Abstract
Rationale
Transmembrane TNF-α (tmTNF-α) is the prime ligand for TNFR2, which has been shown to mediate angiogenic and blood vessel repair activities in mice. We have previously reported that the angiogenic potential of highly proliferative endothelial colony forming cells (ECFCs) can be explained by the absence of senescent cells, which in mature endothelial cells occupy more than 30% of the population, and that exposure to a chronic inflammatory environment induced premature, telomere-independent senescence in ECFCs.
Objective
The goal of this study was to determine the role of transmembrane TNF-α in the proliferation of ECFCs.
Methods and Results
Here we show that tmTNF-α expression on ECFCs selects for higher proliferative potential and when removed from the cell surface promotes ECFC senescence. Moreover, the induction of premature senescence by chronic inflammatory conditions is blocked by inhibition of tmTNF-α cleavage. Indeed, the mechanism of chronic inflammation-induced premature senescence involves an abrogation of tmTNF/TNFR2 signaling. This process is mediated by activation of the tmTNF cleavage metalloprotease TACE via p38 MAP kinase activation and its concurrent export to the cell surface by means of increased iRhom2 expression.
Conclusion
Thus we conclude that tmTNF-α on the surface of highly proliferative ECFCs plays an important role in the regulation of their proliferative capacity.
Keywords: Angiogenesis, endothelium, inflammation, vasculature
INTRODUCTION
Despite improvements in treatment and prevention, cardiovascular disease remains one of the leading causes of death, disability, and health care expenditure in the US. Vascular changes such as the decline in passive compliance of arterial blood vessels and impaired endothelium-dependent vasodilation response are reliable markers for aging1. Mathematical modeling based on known rates of endothelial turnover and proliferation rates have calculated that a reservoir of highly proliferative endothelial progenitor cells is required to maintain vascular function2. Therefore endothelial progenitor cells (EPCs) including non-myeloid highly proliferative endothelial progenitor cells, also described as endothelial colony forming cells (ECFCs) or outgrowth endothelial cells, and bone marrow derived circulating progenitor cells (CPCs) are believed to play an important role in maintenance of a viable endothelial layer in the vascular system3-6. ECFCs define a novel hierarchy of endothelial cells and are nearly identical to mature ECs, with the exception of greatly enhanced proliferative potential3, 7. Deregulation of ECFCs and CPCs have been shown to correlate with vascular diseases and diabetes8-11. Therefore, further investigation into their biology is important to both general vascular biology and investigations into potential cell therapies.
TNF-α is predominately associated with an inflammatory response, vascular dysfunction, and eventually endothelial apoptosis. However, most of the studies on TNF-α have been performed with soluble TNF-α, which is cleaved from precursor transmembrane TNF-α by the matrix metalloproteinase TACE12. Soluble TNF-α binds to both TNFR1 and TNFR2, although in endothelial cells it predominantly signals through TNFR113. In contrast to soluble TNF-α, tmTNF-α binds preferentially to TNFR2 in endothelial cells14. TNFR1 and TNFR2 are quite different in their biology. TNFR1 contains a death signaling domain while TNFR2 does not; this leads to profound differences in terms of cell proliferation and survival in endothelial cells16. In fact, studies comparing TNFR1 and TNFR2 receptor knockout mice demonstrated that TNFR1 is anti-angiogenic, while the tmTNF-α-selective TNFR2 confers a survival signal, mediating angiogenic and blood vessel repair activities17. In addition, transgenic mice that only express tmTNF develop fewer inflammatory atherosclerotic plaques, and transgenic uncleavable transmembrane TNF-α expression in endothelial cells elicits angiogenesis in vivo18, 19.
The proliferative potential of ECs is limited by the processes of senescence20, 21. Endothelial injury in the absence of sufficient circulating progenitor cells may affect the progression of cardiovascular disease, as increases in senescent vascular wall cells may lead to the inability of the endothelium to maintain a continuous functional monolayer2, 22. We have previously shown that ECFCs have extremely low levels of senescence but undergo stress induced cellular senescence when exposed to chronic inflammatory conditions, a process which is independent of telomeric shortening-dependent replicative senescence23.
We show here that ECFCs unexpectedly express high levels of surface tmTNF-α. Moreover, sorting freshly isolated cord blood using tmTNF-specific magnetic beads results in an increase in the number of ECFC colonies recovered, and these colonies contain a greater distribution of highly proliferative cells than unsorted ECFC colonies. We also show that when tmTNF/TNFR2 signaling is perturbed, ECFCs undergo premature senescence, resulting in loss of the highly proliferative phenotype. Furthermore, inflammation-induced premature senescence is blocked by inhibition of tmTNF-α cleavage. Our data show for the first time that transmembrane TNF-α plays an important role in ECFC proliferative capacity.
METHODS
Reagents and cells
Antibodies directed against transmembrane TNF-α, TACE ectodomain, TNFR1, and TNFR2 were purchased from R&D Biotechnologies, and secondary AlexaFluor antibodies were from Invitrogen. p16, p65, and p-Etk antibodies were purchased from Abcam. TACE activity detection kit was from Anaspec and β-gal senescence detection kit was obtained from BioVision (Mountain View, CA). All other reagents were purchased from Sigma.
ECFC were isolated from fresh cord blood as previously described23. Briefly, mononuclear cells (MNC) were isolated using standard Ficoll purification, then grown on collagen-coated tissue culture plates in Endothelial Growth Media-2 (EGM2) supplemented with an additional 10% fetal bovine serum. Medium was changed every 2 days until colonies appeared after 3 weeks. Further purification of MNC was done using magnetic beads (Miltenyi Biotech) prior to plating on collagen. Human microvascular endothelial cells (HMVECs) were used as controls and were obtained from Lonza and maintained in EGM2 MV media.
Isolation of endothelium from arteries
Tibial arteries (diseased) and internal mammary arteries (healthy) were obtained as medical waste from amputations due to critical limb ischemia or cardiac bypass surgeries, respectively. Samples were obtained from males aged 55-68 and matched for comorbidities. There was no significant difference in demographic variables or risk factors associated with critical limb ischemia or heart disease, including smoking, diabetes, hyperlipidemia, and hypertension. Vessels were cut longitudinally, rinsed with saline, and the endothelium was removed by passing gently over the surface with a cell scraper. Recovered cells were washed and then stained for CD31 and tmTNF for flow cytometry analysis.
Immunofluorescence staining and flow cytometry
For detection of tmTNF in vessel walls, healthy internal mammary arteries (healthy) or tibial arteries from patients with peripheral vascular disease (diseased) were obtained. Cells were gently removed from the vessel using a collagenase solution and then stained for tmTNF and CD31.
Cells were fixed with 1% paraformaldehyde (PFA) and blocked with 1% bovine serum albumin in phosphate buffered saline (PBS), then stained with tmTNF-α, TACE, TNFR2, or CD31 and appropriate secondary antibodies. Fluorescence was detected using a FACSCalibur II (Beckton Dickenson) and analyzed using CellQuest software. Percent positive signal was gated based on isotype controls.
For detection of tmTNF+ cells in the CD34+/CD45− fraction, MNCs were stained using a 7 color assay with positive gating based on fluorescent minus one controls as described 24. Data was collected on a BD LSRII and analyzed using Flowjo software.
Staining for senescence-associated β-galactosidase
To assess senescence in cord blood-derived ECFC, SA-β-gal activity was measured using a standard senescence detection kit (Biovision, Mountain View, CA) according to the manufacturer's instructions. Briefly, culture media was removed and cells were washed once with PBS then fixed with the fixation solution for 15 min at room temperature. After two additional washes with PBS, the staining solution containing 1 mg/ml 5-bromo-4-chloro-3-indolyl-d galactoside was added to each well. Cells were incubated at 37°C overnight and then observed under a microscope for development of blue color. The percentage of blue cells vs. total cells was measured by choosing 25 random microscopic fields.
Western blot analysis
For iRhom2 detection, ECFC were treated for up to 6 days with 10 ng/ml soluble TNF-α. Cells were lysed in cell lysis buffer (Cell Signaling) containing protease inhibitor cocktail (ThermoScientific, #88663) with protein concentrations determined by BCA assay. Proteins (20 μg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by immunoblotting using appropriate antibodies. The chemiluminescent signals were quantified by densitometry using Adobe Photoshop.
NFκB and TNFR2 silencing with small interfering RNA
For NFκB gene knockdown by RNAi we used Ambion's Silencer® Select Custom Designed siRNA against NFκB using a protocol as previously described25. TNFR2 siRNA was purchased from Life Technologies. Briefly, cells were transfected with GeneJammer transfection reagent (Aligent Technologies) and after incubation for 2 days at 37°C, total cell lysate was used to determine the knockdown of NFκB or TNFR2 by Western blotting. Cells were then used in proliferation assays.
Proliferation assays
To determine proliferation potential of ECFC, single cells were plated on collagen-coated 96 well plates, 1 cell per well. After 14 days incubation colony size was determined.
Population doubling time (pdt) was determined by seeding cells in 12-well plates. The number of PDs occurring between passages was calculated according to the equation PD = log2(CH/CS), where CH is the number of viable cells at harvest and CS is the number of cells seeded. The PDT was derived using the time interval between cell seeding and harvest divided by the number of PDs for that passage.
For carboxyfluorescein diacetate succinimidyl ester (CFSE) determination of proliferation, cells were labeled with 5 μM CFSE for 15 minutes at 37°C then washed twice with PBS and incubated for 3 days with the appropriate treatments and the percentage of proliferating cells was determined by dilution of CFSE signal as measured by flow cytometry.
Sprouting assay
ECFC sprouting ability was assessed as previously described18. Briefly, cells were seeded onto collagen-coated Cytodex beads and embedded into fibrinogen gel overlaid with growth media. After 5 days the number of sprouts longer than the diameter of the bead were determined.
TACE activation
TACE activity in whole cell lysates was determined with Sensolyte 520 TACE Activity Assay Kit (Anaspec, Fremont, CA) at 490 nm/520 nm according to the manufacturer's instructions.
Statistical analysis
Each experiment was performed in triplicate, with a minimum of three independent experiments. The differences between groups were compared using paired Student T-test or ANOVA with Bonferroni corrections. Where applicable, mean ± SEM of multiple measurements is reported as indicated.
RESULTS
tmTNF correlates with ECFC proliferation
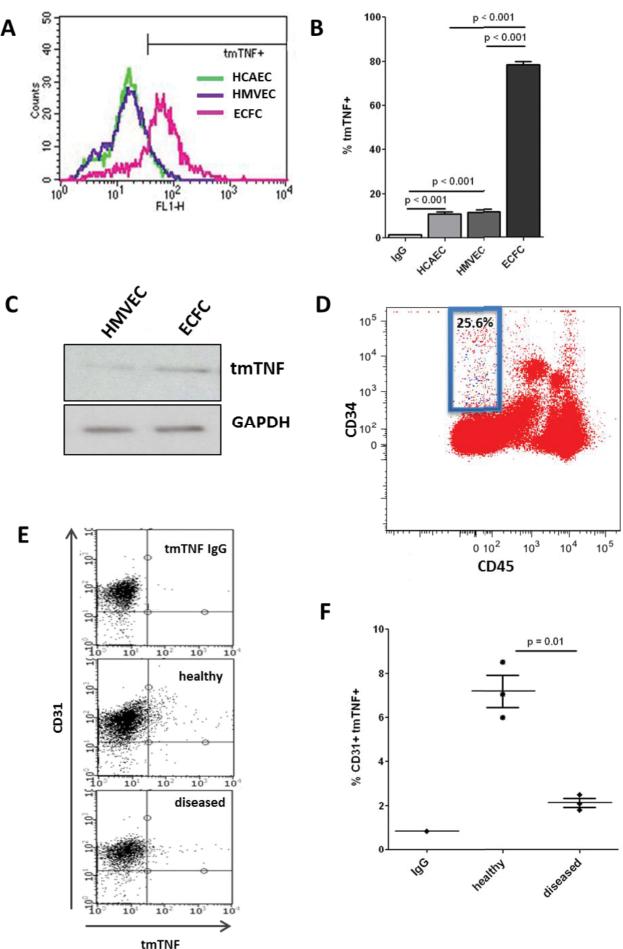
To assess the association of tmTNF with endothelial cell proliferative capacity, we first compared the surface expression levels of tmTNF-α in highly proliferative ECFCs with mature ECs. As shown in Figure 1, ECFCs isolated from cord blood express significant levels of tmTNF-α, whereas human microvascular endothelial cells (HMVECs) or human coronary arterial endothelial cells (HCAECs) exhibit low amounts (Figure 1A-B). We further confirmed this expression by Western blot (Fig. 1C). To confirm that tmTNF-α expression was not an artifact of tissue culture we isolated mononuclear cells (MNCs) from cord blood and stained for CD34, CD45, and tmTNF-α (Fig. 1D). The majority of tmTNF staining (blue dots) was located in the CD34+/CD45− population, which is enriched for ECFCs and circulating angiogenic ECs24. In fact, over 25% of this population was positive for tmTNF-α. Next we obtained sections of arteries from patients with peripheral vascular disease or internal mammary arteries (IMA) as age-matched “healthy” controls. IMA is a particularly good control in our case as we were able to control for age and other demographic variables, yet IMA is seemingly exempt from development of atherosclerosis. We detached the endothelial cells, stained for tmTNF-α with CD31 as an endothelial marker, and determined CD31+tmTNF+ cells by FACS (Fig. 1E). As shown in Figure 1F, CD31/tmTNF-α co-staining in healthy arteries (IMA) showed a small percentage (~6-8%) of endothelial cells expressing tmTNF-α. Interestingly, in diseased arteries (tibial artery from patients with critical limb ischemia) the number of CD31/tmTNF-α double positive cells is lower than in healthy arteries, which correlates with current literature suggesting that ECFCs are decreased in diseased vessels. Together, this data suggests that a small sub-population of endothelial cells expresses tmTNF-α, and that this sub-population correlates with ECFCs.
Figure 1. tmTNF is expressed on a subset of EC.
A-B. ECFC, HCAEC, or HMVEC (passage 3) were stained for tmTNF and analyzed by FACS, with percentage tmTNF+ determined based on IgG controls. C. tmTNF was detected in HMVEC and ECFC cell lysates by Western blot. D. Freshly isolated cord blood MNCs were stained for CD45, CD34, and tmTNF, and the percentage of CD45−/CD34+ cells that were positive for tmTNF was determined (blue dots in blue box ). E-F. Age-matched internal mammary arteries (healthy) were obtained from patients undergoing cardiac bypass surgery and tibial arteries (diseased) from patients with critical limb ischemia. Endothelial lining was gently removed from the vessel wall and co-stained for CD31 and tmTNF-α then analyzed by FACS. Data are representative of 3-4 independent experiments.
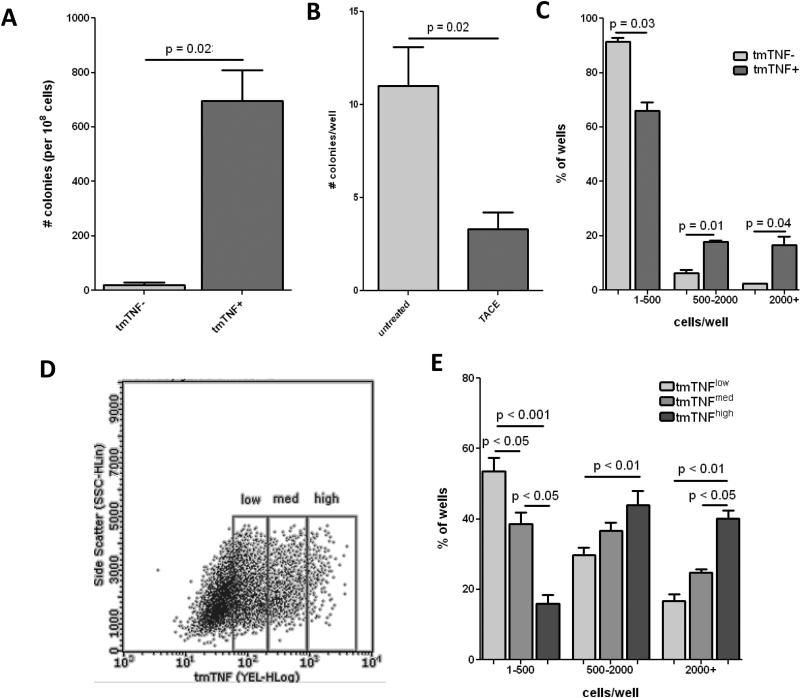
Prior studies suggest that tmTNF-α is associated with increased angiogenesis.18, 19, 26 Because of the known role of ECFCs in angiogenesis we examined the role of tmTNF-α in ECFC proliferation. We separated MNCs from cord blood into tmTNF positive and negative fractions by magnetic bead separation. We determined colony formation, and there were significantly more highly proliferative colonies formed from the tmTNF+ fraction (Fig. 2A) along with a greater number of cells per colony than in the tmTNF− fraction. Conversely, when we added TACE, a metalloprotease that cleaves tmTNF, to the culture media there were significantly fewer colonies recovered (Fig. 2B). Re-seeding single cells from similarly sized colonies showed a marked shift towards greater proliferative potential in cells from tmTNF+ colonies compared to those from tmTNF− colonies (Fig. 2C). Moreover, when we sorted ECFC colonies according to tmTNF intensity (Fig. 2D) and seeded each fraction in single cell assays to determine proliferative potential the stronger tmTNF expressing cells exhibited greater proliferative capacity (Fig. 2E). Thus, our data shows a strong association between tmTNF expression and ECFC proliferative potential.
Figure 2. tmTNF is associated with highly proliferative ECFC.
A. Freshly isolated cord blood MNCs were sorted into tmTNF− or tmTNF+ fractions using MACS beads and plated with equal density on collagen-coated plates. ECFC colony formation was determined after 3 weeks. B. Freshly isolated cord blood MNCs were plated on collagen-coated plates and incubated with or without the addition of TACE. ECFC colony formation was determined after 3 weeks. C. Colonies of equal size were picked from tmTNF− or tmTNF+ plates and reseeded onto 96-well plates with 1 cell/well. After 14 days the number of cells/well were counted to determine low (1-500), medium (500-2000), and high (2000+) proliferative potential. D. Colonies from the tmTNF+ fraction were FACS sorted into tmTNFlow, tmTNFmed, and tmTNFhigh fractions and replated onto 96-well plates with 1 cell/well. E. After 14 days the number of cells/well were counted to determine low, medium, and high proliferative potential. Data are representative of 3-4 independent experiments.
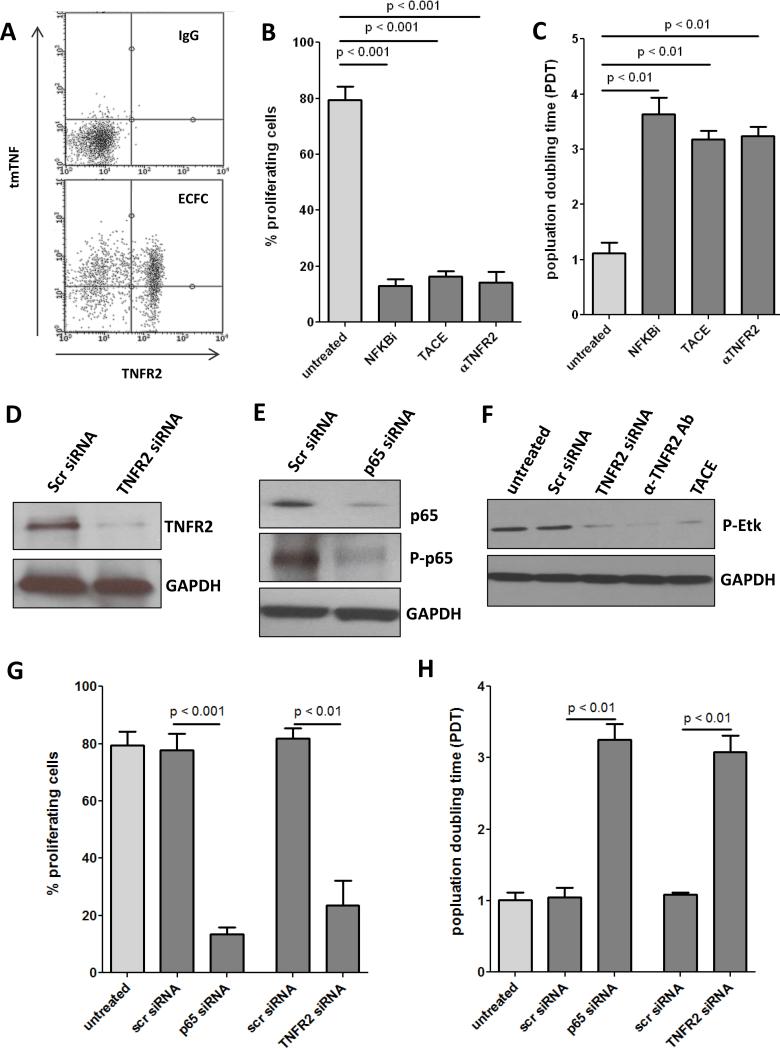
To assess the role of tmTNF signaling in ECFC proliferation we first confirmed that both tmTNF and its preferred receptor, TNFR2, are expressed on ECFCs (Fig. 3A). Next, we determined ECFC proliferative capacity using CFSE, a cell permeable dye which covalently couples to intracellular molecules and is diluted with each cell division, thus allowing quantitative assessment of cell proliferation27 (Fig. 3B), and by determining population doubling times (Fig. 3C) in the presence of TACE or an anti-TNFR2 neutralizing antibody. Because NFκB is known to be a downstream mediator of TNFR2 but not TNFR1, we also included an NFκB inhibitor. In all cases interference with the tmTNF-TNFR2 signaling axis or perturbation of TNFR2 downstream signaling resulted in decreased proliferation, as well as significantly reduced phosphorylation of Etk, a TNFR2-specific kinase (Fig. 3F). To confirm the specificity of this inhibition, we transfected ECFCs with siRNA directed against TNFR2 (Fig. 3D) or the p65 subunit of NFκB (Fig. 3E), both of which resulted in strongly reduced proliferation (Fig. 3G-H).
Figure 3. Loss of tmTNF/TNFR2 axis results in less ECFC proliferation.
A. ECFCs were stained with antibodies for tmTNF and TNFR2 or IgG controls and expression analyzed by FACS. B. ECFCs were labeled with 5 μM CFSE for 10 minutes at RT and incubated for 4 days with NFκB inhibitor (100nM), TACE, or αTNFR2 (500ng/ml). The percentage of proliferating cells was determined by dilution of the CFSE signal. C. ECFCs were seeded into 12-well plates and cultured for 4 days with NFκB inhibitor (100nM), TACE, or αTNFR2 (500ng/ml), after which cell number was determined and the population doubling time (pdt) was calculated. ECFCs were transfected with siRNA targeted to TNFR2 (D) or the p65 subunit of NFκB (E) and knockdown confirmed by Western blot. F. ECFCs were treated with TNFR2 siRNA, αTNFR2, or TACE and phosphorylation of TNFR2-specific Etk determined by Western blot. Percent proliferation (G) and pdt (H) were determined two days after transfection with p65 NFκB siRNA or TNFR2 siRNA. Data are representative of 3-4 independent experiments.
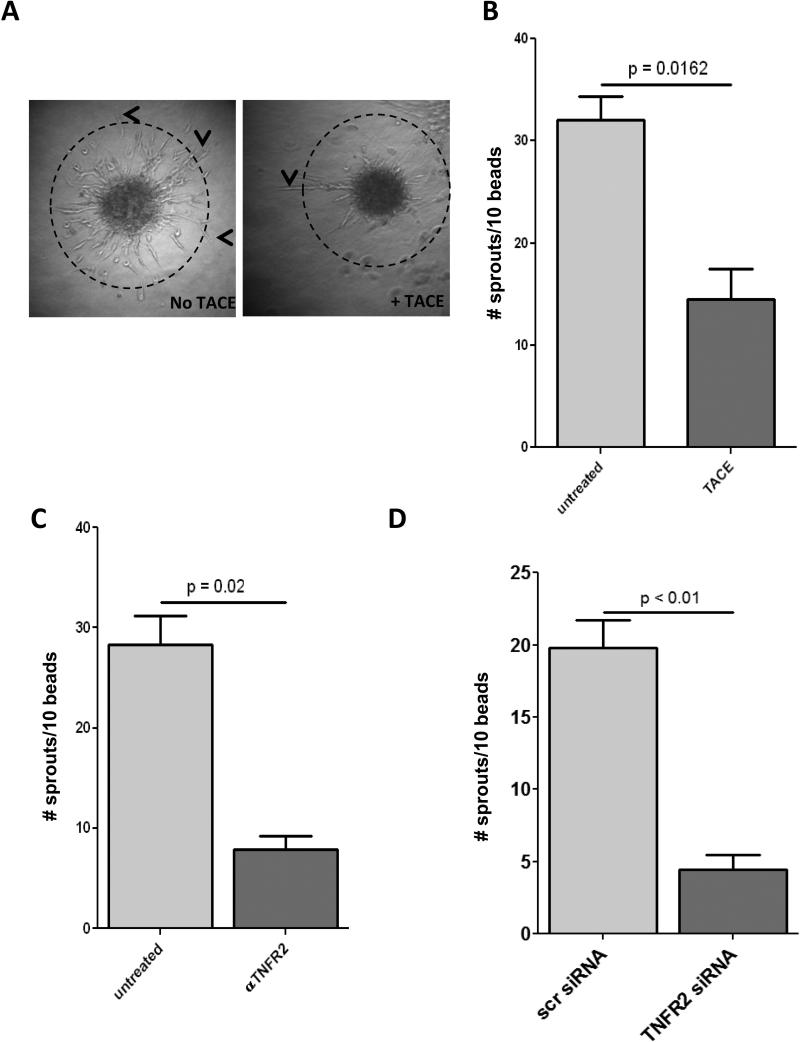
Next, we determined the effect of perturbing tmTNF signaling on the angiogenic capacity of ECFCs. We coated Cytodex beads with ECFCs, embedded them in fibrin gel, and incubated for 5 days with the addition of TACE (Fig. 4A) or an anti-TNFR2 neutralizing antibody (Fig. 4B). Treatment with either TACE or anti-TNFR2 antibodies resulted in significantly fewer sprouts per bead than ECFCs with intact tmTNF-TNFR2 signaling (Fig. 4A, B, representative figure 4C). As further confirmation, we determined sprouting using ECFCs transfected with either TNFR2 siRNA or scr control siRNA and found that knock down of TNFR2 resulted in a similar decrease in angiogenic capacity (Fig. 4D).
Figure 4. Loss of tmTNF/TNFR2 axis diminished the angiogenic capacity of ECFCs.
A-D. ECFCs were grown to confluency on collagen-coated CytoDex beads, embedded in fibrin gel and incubated in ECFC media +/− TACE (0.2 μg/ml) (B) or αTNFR2 (500ng/ml) for 5 days. Sprouts longer than the diameter of the beads (dashed line, A) were counted. Representative pictures of untreated (left panel) and TACE (right panel) sprouts are shown in A. C. Sprouting was determined using ECFCs transfected with TNFR2 siRNA. Data are representative of 3-4 independent experiments.
Loss of tmTNF results in premature senescence
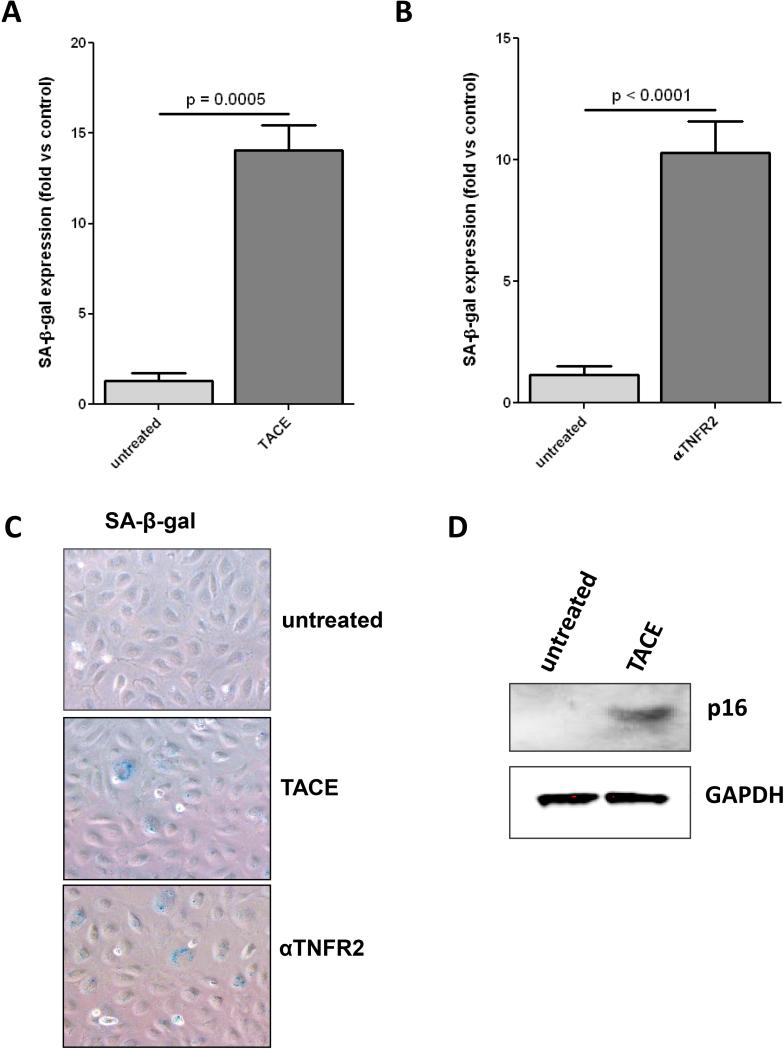
Our previous work has shown that ECFCs undergo premature senescence, resulting in a loss of proliferative potential, when exposed to chronic inflammatory conditions 23. Because of the observed relationship between tmTNF and ECFC proliferative capacity, we next determined the relationship between tmTNF and premature senescence, which is a major cause of decreased proliferation in endothelial cells. We incubated ECFCs with either recombinant TACE (Fig. 5A) or an anti-TNFR2 neutralizing antibody (Fig. 5B) for 6 days and determined development of premature senescence by staining for senescence-associated β-galactosidase (SA-β-gal, Fig. 5C representative). Both cleaving tmTNF and blocking TNFR2 signaling resulted in increased senescence of ECFCs. To confirm this involvement of tmTNF-TNFR2 signaling in the development of senescence, we treated ECFCs with either TACE or anti-TNFR2 neutralizing antibody and detected the presence of p16ink, a senescence-associated cell cycle regulating protein 28 by Western blot (Fig. 5D) and found that p16ink levels increased dramatically over the course of the 6 days treatment, further confirming that loss of the tmTNF/TNFR2 signaling axis results in premature senescence of ECFCs.
Figure 5. Loss of tmTNF/TNFR2 axis induces premature senescence in ECFCs.
A-D. ECFCs were treated with TACE (10 ng/ml) (A.) or αTNFR2 (500ng/ml) (B.) for 6 days, then stained for SA-β-gal and senescent cells were quantified. C. Representative SA-β-gal staining. D. To confirm senescence with another marker of senescence, p16ink (p16) ECFCs were treated with TACE for 6 days and p16 expression was determined by Western blot. Data are representative of 3-4 independent experiments.
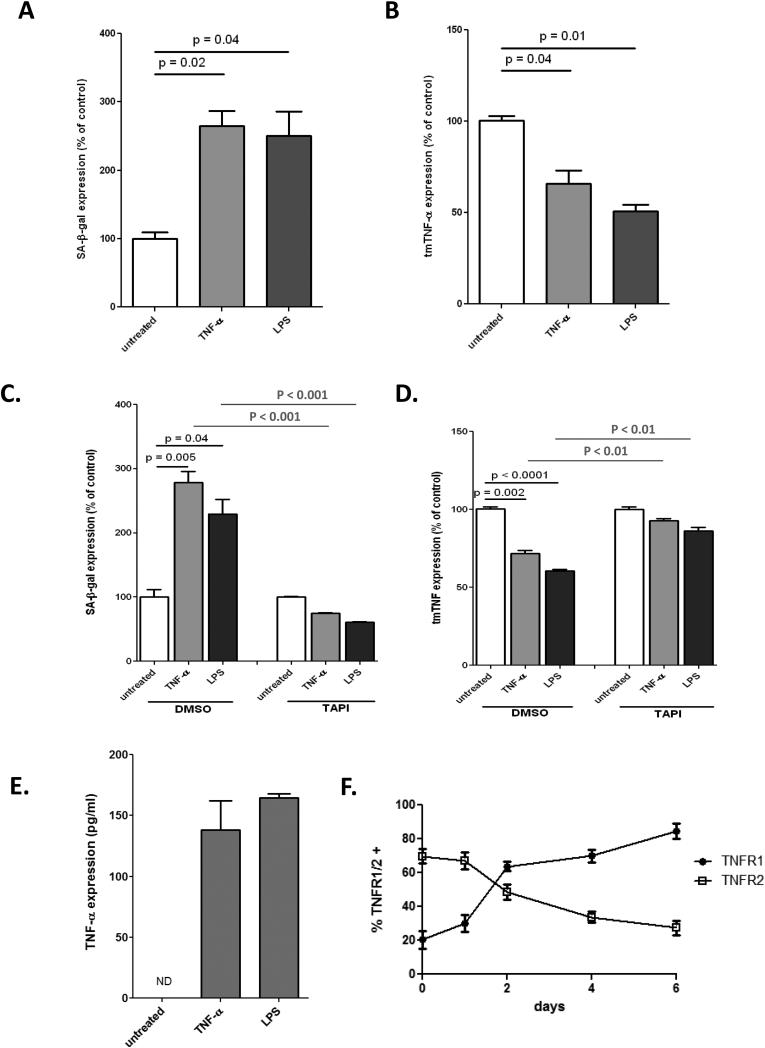
To determine the role of tmTNF-TNFR2 signaling in our previously established inflammation-induced senescence model23 we treated ECFCs with soluble TNF or LPS for 6 days to simulate a chronic inflammatory state and then determined senescence (Fig. 6A) and tmTNF surface expression (Fig. 6B). Interestingly, we found that senescence correlated with a downregulation of tmTNF. Next, we exposed ECFCs to chronic inflammation (soluble TNF or LPS) for 6 days with or without the TACE inhibitor TAPI and determined both tmTNF expression (Fig. 6C) and senescence (Fig. 6D). Importantly, we found that when tmTNF expression is maintained the development of inflammation-induced senescence is blocked even in chronic inflammatory conditions. An ELISA of the ECFC supernatant showed a dramatic increase in soluble TNF-α after 6 days of culture in chronic inflammatory conditions, further demonstrating that these conditions resulted in a loss of tmTNF (Fig. 6E). Moreover, ECFCs experience dramatic changes in TNF receptor expression over the course of chronic inflammation treatment, shifting from a predominantly TNFR2 profile to one strongly expressing TNFR1 (Fig. 6F). Together this data describes a situation in which chronic inflammation dramatically alters TNF signaling, resulting in a shift from tmTNF-TNFR2 signaling to soluble TNF-TNFR1 signaling and concomitant development of premature senescence.
Figure 6. Inflammation-induced premature senescence correlates with loss of tmTNF.
ECFCs were treated with soluble TNF (10 ng/ml) or LPS (100 ng/ml) for 6 days, then stained for SA-β-gal. (A.) or tmTNF expression was determined by FACS (B). Soluble TNF or LPS treatment was carried out in the presence of TAPI (10μM), then stained for SA-β-gal (A) or tmTNF (B.) and normalized to untreated controls. E. Soluble TNF or LPS treatment was carried out and soluble TNF levels in the supernatant determined by ELISA, with final amounts adjusted for the initial TNF treatment. F. ECFCs were treated with soluble TNF and surface expression of TNFR1 and TNFR2 determined by FACS over the course of 6 days. Data are representative of 3-4 independent experiments.
Mechanisms of premature senescence
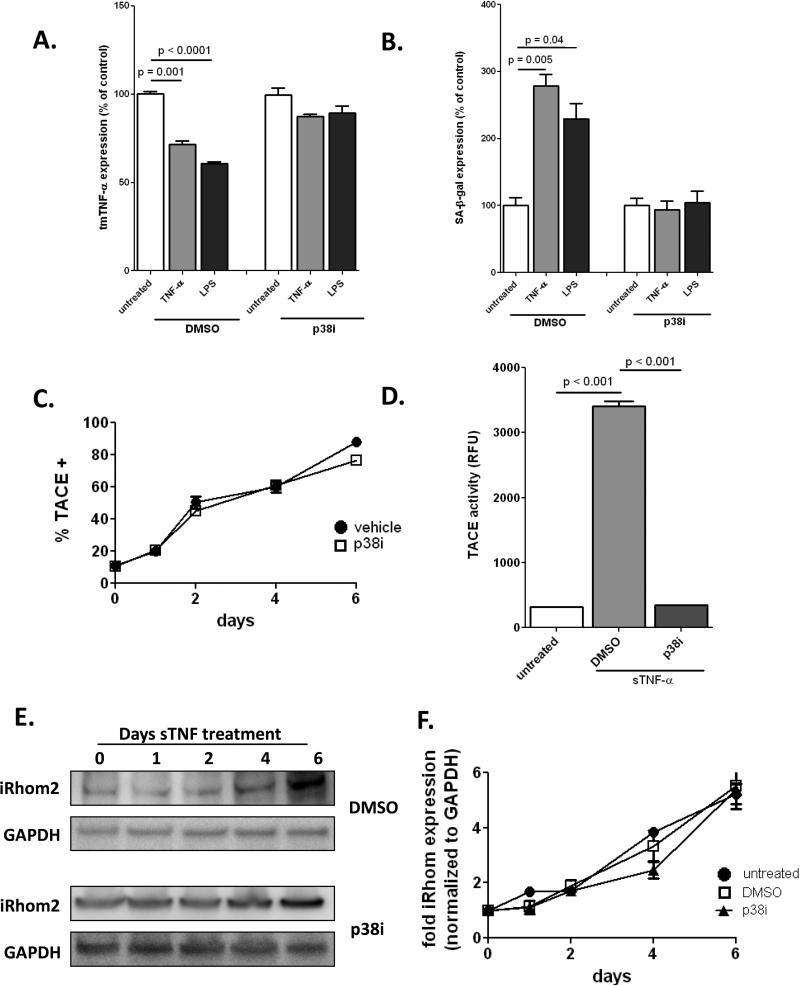
Next we addressed the mechanisms responsible for loss of tmTNF, and therefore premature ECFC senescence. Because of our previous observation that chronic inflammation-induced senescence required p38 MAP kinase activation23, we examined the effect of p38 inhibition on the expression of tmTNF and found that blocking p38 completely prevented the loss of tmTNF and subsequent development of premature senescence (Fig. 7A-B). We then determined surface expression of TACE by FACS over the course of 6 days treatment with soluble TNF (Fig. 7C) and found that TACE expression increased until almost 100% of ECFCs expressed TACE. However, upregulated surface expression of TACE was not p38-dependent, as inclusion of a p38 inhibitor did not prevent this. We determined TACE activity and found that soluble TNF treatment led to increased activation of TACE, corresponding to the same timeframe as TACE translocation to the plasma membrane. TACE activity, in contrast to surface expression, was p38-dependent (Fig. 7D). Endothelial cells do not constitutively express TACE on their surface; instead, upon stimulus the chaperone protein iRhom2 enables its release from the ER and allows transport to the surface29. Therefore, we examined the expression of iRhom2 upon addition of soluble TNF and found that expression increased dramatically over the course of the 6-day treatment (Fig. 7E-F). Increased iRhom2 expression was not p38 dependent, further confirming that translocation of TACE to the cell surface is does not require p38. Taken together, our data indicate that under chronic inflammatory conditions ECFCs lose surface transmembrane TNF due to a combination of iRhom2-dependent TACE surface upregulation and p38-dependent increased TACE activation, resulting in the loss of a tmTNF/TNFR2-dependent proliferative signal and premature development of senescence.
Figure 7. TACE expression is upregulated during chronic inflammatory conditions and required p38 activity and iRhom expression.
ECFC were treated with soluble TNF (10 ng/ml) or LPS (100 ng/ml) for 6 days +/− p38 inhibitor (10nM), then stained for tmTNF (A.) or SA-β-gal (B). C. ECFC were treated with soluble TNF (10 ng/ml) for 6 days +/− p38 inhibitor. Every 2 days cells were harvested and stained for surface TACE and analyzed by FACS. D. ECFC were treated with soluble TNF (10 ng/ml) for 6 days +/− p38 inhibitor and TACE activity was determined using a fluorescence-based kit (Anaspec). E.ECFC were treated with soluble TNF (10 ng/ml) for 6 days +/− p38 inhibitor. Cell lysates were harvested every 2 days and probed for iRhom2 and GAPDH expression. F. Densitometry analysis of iRhom2, normalized to GAPDH. Data are representative of 3-4 independent experiments.
DISCUSSION
Our data address for the first time that transmembrane TNF-α may have an important role in ECFC proliferative capacity. We show here that ECFCs express higher levels of tmTNF than mature endothelial cells. Moreover, the majority of tmTNF signal is found in populations of cord blood that have previously been shown to be enriched for ECFCs and circulating ECs, both of which exhibit high proliferative potential24. Selection of tmTNF+ MNCs from cord blood enriches for ECFCs as shown by an almost 300 fold increase in ECFC colonies per million MNCs over tmTNF− MNCs. Not only were more colonies obtained, but individual cells from these colonies exhibited a greater proportion of highly proliferative ECFCs than cells from the tmTNF− fraction. Finally, we found a positive correlation between the intensity of tmTNF expression and proliferative potential of ECFCs. This is in agreement with previous studies showing that umbilical vein endothelial cells (HUVECs) not only contain a higher percentage of ECFCs than other vessels, but also that the tmTNF expression in the HUVEC population is heterogenous and contains numerous highly positive cells, in contrast to mature endothelium which exhibits extremely low expression of tmTNF. Importantly, detection of tmTNFhigh cells may provide a novel technique for selecting highly proliferative ECFCs for both experimental procedures and potential cell therapies.
Our finding that tmTNF comprises both a marker for and a functional “maintenance” mechanism of ECFCs is surprising and on the first glance appears to be counter-intuitive. This is because the bulk of literature on TNF-α is on its soluble form, which is converted by cleavage from cell surface tmTNF and plays a major role in immunity and inflammation30. Comparably few publications analyze its precursor, tmTNF. Among those are publications demonstrating that in immune and vascular endothelial cells tmTNF binds preferentially to TNFR2, whereas soluble TNF-α binds to both TNFR1 and TNFR2. However, soluble TNF-α binds weakly to TNFR2 and disassociates very quickly resulting in minimal receptor activation. Conversely, tmTNF binds strongly to TNFR2 and disassociates at a much slower rate, resulting in a strong and sustained signal transduction14. One of the functions of the tmTNF-TNFR2 axis in endothelial cells is to protect against atherosclerosis formation and to promote angiogenesis and repair as demonstrated by studies using uncleavable tmTNF transgenic and TNFR2 knockout mice18, 19, 31. Another proposed function of tmTNF is regulating responsiveness to VEGF for the induction of vascular permeability as previously shown by our group's and others’ TNF knockout and in vitro studies32, 33. Interestingly, in a previous study we observed a pronounced upregulation of tmTNF in angiogenic tumor blood vessels32, which is in line with studies demonstrating involvement of endothelial progenitors in tumor angiogenesis, a process also referred to as vasculogenesis34-36. In contrast to the proposed maintenance function of tmTNF in ECFC in vascular repair and in angiogenesis, soluble TNF-α is predominately associated with inflammation, vascular dysfunction, and impaired repair26, and according to our group and others acts overwhelmingly through TNFR1 in endothelial cells13. Our data reported here show that removal of either tmTNF or TNFR2 causes ECFCs to lose their proliferative potential and develop premature senescence, which provides a mechanism for the observed role of TNFR2 in angiogenesis and vascular repair.
Our data demonstrate that NFκB is a key component of ECFC proliferation. This may be of relevance for anti-inflammatory therapies targeting NFκB as aggressive NFκB may reduce repair capacities of progenitor cells. Our findings are also in agreement with previous studies showing that NFκB is a regulator of cell proliferation and cell survival genes37-39 and indeed is upregulated or constitutively active in many cancers40. Importantly, NFκB has been identified previously to be downstream of TNFR241 and is even directly activated by TNFR242. Although NFκB is also downstream of TNFR1 it appears to be anti-apoptotic in this context, as it is activated by the TNF receptor-associated protein with death domain (TRADD)/TNF receptor-associated factor 2 (TRAF 2) signaling41, whereas the prototypical apoptotic caspase cascade associated with TNFR1 is downstream of TRADD/Fas-associated protein with death domain (FADD) activation43. Interestingly, a recent report demonstrates that NFκB signaling is involved in regulating the epigenetic machinery required for the nuclear reprogramming that induces pluripotency in iPSCs44, which may suggest a role for NFκB in the establishment of stemness.
While we show here that TNFR2 signaling is necessary to prevent ECFCs from becoming senescent, further studies into the mechanism behind TNFR2-dependent prevention of senescence are needed and are ongoing in our laboratory. There are several candidate regulators of senescence in endothelial cells and various progenitor cells that could be regulated by tmTNF/TNFR2 signaling, including survivin which modulates cell cycle and proliferation in CD34+ cord blood cells45 and SIRT146 which has been shown to prevent the development of senescence in endothelial cells. In this context, our previous work specifically analyzing tmTNF/TNFR2 regulated genes will be useful47. Importantly, we observed upregulation of several genes which promote angiogenesis such as connective tissue growth factor (Ctgf, or CCN2) and endothelial plasminogen activator inhibitor (Serpin E1), along with several cell signaling molecules which promote proliferation such as Akt1 and p65 NFκB.
Endothelial injury in the absence of sufficient circulating progenitor cells may affect the progression of vascular diseases, as increases in senescent vascular wall cells may lead to the inability of the endothelium to maintain a continuous functional monolayer2, 22. ECFCs normally have low levels of senescence but undergo stress induced cellular senescence when exposed to chronic inflammatory conditions, a process which is independent of telomeric shortening-dependent replicative senescence23. We show here that this process is blocked by inhibition of tmTNF-α cleavage, and indeed the mechanism of chronic inflammation-induced premature senescence involves an abrogation of tmTNF/TNFR2 signaling. This is accomplished by the activation of the tmTNF cleavage metalloprotease TACE via p38 MAP kinase as has been shown in other situations48, along with its concurrent export to the cell surface by means of increased iRhom2 expression (see schematic, Figure 8). Further investigation of this pathway remains to be done, but may involve upregulation of iNOS, as this pathway has been linked to iRhom2 expression in hepatocytes29, 49.
Figure 8. Schematic of tmTNF/TNFR2 regulation in ECFC.
Under normal conditions tmTNF signaling through TNFR2 results in NFκB-dependent proliferation in the presence of growth factor receptor (GFRs) mediated signaling (green arrows). Upon cultivation in chronic inflammatory conditions, signaling through TNFR1 results in an upregulation of iRhom2 and activation of p38 MAPK, which translocate TACE to the cell surface and activate it, respectively. TACE then cleaves tmTNF, resulting in a loss of tmTNF/TNFR2 signaling and subsequent development of senescence (red arrows).
Inflammation is involved at all stages of atherosclerosis, from the initial formation of the plaque to the time of plaque rupture resulting in acute coronary syndrome, which may explain the decreased numbers of ECFCs in diseased arteries50. Furthermore, because of the prevalence of conditions resulting in chronic inflammation such as diabetes, autoimmune diseases, and the clearly documented association between aging and chronic low level systemic inflammation,51 investigation into the mechanism of premature ECFC senescence is extremely relevant and may enable the development of novel therapies for treating vascular disease. Protecting expression of tmTNF-α in vivo could retain a highly proliferative ECFC population even during chronic inflammatory conditions, enabling continued vascular repair and more favorable prognosis in conditions such as diabetes or cardiovascular disease.
Supplementary Material
Novelty and Significance.
What Is Known?
Expression of an uncleavable transmembrane TNF-α (tmTNF) in mice enhances angiogenesis.
TNF receptor 1 (TNFR1) is anti-angiogenic, while the tmTNF-α-selective TNF receptor 2 (TNFR2) confers a survival signal, mediating angiogenic and blood vessel repair activities.
Endothelial colony forming cells (ECFCs) become prematurely senescent upon exposure to chronic inflammatory conditions.
What New Information Does This Article Contribute?
Unlike mature endothelial cells, ECFCs stably express tmTNF, which corresponds to increased proliferative capacity.
Loss of tmTNF expression or tmTNF-TNFR2 signaling results in premature senescence of ECFCs.
The mechanism of chronic inflammation-induced senescence in ECFCs is decreased tmTNF expression due to upregulation of surface expression and activity of the TNF-cleavage enzyme TACE.
ECFCs are believed to play an important role in the maintenance of healthy vasculature, and loss of ECFCs has been linked to development of atherosclerosis. Endothelial injury in the absence of sufficient ECFCs may affect the progression of vascular diseases, as increases in senescent vascular wall cells may lead to the inability of the endothelium to maintain a functional monolayer. Because of the prevalence of conditions resulting in chronic inflammation such as diabetes, autoimmune diseases, and the clearly documented association between aging and chronic low level systemic inflammation, investigation into the mechanism of premature ECFC senescence is extremely relevant and may enable the development of novel therapies for treating vascular disease. We show here that ECFCs stably express tmTNF; that tmTNF-TNFR2 signaling is essential for ECFC proliferation; and that chronic inflammation results in a loss of tmTNF and TNFR2, triggering premature senescence. This work shows a novel aspect of tmTNF biology, pinpoints a key signal for ECFC proliferation, and identifies the molecular mechanisms of inflammation-induced senescence. Protecting expression of tmTNF in patients could retain a highly proliferative ECFC population even during chronic inflammatory conditions, enabling continued vascular repair and more favorable prognosis in conditions such as diabetes or cardiovascular disease.
ACKNOWLEDGEMENTS
We also acknowledge the support of the Richard L. Roudebush Veterans Administration Medical Center.
SOURCES OF FUNDING
This study was supported by T32 HL079995 (LG), and R01HL090950 (MC), from the NIH.
Nonstandard Abbreviations and Acronyms
- tmTNF
transmembrane TNF
- ECFCs
endothelial colony forming cells
- CPC
circulating progenitor cells
- HMVECs
human microvascular endothelial cells
- HCAECs
human coronary arterial endothelial cells
- HUVECs
human umbilical vein endothelial cells
Footnotes
AUTHORSHIP
LG designed experiments, performed most research, analyzed data, and wrote the paper. VN and JM performed research. JC and MY provided valuable insights and reagents. MC and MM designed experiments.
DISCLOSURES
All authors declare to have no financial conflict of interests.
REFERENCES
- 1.Wang YX, Fitch RM. Vascular stiffness: measurements, mechanisms and implications. Curr Vasc Pharmacol. 2004;2:379–84. doi: 10.2174/1570161043385448. [DOI] [PubMed] [Google Scholar]
- 2.Op den Buijs J, Musters M, Verrips T, Post JA, Braam B, van Riel N. Mathematical modeling of vascular endothelial layer maintenance: the role of endothelial cell division, progenitor cell homing, and telomere shortening. Am J Physiol Heart Circ Physiol. 2004;287:H2651–8. doi: 10.1152/ajpheart.00332.2004. [DOI] [PubMed] [Google Scholar]
- 3.Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–60. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 4.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–9. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Issner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 6.Popa ER, Harmsen MC, Tio RA, van der Strate BW, Brouwer LA, Schipper M, Koerts J, De Jongste MJ, Hazenberg A, Hendriks M, van Luyn MJ. Circulating CD34+ progenitor cells modulate host angiogenesis and inflammation in vivo. J Mol Cell Cardiol. 2006;41:86–96. doi: 10.1016/j.yjmcc.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 7.Ingram DA, Mead LE, Moore DB, Woodard W, Fenoglio A, Yoder MC. Vessel wall derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood. 2004 doi: 10.1182/blood-2004-08-3057. [DOI] [PubMed] [Google Scholar]
- 8.Toshner M, Voswinckel R, Southwood M, Al-Lamki R, Howard LS, Marchesan D, Yang J, Suntharalingam J, Soon E, Exley A, Stewart S, Hecker M, Zhu Z, Gehling U, Seeger W, Pepke-Zaba J, Morrell NW. Evidence of dysfunction of endothelial progenitors in pulmonary arterial hypertension. American journal of respiratory and critical care medicine. 2009;180:780–7. doi: 10.1164/rccm.200810-1662OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. The New England journal of medicine. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 10.Alphonse RS, Vadivel A, Fung M, Shelley WC, Critser PJ, Ionescu L, O'Reilly M, Ohls RK, McConaghy S, Eaton F, Zhong S, Yoder M, Thebaud B. Existence, Functional Impairment and Lung Repair Potential of Endothelial Colony Forming Cells in Oxygen-Induced Arrested Alveolar Growth. Circulation. 2014 doi: 10.1161/CIRCULATIONAHA.114.009124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ingram DA, Lien IZ, Mead LE, Estes M, Prater DN, Derr-Yellin E, DiMeglio LA, Haneline LS. In vitro hyperglycemia or a diabetic intrauterine environment reduces neonatal endothelial colony-forming cell numbers and function. Diabetes. 2008;57:724–31. doi: 10.2337/db07-1507. [DOI] [PubMed] [Google Scholar]
- 12.Black RA, Rauch CT, Koziosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner J, Johnson RS, Paxton RJ, March CJ, Cerretti DP. A metalloproteinase disintegrin that releases tumour-necrosis factor-a from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 13.Clauss M, Grell M, Fangmann C, Fiers W, Scheurich P, Risau W. Synergistic induction of endothelial tissue factor by tumor necrosis factor and vascular endothelial growth factor: functional analysis of the tumor necrosis factor receptors. FEBS Lett. 1996;390:334–8. doi: 10.1016/0014-5793(96)00690-4. [DOI] [PubMed] [Google Scholar]
- 14.Grell M, Douni E, Wajant H, Lohden M, Clauss M, Maxeiner B, Georgopoulos S, Lesslauer W, Kollias G, Pfizenmaier K, Scheurich P. The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell. 1995;83:793–802. doi: 10.1016/0092-8674(95)90192-2. [DOI] [PubMed] [Google Scholar]
- 15.Schacker T. The role of secondary lymphatic tissue in immune deficiency of HIV infection. Aids. 2008;22(Suppl 3):S13–8. doi: 10.1097/01.aids.0000327511.76126.b5. [DOI] [PubMed] [Google Scholar]
- 16.Grell M, Becke FM, Wajant H, Mannel DN, Scheurich P. TNF receptor type 2 mediates thymocyte proliferation independently of TNF receptor type 1. European Journal of Immunology. 1998;28:257–63. doi: 10.1002/(SICI)1521-4141(199801)28:01<257::AID-IMMU257>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 17.Luo D, Luo Y, He Y, Zhang H, Zhang R, Li X, Dobrucki WL, Sinusas AJ, Sessa WC, Min W. Differential functions of tumor necrosis factor receptor 1 and 2 signaling in ischemia-mediated arteriogenesis and angiogenesis. The American journal of pathology. 2006;169:1886–98. doi: 10.2353/ajpath.2006.060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajashekhar G, Willuweit A, Patterson CE, Sun P, Hilbig A, Breier G, Helisch A, Clauss M. Continuous endothelial cell activation increases angiogenesis: evidence for the direct role of endothelium linking angiogenesis and inflammation. J Vasc Res. 2006;43:193–204. doi: 10.1159/000090949. [DOI] [PubMed] [Google Scholar]
- 19.Canault M, Peiretti F, Mueller C, Kopp F, Morange P, Rihs S, Portugal H, Juhan-Vague I, Nalbone G. Exclusive expression of transmembrane TNF-alpha in mice reduces the inflammatory response in early lipid lesions of aortic sinus. Atherosclerosis. 2004;172:211–8. doi: 10.1016/j.atherosclerosis.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Murasawa S, Llevadot J, Silver M, Isner JM, Losordo DW, Asahara T. Constitutive human telomerase reverse transcriptase expression enhances regenerative properties of endothelial progenitor cells. Circulation. 2002;106:1133–9. doi: 10.1161/01.cir.0000027584.85865.b4. [DOI] [PubMed] [Google Scholar]
- 21.Assmus B, Urbich C, Aicher A, Hofmann WK, Haendeler J, Rossig L, Spyridopoulos I, Zeiher AM, Dimmeler S. HMG-CoA reductase inhibitors reduce senescence and increase proliferation of endothelial progenitor cells via regulation of cell cycle regulatory genes. Circulation research. 2003;92:1049–55. doi: 10.1161/01.RES.0000070067.64040.7C. [DOI] [PubMed] [Google Scholar]
- 22.Minamino T, Miyauchi H, Yoshida T, Tateno K, Kunieda T, Komuro I. Vascular cell senescence and vascular aging. J Mol Cell Cardiol. 2004;36:175–83. doi: 10.1016/j.yjmcc.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Herbert BS, Rajashekhar G, Ingram DA, Yoder MC, Clauss M, Rehman J. Premature senescence of highly proliferative endothelial progenitor cells is induced by tumor necrosis factor-alpha via the p38 mitogen-activated protein kinase pathway. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2009;23:1358–65. doi: 10.1096/fj.08-110296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mund JA, Estes ML, Yoder MC, Ingram DA, Jr., Case J. Flow cytometric identification and functional characterization of immature and mature circulating endothelial cells. Arterioscler Thromb Vasc Biol. 2012;32:1045–53. doi: 10.1161/ATVBAHA.111.244210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajashekhar G, Traktuev DO, Roell WC, Johnstone BH, Merfeld-Clauss S, Van Natta B, Rosen ED, March KL, Clauss M. IFATS collection: Adipose stromal cell differentiation is reduced by endothelial cell contact and paracrine communication: role of canonical Wnt signaling. Stem Cells. 2008;26:2674–81. doi: 10.1634/stemcells.2008-0277. [DOI] [PubMed] [Google Scholar]
- 26.Luo YQ, Wang LH, Ma XL, Kong JX, Jiao BH. Construction, expression, and characterization of a new targeted bifunctional fusion protein: tumstatin45-132-TNF. IUBMB Life. 2006;58:647–53. doi: 10.1080/15216540600981743. [DOI] [PubMed] [Google Scholar]
- 27.Shi H, Yang W, Cui ZH, Lu CW, Li XH, Liang LL, Song E. Tracking of CFSE-labeled endothelial progenitor cells in laser-injured mouse retina. Chin Med J (Engl) 2011;124:751–7. [PubMed] [Google Scholar]
- 28.Janzen V, Forkert R, Fleming HE, Saito Y, Waring MT, Dombkowski DM, Cheng T, DePinho RA, Sharpless NE, Scadden DT. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;443:421–6. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- 29.Adrain C, Zettl M, Christova Y, Taylor N, Freeman M. Tumor necrosis factor signaling requires iRhom2 to promote trafficking and activation of TACE. Science. 2012;335:225–8. doi: 10.1126/science.1214400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aggarwal BB, Gupta SC, Kim JH. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood. 2012;119:651–65. doi: 10.1182/blood-2011-04-325225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang L, Sivashanmugam P, Wu JH, Brian L, Exum ST, Freedman NJ, Peppel K. Tumor necrosis factor receptor-2 signaling attenuates vein graft neointima formation by promoting endothelial recovery. Arterioscler Thromb Vasc Biol. 2008;28:284–9. doi: 10.1161/ATVBAHA.107.151613. [DOI] [PubMed] [Google Scholar]
- 32.Horiuchi T, Mitoma H, Harashima S, Tsukamoto H, Shimoda T. Transmembrane TNF-alpha: structure, function and interaction with anti-TNF agents. Rheumatology (Oxford) 2010;49:1215–28. doi: 10.1093/rheumatology/keq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clauss M, Sunderkotter C, Sveinbjornsson B, Hippenstiel S, Willuweit A, Marino M, Haas E, Seljelid R, Scheurich P, Suttorp N, Grell M, Risau W. A permissive role for tumor necrosis factor in vascular endothelial growth factor-induced vascular permeability. Blood. 2001;97:1321–9. doi: 10.1182/blood.v97.5.1321. [DOI] [PubMed] [Google Scholar]
- 34.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–6. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 35.Kopp HG, Ramos CA, Rafii S. Contribution of endothelial progenitors and proangiogenic hematopoietic cells to vascularization of tumor and ischemic tissue. Curr Opin Hematol. 2006;13:175–81. doi: 10.1097/01.moh.0000219664.26528.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Critser PJ, Yoder MC. Endothelial colony-forming cell role in neoangiogenesis and tissue repair. Curr Opin Organ Transplant. 2010;15:68–72. doi: 10.1097/MOT.0b013e32833454b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature. 1995;376:167–70. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- 38.May MJ, Ghosh S. Signal transduction through NF-kappa B. Immunol Today. 1998;19:80–8. doi: 10.1016/s0167-5699(97)01197-3. [DOI] [PubMed] [Google Scholar]
- 39.Kanegae Y, Tavares AT, Izpisua Belmonte JC, Verma IM. Role of Rel/NF-kappaB transcription factors during the outgrowth of the vertebrate limb. Nature. 1998;392:611–4. doi: 10.1038/33429. [DOI] [PubMed] [Google Scholar]
- 40.Newton TR, Patel NM, Bhat-Nakshatri P, Stauss CR, Goulet RJ, Jr., Nakshatri H. Negative regulation of transactivation function but not DNA binding of NF-kappaB and AP-1 by IkappaBbeta1 in breast cancer cells. J Biol Chem. 1999;274:18827–35. doi: 10.1074/jbc.274.26.18827. [DOI] [PubMed] [Google Scholar]
- 41.Rothe M, Sarma V, Dixit VM, Goeddel DV. TRAF2-mediated activation of NF-kappa B by TNF receptor 2 and CD40. Science. 1995;269:1424–7. doi: 10.1126/science.7544915. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez M, Cabal-Hierro L, Carcedo MT, Iglesias JM, Artime N, Darnay BG, Lazo PS. NF-kappaB signal triggering and termination by tumor necrosis factor receptor 2. J Biol Chem. 2011;286:22814–24. doi: 10.1074/jbc.M111.225631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chinnaiyan AM, O'Rourke K, Tewari M, Dixit VM. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–12. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 44.Lee J, Sayed N, Hunter A, Au KF, Wong WH, Mocarski ES, Pera RR, Yakubov E, Cooke JP. Activation of innate immunity is required for efficient nuclear reprogramming. Cell. 2012;151:547–58. doi: 10.1016/j.cell.2012.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fukuda S, Foster RG, Porter SB, Pelus LM. The antiapoptosis protein survivin is associated with cell cycle entry of normal cord blood CD34(+) cells and modulates cell cycle and proliferation of mouse hematopoietic progenitor cells. Blood. 2002;100:2463–71. doi: 10.1182/blood.V100.7.2463. [DOI] [PubMed] [Google Scholar]
- 46.Zu Y, Liu L, Lee MY, Xu C, Liang Y, Man RY, Vanhoutte PM, Wang Y. SIRT1 promotes proliferation and prevents senescence through targeting LKB1 in primary porcine aortic endothelial cells. Circulation research. 2010;106:1384–93. doi: 10.1161/CIRCRESAHA.109.215483. [DOI] [PubMed] [Google Scholar]
- 47.Rajashekhar G, Grow M, Willuweit A, Patterson CE, Clauss M. Divergent and convergent effects on gene expression and function in acute versus chronic endothelial activation. Physiol Genomics. 2007;31:104–13. doi: 10.1152/physiolgenomics.00157.2006. [DOI] [PubMed] [Google Scholar]
- 48.Xu P, Derynck R. Direct activation of TACE-mediated ectodomain shedding by p38 MAP kinase regulates EGF receptor-dependent cell proliferation. Mol Cell. 2010;37:551–66. doi: 10.1016/j.molcel.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chanthaphavong RS, Loughran PA, Lee TY, Scott MJ, Billiar TR. A role for cGMP in inducible nitric-oxide synthase (iNOS)-induced tumor necrosis factor (TNF) alpha-converting enzyme (TACE/ADAM17) activation, translocation, and TNF receptor 1 (TNFR1) shedding in hepatocytes. J Biol Chem. 2012;287:35887–98. doi: 10.1074/jbc.M112.365171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 51.Tracy RP. Emerging relationships of inflammation, cardiovascular disease and chronic diseases of aging. Int J Obes Relat Metab Disord. 2003;27(Suppl 3):S29–34. doi: 10.1038/sj.ijo.0802497. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.