binding at EF-hand 2 of DREAM(Δ64) and likely corresponds to the same binding process. The decrease in enthalpy and entropy upon binding of Tb3+ at the site with K1 as well as its stronger binding affinity compared to those of K2 and K3 seems to indicate that this site likely corresponds to the inactivated EF-hand 3. The fact that only small changes are observed upon mutation of glutamic acid at position 12 (−z coordination) to glutamine supports the idea that this site may still be weakly bound to either Ca2+ or Mg2+. Interestingly, these results also show that the E186Q substitution does not affect the binding of Tb3+ at EF-hand 3, which is interesting but not unexpected. This effect is likely due to weaker binding of Ca2+, which in turn facilitates Tb3+ association.
The DREAM(E234Q) construct, in which EF-hand 4 is inactivated, is also able to bind three Tb3+ ions with ranges of affinities similar to those obtained for the DREAM(Δ64) in the presence of Mg2+. In this construct, the site with association constant K2 binds stronger, followed by K3 and then K1 sites. However, the enthalpy for site K2 on DREAM(E234Q) is 5-fold lower than those obtained for K2 on DREAM(Δ64). The sites with a K3 association constant show an enthalpy and an entropy identical to those of the site corresponding to K3 on DREAM(Δ64). In fact, the enthalpy and entropy for sites K2 and K3 are much closer to those obtained for binding of Tb3+ to EF-hand 2 of DREAM(Δ64) in the presence of Mg2+, with a ΔH of ~4 kcal mol−1 and a ΔS of ~10 kcal mol−1. These results suggest that inactivation of EF-hand 4 likely results in a weak binding of Ca2+ or Mg2+, both of which can be easily displaced by Tb3+ (based on higher K2 and K3) with a concomitant lower enthalpic and entropic contribution. Overall, ITC demonstrates that in the presence of saturating amounts of calcium, the Glu → Gln mutation at position 12 of the EF-hand metal binding loop actually facilitates binding of Tb3+.
Luminescence Decay of Tb3+-Bound DREAM Is Sensitive to Ligand Binding
Sensitized emission studies show that Mg2+ is able to induce changes in the emission of bound Tb3+; to further study this effect, we decided to determine if Mg2+ can induce structural changes within the coordination sphere of Tb3+. We employed direct excitation of Tb3+ using the 355 nm line of a Nd:YAG pulsed laser and measured the luminescence decay of terbium bound to the EF-hands of CaM and DREAM constructs. This approach permits analysis of the local environment at the binding site of Tb3+ ions, namely the EF-hand loops. Additionally, this method has been widely used to monitor changes in the coordination sphere of lanthanides as well as the effects of water coordination.46 Following the studies described above, we first characterized the luminescence decay of Tb3+ bound to CaM, and as expected, the luminescence decay shown in Figure 6a follows a monoexponential decay with a τ1 of 1.38 ms. A single-exponential decay of approximately 1 ms has been reported for the Tb3+:CaM complex, and double-exponential decays have been observed for complexes of Eu3+ and calmodulin.30,33
Figure 6.
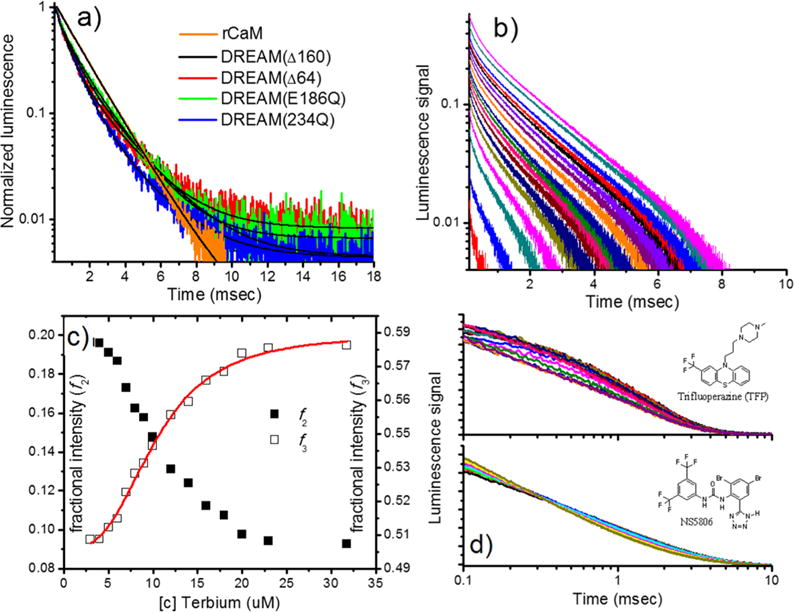
(a) Luminescence decay of Tb3+ bound to CaM and constructs of DREAM, with 4:1 Tb3+:CaM and 2:1 Tb3+:DREAM stoichiometric ratios. Solid lines represent the best fit using Globals software analysis, and recovered parameters listed in Table 3. (b) Luminescence intensity decay profiles as a function of Tb3+ binding to 10 μM DREAM(Δ64). The luminescence decays in panel b were analyzed using a triple discrete model, and the fractional intensity contribution of the two slowest lifetimes is shown in c. The fastest decay component had a lifetime of ~80 μs with a <10% contribution and is not shown. (d) Luminescence decay of Tb3+ bound to CaM and DREAM(Δ64) as a function of the hydrophobic molecules TFP (top) bound to CaM and NS5806 (bottom) bound to DREAM(Δ64).
Luminescence decay of Tb3+ bound to DREAM(Δ64) shows a τ1 lifetime of 0.86 ms (59%) and a τ2 lifetime of 2.20 ms (14%), whereas Tb3+ bound to DREAM(Δ160) decays with a τ1 lifetime of 0.74 ms (54%) and a τ2 lifetime of 2.16 ms (29%). The faster lifetime likely corresponds to a contribution from partially coordinated Tb3+ ions and is similar to the 780 μs decay obtained for Tb3+ bound to NTA in water.34 We and others have determined the lifetime of Tb3+ in buffer at pH 7.4 to be 450 ± 30 μs; therefore, we associate τ1 to represent Tb3+ bound to a weak coordination site on DREAM.47 The second lifetime is much longer than that determined for Tb3+ bound to parvalbumin (1.3 ms) or CaM (1.38 ms) but is similar to that of Eu3+ bound to these proteins or complexes of Tb3+ and bimetallic ligand in which no water coordination is observed.30,34,48 Long-lived excited-state decays are observed for lanthanides with little phonon quenching that often is due to limited water access.47 Interestingly, addition of 100 mM NaCl reduces the fast lifetime to values similar to those of Tb3+ in water and increases the fractional intensity of the long lifetime component ~3-fold (Table 3). The luminescence decay of Tb3+ was not significantly affected by LDAO, a detergent that has been proposed to stabilize DREAM in a single conformation.12 Titration of Tb3+ into DREAM(Δ64) in the presence of 100 mM NaCl and 10 mM LDAO shows that the fractional intensity of the τ2 lifetime increases in a dose-dependent manner and saturates at a stoichiometric ratio of 2:1, whereas the fractional intensity of τ1 decreases (Figure 6b,c).
Table 3.
Terbium(III) Luminescence Decay Parameters Recovered upon Binding to DREAM and DREAM Constructsa
| τ1 (ms) | f1 | τ2 (ms) | f2 | |
|---|---|---|---|---|
| CaM | 1.38 | 1.00 | ||
| CaM with TFPb | 0.46 | 0.31 | 1.36 | 0.69 |
| DREAM(Δ64) | 0.86 | 0.59 | 2.22 | 0.14 |
| DREAM(Δ64) with Mg2+ | 0.74 | 0.54 | 2.05 | 0.24 |
| DREAM(Δ64)(a)c | 0.58 | 0.27 | 2.00 | 0.69 |
| DREAM(Δ64)(b)c | 0.46 | 0.17 | 2.04 | 0.77 |
| DREAM(Δ64)(c)c with NS5806 | 0.43 | 0.58 | 1.83 | 0.39 |
| DREAM(Δ160) | 0.75 | 0.47 | 2.16 | 0.29 |
| DREAM(Δ160) with Mg2+ | 0.63 | 0.41 | 2.03 | 0.37 |
| DREAM(E186Q) | 0.62 | 0.50 | 2.02 | 0.27 |
| DREAM(E186Q) with Mg2+ | 0.48 | 0.47 | 1.58 | 0.42 |
| DREAM(E234Q) | 0.63 | 0.27 | 1.75 | 0.46 |
| DREAM(E234Q) with Mg2+ | 0.45 | 0.31 | 1.70 | 0.48 |
All shown parameters were recovered using a triple or double discrete exponential decay; the error of the reported values is 2.5% on average. All experiments were conducted at room temperature (~20 °C) at protein concentration of 20 μM with addition of 80 μM Tb3+ for CaM and 40 μM Tb3+ for DREAM constructs. An additional lifetime of ~80 μs was resolved for all DREAM constructs, likely due to parasitic light or PMT recovery delay, and is not shown.
Measured in the presence of 131 μM TFP.
The protein concentration for these experiments was 10 μM in the presence of (a) 100 mM NaCl, (b) 100 mM NaCl and 10 mM LDAO, or (c) 100 mM NaCl, 10 mM LDAO, and 31 μM NS5806.
Additional measurements of the luminescence decay of the DREAM construct with inactivated EF-hands show similar bimodal exponential decays. One of the most salient observations is that the decay lifetime of DREAM(E234Q) is significantly shorter [τ2 = 1.75 ms (27%)] than that observed for DREAM(E186Q) [τ2 = 2.02 ms (46%)]. This indicates that binding of Tb3+ at EF-hand 4 induces a restructuring of the coordination sphere similar to that induced when both EF-hands 3 and 4 are bound to Tb3+. On the other hand, the structural arrangement of the binding loop at EF-hand 3 when Tb3+ is bound at this site is significantly different, with a much more solvent-exposed Tb3+ ion. However, it could also be possible that these two lifetimes cannot be separately resolved in the DREAM(Δ64) decays. The lifetime of Tb3+ bound at the solvent-exposed site is identical on both constructs, with τ1 being 0.62 ms (50%) for DREAM(E186Q) and τ1 being 0.63 ms (27%) for DREAM(E234Q), but faster than that observed for the fully active proteins. Interestingly, in the presence of 5 mM Mg2+, a decrease in the decay lifetimes and an increase in the intensity contribution of the long-lived decay are observed for all constructs, except for DREAM(E234Q). The observed changes are largest for DREAM(E186Q) where a decrease of 0.44 ms in τ2 is also accompanied by a 15% increase in the contribution of this decay component. The decay lifetimes, τ1 = 0.74 ms for DREAM(Δ64) and τ1 = 0.63 ms for DREAM-(Δ160), are still longer than those for Tb3+ in water, while those of DREAM(E186Q) and DREAM(E234Q) are identical to that of Tb3+ in water. The effect of Mg2+ on the luminescence data is in good agreement with the changes observed in the sensitized emission titrations (Figure 3d).
Finally, we examined whether Tb3+ luminescence decay would be sensitive to structural changes induced on CaM and DREAM upon binding of small hydrophobic ligands. To test this hypothesis, we chose trifluoperazine (TFP) and a novel biphenyl-urea compound named NS5806. These compounds have been shown to bind at hydrophobic cavities on CaM and DREAM, respectively, in a calcium-dependent manner.20,49 Upon titration of each ligand into Tb3+-bound CaM and DREAM(Δ64), we observe a strong dose-dependent modulation of the luminescence decay (Figure 6d). Global analysis of the luminescence decay of the Tb3+CaM and Tb3+DREAM-(Δ64) complexes reveals that binding of these small hydrophobic ligands induces a decrease in intensity contribution from τ2 on both constructs while also decreasing the long lifetime on DREAM(Δ64). Plotting the change in f2 as a function of TFP or NS5806 concentration yields dissociation constants of ~37 μM for both ligands (data not shown), which is larger than those observed in the Ca2+-bound form of these proteins (Kd ~ 1 μM for TFP, and Kd = 5 μM for NS5806). The discrepancies could be due to secondary sites being populated (TFP has been shown to bind at four sites on CaM with dissociation constants between 1 μM and 5 mM) or to the distinct conformation of the hydrophobic cavity of the proteins in the Tb3+-bound form.
Displacement of Ca2+ by Tb3+ Induces Minimal Changes in the Collisional Cross Section of DREAM
To further study the displacement of Ca2+ ions from the EF-hand domains of DREAM, as well as to determine the magnitude of the structural differences between the Ca2+- and Tb3+-bound DREAM structures, ion mobility measurements were conducted to determine the ion-neutral collisional cross section using a TIMS–MS analyzer (see Table 4). The mass spectrum of DREAM(Δ64) under native nanoESI conditions shows a narrow charge-state distribution (+7 to +9), with multiple Ca2+ and Tb3+ adducts. A closer look at the charge distribution shows the presence of the apo form, [M + nH]n+, as well as three adduct series: [M + Ca2+x=1–3 + (n−x)H]n +, [M + Ca2+x + Tb3+y + (n−x−y)H]n+, and [M + Tb3+x=1–2 + (n−x)H]n+ [see the example of the +8 charge-state distribution of DREAM-(Δ64) in the presence of Ca2+, Ca2+ and Tb3+, and Tb3+ in Figure 7a]. Inspection of the mobility profiles for each adduct series shows that as the charge state increases from +7 to +9, a decrease in mobility (and increase in CCS) is observed as a consequence of the interaction of the molecular ion with the external electric field, which is not necessarily an indication of conformational changes. However, small changes in the CCS are observed as a function of the adduct series within each charge state. For example, a decrease in the CCS is observed for DREAM(Δ64) bound to Tb3+ when compared to that of DREAM(Δ64) bound to Ca2+ and DREAM(Δ64) bound to Ca2+ and Tb3+ (see Figure 7b). Comparison of the observed CCS profiles (CCS+7 – +9 = 2200–2300 Å2) to that of a previously reported 2JUL NMR structure of DREAM7 (CCStheo = 2773 Å2) suggests that the gas-phase conformations are more compact than that observed in solution, probably as a consequence of the interaction of the adduct with the EF-hand domains in the absence of the solvent. In addition, the higher affinity of Tb3+ when compared to that of Ca2+ may induce a more compact structure (smaller CCS) for DREAM(Δ64) bound to Tb3+ than for DREAM(Δ64) bound to Ca2+ and DREAM(Δ64) bound to Ca2+ and Tb3+ in the absence of the solvent.
Table 4.
Experimental and Theoretical Ion-Neutral Collision Cross Sections for the DREAM(Δ64):Ca2+/Tb3+ Molecular Ions
| +7 (Å2) | +8 (Å2) | +9 (Å2) | |
|---|---|---|---|
| theoreticala | |||
| DREAM(Δ64):Ca2+ Ca2+ | 2773 | 2798 | 2825 |
| experimental | |||
| DREAM(Δ64):Ca2+ Ca2+ | 2225 | 2243 | 2297 |
| DREAM(Δ64):Ca2+ Tb3+ | 2205 | 2231 | 2260 |
| DREAM(Δ64):Tb3+ Tb3+ | 2200 | 2228 | 2238 |
Calculated using the IMoS software as described in Materials and Methods.
Figure 7.
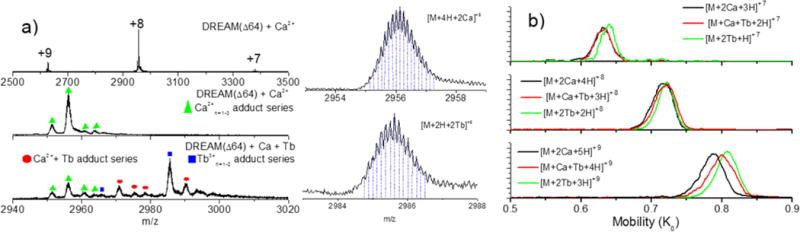
(a) Typical nanoESI mass spectra of DREAM(Δ64) in the presence of Ca2+ and DREAM(Δ64) in the presence of Ca2+ and Tb3+. The isotopic distributions of the DREAM(Δ64):2Ca2+ and DREAM(Δ64):2Tb3+ complexes are shown for the sake of clarity. (b) NanoESI TIMS mobility spectra of DREAM(Δ64) bound to Ca2+ and/or Tb3+.
DISCUSSION
In this report, we present conclusive evidence that Tb3+ is able to bind at the C-terminus of DREAM and displace Ca2+ from the binding loop at EF-hands 3 and 4. Using circular dichroism as well as fluorescence intensity and anisotropy decay of intrinsic fluorescent probes, we demonstrate that Tb3+ is able to induce structural changes on DREAM identical to those observed upon Ca2+ binding. Circular dichroism shows a small deviation of the spectra near 208 nm, a region that is sensitive to the presence of antiparallel β-sheets.50 DREAM is mainly α-helical, and the only region in which small antiparallel β-sheets are formed is between the metal binding loops of each EF-hand pair.7 Therefore, it is possible that association of Tb3+ induces structural changes at the metal binding loop that result in a loss of these short β-sheet regions. Small differences between Tb3+- and Ca2+-bound DREAM were also observed by anisotropy decay measurements. Anisotropy decay revealed that dimers are formed in the Ca2+- or Tb3+-bound form of DREAM, but binding of Tb3+ induces a more dynamic environment near Trp169. Using molecular dynamics, we have previously shown that Trp169 in DREAM(Δ64) can populate two rotamers, and it is possible that Tb3+ binding could enhance this rotamer transition, which would result in an increase in local dynamics.43
As seen for other calcium binding proteins, we observe that aromatic amino acids at the C-terminus are able to transfer energy to the bound Tb3+. Specifically, we show that mutation of Y174 to alanine results in a significant reduction (~60%) in the sensitized emission of Tb3+ at 545 nm, while maintaining identical 280 nm/295 nm ratios compared to that of DREAM(Δ64). Together, these results highlight the presence of an antenna effect, in which W169 is the main donor to Tb3+. Attempts to use sensitized emission and Tb3+ titrations to elucidate the lanthanide binding sequence to EF-hands 3 and 4 on DREAM indicate that both EF-hands bind Tb3+ with a similar affinity [Kd = 5.9 μM for DREAM(E234Q), and Kd = 11 μM for DREAM(E186Q)]. Because these experiments were conducted in the presence of excess calcium and magnesium, inactivation of each EF-hand actually favors binding to the mutated loop (see ITC results). Therefore, we are unable to identify whether the slightly lower affinity of Tb3+ with the DREAM(E186Q) mutant is due to the inherent lower affinity to this hand or because a metal ion (Ca2+ or Mg2+) is loosely bound at this mutated site. Nonetheless, one of the most salient observations of these Tb3+ titration experiments is that in the presence of Mg2+, the sensitized emission of Tb3+ decreases by more than 50%. This decrease in intensity at 545 nm could be due to either Mg2+-induced structural rearrangements of the environment near Trp169 or rearrangement of the Tb3+ binding loop at EF-hands 3 and 4. Indeed, we observe that EF-hand 3, which is next to EF-hand 2, is more sensitive to magnesium binding. These results support the idea that Mg2+ plays a structural role in DREAM and that at physiological concentrations it may act as a functional cofactor through interaction with EF-hand 2.
Isothermal calorimetry experiments show that Tb3+ displacement of Ca2+ from EF-hands 3 and 4 on DREAM(Δ64) in the presence of 5 mM Mg2+ is associated with a ΔG of −14 kcal mol−1, while the displacement energy for the DREAM(Δ160) construct is −12 kcal mol−1. The same displacement process for EF-hands 1–4 in CaM is associated with a free energy ΔG of −18 kcal mol−1. The more favorable displacement of Ca2+ from CaM EF-hands compared to DREAM(Δ64) highlights the stronger association of Ca2+ in the latter.42 Throughout this report, we correlate the K1 thermodynamic parameters with displacement of Ca2+ from EF-hand 3 and K2 with displacement from EF-hand 4 in the DREAM(Δ64), DREAM(E186Q), and DREAM(E234Q) constructs. Even though these correlations are not unequivocally proven by our results, they are supported by the associated changes in enthalpy and entropy under different conditions. Following this assignment, we can approximate the enthalpy and entropy associated with coordination of Tb3+ by Glu at position 12 of EF-hands 3 and 4 by calculating ΔHGlu(Mutant) = ΔHiDREAM(Δ64) − ΔHiDREAM(Mutant) and TΔSGlu(Mutant) = TΔSiDREAM(Δ64) − TΔSiDREAM(Mutant), where the subscript i is 1 for DREAM-(E186Q) and 2 for DREAM(E234Q). These calculations reveal that coordination of Tb3+ by Glu186 of EF-hand 3 is associated with a ΔHGlu(E186Q) of 4.5 kcal mol−1 and a TΔSGlu(E186Q) of 5.0 kcal mol−1. In contrast, Glu234 of EF-hand 4 has a 3-fold larger enthalpy and entropy contribution, with a ΔHGlu(E234Q) of 14 kcal mol−1 and a TΔSGlu(E234Q) of 15 kcal mol−1. In both cases, coordination of the metal ion is driven by favorable entropy contributions, likely due to release of a water molecule upon coordination of Glu at position 12. These results are similar to those observed for Ca2+ binding to CaM and DREAM(Δ64) in which entropy was observed to be the main driving force.,42,44 In contrast, the enthalpic contribution due to metal binding to the EF-hands of CaM and DREAM(Δ64) has been shown to be very small. It is possible that the unfavorable endothermic process observed here is due to additional structural changes associated with Glu12 coordination. The larger enthalpy and entropy associated with coordination of Glu12 on EF-hand 4 highlights the role of this EF-hand in controlling the activation of DREAM. Indeed, Glu12 is positioned on the exiting helix of EF-hand 4 that is immediately adjacent to a hydrophobic cavity that mediates the calcium-regulated co-assembly with potassium channels and small ligands.20,51,52
Another interesting aspect of the recovered thermodynamic parameters shown in Table 2 is that the enthalpy and entropy recovered for all the titrations are highly correlated. This correlation has been observed before for Ca2+ binding to CaM44 as well as in other systems.53 More importantly, the linear relationship of TΔS as a function of ΔH allows us to extrapolate the entropy associated with displacement of Ca2+, prior to any structural change induced by Tb3+ (Figure 8). This is based on the idea that displacement of Ca2+ by Tb3+ is not associated with the formation or breakage of any new bond because the coordinations of Ca2+ and Tb3+ in bulk water are identical. Extrapolating the linear relationship yields a TΔSdisplacement value of 6.9 ± 0.2 kcal mol−1, which is similar to the value obtained for binding of Ca2+ to metal-free CaM in which TΔSbind = 7.2 ± 0.1 kcal mol−1.44 A possible explanation for the entropic gain could be due to an increase in the dynamics of the protein, something that is supported by the anisotropy decay data. The correlation of enthalpy and entropy can also be explained by a process that involves the release of water following the association of two ions of opposite charges in solution. The resulting charge neutralization would facilitate the mobilization of solvent molecules from the surface of the protein into the bulk water.54 This release of water molecules is associated with an unfavorable endothermic process (breakage of water–protein hydrogen bonds) and favorable positive change in entropy. Indeed, Ca2+-bound DREAM(Δ64) (net charge of −3 at pH 7.4) and Ca2+-bound CaM (net charge of −16 at pH 7.4) would be neutralized by 2 and 8 units upon Tb3+ displacement, respectively. This charge neutralization effect could also partially account for the decreased enthalpy and entropy contribution of the DREAM(E186Q) and DREAM(E234Q) mutants, both of which have one less negative charge than DREAM(Δ64) does.
Figure 8.
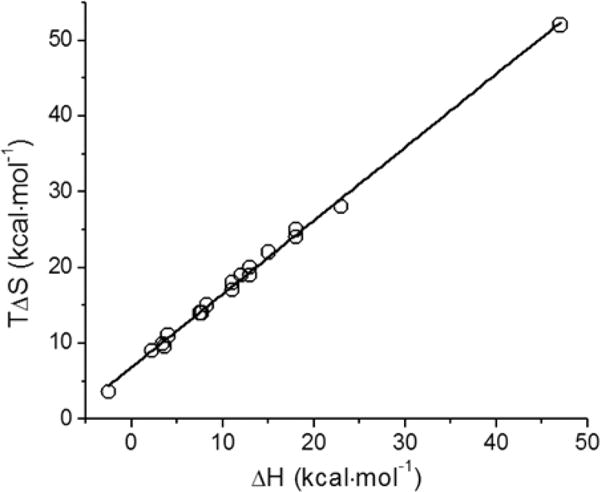
Plot of TΔS vs ΔH for Tb3+ displacement of Ca2+ from DREAM(Δ64), DREAM(Δ161), DREAM(E186Q), DREAM(E234Q), and CaM. The solid line represents the best linear fit to the data.
Sensitized emission, circular dichroism, TIMS–MS, and ITC experiments provide information about global structural changes of the protein upon displacement of Ca2+ by Tb3+. On the other hand, luminescence studies allow us to gain better insight into the immediate coordination sphere of Tb3+ ion, and how this environment is affected by inactivation of EF-hands, Mg2+ binding, ionic strength, and/or ligand binding. Measurements of the luminescence decay of Tb3+ bound to different constructs highlight the idea that Tb3+ bound at EF-hand 3 is more solvent-exposed than Tb3+ bound at EF-hand 4. Nonetheless, the decay observed for DREAM(Δ64) is much slower than that observed for CaM, indicating that the coordination around Tb3+ in DREAM greatly restricts the accessibility of water. We also observe that the luminescence decays are widely affected by addition of excess Mg2+, supporting the idea that some secondary sites of Tb3+ binding, including EF-hand 2 on DREAM, are identical to those of Mg2+ binding. The high sensitivity of EF-hand 3 to Mg2+ binding could also be due to propagated rearrangement of EF-hand 2 upon association of Mg2+. It is also possible that binding of Mg2+ at these secondary sites is responsible for the observed rearrangement of the EF-hand loops. The overall picture that emerges from these observations is that Mg2+ is able to modulate the protein structure and that secondary metal sites may play a role in modulating protein conformation. Indeed, previous work and our unpublished results support the idea that two Ca2+ specific sites and additional secondary sites are found on DREAM(Δ160).10 Lastly, titration of small hydrophobic ligands also reveals that the environment near the metal binding loops of EF-hands 3 and 4 is sensitive to association of ligand with DREAM. Altogether, these observations highlight the idea that association of these small molecules at the hydrophobic surfaces of CaM and DREAM induces conformational changes that not only can distort the coordination geometry of Tb3+ at the binding loops but also could potentially facilitate dissociation of Tb3+ from the protein.
Finally, ion mobility and mass spectrometry measurements support the hypothesis that Tb3+ can displace Ca2+ from EF-hands 3 and 4 and that the resulting folded conformation of the Tb3+-bound DREAM(Δ64) protein is almost identical to that of the calcium-bound protein during native ESI conditions. Comparison of the observed CCS profiles to that of a previously reported NMR structure of DREAM7 suggested that the gas-phase conformations are more compact than that observed in solution, probably as a consequence of the interaction of the adduct with the EF-hand domains in the absence of the solvent. In addition, the higher affinity of Tb3+ when compared to that of Ca resulted in more compact structures (smaller CCS) for DREAM(Δ64) bound to Tb3+ than for DREAM(Δ64) bound to Ca2+ and DREAM(Δ64) bound to Ca2+ and Tb3+ in the absence of the solvent.
CONCLUSION
In this report, we show compelling evidence supporting the specific association of Tb3+ with EF-hands 3 and 4 of DREAM. We also demonstrate that replacement of Ca2+ with Tb3+ leads to an increase in the dynamics of the protein; however, the structural and functional properties between DREAM bound to either metal are highly similar. We show that like the case for CaM, Tb3+ bound to DREAM can be sensitized by aromatic amino acids at the C-terminus, with tryptophan 169 being the main energy donor. The high affinity of the EF-hands for Tb3+ and the fluorescence properties of this lanthanide have allowed us to highlight the role of Mg2+ as a structural cofactor, which can bind to EF-hand 2 and modify the immediate environment near the calcium binding loops of EF-hands 3 and 4. Isothermal calorimetry also highlights the role of EF-hand 4 in mediating calcium-regulated ligand recognition in DREAM. These findings provide structural information about DREAM and will facilitate future structural NMR studies and lanthanide resonance energy transfer experiments aimed at exploring the association of DREAM with other proteins.
Supplementary Material
Acknowledgments
Funding
This work was supported by the National Science Foundation (MCB 1021831, J.M.) and the National Institutes of Health (Grant R00GM106414, F.F.-L.). W.G.G. was fully supported by National Institute of General Medical Sciences Grant R25 GM061347.
ABBREVIATIONS
- DREAM
downstream regulatory element antagonist modulator
- CaM
calmodulin
- fwhm
full width at half-maximum
- KChIP
potassium channel-interacting protein
- DREAM(Δ64)
mouse DREAM construct lacking residues 1–64
- 1,8-ANS
8-anilinonaphthalene-1-sulfonic acid
- CD
circular dichroism
- TIMS–MS
trapped ion mobility spectrometry–mass spectrometry
- TFP
trifluoperazine
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.biochem.6b00067.
Recovered parameters from fitting of the ITC isotherms using an N-set-of-sites model (PDF)
Notes
The authors declare no competing financial interest.
References
- 1.Carrion AM, Link WA, Ledo F, Mellstrom B, Naranjo JR. DREAM is a Ca2+-regulated transcriptional repressor. Nature. 1999;398:80–84. doi: 10.1038/18044. [DOI] [PubMed] [Google Scholar]
- 2.Buxbaum JD, Choi E, Luo Y, Lilliehook C, Crowley AC, Merriam DE, Wasco W. Calsenilin: A calcium-binding protein that interacts with the presenilins and regulates the levels of a presenilin fragment. Nat Med. 1998;4:1177–1181. doi: 10.1038/2673. [DOI] [PubMed] [Google Scholar]
- 3.An WF, Bowlby MR, Betty M, Cao J, Ling H, Mendoza G, Hinson JW, Mattsson KI, Strassle BW, Trimmer JS, Rhodes KJ. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000;403:553–556. doi: 10.1038/35000592. [DOI] [PubMed] [Google Scholar]
- 4.Cheng HM, Pitcher GM, Laviolette SR, Whishaw IQ, Tong KI, Kockeritz LK, Wada T, Joza NA, Crackower M, Goncalves J, Sarosi I, Woodgett JR, Oliveira-dos-Santos AJ, Ikura M, van der Kooy D, Salter MW, Penninger JM. DREAM is a critical transcriptional repressor for pain modulation. Cell. 2002;108:31–43. doi: 10.1016/s0092-8674(01)00629-8. [DOI] [PubMed] [Google Scholar]
- 5.Costigan M, Woolf CJ. No DREAM, no pain: Closing the spinal gate. Cell. 2002;108:297–300. doi: 10.1016/s0092-8674(02)00640-2. [DOI] [PubMed] [Google Scholar]
- 6.Fontán-Lozano Á, Romero-Granados R, del-Pozo-Martín Y, Suárez-Pereira I, Delgado-García JM, Penninger JM, Carrión ÁM. Lack of DREAM protein enhances learning and memory and slows brain aging. Curr Biol. 2009;19:54–60. doi: 10.1016/j.cub.2008.11.056. [DOI] [PubMed] [Google Scholar]
- 7.Lusin JD, Vanarotti M, Li C, Valiveti A, Ames JB. NMR structure of DREAM: Implications for Ca2+-dependent DNA binding and protein dimerization. Biochemistry. 2008;47:2252–2264. doi: 10.1021/bi7017267. [DOI] [PubMed] [Google Scholar]
- 8.da Silva AC, Kendrick-Jones J, Reinach FC. Determinants of ion specificity on EF-hands sites. conversion of the Ca2+/Mg2+ site of smooth muscle myosin regulatory light chain into a ca(2+)-specific site. J Biol Chem. 1995;270:6773–6778. doi: 10.1074/jbc.270.12.6773. [DOI] [PubMed] [Google Scholar]
- 9.Gifford J, Walsh M, Vogel H. Structures and metalion-binding properties of the Ca2 -binding helix-loop-helix EF-hand motifs. Biochem J. 2007;405:199–221. doi: 10.1042/BJ20070255. [DOI] [PubMed] [Google Scholar]
- 10.Craig TA, Benson LM, Venyaminov SY, Klimtchuk ES, Bajzer Z, Prendergast FG, Naylor S, Kumar R. The metal-binding properties of DREAM. J Biol Chem. 2002;277:10955–10966. doi: 10.1074/jbc.M109660200. [DOI] [PubMed] [Google Scholar]
- 11.Kretsinger RH, Nockolds CE. Carp muscle calcium-binding protein. II structure determination and general description. J Biol Chem. 1973;248:3313–3326. [PubMed] [Google Scholar]
- 12.Osawa M, Tong KI, Lilliehook C, Wasco W, Buxbaum JD, Cheng HYM, Penninger JM, Ikura M, Ames JB. Calcium-regulated DNA binding and oligomerization of the neuronal calcium-sensing protein, Calsenilin/DREAM/KChIP3. J Biol Chem. 2001;276:41005–41013. doi: 10.1074/jbc.M105842200. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez WG, Miksovska J. Application of ANS fluorescent probes to identify hydrophobic sites on the surface of DREAM. Biochim Biophys Acta, Proteins Proteomics. 2014;1844:1472–1480. doi: 10.1016/j.bbapap.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Mustafi SM, Mukherjee S, Chary KVR, Del Bianco C, Luchinat C. Energetics and mechanism of Ca2+ displacement by lanthanides in a calcium binding protein. Biochemistry. 2004;43:9320–9331. doi: 10.1021/bi049657d. [DOI] [PubMed] [Google Scholar]
- 15.Martin B, Richardson FS. Lanthanides as probes for calcium in biological systems. Q Rev Biophys. 1979;12:181–209. doi: 10.1017/s0033583500002754. [DOI] [PubMed] [Google Scholar]
- 16.Rao ST, Satyshur KA, Greaser ML, Sundaralingam M. X-ray structures of mn, cd and tb metal complexes of troponin C. Acta Crystallogr Sect D: Biol Crystallogr. 1996;52:916–922. doi: 10.1107/S0907444996006166. [DOI] [PubMed] [Google Scholar]
- 17.Pintacuda G, Park AY, Keniry MA, Dixon NE, Otting G. Lanthanide labeling offers fast NMR approach to 3D structure determinations of Protein-Protein complexes. J Am Chem Soc. 2006;128:3696–3702. doi: 10.1021/ja057008z. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez W, Miksovska J. Characterization of the photophysical, thermodynamic and structural properties of the terbium (III)-KChIP3 complex. Biophys J. 2015;108:218a. doi: 10.1021/acs.biochem.6b00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez WG, Arango AS, Miksovska J. Amphiphilic residues 29–44 of DREAM N-termini mediate calmodulin:DREAM complex formation. Biochemistry. 2015;54:4391–4403. doi: 10.1021/acs.biochem.5b00251. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez WG, Pham K, Miksovska J. Modulation of the voltage-gated potassium channel (Kv4.3) and the auxiliary protein (KChIP3) interactions by the current activator NS5806. J Biol Chem. 2014;289:32201–32213. doi: 10.1074/jbc.M114.577528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.George SE, Su Z, Fan D, Means AR. Calmodulin-cardiac troponin C chimeras. effects of domain exchange on calcium binding and enzyme activation. J Biol Chem. 1993;268:25213–25220. [PubMed] [Google Scholar]
- 22.Fernandez-Lima F, Kaplan D, Park M. Note: Integration of trapped ion mobility spectrometry with mass spectrometry. Rev Sci Instrum. 2011;82:126106. doi: 10.1063/1.3665933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernandez-Lima F, Kaplan DA, Suetering J, Park MA. Gas-phase separation using a trapped ion mobility spectrometer. Int J Ion Mobility Spectrom. 2011;14:93–98. doi: 10.1007/s12127-011-0067-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molano-Arevalo JC, Hernandez DR, Gonzalez WG, Miksovska J, Ridgeway ME, Park MA, Fernandez-Lima F. Flavin adenine dinucleotide structural motifs: From solution to gas phase. Anal Chem. 2014;86:10223–10230. doi: 10.1021/ac5023666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernandez DR, DeBord JD, Ridgeway ME, Kaplan DA, Park MA, Fernandez-Lima F. Ion dynamics in a trapped ion mobility spectrometer. Analyst. 2014;139:1913–1921. doi: 10.1039/c3an02174b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDaniel EW, Mason EA. The Mobility and Diffusion of Ions in Gases. Wiley; New York: 1973. [Google Scholar]
- 27.Larriba C, Hogan CJ., Jr Ion mobilities in diatomic gases: Measurement versus prediction with non-specular scattering models. J Phys Chem A. 2013;117:3887–3901. doi: 10.1021/jp312432z. [DOI] [PubMed] [Google Scholar]
- 28.Larriba C, Hogan CJ. Free molecular collision cross section calculation methods for nanoparticles and complex ions with energy accommodation. J Comput Phys. 2013;251:344–363. [Google Scholar]
- 29.Ouyang H, Larriba-Andaluz C, Oberreit DR, Hogan CJ., Jr The collision cross sections of iodide salt cluster ions in air via differential mobility analysis-mass spectrometry. J Am Soc Mass Spectrom. 2013;24:1833–1847. doi: 10.1007/s13361-013-0724-8. [DOI] [PubMed] [Google Scholar]
- 30.Mulqueen P, Tingey JM, Horrocks WD. Characterization of lanthanide(III) ion binding to calmodulin using luminescence spectroscopy. Biochemistry. 1985;24:6639–6645. doi: 10.1021/bi00344a051. [DOI] [PubMed] [Google Scholar]
- 31.Chaudhuri D, Horrocks WD, Jr, Amburgey JC, Weber DJ. Characterization of lanthanide ion binding to the EF-hand protein S100 beta by luminescence spectroscopy. Biochemistry. 1997;36:9674–9680. doi: 10.1021/bi9704358. [DOI] [PubMed] [Google Scholar]
- 32.Kilhoffer M, Gerard D, Demaille JG. Terbium binding to octopus calmodulin provides the complete sequence of ion binding. FEBS Lett. 1980;120:99–103. doi: 10.1016/0014-5793(80)81055-6. [DOI] [PubMed] [Google Scholar]
- 33.Austin RH, Stein DL, Wang J. Terbium luminescence-lifetime heterogeneity and protein equilibrium conformational dynamics. Proc Natl Acad Sci U S A. 1987;84:1541–1545. doi: 10.1073/pnas.84.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horrocks WD, Sudnick DR. Lanthanide ion probes of structure in biology. laser-induced luminescence decay constants provide a direct measure of the number of metal-coordinated water molecules. J Am Chem Soc. 1979;101:334–340. [Google Scholar]
- 35.Hagan AK, Zuchner T. Lanthanide-based time-resolved luminescence immunoassays. Anal Bioanal Chem. 2011;400:2847–2864. doi: 10.1007/s00216-011-5047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fisher JR, Sharma Y, Iuliano S, Piccioti RA, Krylov D, Hurley J, Roder J, Jeromin A. Purification of myristoylated and nonmyristoylated neuronal calcium sensor-1 using single-step hydrophobic interaction chromatography. Protein Expression Purif. 2000;20:66–72. doi: 10.1006/prep.2000.1298. [DOI] [PubMed] [Google Scholar]
- 37.Drabikowski W, Brzeska H, Venyaminov SYu. Tryptic fragments of calmodulin. Ca2+- and Mg2+-induced conformational changes. J Biol Chem. 1982;257:11584–11590. [PubMed] [Google Scholar]
- 38.Hogue CW, MacManus JP, Banville D, Szabo AG. Comparison of terbium (III) luminescence enhancement in mutants of EF hand calcium binding proteins. J Biol Chem. 1992;267:13340–13347. [PubMed] [Google Scholar]
- 39.Brittain HG, Richardson FS, Martin RB. Terbium(III) emission as a probe of calcium(II) binding sites in proteins. J Am Chem Soc. 1976;98:8255–8260. doi: 10.1021/ja00441a060. [DOI] [PubMed] [Google Scholar]
- 40.Kleinerman M. Energy migration in lanthanide chelates. J Chem Phys. 1969;51:2370–2381. [Google Scholar]
- 41.Wallace RW, Tallant EA, Dockter ME, Cheung WY. Calcium binding domains of calmodulin. sequence of fill as determined with terbium luminescence. J Biol Chem. 1982;257:1845–1854. [PubMed] [Google Scholar]
- 42.Osawa M, Dace A, Tong KI, Valiveti A, Ikura M, Ames JB. Mg2+ and Ca2+ differentially regulate DNA binding and dimerization of DREAM. J Biol Chem. 2005;280:18008–18014. doi: 10.1074/jbc.M500338200. [DOI] [PubMed] [Google Scholar]
- 43.Pham K, Dhulipala G, Gonzalez WG, Gerstman BS, Regmi C, Chapagain PP, Miksovska J. Ca2+ and Mg2+ modulate conformational dynamics and stability of downstream regulatory element antagonist modulator. Protein Sci. 2015;24:741–751. doi: 10.1002/pro.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gilli R, Lafitte D, Lopez C, Kilhoffer M, Makarov A, Briand C, Haiech J. Thermodynamic analysis of calcium and magnesium binding to calmodulin. Biochemistry. 1998;37:5450–5456. doi: 10.1021/bi972083a. [DOI] [PubMed] [Google Scholar]
- 45.Wang CL, Aquaron RR, Leavis PC, Gergely J. Metal-binding properties of calmodulin. Eur J Biochem. 1982;124:7–12. doi: 10.1111/j.1432-1033.1982.tb05900.x. [DOI] [PubMed] [Google Scholar]
- 46.Hungerford G, Hussain F, Patzke GR, Green M. The photophysics of europium and terbium polyoxometalates and their interaction with serum albumin: A time-resolved luminescence study. Phys Chem Chem Phys. 2010;12:7266–7275. doi: 10.1039/b925547h. [DOI] [PubMed] [Google Scholar]
- 47.Bünzli JCG, Eliseeva SV. Lanthanide Luminescence. Springer; Berlin: 2011. Basics of lanthanide photophysics; pp. 1–45. [Google Scholar]
- 48.Deiters E, Song B, Chauvin A, Vandevyver C, Gumy F, Bünzli J. Luminescent bimetallic lanthanide bioprobes for cellular imaging with excitation in the visible-light range. Chem – Eur J. 2009;15:885–900. doi: 10.1002/chem.200801868. [DOI] [PubMed] [Google Scholar]
- 49.Cook WJ, Walter LJ, Walter MR. Drug binding by calmodulin: Crystal structure of a calmodulin-trifluoperazine complex. Biochemistry. 1994;33:15259–15265. doi: 10.1021/bi00255a006. [DOI] [PubMed] [Google Scholar]
- 50.Kelly SM, Jess TJ, Price NC. How to study proteins by circular dichroism. Biochim Biophys Acta, Proteins Proteomics. 2005;1751:119–139. doi: 10.1016/j.bbapap.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 51.Wang H, Yan Y, Liu Q, Huang Y, Shen Y, Chen L, Chen Y, Yang Q, Hao Q, Wang K, Chai J. Structural basis for modulation of Kv4 K+ channels by auxiliary KChIP subunits. Nat Neurosci. 2007;10:32–39. doi: 10.1038/nn1822. [DOI] [PubMed] [Google Scholar]
- 52.Pioletti M, Findeisen F, Hura GL, Minor DL. Three-dimensional structure of the KChIP1-Kv4.3 T1 complex reveals a cross-shaped octamer. Nat Struct Mol Biol. 2006;13:987–995. doi: 10.1038/nsmb1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuroki R, Yutani K. Structural and thermodynamic responses of mutations at a Ca2+ binding site engineered into human lysozyme. J Biol Chem. 1998;273:34310–34315. doi: 10.1074/jbc.273.51.34310. [DOI] [PubMed] [Google Scholar]
- 54.Bowman-James K, Bianchi A, García-Espana E. Anion Coordination Chemistry. John Wiley & Sons; New York: 2012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


