Figure 2.
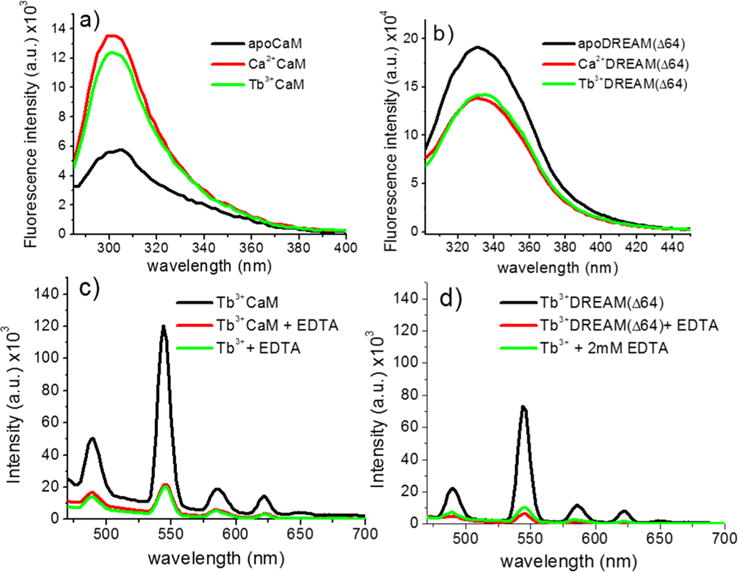
Intrinsic fluorescence changes of 40 μM (a) CaM and (b) DREAM(Δ64) upon binding of 1 mM calcium or 160 μM Tb3+ on CaM or 80 μM Tb3+ for DREAM(Δ64), excited at 280 ± 4 nm. The observed tyrosine fluorescence change upon binding of Tb3+ to CaM shows a small deviation from that observed for Ca2+, likely due to quenching of the fluorescence by energy transfer to Tb3+. The Tb3+-induced transition for DREAM(Δ64) is identical to that observed in the presence of Ca2+. Sensitized emission of (c) 160 μM Tb3+ bound to CaM and (d) 80 μM Tb3+ bound to DREAM(Δ64), excited at 280 nm with and without 2 mM EDTA. The background emission of the Tb3+:EDTA complex is shown as a reference. The observed sensitized emission of Tb3+ shows the characteristic sharp bands at 489, 544 (major), 586, and 622 nm due to the 5D4 → 7F6, 5D4 → 7F5, 5D4 → 7F4, and 5D4 → 7F3 transitions of Tb3+, respectively. The major peak at 545 nm for DREAM(Δ64) is 40% smaller than that observed for CaM. Addition of 2 mM EDTA to either CaM or DREAM(Δ64) resulted in an emission identical to that of Tb3+ in solution.
