Abstract
An important aspect in alcohol abuse associated immune suppression is the loss of T helper CD4+ lymphocytes leading to an impairment of multiple immune functions. Our work has shown that ethanol can sensitize CD4+ T lymphocytes to activation-induced, caspase-3 dependent cell death (AICD). It has been demonstrated that formation of S-adenosylmethionine (SAMe) catalyzed by methionine adenosyltransferase II (MAT II) is essential for CD4+ T cell activation and proliferation. Since ethanol is known to affect SAMe metabolism in hepatocytes, we investigated the effect of ethanol on MAT II activity/expression, SAMe biosynthesis and cell survival in CD4+ T lymphocytes. We demonstrate for the first time that ethanol at a physiologically relevant concentration (25mM) substantially decreased the enzymatic activity of MAT II in T lymphocytes. Ethanol was observed to decrease the transcription of MAT2A, which encodes the catalytic subunit of MAT II and is vital for MAT II activity and SAMe biosynthesis. Further, correspondent to its effect on MAT II, ethanol decreased intracellular SAMe levels and enhanced caspase-3 dependent AICD. Importantly, restoration of intracellular SAMe levels by exogenous SAMe supplementation considerably decreased both caspase-3 activity and apoptotic death in T lymphocytes. In conclusion, our data shows that MAT II and SAMe are critical molecular components essential for CD4+ T cell survival which are affected by ethanol leading to enhanced AICD. Furthermore, these studies provide a clinical paradigm for the development of the much needed therapy using SAMe supplementation in the treatment of immune dysfunction induced by alcohol abuse.
Keywords: Ethanol, methionine adenosyltransferase, S-adenosylmethionine, CD4+ T cells, caspase 3, Activation-induced cell death
INTRODUCTION
Excessive alcohol consumption is known to have deleterious effects on the immune system (1–5). Chronic alcohol administration in experimental animal systems leads to a decrease in the absolute numbers of CD4+ T lymphocytes from the periphery and the spleen as well as a reduction in their immune function (6–12).
In human studies, alcoholic patients have significantly reduced numbers of CD4+ T lymphocytes and recovery of the CD4+ T lymphocyte count has been noted in several studies after alcohol withdrawal, suggesting that ethanol can directly affect CD4+ T lymphocyte survival (13–18). CD4+ T lymphocytes are the central regulators of the immune system, controlling both cell-mediated and humoral immunity. While experimental and clinical studies have documented that alcohol intake can cause depletion of CD4+ T lymphocytes, the mechanisms underlying this alcohol effect are only beginning to be understood. Recently, work done by us has shown that in vitro exposure of human CD4+ T cells to physiologically relevant concentrations of ethanol enhances their susceptibility to Fas -induced as well as activation-induced cell death (AICD) by augmenting FasR and TCR-CD3 mediated caspase-3 activation (19,20). The present work was carried out to further clarify the mechanisms and identify the molecular components involved in the ethanol-mediated enhancement of AICD of CD4+ T cells which are the central regulators of the immune system, controlling both cell-mediated and humoral immunity.
Intracellular S-Adenosyl-L-methionine (SAMe) is known to be critical for normal cell development and function. SAMe levels vary under different biological conditions of differentiation and proliferation, and are regulated by its biosynthesis and utilization (21). SAMe is of pivotal importance in cellular metabolism and serves as the principal biologic donor of methyl groups in transmethylation reactions, thereby supporting the synthesis and modification of several key cellular components including proteins, lipids, RNA and DNA. Moreover, SAMe controls essential metabolic pathways by regulating several important enzymatic reactions including those involved in the polyamine biosynthesis and single carbon metabolism (21). Methionine adenosyltransferase (MAT) is a key enzyme in cellular metabolism because it catalyzes the only reaction that generates SAMe from L-methionine (L-Met) and ATP (21).
An important consequence of chronic alcohol abuse is abnormal metabolism of hepatic S-adenosylmethionine (SAMe) due to decreased hepatic methionine adenosyl transferase (MAT – MAT I) expression and activity, resulting in hepatic SAMe deficiency and hepatotoxicity (22). However, the effects of ethanol on non-hepatic MAT (MAT II) and SAMe levels in T lymphocytes have not been examined. In the case of T lymphocytes, both the SAMe pool size and the rate of SAMe utilization are known to increase upon T- cell activation (23). A key mechanism for the increase in SAMe biosynthesis in T lymphocytes is increased transcription of MAT2A, which encodes the catalytic subunit of MAT II and is vital for MAT II activity and SAMe biosynthesis (24, 25). MAT2A is constitutively expressed in the actively dividing/proliferating T-cells and its expression is inducible upon T-cell activation (26). Hence, in the present work we examined the effect of ethanol on MAT II activity expression and SAMe biosynthesis, and its consequent impact on activation-induced CD4+ T cell death. Our data show for the first time that MAT II activity and SAMe biosynthesis are essential for T cell survival and their down-regulation by ethanol leads to the enhancement of caspase-3 dependent apoptotic cell death. Importantly, exogenous SAMe supplementation significantly attenuates the ethanol-induced enhancement of caspase-3 dependant apoptotic cell death indicating its potential therapeutic use in the treatment of alcohol induced immune suppression.
METHODS AND MATERIALS
Cell culture and Treatment
Jurkat T cells (Clone E6-1, ATCC, Rockville, MD, USA) and MOLT-4 T cells (ATCC, Rockville, MD, USA) were cultured in RPMI 1640 medium (GIBCO BRL, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS), 10 units/ml of penicillin, 10ug/ml; of streptomycin (Invitrogen Corporation, Carlsbad, CA, U.S.A) in a 37° C and 5% CO2 environment. Jurkat and MOLT-4 cells were resuspended at 1 × 106 cells/ml prior to treatment.
Peripheral blood lymphocyte (PBL) isolation
Fresh, whole blood was drawn with informed consent from healthy non-alcoholic donors into vacutainer tubes (Becton Dickinson Vacutainer; Franklin Lakes, NJ) containing EDTA. Peripheral blood mononuclear cells (PBMC) were isolated using Histopaque-1077 (Sigma-Aldrich Chemicals, St. Louis, MO). 5 × 106 PBMC per well were distributed into 6-well plates (Corning Inc. Costar, NY, USA), and allowed to adhere in a 5% CO2 incubator at 37°C for 2 hr in 2 ml of RPMI-1640. Non-adherent PBLs were removed from the adherent cells (monocytes) and were washed carefully, twice, with pre-warmed RPMI 1640 and resuspended at a concentration of 2 × 106 cells/ml.
Reagents and Antibodies
Cell culture reagents were obtained from Invitrogen (Invitrogen Corporation, Carlsbad, CA, U.S.A). Ethyl alcohol and phytohemoagglutinin (PHA) were obtained from Sigma (St. Louis, MO, USA). S-adenosylmethionine, as its 1,4-butanedisulphonate salt, was provided by Dr. R. O’Brian (Knoll Pharmaceuticals, Piscataway, NJ) and by Dr. G. Stramentionoli (Knoll Farmaceutici, Milan, Italy).
RT- PCR and Real Time PCR
Reverse transcriptase polymerase chain reaction (RT-PCR) assays were used to assess MAT2A mRNA levels in Jurkat cells. Total RNA was isolated from treated cells after 3 h, using TRIZOL (Invitrogen, Carlsbad, CA) and real time PCR was performed as described elsewhere (27). The specific primers were designed for human GAPDH and MAT2A using Primer3 software program. The following primers were used in real time PCR:
GAPDH-RT-FP: 5′- TGGGCTACACTGAGCACCAG - 3′ and
GAPDH-RT-RP: 5′ –GGGTGTCGCTGTTGAAGTCA - 3′
MAT2A–RT-FP: 5′ –ACAATCTACCACCTACAGCCAAGT-3′ and
MAT2A-RT- RP: 5′ – GCATAAGAGACCTGAACAAGAACC - 3′
The parameter Ct (threshold cycle) was defined as the fraction cycle number at which the fluorescence passed the threshold. The relative gene expression of MAT2A was analyzed using 2−ΔΔCt method (28) by normalizing with GAPDH gene expression in all the experiments.
MAT II Activity and S-adenosylmethionine (SAMe) levels
MAT II activity was assayed in extracts prepared from cell pellets by three cycles of freeze thawing, as per the procedure described elsewhere (29) with minor modifications. MAT activity was assayed by using 20 μM L-Met, 5mM ATP in 50mM TES buffer, pH 7.4, 50mM KCl, 15mM MgCl2, 0.3mM EDTA, and 4mM DTT. One unit of MAT activity was defined as the amount of enzyme that catalyzes the formation of 1nmol of SAMe in 1 h. SAMe catalyzed by MAT II as well as intracellular SAMe levels were assayed by reverse-phase HPLC from deproteinized extracts prepared by using 4% metaphosphoric acid (MPA) as described elsewhere (30).
DNA Fragmentation ELISA Analysis
Treated Jurkat, MOLT-4 cells or PBLs were lysed after 8 h to measure apoptosis. DNA fragmentation was quantitated using the Cell Death ELISA kit (Roche, Indianapolis, IN, USA) by a method described elsewhere (27).
Caspase-3 activity Assay
Caspase-3 activity was quantified colorimetrically using cytoplasmic extracts prepared from Jurkat cells treated for 8 h. Caspase- 3 like activity was analyzed using the Caspase-3 Fluorometric Assay Kit (R&D Systems, Inc. Minneapolis, MN) as directed by the manufacturer.
Statistical Analyses
Summary statistics are depicted as means ± S.E.M. Analysis of Variance (ANOVA) was used to investigate the influence of ethanol levels on all outcome variables. For post-hoc pairwise comparisons the Tukey–Kramer multiple comparison test was used (all Figures except Fig 3) to determine significance (P value) of the effect of ethanol pretreatment on AICD. Differences were considered statistically significant for P < 0.05. Since intracellular levels of SAMe may not follow a Gaussian distribution, percent SAMe changes were measured (Fig. 3) and these data were examined using additional non-parametric tests (Friedman’s One-way ANOVA) with the test significance level set at 0.05.
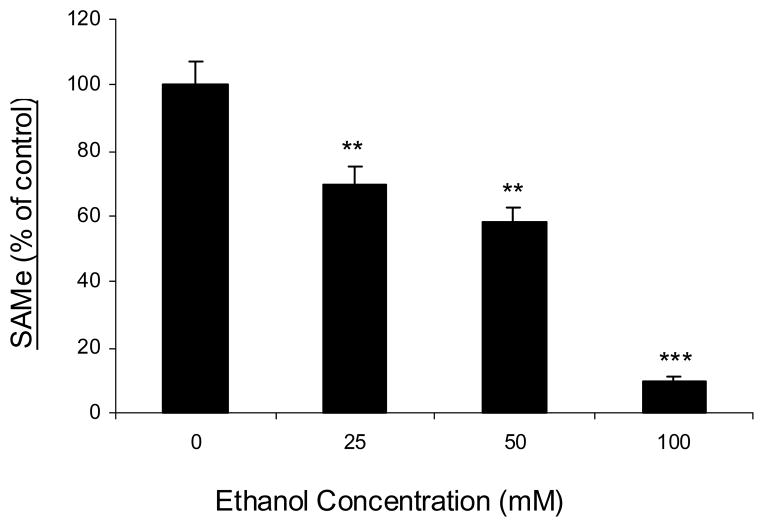
Fig. 3. Ethanol decreases intracellular SAMe levels in a dose-dependant manner.
Jurkat cells were untreated (0) or treated with increasing concentrations of ethanol (25mM, 50mM and 100mM) for 24h. Cell extracts were prepared and SAMe levels were measured by a process as described in the ‘Methods’ section. Results are represented as mean ± SEM from three separate experiments. P values: ** P < 0.01 while *** P < 0.001 when comparing to untreated (0).
RESULTS
Effect of ethanol exposure on MAT II, SAMe biosynthesis and AICD in CD4+ T cells was primarily carried out at a physiologically relevant ethanol level of 25mM. Blood ethanol concentration of 25mM represents legally intoxicating levels and is easily achieved during human alcohol consumption. In all the experiments involving ethanol treatment, ethanol concentrations were routinely measured post-treatment using the Sigma Diagnostics Alcohol Reagent (Sigma Diagnostics, St. Louis, MO). Typically, under the experimental conditions used, a reduction in ethanol concentration by approximately 15 to 20 % was consistently observed over the incubation period of 24 h (data not shown).
Effect of ethanol on MAT II activity and intracellular SAMe levels
Ethanol decreases MAT II activity
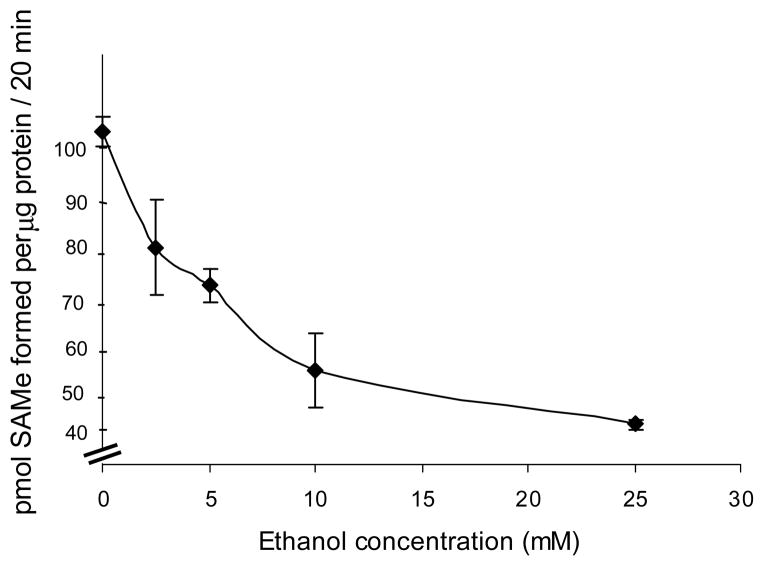
Considering the importance of MAT II and SAMe biosynthesis in T cell proliferation and function, the effect of ethanol exposure on MAT II activity in CD4+ T cells was examined. Cells were untreated (control) or treated with varying concentrations of ethanol (2.5mM, 5mM, 10mM and 25mM) for 24 h. Cell extracts were examined for MAT II activity using a MAT enzymatic assay (as described in the methods) and expressed as pmoles of SAMe formed per μg of (extract) protein. Ethanol was observed to decrease MAT II activity in the T cells in a dose-dependant manner with approximately 50% reduction occurring at 25mM, which represents the physiologically relevant concentration of ethanol (Fig. 1).
Fig. 1. Ethanol decreases MAT II activity.
Jurkat cells were untreated (0) or treated with the indicated concentrations of ethanol for 24 hours. Cell extracts were prepared and MAT II activity was measured as described in the ‘Methods’ section. Each point represents mean ± SEM of three separate experiments.
Ethanol decreases MAT2A mRNA levels
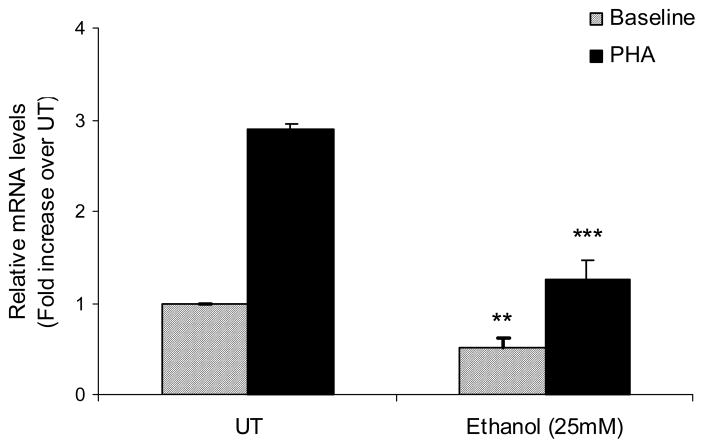
Upon T cell activation, a key mechanism for the increase in SAMe biosynthesis is increased transcription of MAT2A, which is the inducible gene partly responsible for increased MAT II activity. Since ethanol was observed to decrease the MAT II activity, effects of ethanol on the MAT2A mRNA levels were evaluated before and after T cell activation. Total RNA was isolated from the cells exposed to ethanol (25mM) with and without stimulation with PHA (5μg/ml) for 3h. MAT2A mRNA levels were assessed by real time PCR. Ethanol was found to significantly decrease both the constitutive as well as the PHA-inducible MAT2A mRNA expression (Fig. 2).
Fig. 2. Ethanol decreases MAT2A gene expression.
Quantification of MAT2A mRNA levels by real-time PCR. Jurkat cells were either untreated (UT) or treated with 25mM ethanol with or without stimulation by PHA (5μg/ml, solid bars) for 3h. Results are expressed as mean ± SEM from three separate experiments. ** P value <0.05 when comparing ethanol alone to untreated (UT), *** P value < 0.001 when comparing Ethanol + PHA to PHA alone.
Ethanol induces SAMe deficiency in CD4+ T lymphocytes
Since MAT is a key enzyme that catalyzes the only reaction that generates SAMe, we then examined the effect of ethanol on the intracellular levels of SAMe. Deproteinized extracts were prepared from cells exposed for 24 h to varying concentrations of ethanol (25mM, 50mM and 100mM), and SAMe levels determined by HPLC (Fig. 3). Since intracellular levels of SAMe may not follow a Gaussian distribution, percent SAMe changes were measured and analyzed using additional non-parametric tests (Friedman’s One-way ANOVA). There was a significant difference in the percent SAMe levels between no ethanol (0mM) and increasing concentrations of ethanol (25–100mM) (P < 0.05). All results held consistent; importantly, a mean decrease of 30% in the intracellular SAMe levels was observed in the T cells exposed to physiologically relevant concentration (25mM) of ethanol after a period of 24 h. These results showed that correspondent to its effect on MAT II activity and expression, ethanol exposure led to a dose-dependant decrease in the intracellular SAMe concentrations, causing SAMe deficiency in the T cells (Fig. 3).
Effect of pharmacologic inhibition of MAT II on intracellular SAMe levels and T lymphocyte survival
MAT II inhibition and SAMe deficiency enhances T lymphocyte AICD
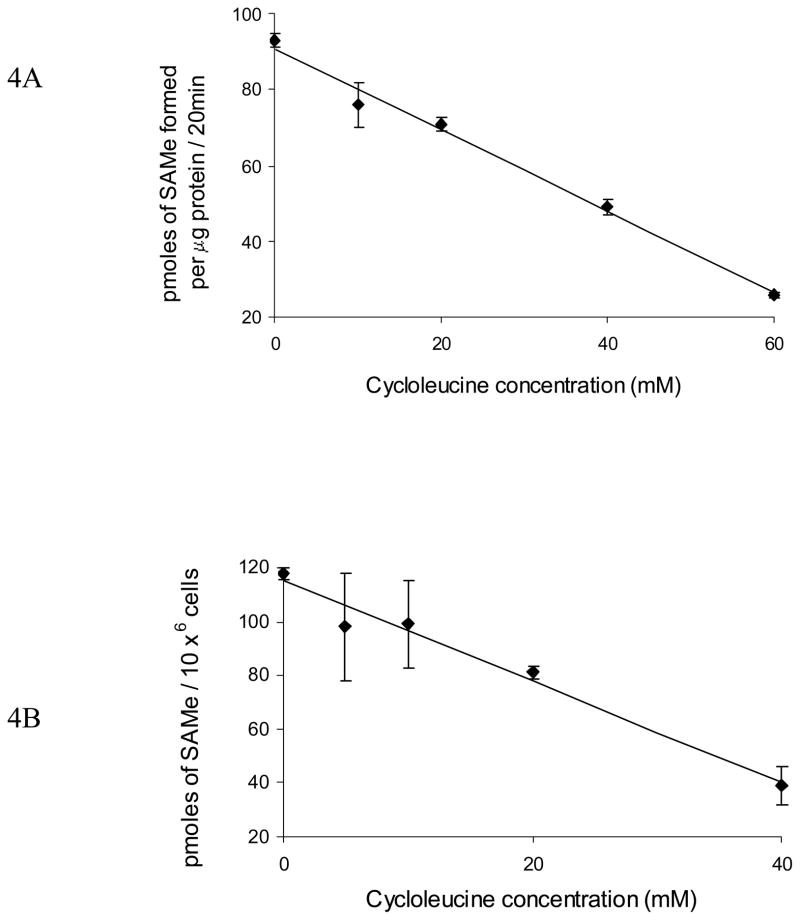
Our previous work has shown that physiologically relevant concentrations of ethanol (25mM) enhance caspase-3 dependent AICD of CD4+ T lymphocytes. Therefore, to establish the causative role of MAT II inhibition and resultant SAMe deficiency in this ethanol-mediated enhancement of T-cell apoptotic death, MAT II activity was inhibited using cycloleucine (1-aminocyclopentane-1-carboxylic acid), a cyclic analogue of methionine, which acts as a specific inhibitor of MAT (31–33). Cells were untreated (control) or treated with varying concentrations of cycloleucine (10 – 60mM) for 4 h. MAT II activity was measured in cell extracts. Further intracellular SAMe levels were measured in control cells (untreated) or in cells treated with cycloleucine (10, 20 and 40 mM) for 4 h. SAMe levels were measured by HPLC. As expected cycloleucine treatment inhibited MAT II activity in a dose dependent manner (Fig. 4A) and consequently decreased cellular SAMe levels (Fig. 4B). This inhibition of MAT II activity and lowering of cellular SAMe levels was observed to significantly induce and enhance AICD of T lymphocytes as documented by the quantitative DNA fragmentation analysis (Fig. 5). These data show that similar to alcohol; pharmacologic induction of SAMe deficiency sensitizes CD4+ T cells to undergo enhanced AICD. Taken together, these data strongly implicate that MAT II activity and SAMe are critical components of T lymphocyte survival that are affected by ethanol.
Fig. 4. Pharmacologic inhibition of MAT II and SAMe biosynthesis enhances T lymphocyte AICD.
A, Cycloleucine decreases MAT II activity in dose-dependant manner. Jurkat cells were untreated (0) or treated with indicated concentrations of cycloleucine for 4h. Cell extracts were prepared and MAT II activity was measured as described in the ‘Methods’. Each point represents mean ± SEM of three separate experiments. B, Cycloleucine induces SAMe deficiency in dose-dependant manner. Cell (Jurkat) extracts were prepared from cells that were either untreated (0) or treated with indicated concentrations of cycloleucine for 4h and intracellular SAMe levels were measured. Results are shown as mean ± SEM from three separate experiments.
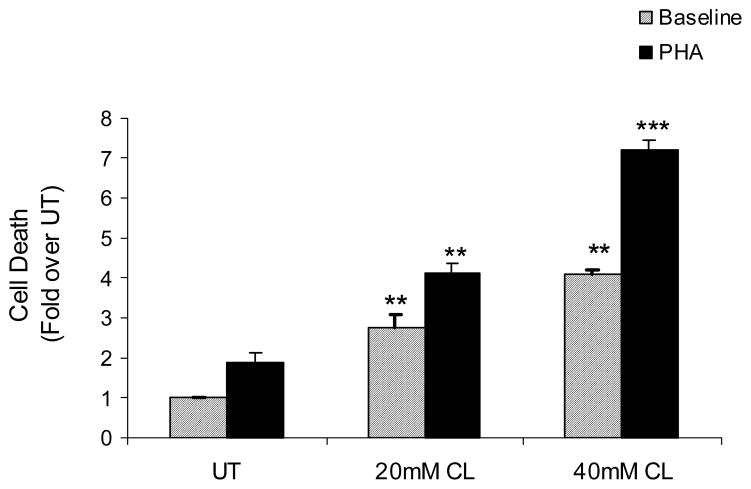
Fig. 5. Inhibition of MAT II by cycloleucine leads to activation-induced apoptotic death of CD+ 4 T- Lymphocytes.
DNA Fragmentation analysis of Jurkat cells untreated (UT) or pretreated with cylcoleucine (20mM CL and 40mM CL) for 4h with or without stimulation by PHA (5μg/ml, solid bars) for 6h. Data are normalized to untreated and expressed as mean ± SEM from three separate experiments. ** P value < 0.01 when comparing cycloleucine (CL) alone treatments to untreated (UT); *** P value < 0.001 comparing CL + PHA treatments with PHA alone.
Effect of SAMe supplementation on ethanol-mediated enhancement of activation-induced T lymphocyte apoptotic death
Exogenous SAMe supplementation increases cellular SAMe levels
Data obtained from ethanol and cycloleucine treated cells showed that decline in MAT II activity and consequent SAMe deficiency can significantly enhance the susceptibility of T cells to apoptotic death. Accordingly, effects of SAMe supplementation in ethanol treated CD4+ T lymphocytes were evaluated. Initially, the effect of exogenous SAMe supplementation on the intracellular SAMe levels was examined. Cells were treated with increasing concentrations of SAMe (0.25mM, 0.5mM and 1.0mM) for 4 h and intracellular SAMe pools were measured in the deproteinized extracts by HPLC. Exogenous SAMe supplementation significantly increased the intracellular SAMe levels in a dose dependent manner with maximal concentrations achieved at 1.0mM (Fig. 6). Hence subsequent studies evaluating the effect of SAMe supplementation on ethanol treated cells were carried out at 1.0mM.
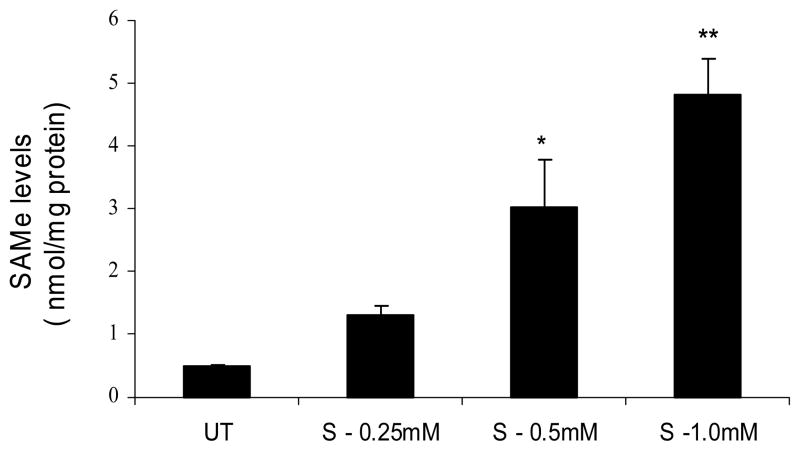
Fig. 6. Exogenous SAMe supplementation increases cellular SAMe levels.
Jurkat cells were untreated (UT) or treated with increasing concentrations of SAMe (0.25mM, 0.5mM and 1mM) for 4h. Cell extracts were prepared and SAMe levels were measured by HPLC. Results are represented as mean ± SEM from three separate experiments. P values: * P < 0.05 while ** P < 0.01 when comparing to untreated (UT).
SAMe supplementation decreases ethanol-mediated increase in caspase-3activity and attenuates AICD in T lymphocytes
Our earlier data showed that ethanol induced enhancement in the susceptibility of CD4+ T lymphocytes to AICD is mediated by an increase in caspase-3 activation. Hence, to further elucidate the role of SAMe deficiency as an underlying cause in the ethanol induced CD4+ T cell toxicity, effect of SAMe supplementation was assessed.
Cells were pretreated with SAMe (1.0mM) for 4 h. followed by ethanol treatment (25mM) for 24 h with or without PHA stimulation for 8 h. As seen before, ethanol augmented the PHA inducible caspase-3 activity, which was significantly attenuated by SAMe supplementation (Fig. 7A). Additionally, SAMe supplementation significantly attenuated the ethanol-mediated enhancement in activation-induced cell death as documented by the DNA fragmentation analysis (Figs. 7B). To ensure that the induction of apoptosis by MAT II inhibition was not cell line specific (i.e. restricted to Jurkat T cells), we tested the effect of MAT II inhibition on another human leukemic T-cell line, namely, MOLT-4. Similar attenuation of ethanol mediated apoptotic death by SAMe was observed in MOLT-4 T cells (Fig. 7C).
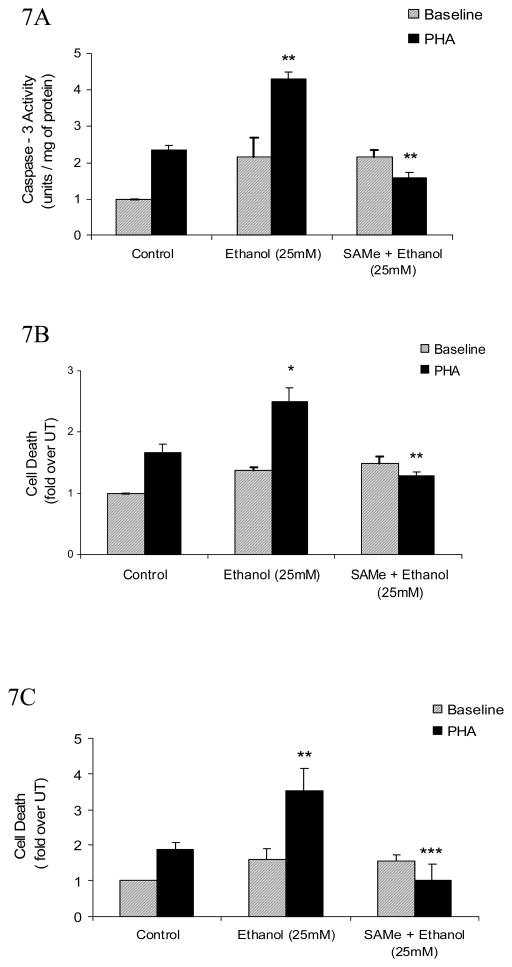
Fig. 7. SAMe supplementation decreases ethanol-mediated increase in caspase-3 and attenuates AICD in T lymphocytes.
A, Quantification of ethanol- mediated caspase-3 activity. Jurkat cells were untreated (control) or pretreated with SAMe (1mM) for 4h and then exposed to 25mM Ethanol for 24h with or without stimulation by PHA (5μg/ml, solid bars) for 8h. Cytoplasmic extracts were isolated and assayed for caspase-3 activity. Results are represented as mean ± SEM from three separate experiments and expressed as fold over untreated (which is set to 1). P values: ** P value < 0.01 when comparing Ethanol + PHA to PHA alone, and when comparing SAMe + Ethanol + PHA to Ethanol + PHA. B, DNA Fragmentation analysis of T (Jurkat) cells that were treated in the similar manner as mentioned above (Fig 7A). Results are represented as mean ± SEM from three separate experiments and expressed as fold over untreated (which is set to 1). P values: * P value < 0.05 when comparing Ethanol + PHA to PHA alone; ** P value < 0.01 when comparing SAMe + Ethanol + PHA to Ethanol + PHA. C, Exogenous SAMe supplementation significantly attenuates the ethanol – mediated AICD in MOLT-4 T cells. Cells (MOLT-4) were untreated (control) or pretreated with SAMe (1mM) for 4h and then exposed to 25mM Ethanol for 24h with or without stimulation by PHA (5μg/ml, solid bars) for 8h. AICD was quantified from cell extracts using the Cell Death detection ELISA assay. Results are represented as mean ± SEM from three separate experiments and expressed as fold over untreated (which is set to 1). P values: ** P value < 0.01 when comparing Ethanol + PHA to PHA alone; *** P value < 0.001 when comparing SAMe + Ethanol + PHA with Ethanol + PHA.
SAMe supplementation attenuates ethanol-induced enhancement of AICD in human PBLs
Since ethanol exposure enhances AICD in primary human lymphocytes, the effect of SAMe supplementation was also evaluated in human PBLs isolated from six healthy (non-alcoholic) volunteers. PBLs were pretreated with SAMe (1.0mM) for 4 h. followed by ethanol treatment (25mM) for 24 h with or without stimulation with plate bound anti-CD3 antibody (5μg/ml, solid bars) for 8 h. AICD in human PBLs was measured by quantifying DNA fragmentation with the Cell Death detection ELISA assay. In agreement with our earlier findings, ethanol exposure enhanced AICD in human PBLs. Importantly, SAMe pretreatment, similar to both Jurkat and MOLT-4 CD4+ T cells, significantly attenuated AICD in primary human PBLs (Fig 8). Additionally, matching results were obtained in ethanol and SAMe treated human PBLs that were activated with PHA (5μg/ml) (data not shown). These findings strongly indicate that the inhibition of MAT II activity/expression and resultant SAMe deficiency are critical molecular events mediated by ethanol exposure that lead to the augmentation of activation-induced apoptotic death in CD4+ T lymphocytes.
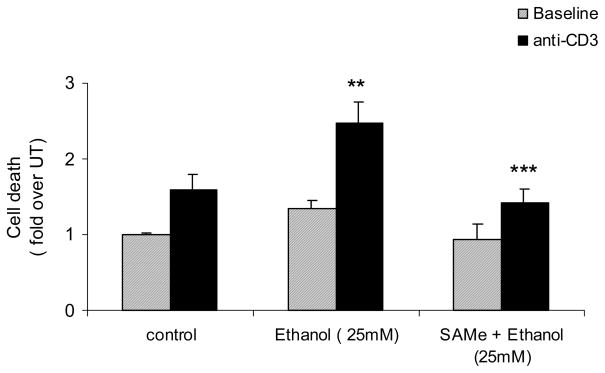
Fig 8. SAMe supplementation attenuates ethanol-induced enhancement of AICD in human peripheral blood lymphocytes (PBLs).
PBLs were untreated (control) or pretreated with SAMe (1mM) for 4h and then exposed to 25mM Ethanol for 24h with or without stimulation with plate bound anti-CD3 antibody (solid bars) for 8h. AICD was measured by quantifying DNA fragmentation with the Cell Death detection ELISA assay. Results are represented as mean ± SEM from six healthy (non-alcoholic) volunteers and expressed as fold over untreated (which is set to 1). P values: ** P value < 0.01 when comparing Ethanol + PHA to PHA alone; *** P value < 0.001 when comparing SAMe + Ethanol + PHA with Ethanol + PHA.
DISCUSSION
A critical factor in alcohol-induced immunosuppression is the loss of CD4+ T lymphocytes that could lead to impairment of multiple immune functions. While experimental and clinical studies have documented that chronic alcohol intake can cause reduction in numbers of CD4+ T lymphocytes, the mechanism(s) underlying are only beginning to be understood. Our earlier studies showed that physiologically relevant concentrations of ethanol could induce and enhance caspase-3 dependant apoptotic death in human CD4+ T lymphocytes by sensitizing them to AICD (19). The present work was aimed at identifying the molecular events that could be involved in this ethanol-mediated sensitization of CD4+ T lymphocytes to AICD.
The results from this study document for the first time that exposure of CD4+ T lymphocytes to ethanol can significantly decrease MAT II activity in a dose-dependant manner with a concomitant decrease in the intracellular SAMe levels. Further analysis showed that ethanol at physiologically relevant concentrations (25mM) substantially decrease the transcription of MAT 2A, which encodes the catalytic subunit of MAT II and is the vital component of MAT II activity and SAMe biosynthesis. These results are clinically significant in the context of immunotoxicity observed in alcoholic patients, who also have hepatic SAMe deficiency and hepatotoxicity due to decreased hepatic methionine adenosyl transferase (MAT I/III) activity and expression.
T-cell activation leads to an increment in both the SAMe pool size and SAMe utilization and inhibition of SAMe synthesis blocks T-cell proliferation (34). Additionally, ethanol enhances caspase-3 dependant apoptotic death in T lymphocytes. Taken together, our present data strongly suggests that ethanol mediated decrease in MAT II expression and activity and consequent SAMe deficiency plays a significant role in the development of ethanol mediated susceptibility of CD4+ T cells to activation-induced cell death. This is further supported by the observations that inhibition of MAT II activity and decrease in intracellular SAMe levels by a selective pharmacologic inhibitor, cycloleucine, also induces and enhances activation-induced apoptotic death in T lymphocytes. The causal role of MAT II inhibition and ensuing SAMe deficiency in ethanol mediated CD4+ T-cell death, is further supported by the data obtained from the SAMe supplementation studies. Restoration of intracellular SAMe levels achieved by exogenous SAMe supplementation, which bypasses the requirement of MAT II activity, was observed to considerably counter the cytotoxic effects of ethanol by decreasing both caspase-3 activity and AICD in T-cells. Importantly, the protective effects of SAMe supplementation observed in two different CD4+ T cell lines (Jurkat and MOLT-4) were also recapitulated in human PBLs.
Overall, these data strongly implicate a survival role for MAT II and SAMe in activated T-cells. Moreover, the present work identifies MAT2A gene to be directly associated with T-cell survival and supports the notion that MAT II dependent SAMe biosynthesis and the consequent trans-methylation reactions controlled by SAMe, either directly or indirectly, prevents the activation-induced apoptotic signaling that leads to caspase-3 activation and T cell death. The mechanisms underlying SAMe mediated regulation of apoptotic signaling and activation induced T cell death are currently under investigation.
In conclusion, we have identified MAT II and SAMe as critical molecular components required for CD4+ T cell survival which are diminished by ethanol, leading to an increase in activation induced apoptotic death. Based on these findings, we postulate that investigation of the effects of alcohol on CD4+ T cell MAT II activity, SAMe levels and survival will provide important information concerning mechanisms of alcohol-induced immunosuppression. Additionally, attenuation of ethanol induced CD4+ T cell apoptotic death by exogenous SAMe supplementation provides “proof of concept” for the potential therapeutic use of SAMe in treating immune dysfunction associated with chronic alcohol abuse in alcoholics with and without other immnunosuppressive disorders such as HIV/HCV and diabetes.
Acknowledgments
National Institutes of Health grants AA014371 (Barve), AA015970 (McClain), and Office of Dietary Supplements, NIH
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adams HG, Jordan C. Infections in the alcoholic. Med Clin North Am. 1984;68:179–200. doi: 10.1016/s0025-7125(16)31249-4. [DOI] [PubMed] [Google Scholar]
- 2.MacGregor RR. Alcohol and immune defense. JAMA. 1986;19:1474–9. [PubMed] [Google Scholar]
- 3.Smith FE, Palmer DL. Alcoholism, infection and altered host defenses: a review of clinical and experimental observations. J Chronic Dis. 1976;29:35–49. doi: 10.1016/0021-9681(76)90066-7. [DOI] [PubMed] [Google Scholar]
- 4.Spies CD, von Dossow V, Eggers V, Jetschmann G, El-Hilali R, Egert J, Fischer M, Schroder T, Hoflich C, Sinha P, Paschen C, Mirsalim P, Brunsch R, Hopf J, Marks C, Wernecke KD, Pragst F, Ehrenreich H, Muller C, Tonnesen H, Oelkers W, Rohde W, Stein C, Kox WJ. Altered cell-mediated immunity and increased postoperative infection rate in long-term alcoholic patients. Anesthesiology. 2004;100:1088–100. doi: 10.1097/00000542-200405000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Waldschmidt TJ, Cook RT, Kovacs EJ. Alcohol and inflammation and immune responses: summary of the 2005 Alcohol and Immunology Research Interest Group (AIRIG) meeting. Alcohol. 2006;38:121–5. doi: 10.1016/j.alcohol.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Helm RM, Wheeler G, Burks AW, Hakkak R, Badger TM. Flow cytometric analysis of lymphocytes from rats following chronic ethanol treatment. Alcohol. 1996;13:467–71. doi: 10.1016/0741-8329(96)00036-5. [DOI] [PubMed] [Google Scholar]
- 7.Chadha KC, Stadler I, Albini B, Nakeeb SM, Thacore HR. Effect of alcohol on spleen cells and their functions in C57BL/6 mice. Alcohol. 1991;8:481–5. doi: 10.1016/s0741-8329(91)90187-2. [DOI] [PubMed] [Google Scholar]
- 8.Shellito JE, Olariu R. Alcohol decreases T-lymphocyte migration into lung tissue in response to Pneumocystis carinii and depletes T-lymphocyte numbers in the spleens of mice. Alcohol Clin Exp Res. 1998;22:658–63. doi: 10.1111/j.1530-0277.1998.tb04308.x. [DOI] [PubMed] [Google Scholar]
- 9.Peterson JD, Herzenberg LA, Vasquez K, Waltenbaugh C. Glutathione levels in antigen-presenting cells modulate Th1 versus Th2 response patterns. Proc Natl Acad Sci U S A. 1998;17:3071–6. doi: 10.1073/pnas.95.6.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saad AJ, Jerrells TR. Flow cytometric and immunohistochemical evaluation of ethanol-induced changes in splenic and thymic lymphoid cell populations. Alcohol Clin Exp Res. 1991;15:796–803. doi: 10.1111/j.1530-0277.1991.tb00603.x. [DOI] [PubMed] [Google Scholar]
- 11.Jerrells TR, Saad AJ, Domiati-Saad R. Effects of ethanol on parameters of cellular immunity and host defense mechanisms to infectious agents. Alcohol. 1992;9:459–63. doi: 10.1016/0741-8329(92)90081-k. [DOI] [PubMed] [Google Scholar]
- 12.Padgett EL, Sibley DA, Jerrells TR. Effect of adrenalectomy on ethanol-associated changes in lymphocyte cell numbers and subpopulations in thymus, spleen, and gut-associated lymphoid tissues. Int J Immunopharmacol. 2000;22:285–98. doi: 10.1016/s0192-0561(99)00083-1. [DOI] [PubMed] [Google Scholar]
- 13.Young GP, Van der Weyden MB, Rose IS, Dudley FJ. Lymphopenia and lymphocyte transformation in alcoholics. Experientia. 1979;35:268–9. doi: 10.1007/BF01920656. [DOI] [PubMed] [Google Scholar]
- 14.Roselle GA, Mendenhall CL, Grossman CJ, Weesner RE. Lymphocyte subset alterations in patients with alcoholic hepatitis. J Clin Lab Immunol. 1988;26:169–73. [PubMed] [Google Scholar]
- 15.Pol S, Artru P, Thepot V, Berthelot P, Nalpas B. Improvement of the CD4+ cell count after alcohol withdrawal in HIV-positive alcoholic patients. AIDS. 1996;10:1293–4. doi: 10.1097/00002030-199609000-00019. [DOI] [PubMed] [Google Scholar]
- 16.Laso FJ, Madruga JI, Lopez A, Ciudad J, Alvarez-Mon M, San Miguel J, Orfao A. Distribution of peripheral blood lymphoid subsets in alcoholic liver cirrhosis: influence of ethanol intake. Alcohol Clin Exp Res. 1996;20:1564–8. doi: 10.1111/j.1530-0277.1996.tb01700.x. [DOI] [PubMed] [Google Scholar]
- 17.Gheorghiu M, Bara C, Pasarica D, Brasoveanu L, Bleotu C, Toparceanu F, Trandafir T, Diaconu CC. Ethanol-induced dysfunction of hepatocytes and leukocytes in patients without liver failure. Roum Arch Microbiol Immunol. 2004;63:5–33. [PubMed] [Google Scholar]
- 18.Kazbariene B, Krikstaponiene A, Monceviciute-Eringiene E. Disturbance of human immunohomeostasis by environmental pollution and alcohol consumption. Acta Microbiol Immunol Hung. 2006;53:209–18. doi: 10.1556/AMicr.53.2006.2.7. [DOI] [PubMed] [Google Scholar]
- 19.Kelkar S, Dong Q, Xiao Y, Joshi-Barve S, McClain CJ, Barve SS. Ethanol enhances activation-induced caspase-3 dependent cell death in T lymphocytes. Alcohol Clin Exp Res. 2002;26:363–70. [PubMed] [Google Scholar]
- 20.Donohue TM, Jr, Clemens DL, Galli A, Crabb D, Nieto N, Kato J, Barve SS. Use of cultured cells in assessing ethanol toxicity and ethanol-related metabolism. Alcohol Clin Exp Res. 2001;25:87S–93S. doi: 10.1097/00000374-200105051-00016. [DOI] [PubMed] [Google Scholar]
- 21.Mato JM, Corrales FJ, Lu SC, Avila MA. S-Adenosylmethionine: a control switch that regulates liver function. FASEB J. 2002;16:15–26. doi: 10.1096/fj.01-0401rev. [DOI] [PubMed] [Google Scholar]
- 22.Cabrero C, Duce AM, Ortiz P, Alemany S, Mato JM. Specific loss of the high-molecular-weight form of S-adenosyl-L-methionine synthetase in human liver cirrhosis. Hepatology. 1988;8:1530–4. doi: 10.1002/hep.1840080610. [DOI] [PubMed] [Google Scholar]
- 23.German DC, Bloch CA, Kredich NM. Measurements of S-adenosylmethionine and L-homocysteine metabolism in cultured human lymphoid cells. J Biol Chem. 1983;258:10997–11003. [PubMed] [Google Scholar]
- 24.LeGros HL, Jr, Geller AM, Kotb M. Differential regulation of methionine adenosyltransferase in superantigen and mitogen stimulated human T lymphocytes. J Biol Chem. 1997;272:16040–7. doi: 10.1074/jbc.272.25.16040. [DOI] [PubMed] [Google Scholar]
- 25.Zeng Zeng Z, Yang H, Huang ZZ, Chen C, Wang J, Lu SC. The role of c-Myb in the up-regulation of methionine adenosyltransferase 2A expression in activated Jurkat cells. Biochem J. 2001;353:163–168. [PMC free article] [PubMed] [Google Scholar]
- 26.Kotb M, Kredich NM. S-Adenosylmethionine synthetase from human lymphocytes. Purification and characterization. J Biol Chem. 1985;260:3923–3930. [PubMed] [Google Scholar]
- 27.Uriarte SM, Joshi-Barve S, Song Z, Sahoo R, Gobejishvili L, Jala VR, Haribabu B, McClain C, Barve S. Akt inhibition upregulates FasL, downregulates c-FLIPs and induces caspase-8-dependent cell death in Jurkat T lymphocytes. Cell Death Differ. 2005;12:233–42. doi: 10.1038/sj.cdd.4401549. [DOI] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 29.De La Rosa J, LeGros, Geller AM, Kotb M. Changes in the relative amount of subunits of methionine adenosyltransferase in human lymphocytes upon stimulation with a polyclonal T cell mitogen. J Biol Chem. 1992;267:10699–704. [PubMed] [Google Scholar]
- 30.Song Z, Chen T, Deaciuc IV, Uriarte S, Hill D, Barve S, McClain CJ. Modulation of endotoxin stimulated interleukin-6 production in monocytes and Kupffer cells by S-adenosylmethionine (SAMe) Cytokine. 2004;28:214–23. doi: 10.1016/j.cyto.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Lombardini JB, Coulter AW, Talalay P. Analogues of methionine as substrates and inhibitors of the methionine adenosyltransferase reaction. Deductions concerning the conformation of methionine. Mol Pharmacol. 1970;6:481–99. [PubMed] [Google Scholar]
- 32.Sufrin JR, Coulter AW, Talalay P. Structural and conformational analogues of L-methionine as inhibitors of the enzymatic synthesis of S-adenosyl-L-methionine. IV. Further mono-, bi- and tricyclic amino acids. Mol Pharmacol. 1979;15:661–77. [PubMed] [Google Scholar]
- 33.Lombardini JB, Sufrin JR. Chemotherapeutic potential of methionine analogue inhibitors of tumor-derived methionine adenosyltransferases. Biochem Pharmacol. 1983;32:489–95. doi: 10.1016/0006-2952(83)90528-2. [DOI] [PubMed] [Google Scholar]
- 34.Kotb M, Dale JB, Beachey EH. Stimulation of S-adenosylmethionine synthetase in human lymphocytes by streptococcal M protein. J Immunol. 1987;139:202–6. [PubMed] [Google Scholar]