Abstract
Rationale
CD4 T cells are involved in the pathogenesis of atherosclerosis, but atherosclerosis-specific CD4 T cells have not been described. Moreover, the chemokine(s) that regulate T cell trafficking to the atherosclerotic lesions are also unknown.
Objective
In Apoe−/− mice with mature atherosclerotic lesions (5 months of high fat diet), we find that most aortic T cells express CCR5 and IFN-γ with a unique combination of cell surface markers (CD4+CD25−CD44hiCD62Llo) and transcription factors (FoxP3+T-bet+). We call these cells CCR5Teff. We investigated the role of CCR5 in regulating T cell homing to the atherosclerotic aorta and the functionality of the CCR5Teff cells.
Methods and Results
CCR5Teff cells are exclusively found in the aorta and para-aortic lymph nodes (paLNs) of Apoe−/− mice. They do not suppress T cell proliferation in vitro and are less potent than regulatory T cells (Tregs) at inhibiting cytokine secretion. Blocking or knocking out CCR5 or its ligand CCL5 significantly blocks T cell homing to atherosclerotic aortas. Transcriptomic analysis shows that CCR5Teff cells are more similar to effector T cells than to Tregs. They secrete IFN-γ, IL-2, IL-10 and TNF. Adoptive transfer of these CCR5Teff cells significantly increases atherosclerosis.
Conclusions
CCR5 is specifically needed for CD4 T cell homing to the atherosclerotic plaques. CCR5+CD4 T cells express an unusual combination of transcriptional factors, FoxP3 and T-bet. Although CCR5Teff express FoxP3, we showed that they are not regulatory and adoptive transfer of these cells exacerbates atherosclerosis.
Keywords: Atherosclerosis, T cell, CCL5, CCR5, chemokine/chemokine receptor, CCR5Teff, Treg, Foxp3+IFN-γ+, inflammation, vascular disease
INTRODUCTION
Atherosclerosis is caused by accumulation of low-density lipoprotein (LDL) in the intima of large arteries, leading to infiltration by monocytes. Atherosclerosis has long been known to have an immune component that modulates plaque burden and disease progression1–4. CD4 T cells are the most abundant adaptive immune cells in atherosclerotic plaque in humans5–7 and mice8. Many plaque CD4 T cells secrete interferon (IFN)-γ9, a T helper 1 (Th1) pro-inflammatory cytokine that is known to advance atherosclerosis10. The defining transcription factor of these IFN-γ-secreting Th1 cells is T-bet11. Some CD4 T cells in atherosclerotic plaques have been described to express FoxP3, the defining transcription factor of regulatory T cells12,3. Tregs are thought to be atheroprotective13–16, but there is evidence that the number of Tregs declines as atherosclerosis progresses17–20.
CD4 T cells are found in atherosclerotic plaque, but it is not clear how they get there. Adoptive transfer experiments have shown that naïve CD4 T cells use L-selectin to home to the adventitia of normal and atherosclerotic mice21. However, most CD4 T cells in atherosclerotic plaque are not naïve but have an effector phenotype and interact with antigen-presenting cells9,22. In a previous study from this laboratory, we showed that the chemokine receptor CXCR6 is required for the homing of a small subset of T cells23. Other candidate chemokine receptors that could be responsible for Th1 cell homing are CXCR3 and CCR5. CXCR3 has been shown to be responsible for T cell homing in airway inflammation24 and adipose tissue25 inflammation in an obesity model. Although CCR5 is known to be involved in atherosclerosis and recruitment of classical monocytes26, 27, its potential role in T cell homing to atherosclerotic arteries has not been studied.
Based on initial findings that CCR5, but not CXCR3 was expressed on many T cells found in atherosclerotic arteries, we focused on CCR5 and its ligand CCL5. CCR5 is expressed on monocytes, macrophages, and Th1 cells26. Classical monocytes (Ly-6Chi in mice) use CCR5 (among other chemokine receptors) to reach atherosclerotic lesions28, 29. Its ligand CCL5 is expressed by monocytes, macrophages, smooth muscle cells and platelets. Platelets can deposit CCL5 on the endothelial surface and promote arrest (adhesion) from rolling30, 31. Genetic deletion studies in Apoe−/− mice suggest that CCR5 has a pro-atherogenic role in neointimal plaque formation. Ccr5−/−Apoe−/− mice are protected from diet-induced atherosclerosis and show a more stable plaque phenotype, reduced mononuclear cell infiltration, reduced T cell infiltration, reduced Th1-type immune responses, and increased IL-10 expression26, 32. TAK-779, an inhibitor of both CCR5 and CXCR3, dramatically reduced atherosclerosis in the aortic root and carotid arteries of Ldlr−/− mice. The number of T cells in the plaque was reduced by 95%, concurrently with a 98% reduction in area staining for IFN-γ33, suggesting a role of CCR5 and CXCR3 in regulating Th1 cell homing to the aorta. CCL5 antagonist treatment in Ldlr−/− mice similarly showed inhibited progression of established atherosclerosis and decreased CD4 T cell infiltration in the aorta30, 34. However, studies with global knockout or inhibition of CCR5 cannot address the role of CCR5 in T cell homing, because CCR5 is broadly expressed.
The present study was designed to investigate mechanisms of T cell homing to atherosclerotic arteries using ex vivo and in vivo adoptive transfer systems analyzed by flow cytometry, confocal immunofluorescence and multiphoton microscopy. In the course of these experiments, we discovered that almost all CCR5+ T cells express FoxP3, T-bet and IFN-γ. Effector T cell (Teff) proliferation assays show that these FoxP3+ T-bet+ IFN-γ+ T cells lack the ability to regulate Teff proliferation. Adoptive transfer of CCR5+ CD4 T cells significantly increases atherosclerosis.
METHODS
An expanded Methods section is available in the online Supplement Material.
In vitro T cell homing assay
The aortic arch, the thoracic aorta, and part of the abdominal aorta (above the renal arteries) were surgically removed from Apoe−/− WD mice. All para-aortic lymph nodes and adipose tissue were carefully removed without disturbing the aortic wall itself. Sorted effector (CD4+CD25−CD44hiCD62L−) or naïve (CD4+CD25−CD44loCD62L+) T cells from Apoe−/− WD mice were incubated with the explanted aorta for 12–18 hours in complete RPMI 1640 media containing 10% FBS, penicillin/streptomycin, l-glutamine, NEAA, HEPES, and sodium pyruvate in the presence or absence of CCL5 neutralizing antibody (clone MAB478; R&D), CCR5 neutralizing antibody (clone MC-68 obtained from Dr. Matthias Mack, University Hospital Regensburg, Germany), PTX (List Biological), or IgG control antibody.
In vivo T cell homing assay
Splenocytes from Ccr5−/−Apoe−/− WD mice and dsRedApoe−/− mice were harvested and red blood cells were lyzed (eBioscience). The leukocytes were mixed 1:1 ratio and 60 million cells were injected retro-orbitally into each recipient CD45.1Apoe−/− WD mice. 24 hrs later, the recipient mice were sacrificed and aorta, paLNs, spleen, blood, inguinal lymph nodes and axillary lymph nodes were harvested. The aortas were digested as described22. Cells were analyzed by flow cytometry. The percentage of each recovered population was normalized to the percentage in the input mix.
RESULTS
T cells in the aorta of Apoe−/− western diet (WD) mice express CCR5/
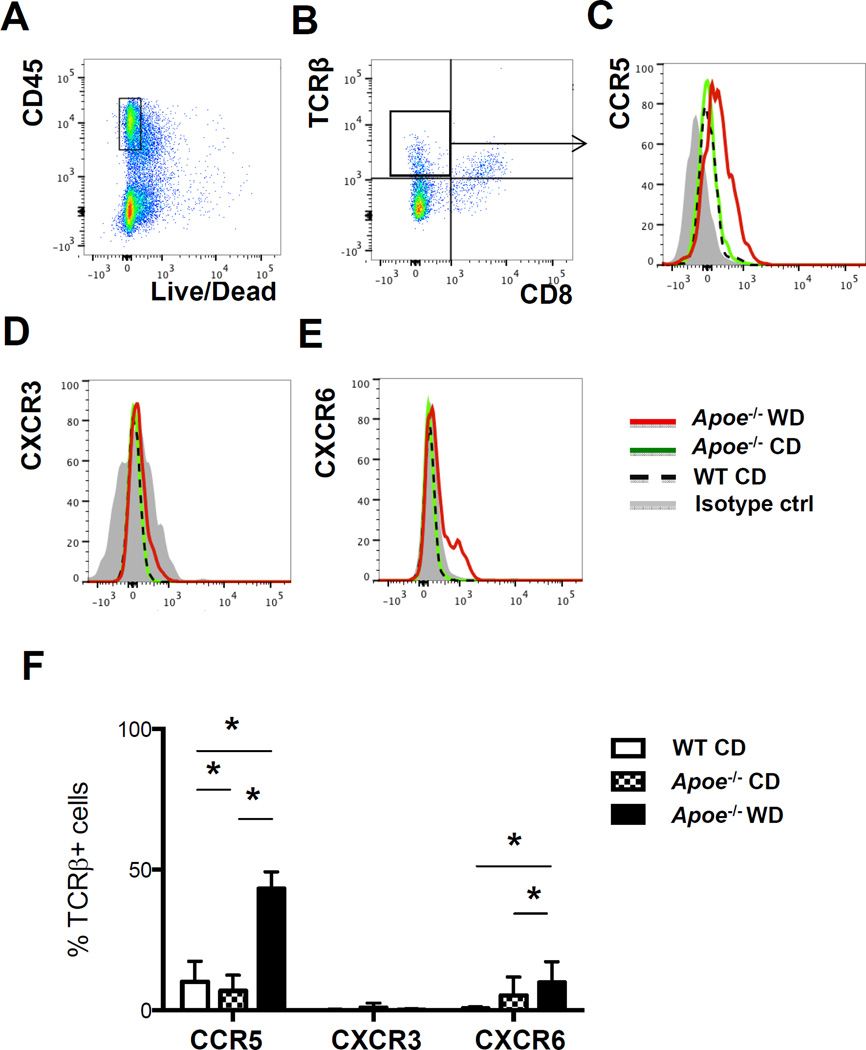
Aortas from Apoe−/− mice on WD for 3–5 months were digested as described21, 22 and prepared for flow cytometry. In all experiments, leukocytes were identified by CD45 expression and dead cells were excluded (Figure 1A). Cells were gated for singlets by forward scatter impulse width and side scatter impulse width (not shown). Because CD4 expressed on T cell surface is lost after the aorta digestion process (Online Figure I), we detected T cells in the aorta by antibodies against TCRβ and CD8. αβ T cells as detected by staining for TCRβ (Figure 1B) accounted for 18–40% of all live CD45+ singlets. We focused on CCR5, because this chemokine receptor was highly and reproducibly induced in aortic αβ T cells of Apoe−/− mice on WD (Figure 1C), but not in control mice. The appearance of CCR5+ T cells correlated with the duration of WD and peaked at 5 months (Online Figure II). CD8+ αβ T cells did not express CCR5 (data not shown). αβ T cells in aortas of Apoe−/− mice on chow diet (CD) or wild-type (WT) C57BL/6 mice expressed low levels of CCR5 (Figure 1C). Interestingly, these cells expressed no CXCR3 (Figure 1D) (positive control in Online Figure III). A subset of about 1–10% of aortic T cells in Apoe−/− mice on WD, but not on CD, expressed CXCR6 (Figure 1E), which is similar to findings in a previous report23. A much larger percentage (43±6 %) of aortic αβ T cells expressed CCR5 (Figure 1C, F). This was significantly higher than the fraction of CCR5-expressing αβ T cells in aortas of wild-type C57BL/6 mice (10±7 %) or in Apoe−/− mice on CD (7±6%). We found some CCR5+CD4+ T cells in the draining paLNs and the spleen, but this fraction did not increase significantly in Apoe−/− mice on CD or WD (Online Figure IV).
Figure 1. CCR5 and CXCR6 expression on aortic T cells is increased in Apoe−/− mice on WD.
Aortas from Apoe−/− on WD (n=8) or CD (n=4) mice and C56BL/6 WT mice (n=4) were digested and analyzed by multi-color flow cytometry. A, Cells gated on live CD45+ and B, on CD4 T cells (TCRβ+CD8−). C, CCR5 D, CXCR3 and E, CXCR6 on aortic CD4 T cells of Apoe−/− mice on WD (red) or CD (green) or WT mice (dotted black); isotype controls in grey. F, Mean±SD of CCR5, CXCR3 and CXCR6 expression on CD4 T cells. * p<0.05 by t-test.
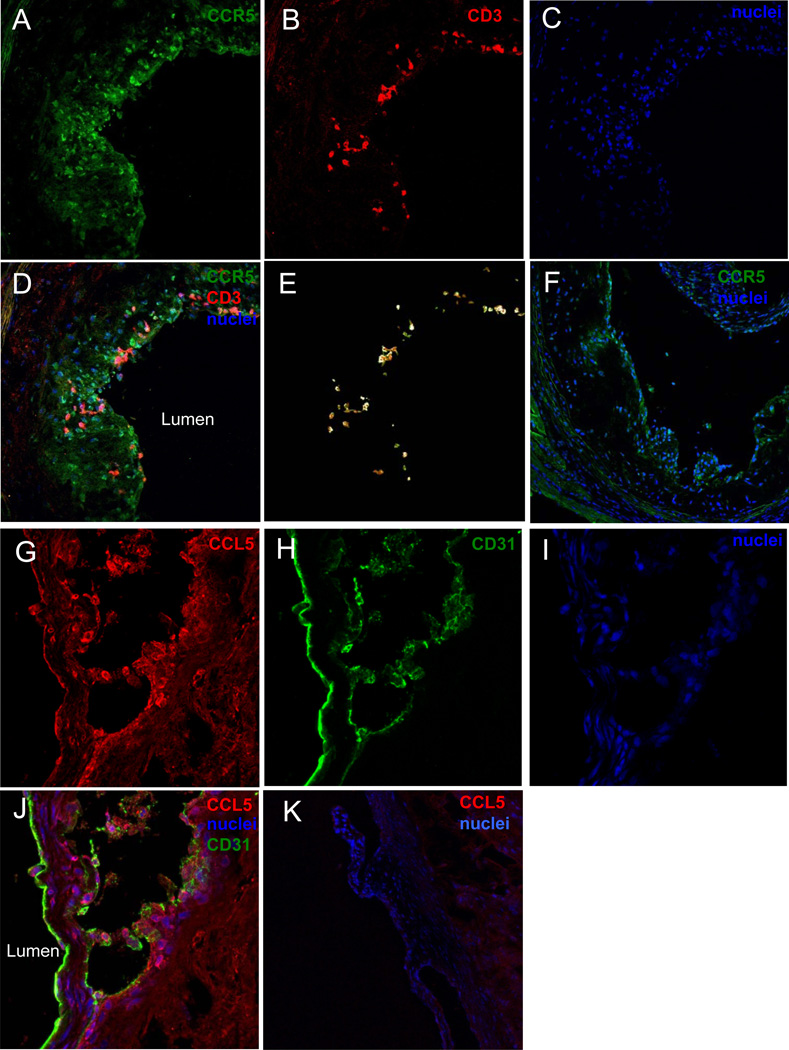
To assess the localization of CCR5+ T cells, we stained aortic root sections from Apoe−/− mice for CCR5 and CD3. As expected, we found many more CCR5+ than CD3+ cells (Figure 2A–E). This is consistent with the known expression of CCR5 on myeloid cells. Almost all CD3+ T cells also expressed CCR5 and localized in the atherosclerotic lesions near the lumen (Figure 2E). The negative control for CCR5 is shown in Figure 2F. The main ligand for CCR5 is CCL5 (RANTES)35. Immunostaining aortic roots for CCL5 was diffuse as expected (Figure 2G), with some CCL5 deposited on the endothelium (Figure 2H,I,J) as described before36. Isotype control for CCL5 is shown in Figure 2K.
Figure 2. Localization of CCR5+ T cells and CCL5 in the aorta root of Apoe−/− mice on WD.
Fresh frozen aortic root sections from Apoe−/− WD mice were fixed and stained with fluorescently labeled antibodies to A, CCR5 (green), B, CD3 (red), C, nuclei (blue), D, merge. E, Colocolization between CCR5 and CD3 was analyzed by creating a mask of CD3 and apply it to CCR5 (image J). F, CCR5 and nuclei staining on aorta root sections from CCR5−/−Apoe−/− mice. G, CCL5 (red), H, CD31 (green), I, nuclei (blue) and J, merge. K, CCL5 and nuclei staining on aorta root sections from CCL5−/− mice.
CCR5 and its ligand CCL5 regulate T cell homing to the atherosclerotic aorta
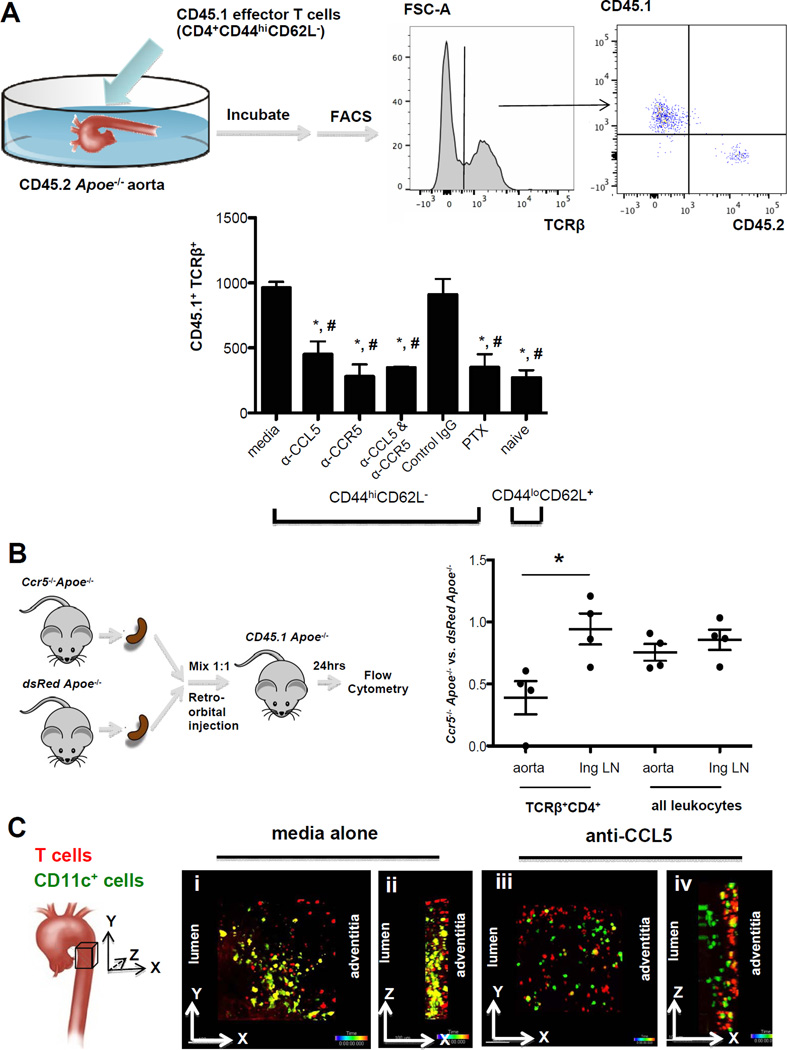
Since almost half of aortic T cells expressed CCR5 (Figure 1 F), and CCL5 was abundantly found in lesions (Figure 2 G), we reasoned that CCL5 and CCR5 might be involved in homing of antigen-experienced CD4 T cells to atherosclerotic lesions. To test this, we used three assays: T cell infiltration into the explanted aorta, multiphoton microscopy22, and competitive homing37. Explanted aortas harvested from Apoe−/− mice were incubated overnight with effector CD4 T cells (CD44+CD25−CD62L−) harvested from Cd45.1 Apoe−/− mice (Figure 3A). Among the TCRβ+ T cells recovered from each explanted aorta, around 80% (~ 1,000 cells) were donor-derived, with the balance being CD45.2+ (host-derived) (Figure 3A, right panel). Pre-treating the T cells with a blocking monoclonal antibody to CCL5 reduced the infiltration by more than 50% (Figure 3A, bar graph). Similar effects were seen with a blocking monoclonal antibody to CCR5. The combined blockade of CCL5 and CCR5 had no additional effect, suggesting that CCL5 acted on CCR5 to attract these cells into the vessel wall. Also, treating the T cells with pertussis toxin (PTX) to block signaling through all Gαi-coupled chemokine receptors had the same effect. These findings suggest that the CCL5-CCR5 pathway is the major receptor-ligand pair driving CD4 T cells into the aortic tissue. Consistent with the finding that not all aortic T cells expressed CCR5, there was residual T cell infiltration into aortas even when CCR5, CCL5, both or all chemokine receptors were blocked, and this background level was similar to that of naïve T cells (CD44loCD62L+: Figure 3A, last bar in bar graph).
Figure 3. Neutralizing CCL5 or CCR5 inhibits T cell infiltration into the Apoe−/− aorta.
A, Effector T cells (CD4+CD25−CD44hiCD62L−) or naïve CD4 T cells (CD4+CD25−CD44lo CD62L+) from spleens of Cd45.1Apoe−/− WD mice were incubated with aortas from Cd45.2Apoe−/− WD mice with media alone, anti-CCL5 (1ug/ml), anti-CCR5 (10ug/ml), IgG control antibody (10ug/ml) or pertussis toxin (PTX, 300ng/ml) over night. CD45.1+ T cells infiltrating into aorta and left in the supernatant were quantified by flow cytometry (right panels). n=5 in each group. Bar graph: mean±SE. *p<0.05 vs media, # p<0.05 vs IgG. t-test. B, Impaired CCR5 deficient T cells homing to the Apoe−/− aorta. 6×107 splenocytes from dsRedApoe−/− mice and Ccr5−/−Apoe−/− mice were transferred at 1:1 ratio to CD45.1Apoe−/− WD mice by retro-orbital injection. 24 hrs later, cells from the aorta and inguinal LNs (Ing) of the recipients were analyzed by flow cytometry. The percentage of transferred TCRβ+CD4+ T cells and all CD45.2 leukocytes was normalized by its percentage in the input. Data shown is the ratio of Ccr5−/−Apoe−/− vs. dsRedApoe−/− cells. * p<0.05, n=5, t-test. C, Two photon imaging of isolated aorta co-cultured with effector T cells. Effector T cells from Apoe−/− WD mice were labeled with SNARF and then incubated with aorta from CD11c-YFP Apoe−/− WD mice without (i and ii) or with (iii and iv) anti-CCL5. Maximum intensity projection along z axis (top view) (i and iii) and axis (side view) (ii and iv) through the image stacks from the adventitia of aorta are shown.
To directly test whether CCR5 was involved in T cell homing to the aorta in vivo, we adoptively transferred splenocytes from Apoe−/− Ccr5−/− mice mixed 1:1 with splenocytes from dsRedApoe−/− mice into CD45.1 Apoe−/− recipients. Splenocytes from Apoe−/− Ccr5−/− mice contained similar numbers of CD4 and CD8 T cells as dsRedApoe−/− mice (Online Figure V). When aortas were harvested 24 hours later, digested and analyzed by flow cytometry, we found significantly fewer Apoe−/− Ccr5−/− CD4 T cells than dsRed Apoe−/− CD4 T cells (Figure 3B). In one recipient aorta, no Apoe−/− Ccr5−/− CD4 T cells were found, and in the other three recipient aortas, only half as many Apoe−/− Ccr5−/− cells as dsRed Apoe−/− CD4 T cells were found. Only the homing of CD4 T cells to aortas was strongly CCR5-dependent. There was no significant difference in the homing of all leukocytes harvested from Apoe−/− Ccr5−/− cells as compared to dsRedApoe−/− (Figure 3B). As a negative control, we investigated inguinal lymph nodes (Figure 3B) as well as other lymphoid organs (Online Figure VI) by flow cytometry and found no effect of absence of CCR5 on the homing of CD4 T cells or all leukocytes, as expected. These in vivo data show that CD4 T cells uniquely depend on CCR5 and its ligand CCL5 for their homing to the atherosclerotic aorta.
To obtain a visual representation of the proximity of T cells to antigen-presenting cells, we investigated aortas from CD11c-YFP Apoe−/− mice22 incubated with labeled effector T cells by multiphoton microscopy (Figure 3C i–iv). We found that untreated T cells infiltrated the explanted aortas and reached the YFP+ antigen presenting cells (Figure 3c ii). This infiltration was abolished by pre-treatment with anti-CCL5 (Figure 3C iv). Taken together, flow cytometry, in vivo competitive homing and ex vivo imaging results all show that CCL5 and its receptor CCR5 are required for CD4 T cell homing to atherosclerotic mouse aortas.
Flow cytometry of CCR5+ CD4 T cells in the aorta and paLNs of Apoe−/− WD mice
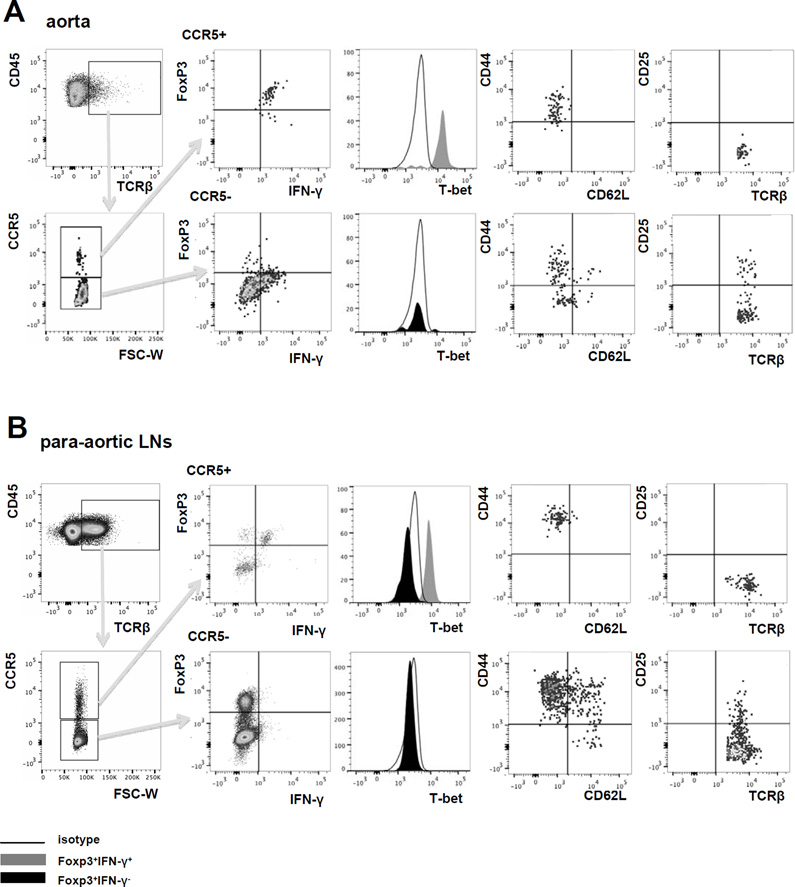
Aortas of Apoe−/− mice38 and humans39 are known to contain CD4 T cells and a smaller number of CD8 T cells. Most of these T cells express IFN-γ, identifying them as Th1 cells9. A smaller subset of CD4 T cells express FoxP316, a transcription factor found in Tregs40, 41. When we interrogated αβ T cells in aortas from Apoe−/− mice for expression of CCR5, we found that almost all of the CCR5+ T cells co-expressed IFN-γ, the Th1 transcription factor T-bet and the Treg transcription factor FoxP3 (Figure 4A). These cells were CD44hiCD62Llo and did not express CD25 (Figure 4A, right panels). By contrast, the CCR5− T cell population did not contain any IFN-γ+ FoxP3+ cells, but did contain a small population of FoxP3+T-bet− T cells (Figure 4A) that were mixed for CD44 and CD62L and some of which expressed CD25 (Figure 4A, right panel). The phenotype of FoxP3+CD25+IFN-γ−T-bet− cells in the CCR5− gate is consistent with Tregs. The percentage of CCR5+ T cells shown in Figure 4 is less than that shown in Figure 1 because permeabilization reduced the detectable CCR5 on T cell surface (Online Figure VII).
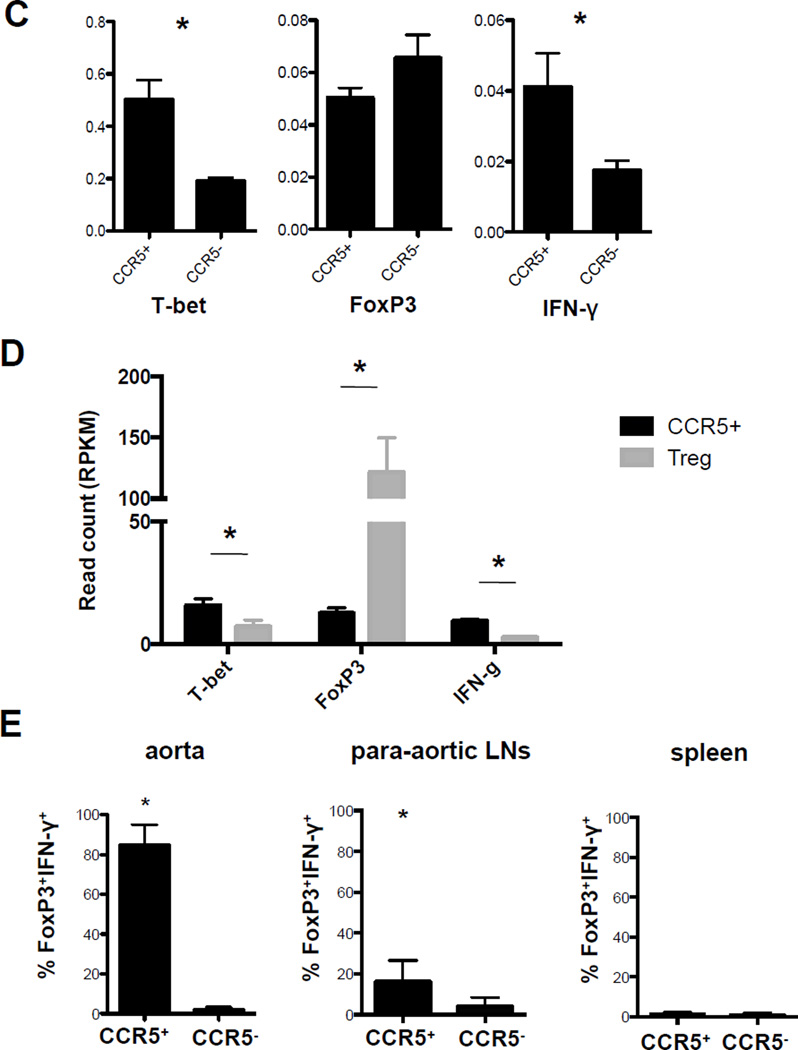
Figure 4. CCR5+ T cells express FoxP3, IFN-γ and T-bet (CCR5Teff). T cells from A, aorta and B, paLNs of Apoe−/− mice on WD for > 5 months were analyzed by multi-color flow cytometry.
Cells were gated on live CD45+ singlets TCRβ+. Gates are set based on FMO and isotype controls. One representative experiment is shown. C, CD4+CCR5+CD25− (CCR5+, enriched CCR5Teff) and CD4+CCR5− (CCR5−) T cells were sorted from the paLNs of Apoe−/− mice on WD. mRNA was extracted and gene expression was analyzed for T-bet, FoxP3 and IFN-γ by rear-time PCR. *p<0.05, n=5, T-test. D, CD4+CCR5+CD25− (CCR5+) and CD4+CD25+CCR5− (Treg) were sorted from paLNs of Apoe−/− mice on WD >5months. mRNA were extracted for next-generation sequencing. Normalized read count of T-bet, FoxP3, and IFN-γ were shown. *p<0.05, T-test. E, Percentage of Foxp3+IFN-γ+ cells among CCR5+ and CCR5− T cells, mean ± SEM, *p<0.05 by 2 way ANOVA, n=5 in aorta, n=4 in paLNs and spleen.
The abdominal aorta is drained by the paLNs. We reasoned that effector T cells may traffic from the aorta to its draining lymph nodes (and back). Therefore, we tested αβ T cells in paLNs for expression of CCR5. Indeed, we found that a subset (about 2.5% of all αβ T cells) expressed CCR5. Among the CCR5+ T cells, we found both FoxP3+ IFN-γ+ and FoxP3+ IFN-γ− cells (Figure 4B). Only the FoxP3+IFN-γ+ cells and not the FoxP3+IFN-γ− cells expressed T-bet (Figure 4B, histograms). FoxP3, T-bet and IFN-γ expression was confirmed by real-time PCR and RNA sequencing (Figure 4 C, D). The FoxP3+IFN-γ+T-bet+ cells were CD44hiCD62Llo and did not express CD25, the same phenotype as CCR5+ T cells in the aorta. Among the CCR5− αβ T cells in the paLNs, we found a substantial number of FoxP3+ IFN-γ− cells that did not express T-bet. These cells were mixed for CD44 and CD62L and some expressed CD25 (Figure 4B, lower row). This is consistent with the phenotype of Tregs. Among the few CCR5+ αβ T cells found in spleens of Apoe−/− mice on WD, no FoxP3+IFN-γ+T-bet+ cells were found (Online Figure VIII). In the aortas of Apoe−/− mice on WD, more than 80% of all CCR5+ αβ T cells expressed FoxP3 and IFN-γ. This fraction was still more than 15% in paLNs (Figure 4E), but FoxP3+IFN-γ+ T cells were undetectable in the spleen.
Taken together, these data show that the CCR5Teff cells are concentrated in the aorta, with some making their way to the paLNs, but not (at detectable levels) to the spleen. We emphasize that, as in previous studies42, we find that the spleen does not reflect the lymphocyte composition of the aorta.
CCR5Teff do not suppress T cell proliferation
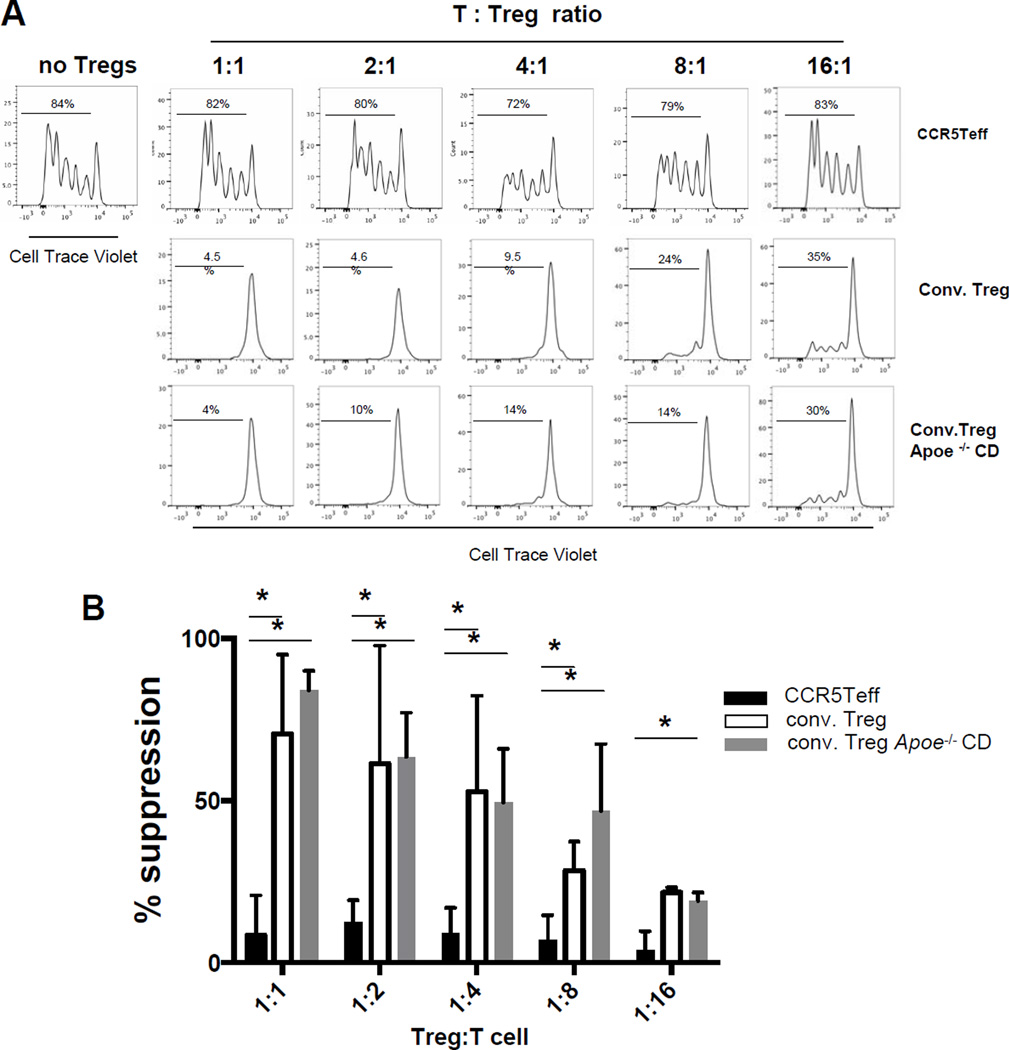
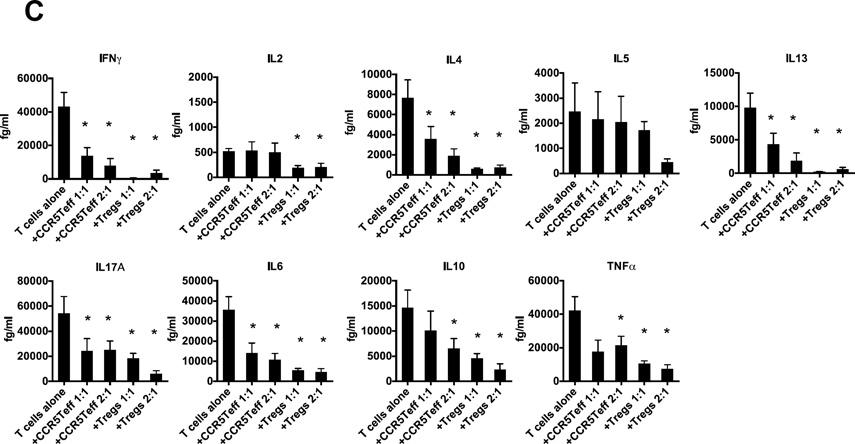
To test the functionality of the newly discovered atherosclerosis-specific T cell subset, we conducted an in vitro Treg assay. As positive controls, we used conventional Tregs sorted from FoxP3-GFP Apoe−/− mice43, 44 on WD for 5 months (Online Figure IX) or CD4+CD25+ conventional Tregs from Apoe−/− mice on CD. Tregs from both types of mice effectively suppressed effector T cell proliferation on anti-CD3 already at a 16:1 Teff:Treg ratio and completely suppressed at 4:1 or higher (Figure 5A). However, the CD4+CCR5+ cells from paLNs were completely unable to suppress Teff proliferation at any ratio, up to 1:1 (Figure 5 A, B). Although CCR5Teff cells did not inhibit T cell proliferation, they did inhibit T cell cytokine secretion (Figure 5C). When cultured at 1:1 or 2:1 ratios to T cells, CCR5Teff cells significantly decreased IFNγ, IL-4, IL-13, IL-6 and IL17A secretion, but not IL-2 or IL-5. At the 2:1 ratio, they also reduced IL-10 and TNF secretion. However, in all cases, the suppressive effect of the CCR5Teffs is less than that of Tregs. The finding that CCR5Teff cannot suppress IL-2 secretion probably explains why CCR5Teff cells are completely unable to suppress Teff proliferation (Figure 5A, B).
Figure 5. CCR5Teff cells do not suppress T cell proliferation but reduce T cell cytokine secretion.
A, Splenic CellTrace Violet labeled CD4+CD25− T cells (1×104 per well) isolated from Apoe−/− CD mice were cultured with anti-CD3 mAb in the presence of 5×104 irradiated T-depleted splenic APCs and decreasing numbers of CD4+CCR5+ cells (top row) or CD4+CCR5−GFP+ conventional Tregs sorted from paLNs of FoxP3GFPApoe−/− WD>5mth mice (middle row) or from Apoe−/−CD mice (bottom row). After 4 days of culture, proliferation was measured by CellTrace Violet signal using flow cytometry. B, Mean±SD of suppression of proliferation (n=3 experiments).* p<0.05 by t-test. C. Cytokine secretion from CD4+CD25− T cells (1×104 per well) cultured with CCR5Teff or conventional Treg sorted from WD mice were measured by cytometric bead array. n=5. Mean±SD. * p<0.05 vs. T cells alone, t-test.
Transcriptomic analysis shows that CCR5Teff is similar to Teff
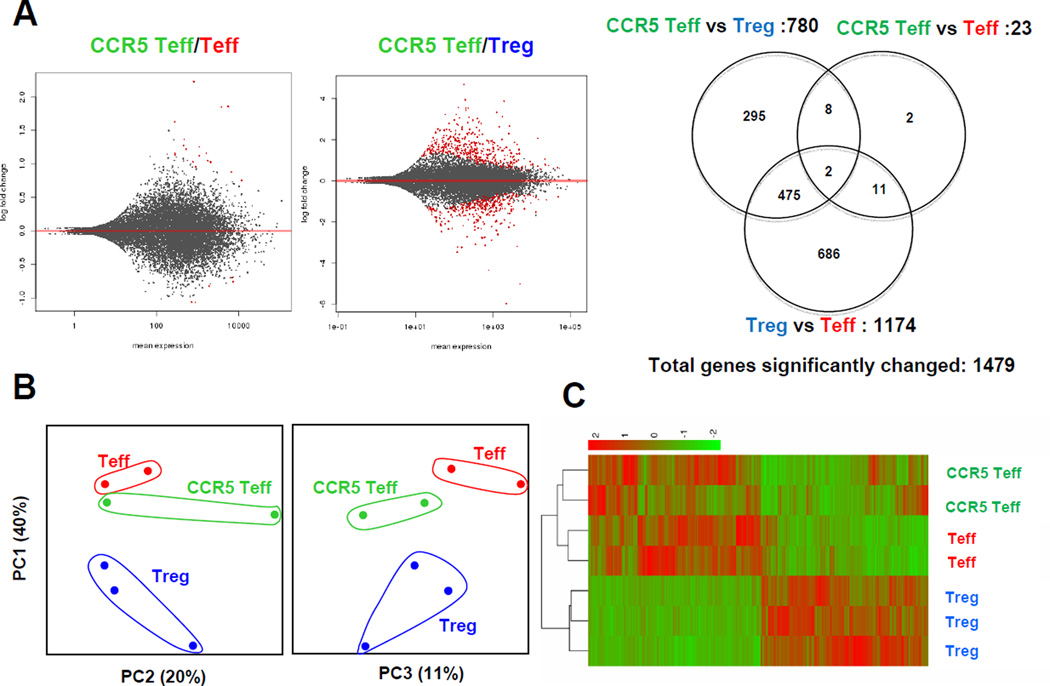
To analyze genome-wide CCR5Teff cell gene expression, we performed RNA sequencing using sorted CCR5Teff, Treg and Teff from the paLNs from Apoe−/− mice on WD for more than 5 months. We found only 23 genes that were significantly differentially expressed in CCR5Teff vs. Teff, whereas 780 genes were differentially expressed between CCR5Teff and Treg (Figure 6 and online table). This result confirms the functional result (Figure 5) that CCR5Teff are similar to Teff but not to Treg. The similarity between CCR5Teff and Teff was confirmed by heatmap and PCA analysis (Figure 6 B, C). Although CCR5Teff express FoxP3 as seen by flow cytometry, real-time PCR and confirmed by RNA sequencing, their read counts reflecting FoxP3 mRNA are much lower than in Treg (Figure 4 D). They express more T-bet and IFN-γ than Tregs (Figure 4 D). These findings suggest that CCR5Teff are not regulatory and may play a pathogenic role in the pathogenesis of atherosclerosis.
Figure 6. CCR5Teff cells are similar to effector T cells but not Tregs.
CCR5Teff (CD4+CCR5+CD25−), Tregs (CD4+CD25+) and Teff (CD4+CD25−CCR5−CD44hiCD62Llo) were sorted from the paLNs from Apoe−/− mice on WD for >5 months. RNA was extracted from the three populations, amplified, reverse transcribed and subjected to next generation sequencing (Illumina). A, The MA-plot shows the log fold changes as a function of the mean of normalized read counts. Genes with an adjusted p-value below 0.05 are shown in red. Venn diagram represents a summary of differentially expressed genes in the three contrasts as indicated. B, Principal component analysis of normalized read counts (n=14,839) shows the relatedness of samples. The first three principal components accounted for more than 70% of the total variance. C, Unsupervised clustering of the 1,479 differentially expressed genes also separates CCR5 Teff, Teff and Treg from all samples. The colors of the heatmap represent normalized gene counts (red as high).
Adoptive transfer
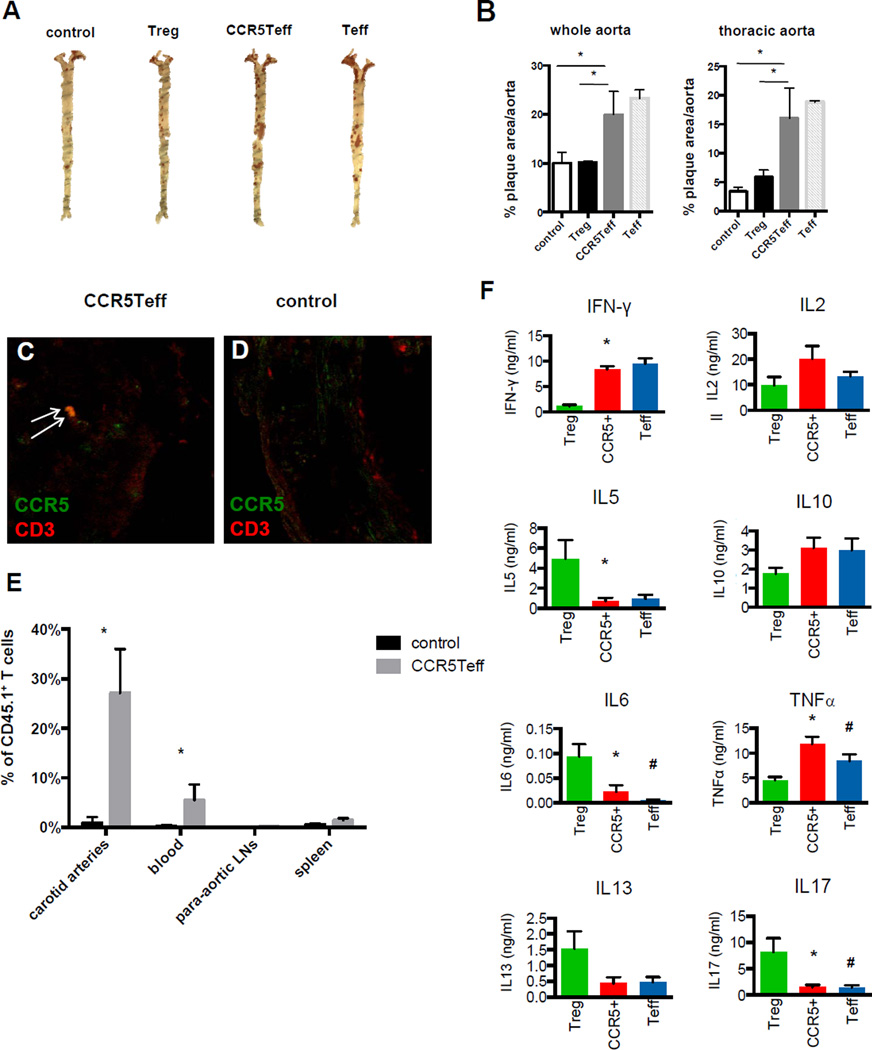
To test the functionality of the newly discovered CCR5Teff T cell subset in vivo, we adoptively transferred sorted CCR5+CD4+CD25− T cells, Tregs and CCR5− effector T cells isolated from paLNs into Ccr5−/−Apoe−/− WD mice and quantified aortic plaque area of the recipients 12 weeks after adoptive transfer. The mice that received CCR5Teff showed significantly increased plaque area in the whole aorta and thoracic aorta as that in the mice received CCR5− effector T cells (Figure 7 A, B), and transferred CCR5+ T cells were detected in the aortic roots by immunofluorescence indicated by white arrows (Figure 7 C, D). Flow cytometry analysis of the carotid arteries, blood, paLNs and spleen of the recipient mice showed that CCR5Teff cells preferentially homed to the carotid arteries (Figure 7 E). Cytometric bead array analysis of sorted cells showed that the cytokine production profile of the CCR5Teff T cell subset is more similar to the effector T cells than the Tregs. CCR5Teff cells produce high levels of IFN-γ, TNFα, IL-10 and IL-2, but not IL-5, IL-6, IL-13 or IL-17 upon stimulation (Figure 7 F). IFN-γ and TNF are known pro-atherogenic cytokines9, 10, 45, 46. Although M1 macrophage markers like iNOS and CD11c are not increased in the aorta root of mice received CCR5Teff (Online Figure X), high expression of IFN-γ and TNF at mRNA and protein level suggests that the CCR5Teff T cells promote atherosclerosis by secreting these cytokines.
Figure 7. Adoptive transfer CD4+CCR5+CD25− T cells increases atherosclerosis.
A, Sudan IV stained aortas (whole aorta and thoracic aorta) from Ccr5−/−Apoe−/− mice that received 2×105 enriched CCR5Teff, Treg or Teff isolated from paLNs of Cd45.1Apoe−/− mice on WD for 5 month or saline (control) i.v. 12 weeks before analysis. B, Quantification of plaque area as % of aortic surface. n=5, * p<0.05, t-test. C and D, IHC of aorta root sections of the recipients that received CCR5Teff (C) or saline (D) stained with CCR5 (green) and CD3 (green). The CCR5+CD3+ T cells were indicated by arrows. E, Flow cytometry analysis of the carotid arteries, blood, paLNs and spleen of the Ccr5−/−Apoe−/− recipient mice that received 2×105 CCR5Teff or saline. The CD45.1+ TCRβ+ donor T cells were gated from live CD45+ singlets TCRβ+. Percent CD45.1+ among all TCRβ+ cells indicated above gate. n=5, Mean±SE, t-test, *p<0.05. F, CCR5Teff (CCR5+), conventional Tregs (Treg) and effector T cells (Teff) were sorted directly into PMA /ionomycin (8,000 to 25,000 cells/well) and cytokine secretion was measured by cytometric bead array and expressed as ng/ml per million cells. n=4, Mean±SE, t-test, *p<0.05 CCR5+ vs. Treg, # p<0.05 Teff vs. Treg.
DISCUSSION
Here, we report that CCR5 and its ligand CCL5 is a major chemokine receptor-ligand pair required for CD4 T cell homing to atherosclerotic aortas in Apoe−/− mice. The CCR5+ CD4 T cells in the aorta, but not elsewhere, have a striking phenotype in that they express FoxP3, IFN-γ and T-bet, but not CD25. We propose to call this new CCR5+CD4+CD44hiCD62LloFoxP3lowT-bet+IFN- γ+ T cell subset CCR5Teff. Although previous studies had shown that blocking CCR5 was protective33, 47, our data are the first to unequivocally establish CCR5 as the major homing receptor for CD4 T cells into atherosclerotic lesions in Apoe−/− mice.
A previous study from this laboratory has shown that effector CD4 T cells (CD44hiCD62Llo) are present in atherosclerotic, but not control mice22. These cells can interact with CD11c+ antigen presenting cells in the aortic wall in an MHC-II-restricted manner without adding exogenous antigen. Therefore, “atherosclerosis antigens” are present in the diseased, but not the normal aorta and their presentation induces a recall response with production of IFN-γ and TNF22. However, the phenotype of endogenous CD4 T cells was not analyzed beyond CD44 and CD62L in that study. It is likely that the aorta-homing T cells described here are the producers of IFN-γ seen in22. IFN-γ is known to drive atherosclerosis9, 10, 48.
FoxP3 is considered the lineage-defining transcription factor for Tregs40, 49. Recently, subsets of regulatory T cells have been described that express FoxP3 and T-bet (for Th1) or GATA3 (for Th2) or ROR-γt (for Th17) or Bcl6 (for TFH)50–54. T-bet+FoxP3+ CD4 cells have been called “Th1-like Treg”. Even though Th1-likeTregs can produce IFN-γ, they retain the capacity to regulate effector T cell expansion in the standard in vitro suppression assay51, 54. In the present study, the absence of suppressive activity of CCR5Teff formally rules out the possibility that the CCR5Teff cells found in atherosclerotic aortas and paLNs are “Th1-like Tregs” as described54–58. This leaves two possibilities: The CCR5Teff cells could be ex-Tregs that have lost (most) FoxP3 expression and the ability to regulate, or they could be effector T cells that transiently express (some) FoxP3.
That FoxP3 is not only expressed in Tregs was discovered by Steven Ziegler’s group in 200359. FoxP3 can be expressed in human CD4+CD25− T cells, a population that is not thought to contain Tregs. This was confirmed in another study60, suggesting that FoxP3 is not a sufficient marker to identify Tregs in humans. Several later studies confirmed this. However, there is no published evidence that FoxP3 can be expressed in Teff cells in mice. In a lethal model of oral infection with toxoplasma gondii, a collapse of Treg numbers and increased expression of IFN-γ by former Tregs was described51. This was accompanied by a reduced number of Tregs. However, we did not observe a change in the number of conventional CD4+CD25+FoxP3+ Tregs in our atherosclerosis model. Instead, we saw the emergence of a new subset of T cells that are CD4+CCR5+T-bet+IFN-γ+FoxP3lowCD25− and cannot regulate Teff proliferation but exacerbate atherosclerosis. The gene expression profile of these CCR5Teff cells is similar to that expressed in Teff. Flavell’s group observed lower levels of FoxP3 in tissue-resident pancreatic Tregs of diabetic mice compared to normal mice61. This phenotype is reminiscent of the lower level of FoxP3 observed here in CCR5+ CD4 T cells compared to Tregs.
Ccr5−/−Apoe−/− mice have been reported to develop reduced atherosclerosis26, 32. This finding was attributed to reduced monocyte recruitment, but our data suggest that there is likely a contribution of the absence of CCR5Teff cells. Other studies have shown that blocking CCR527 or its ligand CCL5 by gene targeting or by peptide inhibitors30 is atheroprotective. Again, in these studies the protective effect cannot be attributed to reduced T cell homing because recruitment of inflammatory monocytes is also reduced28, 29. Here, we show that half of the CD4 T cells homing into the atherosclerotic aorta require CCR5. In a previous study, we have shown that another, smaller subset uses CXCR6 for homing to the aorta23. Together, these two chemokine-receptor systems, CCR5-CCL5 and CXCR6-CXCL16, explain most T cell homing to atherosclerotic aortas in mice.
The CCR5Teff promote atherosclerosis by secreting TNF and IFN-γ, both known pro-atherogenic cytokines9,10,45, 46. IFN-γ activates macrophages62, inducing and reinforcing the M1 phenotype63) that is known to drive atherosclerosis64,65. The role of TNF in atherosclerosis is less clear, but blocking TNF has been reported to limit atherosclerosis in mice45, 46. We have previously shown that TNF is produced when antigen-experienced CD4 T cells are incubated with explanted atherosclerotic aortas22. Taken together, the CCR5Teff cells appear to be a very significant source of pro-atherogenic cytokines.
We conclude that CCR5 fulfills a non-redundant, essential role for the homing of CD4 T cells to the atherosclerotic aortic wall where they exacerbate atherosclerosis. This identifies CCR5 and its ligand CCL5 as the most important homing receptor-ligand pair for T cells in atherosclerosis. Almost all CCR5+ T cells are CD4+CD44hiCD62L−FoxP3lowIFNγ+T-bet+CD25− and thus represent an atherosclerosis-specific subset of T cells that has not been described before in atherosclerosis or in other diseases. Interestingly, these CCR5Teff cells home to and are highly enriched in the atherosclerotic aorta, suggesting they are a local pool of memory T cells.
Supplementary Material
Novelty and Significance.
What Is Known?
T cell is the second large leukocyte population after macrophage in atherosclerotic plaques.
Th1 cells are abundant and pathogenic in atherosclerosis.
What New Information Does This Article Contribute?
A novel CCR5+CD4+CD44hiCD62LloFoxP3+T-bet+IFN- γ+ subset was found in aortas of Apoe−/− mice.
These cells are found exclusively in the aorta and the draining lymph nodes.
CCR5 and its ligand CCL5 regulate T cell homing to the atherosclerotic aorta.
These cells exacerbate atherosclerosis and are more similar to effector T cells than to Tregs
CD4 T cells are found in atherosclerotic lesions, but their homing mechanism was unknown. Here, we show that the chemokine receptor CCR5 and its ligand CCL5 are necessary. Many of the CD4 T cells in the aorta of atherosclerotic mice show a unique co-expression of the transcription factors FoxP3 (Treg) and T-bet (Th1). Although these cells retain some regulatory function (reduce cytokine secretion by effector T cells), their net effect is pro-atherogenic.
Acknowledgments
We thank Dr. Matthias Mach from University of Regensburg, Germany for providing the CCR5 neutralizing antibody; Dr. Catherine Hedrick from La Jolla Institute for providing FoxP3GFPApoe−/− mice.
SOURCES OF FUNDING
This work was supported by NIH R01 HL115232 and HL121697 to KL. JL was supported by an AHA postdoctoral fellowship.
Nonstandard Abbreviations and Acronyms
- paLNs
Para-aortic lymph nodes
- Tregs
Regulatory T cells
- LDL
Low-density lipoprotein
- IFN
Interferon
- Th1
T helper 1
- Teff
Effector T cell
- WD
Western diet
- CD
Chow diet
- WT
Wild type
- PTX
Pertussis toxin
Footnotes
DISCLOSURES
The authors have declared that no conflict of interest exists.
REFERENCES
- 1.Lichtman AH, Binder CJ, Tsimikas S, Witztum JL. Adaptive immunity in atherogenesis: New insights and therapeutic approaches. J Clin Invest. 2013;123:27–36. doi: 10.1172/JCI63108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Libby P, Lichtman AH, Hansson GK. Immune effector mechanisms implicated in atherosclerosis: From mice to humans. Immunity. 2013;38:1092–1104. doi: 10.1016/j.immuni.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tse K, Tse H, Sidney J, Sette A, Ley K. T cells in atherosclerosis. International immunology. 2013;25:615–622. doi: 10.1093/intimm/dxt043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J, Ley K. Lymphocyte migration into atherosclerotic plaque. Arterioscler Thromb Vasc Biol. 2015;35:40–49. doi: 10.1161/ATVBAHA.114.303227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hansson GK, Holm J, Jonasson L. Detection of activated t lymphocytes in the human atherosclerotic plaque. Am J Pathol. 1989;135:169–175. [PMC free article] [PubMed] [Google Scholar]
- 6.Stemme S, Holm J, Hansson GK. T lymphocytes in human atherosclerotic plaques are memory cells expressing cd45ro and the integrin vla-1. Arteriosclerosis and thrombosis : a journal of vascular biology / American Heart Association. 1992;12:206–211. doi: 10.1161/01.atv.12.2.206. [DOI] [PubMed] [Google Scholar]
- 7.Hosono M, de Boer OJ, van der Wal AC, van der Loos CM, Teeling P, Piek JJ, Ueda M, Becker AE. Increased expression of t cell activation markers (cd25, cd26, cd40l and cd69) in atherectomy specimens of patients with unstable angina and acute myocardial infarction. Atherosclerosis. 2003;168:73–80. doi: 10.1016/s0021-9150(03)00024-8. [DOI] [PubMed] [Google Scholar]
- 8.Kolbus D, Ramos OH, Berg KE, Persson J, Wigren M, Bjorkbacka H, Fredrikson GN, Nilsson J. Cd8+ t cell activation predominate early immune responses to hypercholesterolemia in apoe(−)(/)(−) mice. BMC immunology. 2010;11:58. doi: 10.1186/1471-2172-11-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frostegard J, Ulfgren AK, Nyberg P, Hedin U, Swedenborg J, Andersson U, Hansson GK. Cytokine expression in advanced human atherosclerotic plaques: Dominance of pro-inflammatory (th1) and macrophage-stimulating cytokines. Atherosclerosis. 1999;145:33–43. doi: 10.1016/s0021-9150(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 10.Buono C, Come CE, Stavrakis G, Maguire GF, Connelly PW, Lichtman AH. Influence of interferon-gamma on the extent and phenotype of diet-induced atherosclerosis in the ldlr-deficient mouse. Arterioscler Thromb Vasc Biol. 2003;23:454–460. doi: 10.1161/01.ATV.0000059419.11002.6E. [DOI] [PubMed] [Google Scholar]
- 11.Buono C, Binder CJ, Stavrakis G, Witztum JL, Glimcher LH, Lichtman AH. T-bet deficiency reduces atherosclerosis and alters plaque antigen-specific immune responses. Proc Natl Acad Sci U S A. 2005;102:1596–1601. doi: 10.1073/pnas.0409015102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gotsman I, Grabie N, Gupta R, Dacosta R, MacConmara M, Lederer J, Sukhova G, Witztum JL, Sharpe AH, Lichtman AH. Impaired regulatory t-cell response and enhanced atherosclerosis in the absence of inducible costimulatory molecule. Circulation. 2006;114:2047–2055. doi: 10.1161/CIRCULATIONAHA.106.633263. [DOI] [PubMed] [Google Scholar]
- 13.Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S, Flavell RA, Hansson GK, Klatzmann D, Tedgui A, Mallat Z. Natural regulatory t cells control the development of atherosclerosis in mice. Nature medicine. 2006;12:178–180. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 14.Mallat Z, Ait-Oufella H, Tedgui A. Regulatory t-cell immunity in atherosclerosis. Trends Cardiovasc Med. 2007;17:113–118. doi: 10.1016/j.tcm.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Herbin O, Ait-Oufella H, Yu W, Fredrikson GN, Aubier B, Perez N, Barateau V, Nilsson J, Tedgui A, Mallat Z. Regulatory t-cell response to apolipoprotein b100-derived peptides reduces the development and progression of atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2012;32:605–612. doi: 10.1161/ATVBAHA.111.242800. [DOI] [PubMed] [Google Scholar]
- 16.Klingenberg R, Gerdes N, Badeau RM, Gistera A, Strodthoff D, Ketelhuth DF, Lundberg AM, Rudling M, Nilsson SK, Olivecrona G, Zoller S, Lohmann C, Luscher TF, Jauhiainen M, Sparwasser T, Hansson GK. Depletion of foxp3+ regulatory t cells promotes hypercholesterolemia and atherosclerosis. J Clin Invest. 2013;123:1323–1334. doi: 10.1172/JCI63891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han SF, Liu P, Zhang W, Bu L, Shen M, Li H, Fan YH, Cheng K, Cheng HX, Li CX, Jia GL. The opposite-direction modulation of cd4+cd25+ tregs and t helper 1 cells in acute coronary syndromes. Clin Immunol. 2007;124:90–97. doi: 10.1016/j.clim.2007.03.546. [DOI] [PubMed] [Google Scholar]
- 18.Mor A, Luboshits G, Planer D, Keren G, George J. Altered status of cd4(+)cd25(+) regulatory t cells in patients with acute coronary syndromes. European heart journal. 2006;27:2530–2537. doi: 10.1093/eurheartj/ehl222. [DOI] [PubMed] [Google Scholar]
- 19.Ji QW, Guo M, Zheng JS, Mao XB, Peng YD, Li SN, Liang ZS, Dai ZY, Mao Y, Zeng QT. Downregulation of t helper cell type 3 in patients with acute coronary syndrome. Archives of medical research. 2009;40:285–293. doi: 10.1016/j.arcmed.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Maganto-Garcia E, Tarrio ML, Grabie N, Bu DX, Lichtman AH. Dynamic changes in regulatory t cells are linked to levels of diet-induced hypercholesterolemia. Circulation. 2011;124:185–195. doi: 10.1161/CIRCULATIONAHA.110.006411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galkina E, Kadl A, Sanders J, Varughese D, Sarembock IJ, Ley K. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially l-selectin dependent. The Journal of experimental medicine. 2006;203:1273–1282. doi: 10.1084/jem.20052205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koltsova EK, Garcia Z, Chodaczek G, Landau M, McArdle S, Scott SR, von Vietinghoff S, Galkina E, Miller YI, Acton ST, Ley K. Dynamic t cell-apc interactions sustain chronic inflammation in atherosclerosis. J Clin Invest. 2012 doi: 10.1172/JCI61758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galkina E, Harry BL, Ludwig A, Liehn EA, Sanders JM, Bruce A, Weber C, Ley K. Cxcr6 promotes atherosclerosis by supporting t-cell homing, interferon-gamma production, and macrophage accumulation in the aortic wall. Circulation. 2007;116:1801–1811. doi: 10.1161/CIRCULATIONAHA.106.678474. [DOI] [PubMed] [Google Scholar]
- 24.Mikhak Z, Farsidjani A, Luster AD. Endotoxin augmented antigen-induced th1 cell trafficking amplifies airway neutrophilic inflammation. J Immunol. 2009;182:7946–7956. doi: 10.4049/jimmunol.0803522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rocha VZ, Folco EJ, Ozdemir C, Sheikine Y, Christen T, Sukhova GK, Tang EH, Bittencourt MS, Santos RD, Luster AD, Cohen DE, Libby P. Cxcr3 controls t-cell accumulation in fat inflammation. Arterioscler Thromb Vasc Biol. 2014;34:1374–1381. doi: 10.1161/ATVBAHA.113.303133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braunersreuther V, Zernecke A, Arnaud C, Liehn EA, Steffens S, Shagdarsuren E, Bidzhekov K, Burger F, Pelli G, Luckow B, Mach F, Weber C. Ccr5 but not ccr1 deficiency reduces development of diet-induced atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2007;27:373–379. doi: 10.1161/01.ATV.0000253886.44609.ae. [DOI] [PubMed] [Google Scholar]
- 27.Soehnlein O, Drechsler M, Doring Y, Lievens D, Hartwig H, Kemmerich K, Ortega-Gomez A, Mandl M, Vijayan S, Projahn D, Garlichs CD, Koenen RR, Hristov M, Lutgens E, Zernecke A, Weber C. Distinct functions of chemokine receptor axes in the atherogenic mobilization and recruitment of classical monocytes. EMBO molecular medicine. 2013;5:471–481. doi: 10.1002/emmm.201201717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Combadiere C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, Merval R, Proudfoot A, Tedgui A, Mallat Z. Combined inhibition of ccl2, cx3cr1, and ccr5 abrogates ly6c(hi) and ly6c(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–1657. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 29.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ ccr2, ccr5, and cx3cr1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braunersreuther V, Steffens S, Arnaud C, Pelli G, Burger F, Proudfoot A, Mach F. A novel rantes antagonist prevents progression of established atherosclerotic lesions in mice. Arterioscler Thromb Vasc Biol. 2008;28:1090–1096. doi: 10.1161/ATVBAHA.108.165423. [DOI] [PubMed] [Google Scholar]
- 31.Hugues S, Fetler L, Bonifaz L, Helft J, Amblard F, Amigorena S. Distinct t cell dynamics in lymph nodes during the induction of tolerance and immunity. Nat Immunol. 2004;5:1235–1242. doi: 10.1038/ni1134. [DOI] [PubMed] [Google Scholar]
- 32.Zernecke A, Liehn EA, Gao JL, Kuziel WA, Murphy PM, Weber C. Deficiency in ccr5 but not ccr1 protects against neointima formation in atherosclerosis-prone mice: Involvement of il-10. Blood. 2006;107:4240–4243. doi: 10.1182/blood-2005-09-3922. [DOI] [PubMed] [Google Scholar]
- 33.van Wanrooij EJ, Happe H, Hauer AD, de Vos P, Imanishi T, Fujiwara H, van Berkel TJ, Kuiper J. Hiv entry inhibitor tak-779 attenuates atherogenesis in low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2005;25:2642–2647. doi: 10.1161/01.ATV.0000192018.90021.c0. [DOI] [PubMed] [Google Scholar]
- 34.Veillard NR, Kwak B, Pelli G, Mulhaupt F, James RW, Proudfoot AE, Mach F. Antagonism of rantes receptors reduces atherosclerotic plaque formation in mice. Circulation research. 2004;94:253–261. doi: 10.1161/01.RES.0000109793.17591.4E. [DOI] [PubMed] [Google Scholar]
- 35.Weber C, Alon R, Moser B, Springer TA. Sequential regulation of alpha 4 beta 1 and alpha 5 beta 1 integrin avidity by cc chemokines in monocytes: Implications for transendothelial chemotaxis. The Journal of cell biology. 1996;134:1063–1073. doi: 10.1083/jcb.134.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Hundelshausen P, Weber KS, Huo Y, Proudfoot AE, Nelson PJ, Ley K, Weber C. Rantes deposition by platelets triggers monocyte arrest on inflamed and atherosclerotic endothelium. Circulation. 2001;103:1772–1777. doi: 10.1161/01.cir.103.13.1772. [DOI] [PubMed] [Google Scholar]
- 37.Arbones ML, Ord DC, Ley K, Ratech H, Maynard-Curry C, Otten G, Capon DJ, Tedder TF. Lymphocyte homing and leukocyte rolling and migration are impaired in l-selectin-deficient mice. Immunity. 1994;1:247–260. doi: 10.1016/1074-7613(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 38.Roselaar SE, Kakkanathu PX, Daugherty A. Lymphocyte populations in atherosclerotic lesions of apoe −/− and ldl receptor −/− mice. Decreasing density with disease progression. Arterioscler Thromb Vasc Biol. 1996;16:1013–1018. doi: 10.1161/01.atv.16.8.1013. [DOI] [PubMed] [Google Scholar]
- 39.Jonasson L, Holm J, Skalli O, Bondjers G, Hansson GK. Regional accumulations of t cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986;6:131–138. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- 40.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory t cells: Mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohkura N, Kitagawa Y, Sakaguchi S. Development and maintenance of regulatory t cells. Immunity. 2013;38:414–423. doi: 10.1016/j.immuni.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 42.Koltsova EK, Kim G, Lloyd KM, Saris CJ, von Vietinghoff S, Kronenberg M, Ley K. Interleukin-27 receptor limits atherosclerosis in ldlr−/− mice. Circulation research. 2012;111:1274–1285. doi: 10.1161/CIRCRESAHA.112.277525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein e. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- 44.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory t cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 45.Tam LS, Kitas GD, Gonzalez-Gay MA. Can suppression of inflammation by anti-tnf prevent progression of subclinical atherosclerosis in inflammatory arthritis? Rheumatology. 2014;53:1108–1119. doi: 10.1093/rheumatology/ket454. [DOI] [PubMed] [Google Scholar]
- 46.Elhage R, Maret A, Pieraggi MT, Thiers JC, Arnal JF, Bayard F. Differential effects of interleukin-1 receptor antagonist and tumor necrosis factor binding protein on fatty-streak formation in apolipoprotein e-deficient mice. Circulation. 1998;97:242–244. doi: 10.1161/01.cir.97.3.242. [DOI] [PubMed] [Google Scholar]
- 47.Potteaux S, Combadiere C, Esposito B, Lecureuil C, Ait-Oufella H, Merval R, Ardouin P, Tedgui A, Mallat Z. Role of bone marrow-derived cc-chemokine receptor 5 in the development of atherosclerosis of low-density lipoprotein receptor knockout mice. Arterioscler Thromb Vasc Biol. 2006;26:1858–1863. doi: 10.1161/01.ATV.0000231527.22762.71. [DOI] [PubMed] [Google Scholar]
- 48.McLaren JE, Ramji DP. Interferon gamma: A master regulator of atherosclerosis. Cytokine & growth factor reviews. 2009;20:125–135. doi: 10.1016/j.cytogfr.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 49.Hori S, Nomura T, Sakaguchi S. Control of regulatory t cell development by the transcription factor foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 50.Ayyoub M, Deknuydt F, Raimbaud I, Dousset C, Leveque L, Bioley G, Valmori D. Human memory foxp3+ tregs secrete il-17 ex vivo and constitutively express the t(h)17 lineage-specific transcription factor rorgamma t. Proc Natl Acad Sci U S A. 2009;106:8635–8640. doi: 10.1073/pnas.0900621106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oldenhove G, Bouladoux N, Wohlfert EA, Hall JA, Chou D, Dos Santos L, O'Brien S, Blank R, Lamb E, Natarajan S, Kastenmayer R, Hunter C, Grigg ME, Belkaid Y. Decrease of foxp3+ treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity. 2009;31:772–786. doi: 10.1016/j.immuni.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wohlfert EA, Grainger JR, Bouladoux N, Konkel JE, Oldenhove G, Ribeiro CH, Hall JA, Yagi R, Naik S, Bhairavabhotla R, Paul WE, Bosselut R, Wei G, Zhao K, Oukka M, Zhu J, Belkaid Y. Gata3 controls foxp3(+) regulatory t cell fate during inflammation in mice. J Clin Invest. 2011;121:4503–4515. doi: 10.1172/JCI57456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, Wang YH, Lim H, Reynolds JM, Zhou XH, Fan HM, Liu ZM, Neelapu SS, Dong C. Follicular regulatory t cells expressing foxp3 and bcl-6 suppress germinal center reactions. Nature medicine. 2011;17:983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stock P, Akbari O, Berry G, Freeman GJ, Dekruyff RH, Umetsu DT. Induction of t helper type 1-like regulatory cells that express foxp3 and protect against airway hyper-reactivity. Nat Immunol. 2004;5:1149–1156. doi: 10.1038/ni1122. [DOI] [PubMed] [Google Scholar]
- 55.Koenecke C, Lee CW, Thamm K, Fohse L, Schafferus M, Mittrucker HW, Floess S, Huehn J, Ganser A, Forster R, Prinz I. Ifn-gamma production by allogeneic foxp3+ regulatory t cells is essential for preventing experimental graft-versus-host disease. J Immunol. 2012;189:2890–2896. doi: 10.4049/jimmunol.1200413. [DOI] [PubMed] [Google Scholar]
- 56.Feng T, Cao AT, Weaver CT, Elson CO, Cong Y. Interleukin-12 converts foxp3+ regulatory t cells to interferon-gamma-producing foxp3+ t cells that inhibit colitis. Gastroenterology. 2011;140:2031–2043. doi: 10.1053/j.gastro.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stroopinsky D, Avivi I, Rowe JM, Avigan D, Katz T. Allogeneic induced human foxp3(+)ifn-gamma(+) t cells exhibit selective suppressive capacity. European journal of immunology. 2009;39:2703–2715. doi: 10.1002/eji.200839097. [DOI] [PubMed] [Google Scholar]
- 58.McClymont SA, Putnam AL, Lee MR, Esensten JH, Liu W, Hulme MA, Hoffmuller U, Baron U, Olek S, Bluestone JA, Brusko TM. Plasticity of human regulatory t cells in healthy subjects and patients with type 1 diabetes. J Immunol. 2011;186:3918–3926. doi: 10.4049/jimmunol.1003099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walker MR, Kasprowicz DJ, Gersuk VH, Benard A, Van Landeghen M, Buckner JH, Ziegler SF. Induction of foxp3 and acquisition of t regulatory activity by stimulated human cd4+cd25− t cells. J Clin Invest. 2003;112:1437–1443. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Allan SE, Crome SQ, Crellin NK, Passerini L, Steiner TS, Bacchetta R, Roncarolo MG, Levings MK. Activation-induced foxp3 in human t effector cells does not suppress proliferation or cytokine production. International immunology. 2007;19:345–354. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- 61.Wan YY, Flavell RA. Regulatory t-cell functions are subverted and converted owing to attenuated foxp3 expression. Nature. 2007;445:766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 62.Nathan CF, Murray HW, Wiebe ME, Rubin BY. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. The Journal of experimental medicine. 1983;158:670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mills CD, Ley K. M1 and m2 macrophages: The chicken and the egg of immunity. Journal of innate immunity. 2014 doi: 10.1159/000364945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hanna RN, Shaked I, Hubbeling HG, Punt JA, Wu R, Herrley E, Zaugg C, Pei H, Geissmann F, Ley K, Hedrick CC. Nr4a1 (nur77) deletion polarizes macrophages toward an inflammatory phenotype and increases atherosclerosis. Circulation research. 2012;110:416–427. doi: 10.1161/CIRCRESAHA.111.253377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wolfs IM, Donners MM, de Winther MP. Differentiation factors and cytokines in the atherosclerotic plaque micro-environment as a trigger for macrophage polarisation. Thrombosis and haemostasis. 2011;106:763–771. doi: 10.1160/TH11-05-0320. [DOI] [PubMed] [Google Scholar]
- 66.Picelli S, Faridani OR, Bjorklund AK, Winberg G, Sagasser S, Sandberg R. Full-length rna-seq from single cells using smart-seq2. Nature protocols. 2014;9:171–181. doi: 10.1038/nprot.2014.006. [DOI] [PubMed] [Google Scholar]
- 67.Trapnell C, Pachter L, Salzberg SL. Tophat: Discovering splice junctions with rna-seq. Bioinformatics. 2009;25:1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schmieder R, Edwards R. Quality control and preprocessing of metagenomic datasets. Bioinformatics. 2011;27:863–864. doi: 10.1093/bioinformatics/btr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R Genome Project Data Processing S. The sequence alignment/map format and samtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anders S, Pyl PT, Huber W. Htseq-a python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for rna-seq data with deseq2. Genome biology. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. 1995;Ser. B:289–300. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.