Abstract
Rationale
Endothelial injury is an initial mechanism mediating cardiovascular disease.
Objective
Here, we investigated the effect of hyperhomocysteinemia (HHcy) on programed cell death in endothelial cells (EC).
Methods and Results
We established a novel flow-cytometric gating method to define pyrotosis (Annexin V−/Propidium iodide+). In cultured human EC, we found that: 1). Hcy and Lipopolysaccharide (LPS) individually and synergistically induced inflammatory pyroptotic and non-inflammatory apoptotic cell death. 2). Hcy/LPS induced caspase-1 activation prior to caspase-8, -9, -3 activations. 3). Caspase-1/3 inhibitors rescued Hcy/LPS-induced pyroptosis/apoptosis, but caspase-8/9 inhibitors had differential rescue effect. 4). Hcy/LPS induced NLRP3 protein, caused NLRP3-containing inflammasome assembly, caspase-1 activation and IL-1β cleavage/activation. 5). Hcy/LPS elevated intracellular reactive oxidative species (ROS). 6). Intracellular oxidative gradient determined cell death destiny as intermediate intracellular ROS levels are associated with pyroptosis, whereas, high ROS corresponded to apoptosis. 7). Hcy/LPS induced mitochondrial membrane potential collapse and cytochrome-c release, and increased Bax/Bcl-2 ratio which were attenuated by antioxidants and caspase-1 inhibitor. 8). Antioxidants extracellular superoxide dismutase and catalase prevented Hcy/LPS-induced caspase-1 activation, mitochondrial dysfunction and pyroptosis/apoptosis. In cystathionine β-synthase deficient (Cbs−/−) mice, severe HHcy induced caspase-1 activation in isolated lung EC and caspase-1 expression in aortic endothelium, and elevated aortic caspase-1,9 protein/activity and Bax/Bcl-2 ratio in Cbs−/− aorta and HUVEC. Finally, Hcy-induced DNA fragmentation was reversed in caspase-1−/− EC. HHcy-induced aortic endothelial dysfunction was rescued in caspase-1−/− and NLRP3−/− mice.
Conclusion
HHcy preferentially induces EC pyroptosis via caspase-1-dependent inflammasome activation leading to endothelial dysfunction. We termed caspase-1 responsive pyroptosis and apoptosis as pyrop-apoptosis.
Keywords: Homocysteine, endothelial cells, pyroptosis, apoptosis, caspase-1, caspase activation
INTRODUCTION
Hyperhomocysteinemia (HHcy) is an independent risk factor for cardiovascular disease (CVD).1–3 We and others have reported that HHcy promotes atherosclerosis4 via inhibiting endothelial cell (EC) growth,5 leading to impaired post-injury endothelial repair, vascular remodeling, atherosclerosis,4–7 and endothelial dysfunction8, inhibits HDL biosynthesis and promotes inflammatory monocyte differentiation.9–11
Endothelial injury is the initial event of atherogenesis.12 Homocysteine (Hcy) may cause apoptosis in ECs,13, 14 which disrupts the integrity of the endothelium. It is believed that apoptosis can be mediated by three pathways, including 1) intrinsic (mitochondrial-mediated), 2) extrinsic (death receptor-mediated), and 3) endoplasmic reticulum (ER) stress-derived signaling pathway.15, 16,17 All these three pathways lead to caspase-3 activation and finally apoptosis.16
Recently, pyroptosis is recognized as a new type of inflammatory programed cell-death (PCD). Unlike apoptosis, pyroptosis responses to pathogen associated molecular patterns (PAMPs), results in the release of cytokines and is initially described as antimicrobial reaction in immune cells.18, 19 Pyroptosis is initiated by the binding of intracellular pathogen to NOD-like receptors (NLRs), leading to the formation of inflammasome, in which pro-caspase-1 complexes with inflammasome-adaptor protein adaptor-apoptosis-associated speck-like protein containing a CARD (ASC) and become activated caspase-1. Activated caspase-1 can cleave proinflammatory cytokines interleukin (IL)-1β and IL-18 resulting in their maturation.20, 21 Inflammasome can also sense danger associated molecular patterns (DAMPs) in the absence of pathogenic challenge, such as glucose,22 oxidized low density lipoprotein and cholesterol crystals,23 or ion channel efflux,24 and lysosome destabilization.25 However, how metabolic DAMPs cause pyroptosis is largely unknown. It was reported that Hcy induced apoptosis in EC.26 HHcy-induced pyroptosis has not been studied.
We and others have demonstrated that HHcy inhibits EC growth and vascular repair, and causes vascular inflammation by promoting inflammatory monocytes differentiation and infiltration.5–7, 9, 10, 27 The contribution of endothelial inflammation to vascular diseases in HHcy is unknown. In this study, we investigated the effect of Hcy on PCD. Our study support the hypothesis that Hcy is as an endogenous metabolic stress stimuli and is able to activate inflammatory pyroptosis and non-inflammatory apoptosis in ECs.
METHODS
(Details in Supplement Methods).
Mouse model
All mice are in C57B/L6 background. hCBC/Cbs−/− mice were used as an HHcy mouse model.9 Caspase-1 and NLRP3 deficient mice (Casp-1−/−, NLRP3−/−) were provided by Dr. Richard Flavell (Yale University School of Medicine).
Hcy plasma level measurement
Plasma Hcy level was analyzed by LC-ESI-MS/MS.11
Cell culture
HUVEC28 and mouse endothelial cell were used.8
Cell viability was determined by crystal violet staining.26
Immunostaining and Immunoblot
Mouse aorta was collected for cryostat sections (immunostaining) or West Blotting.29
Co-Immunoprecipitation
HUVEC extracts were used for co-immunoprecipitation.30
Mitochondrial potential measurement
Mitochondrial membrane potential (Δψm) was assessed by JC-1 staining31 and cytochrome-c releasing.32
Pyroptosis/apoptosis, and ROS levels
Flow cytometry (FCM) was utilized to detect pyroptosis/apoptosis by Annexin V/PI/7-AAD staining and intracellular reactive oxygen species (ROS) by Dihydroethidium (DHE) staining.
Caspase activity was assayed with APO LOGIX kit (Cell Technology).33
Rescue efficacy score of caspase inhibitors
Cells was pretreated by caspase inhibitors 2hr prior to Hcy/LPS treatment. Rescue efficacy score were calculated.
Vascular relaxation responses was determined as described previously.8, 28, 34
RESULTS
Pyroptosis cells exhibited low levels of caspase-9 activation and were Annexin V
We featured PCD by using Annexin V and 7-amino-actinomycin (7AAD) staining and justified with caspase-1/-9 activity staining (Fig. 1A). Necrosis were excluded from intact cells based on small size (FSC) and minimal DNA florescence, which justify debris and residuals of necrotic cells (Fig. 1A, online Figure II).36 Annexin V labels phophatidylserine, an internal membrane component flipped to the external membrane at early stage of apoptosis (Q2) while cell membrane integrity maintained as 7AAD or propidium iodide (PI, equivalent to 7AAD) negative. In the later stage of apoptosis (Q3), apoptotic cells loss cell membrane integrity and are stained for 7AAD or PI, as it enters through un-intact plasma membranes to nuclear and binds to double stranded DNA. We found that caspase-9 activation, an established marker for apoptosis and core component of apoptosome,37 was significantly higher in Q2 in control and H2O2 treated cells (531% and 557%), further increased in Q3 (1090% and 1116%), but much lower in Q4 (191% and 237%). However, caspase-1 activation, an essential feature for pyroptosis and also observed in apoptosis,38 was high in Q4, Q3 and Q2 (Q4=426% and 662%, Q3=693% and 864%, and Q2=306% and 369% in control and H2O2 treated cells). Based on the differential levels of caspase-9 activation, we defined the Q4 cells as pyroptosis (low caspase-9 activity), and the Q2 and Q3 cells (high caspase-9 activity) apoptosis. Our data support the notion that caspase-1 activation is a common feature of PCD (pyroptosis and apoptosis), and that high levels of caspase-9 activation is a distinguished functional marker of pyroptosis. The Q4 pyroptosis cells are Annexin V− with phophatidylserine preserved at the internal membrane.
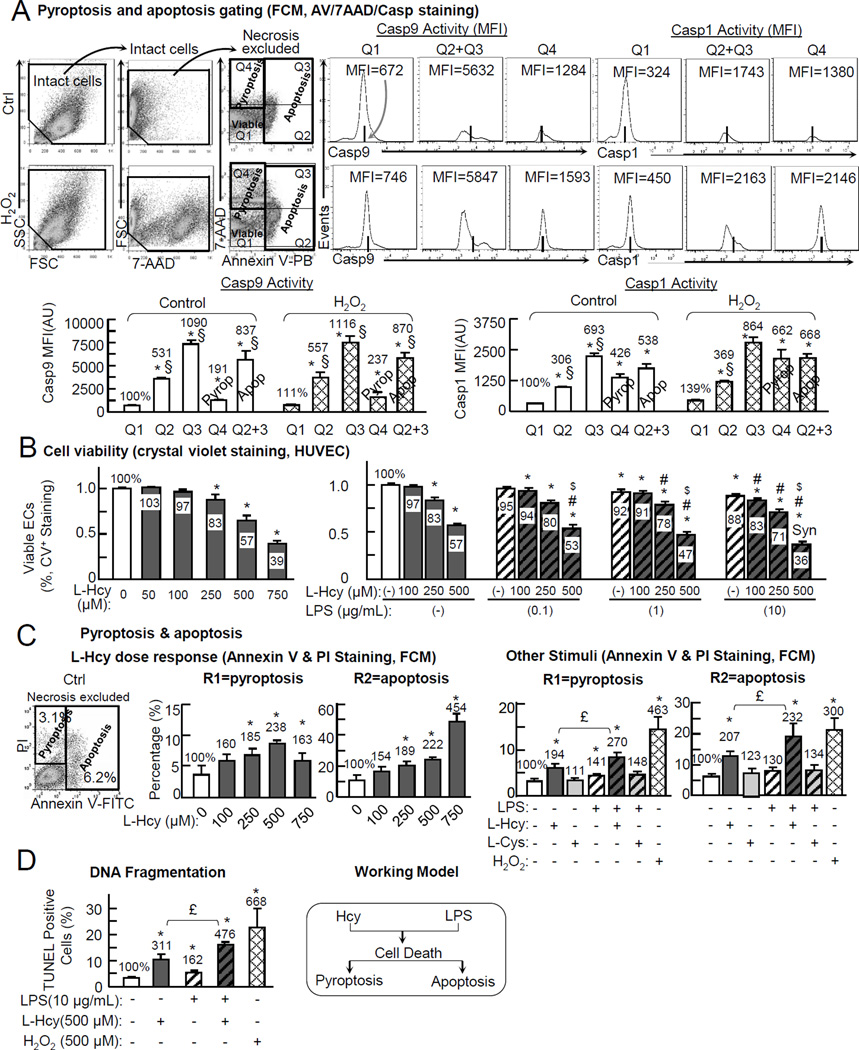
Figure 1. Hcy suppresses cell viability, induces pyroptosis/apoptosis in ECs. HUVECs were cultured to 80% confluence, synchronized (serum free, 6hr), and then switched to 0.5%FBS medium containing L-Hcy, L-Cys, LPS, and H2O2 (500µM) for 24hr as indicated.
A, Pyroptosis and apoptosis gating (FCM, AV/7AAD/Casp staining). Cells were treated and stained by AnnexinV-Pacific Blue and 7AAD, and Casp1/9 activity kit for FCM analysis (dot plot shown in online Figure II). Necrotic cells were excluded from intact cells by their content of nuclear debris. Casp9 activity was used as a marker of apoptosis. Q2+Q3 cells were define as apoptosis. Q4 was defined as pyroptosis. B, Cell viability. Cells in 96-well plate were fixed, stained by crystal violet for cell viability. C, Hcy/LPS induced Pyroptosis/apoptosis (dot plot shown in online Figure IV). Cell death forms were identified by FCM using Annexin V-FITC/PI staining (dot plot shown in online Figure II). D, DNA fragmentation. DNA fragmentation was determined by TUNEL staining (images shown in online Figure V). Data are representative of 4 separated experiments and presented as Mean±SEM. Value on the top of bars are normalized by the mean of the control or Q1. *, P<0.05 vs control; #, P<0.05 vs LPS alone in same group; $, P<0.05 vs same dose of Hcy alone; Syn is synergy effect. £, p<0.05. §, p<0.05 vs Q4. FCM, flow cytometry.
Hcy- and/or LPS- induced pyroptosis and apoptosis in EC
We found that Hcy (250, 500 and 750 µM) reduced cell viability to 83%, 57% and 39% dose sensitively in HUVEC. Cysteine, a sulfhydryl (thiol) amino acid control, had no such effect (Fig. 1B, online Figure III). Lipopolysaccharide (LPS), a pro-inflammatory endotoxin reported to induce endothelial inflammation and cell death,35 is less potent and reduced cell viability to 92% and 83% at the concentration of 1 and 10 µg/ml. The addition of LPS worsened Hcy cytotoxicity. The combination of Hcy (500 µM) and LPS (10 µg/ml) synergistically reduced cell viability to 36% compared to their individual effect (57% and 88%) (Fig. 1B).
Using our novel getting strategy, we observed that Hcy (100, 250, and 500µM) induced pyroptosis/apoptosis dose sensitively in HUVEC. 500 µM Hcy induced pyroptosis from 3.7% to 8.8% (238%), and apoptosis cell population from 10.8% to 23.9% (222%) (Fig. 1C, dot plots shown in online Figure IV). Higher dose of Hcy (750µM) further increased apoptosis to 48.9% (454%), but had less effect on pyroptosis compared with 500µM Hcy. We used the dosage of 500 µM Hcy and 10 µg/ml LPS for the following mechanistic study as this combination caused synergistic suppressive effect on cell viability (Fig. 1B). Hcy induced pyroptosis/apoptosis to 194%/207%. LPS slightly induced pyroptosis/apoptosis to 141%/130%. Hcy+LPS further increased pyroptosis/apoptosis to 270%/230%. H2O2 (500 µM), as a positive control, induced pyroptosis (463%) and apoptosis (300%) (Fig. 1C).
We also examined DNA fragmentation, a common feature of late stage of pyroptosis and apoptosis, with TUNEL assay.39 DNA fragmentation was increased to 311% by Hcy, to 162% by LPS and to 476% by Hcy+LPS (Fig. 1D, images shown in online Figure V).
Hcy/LPS induced caspase-1 activation prior to caspase-8/9/3 activations in EC
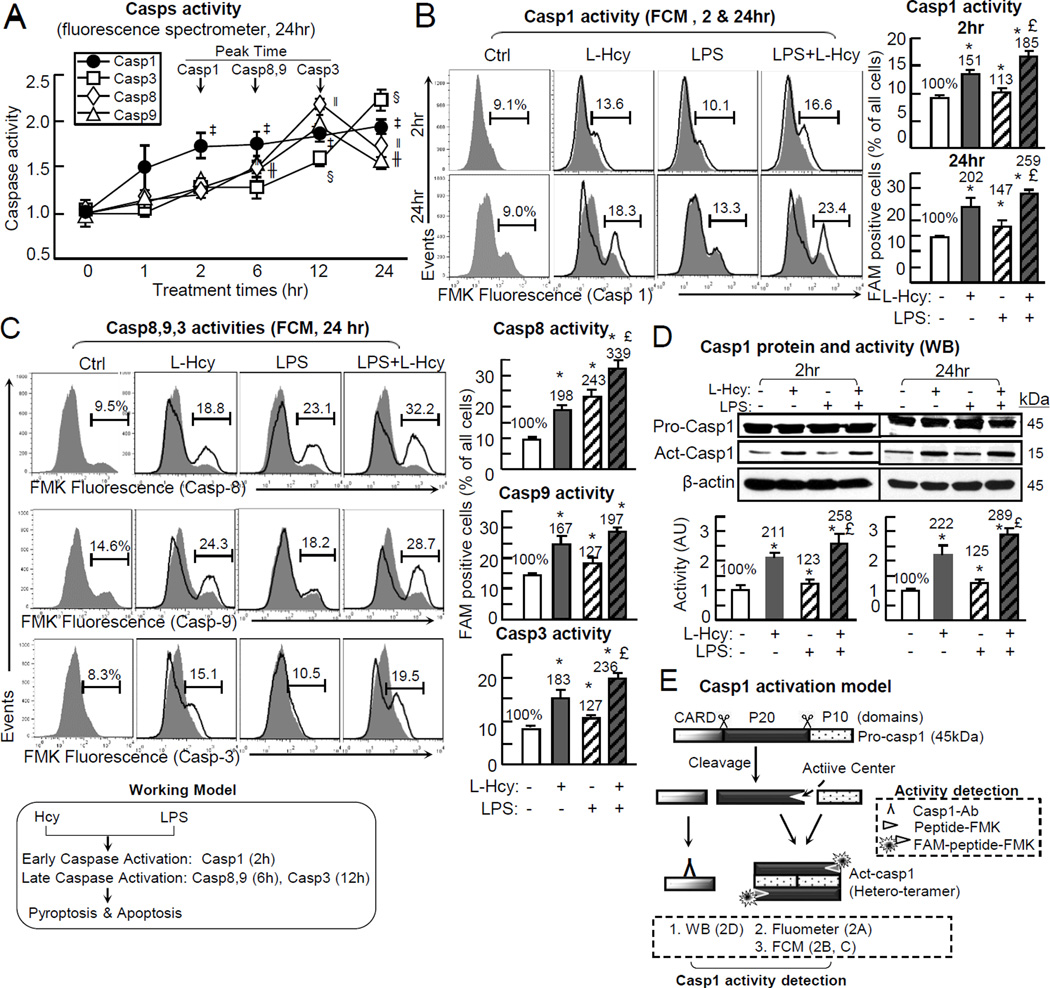
Pyroptosis and apoptosis are both potentially dependent on caspases activation.15, 38 We examined the individual and combinatory effect of Hcy and/or LPS on caspase-1/8/9/3 activation by using three alternative approaches, fluorimeter and flow cytometry using FAM-peptide-FMK staining, and immunoblotting using antibody recognizing cleaved caspase-1.
We modeled caspase activation using caspase-1 as an example. Pro-caspase-1 protein zymogen has 404 amino acid (45KD), and can be cleaved to generate a P20 and a P10 subunits, which form a hetero-tetrameric active enzymatic center (Fig. 2E).40 Caspase activity can be detected either by using a FAM-peptide-FMK probe (Fig. 2B/2D/2E) or by domain recognizing antibodies (Fig. 2C). The peptide binds to active center of caspase. The fluoromethyl ketone (FMK) moiety interacts with the cysteine located in the active center. The carboxyfluorescein (FAM) labels peptide-FMK and irreversibly generates green fluorescence (images shown in online Figure VI).40 We found Hcy+LPS induced caspase-1 activation at 2hr of the treatment and persisted till 24hr of treatment. Caspase-8 and -9 activation appeared at 6hr and peaked at 12hr. Caspase-3 activation started at 12hr and further increased at 24hr (Fig. 2A). Early and long lasting caspase-1 activation was confirmed by immunoblotting analysis (Fig. 2D). Hcy, LPS and Hcy+LPS increased the cleaved/activated caspase-1 to 211%, 123% and 258% at 2hr, and to 222%, 125% and 289% at 24hr of treatment (Fig. 2D). Alternatively, by using flow cytometry with FAM-peptide-FMK staining, we confirmed caspase-1 activation in identical condition to similar extend (Fig. 2B). Hcy induced caspase-1 activation in 13.6% of EC (a 151% induction) compared to 9.1% (100%) in the control. LPS induced caspase-1 activation to 10.1% (113%) and Hcy+LPS to 16.6% of EC (185%) by 2hr. At 24hr treatment, caspase-1 activation was further increased to 18.3% by Hcy (202%), to 13.3% of EC (147%) by LPS and to 23.4% (259%) by Hcy+LPS (Fig. 2B). Hcy, LPS, and Hcy+LPS induced caspase-8 activation to 18.8%, 23.1% and 32.2% compared to 9.5% in the control. Caspase-9 activation was increased to 24.3%, 18.2% and 28.7% of EC compared to 14.6% in the control. Caspase-3 activation was increased to 15.1%, 10.5% and 19.5% of EC compared to 8.3% in the control (Fig. 2C).In addition, we observed that Hcy and H2O2 induced caspase-1 activation in a dose sensitively manner (online Figure VII).
Figure 2. Hcy activates endothelial casp1 prior to casp8,9,3 activations in the presence/absence of LPS.
HUVECs were cultured and treated with L-Hcy (500µM) and/or LPS (10 µg/mL) as described in Fig. 1. Casp1,8,9,3 activities were measured by using a manufacture’s kit and Western Blotting respectively. Caspase activated cells were FAM positive and labeled by green fluorescence (images shown in online Figure VI). A, Casps Activity (24hr). Casp1,8,9,3 activities were detected by fluorescence spectrometry through a 0~24hr time-course. See supplement Table 1 for values and statistical analysis. B, Casp1 activity (FCM, 2 & 24Hr). Casp1 activity was examined after 2hr and 24hr treatment by FCM. C, Casp8,9,3 activities (FCM, 24 Hr). Casp8,9,3 activities were detected by FCM. D, Casp1 protein and activity. A 20kDa cleavage fragment indicated activated Casp1 and detected by WB, after 2hr and 24hr L-Hcy incubation, with or without LPS presence. E, Schematic sketch show casp1 activation and detection by WB, Fluorometer or FCM. *, p<0.05 vs control; £, p<0.05 vs L-Hcy treatment alone. In A, ‡, p<0.05 vs Casp1 activity at 0hr; ‖, p<0.05 vs Casp8 activity at 0hr; ╫, p<0.05 vs Casp9 activity at 0hr; §, p<0.05 vs Casp3 activity at 0hr. Values are Mean±SEM; n=4.
Caspase-1 mediates Hcy/LPS- induced pyroptosis/apoptosis and the downstream caspase-9 and -3 activation in EC
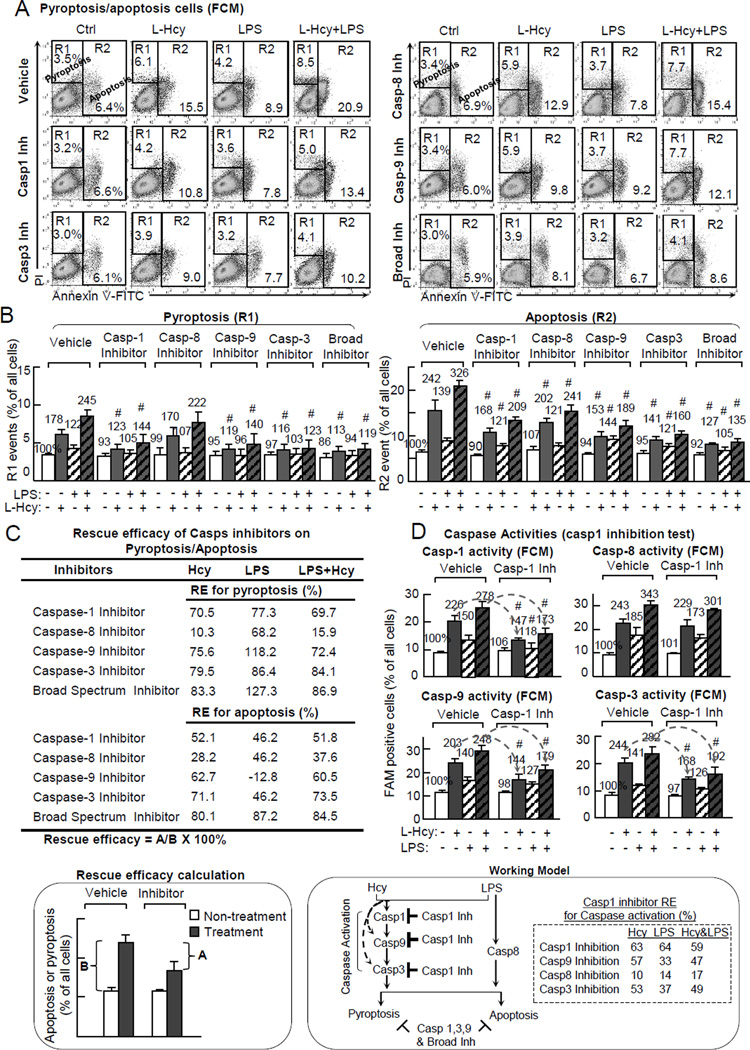
To define the role of each caspase on Hcy-induced pyroptosis/apoptosis, we tested the effect of caspase inhibitors on preventing Hcy-induced cell death. As shown in Fig. 3A & B, caspase-1 inhibitor decreased Hcy-induced pyroptosis from 178% to 123% and apoptosis from 242% to 168%, and Hcy+LPS-induced pyroptosis from 245% to 144% and apoptosis from 326% to 209%. Caspase-9 inhibitor rescued Hcy-induced pyroptosis from 178% to 119% and apoptosis from 242% to 144%, and reduced Hcy+LPS-induced pyroptosis from 245% to 140% and apoptosis from 326% to 189%. Caspase-3 inhibitor reversed Hcy-induced pyroptosis from 178% to 116% and apoptosis from 242% to 141%, and Hcy+LPS-induced pyroptosis from 245% to 123% and apoptosis from 326% to 160%. A broad spectrum inhibitor almost eliminated Hcy- and/or LPS-induced pyprotosis/apoptosis. It decreased Hcy-induced pyroptosis from 178% to 113% and apoptosis from 242% to 127%, and Hcy+LPS-induced pyroptosis from 245% to 119% and apoptosis from 326% to 135%. Notably, caspase-8 inhibitor has much less or no effect on Hcy-, LPS- or Hcy+LPS-induced pyroptosis/apoptosis.
Figure 3. Caspase-1 mediates Hcy induced pyroptosis/apoptosis and casp9/3 activities in presence/absence of LPS.
HUVEC was pretreated by indicated caspase inhibitors for 30m, and then treated with L-Hcy (500µM) and/or LPS (10 µg/mL) for additional 24hr as described in Fig. 1. Cell death forms and caspase activities were determined as described in Fig. 1/2. A, Pyroptosis/apoptosis population (caspase inhibition test). Representative dot plots of Annexing V-FITC/PI staining. R1 was considered as pyroptosis, and R2 as apoptosis. B, Quantification. C, Rescue efficacy on Hcy/LPS-induced pyroptosis/apoptosis (RE=A/B × 100%). Rescue efficacy (RE) for each inhibitor was calculated for their inhibitory capacity to pyrotosis/apoptosis. D, Caspase activities (Casp1 inhibitor). Cells were pretreated with casp1 inhibitor and assayed for casp1,8,9,3 activities by FCM. Numbers above each bar is the percentage normalized by the mean of control. #, P<0.05 vs vehicle with same treatment. Arrows indicate the direction of significant changes. Values are Mean±SEM; n=4. RE, rescue efficacy.
We established a parameter of rescue efficacy (RE) to quantitatively address the capacity of inhibitor on rescuing pyroptosis/apoptosis (Fig. 3C). The RE of broad spectrum inhibitor on Hcy-, LPS-, and LPS-induced pyroptosis/apoptosis are 83.9%/80.1%, 127.3%/87.2%, and 86.9%/84.5%, respectively. Caspases-1, -9, and -3 inhibitors are less potent but still effectively prevented Hcy-, LPS-, and LPS-induced pyroptosis/apoptosis (RE: 70.5%/52.1%, 75.6%/62.7%, and 79.2%/71.1% for Hcy-induced pyroptosis/apoptosis, 77.3%/46.2%, 118.2%/-12.8%, and 86.4%/49.2% for LPS-induced pyroptosis/apoptosis, and 69.7%/51.8%, 72.4%/60.5%, and 84.1%/73.5% for Hcy+LPS-induced pyroptosis/apoptosis). Caspase-8 had minimal RE, ranging from 10.3%/28.2% to 15.9%/37.69%, for Hcy and Hcy+LPS induced pyroptosis/apoptosis.
To further confirm that caspase-1 activation is at the upstream of caspase cascade, we tested the effect of caspase-1 inhibitor on Hcy and Hcy+LPS-induced caspase activation (Fig. 3D). Caspase-1 inhibitor suppressed Hcy-, LPS-, and Hcy+LPS-induced caspase-9 activation from 203%/140%/248% to 144%/127%/179%, caspase-3 activation from 244%/141%/282% to 168%/126%/192%, and had no effect on casapse-8 activation (243%/185%/343% to 229%/173%/301%).
Hcy/LPS induces inflammasome assembly, leading to caspase-1 activation, and IL-1β cleavage in EC
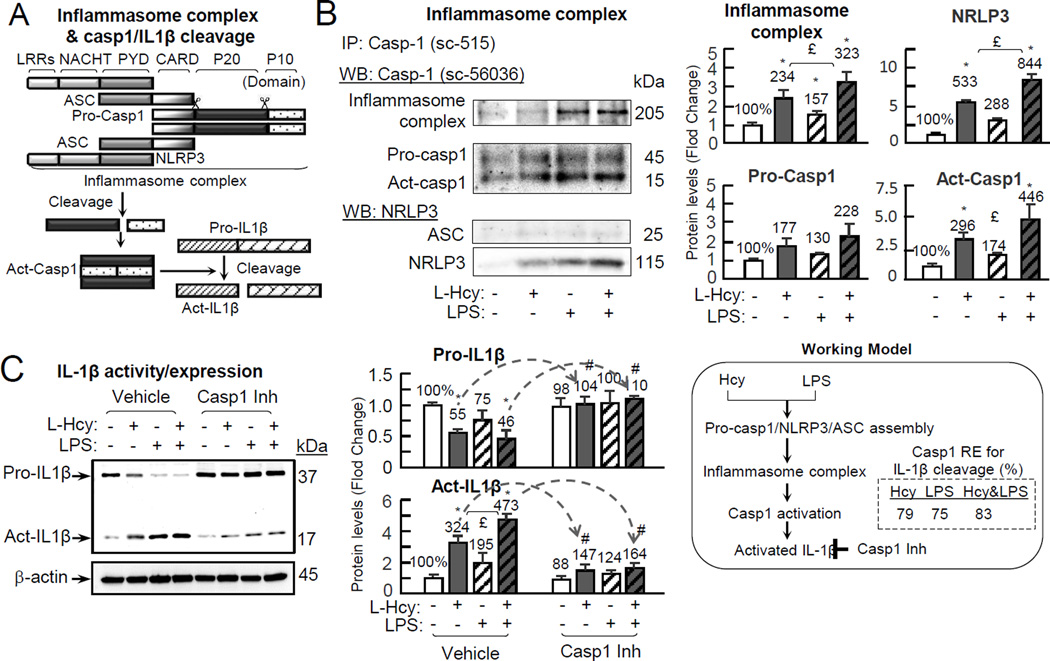
To assess whether Hcy/LPS induce inflammasome assembling and caspase-1 activation, we characterized caspase-1 complex by co-immunoprecipitation. Inflammasome is a protein complex containing pro-caspase-1 which binds to ASC, which binds to Nucleotide-binding oligomerization domain, and Leucine rich Repeat and Pyrin domain containing Protein 3 (NLRP3) to form a protein complex (≥205kDa) (Fig. 4A).21 As described in Fig. 2E, inflammasome performs autocatalytic function to cleave pro-caspase-1 into an 11 kDa pro-domain (aa 1-103), a 20 kDa (p20) and a 10 kDa (p10) subunits. Hcy, but not LPS, increased a 205 kDa caspase-1 protein complex/inflammasome (234% induction), and a 20 kDa activated caspase-1 (296% induction) in caspase-1 complex. LPS potentiated Hcy effects on inflammasome assembling and caspase-1 activation as that was increased to 234% and 323% (Fig. 4B). Hcy, LPS and Hcy+LPS increased NLRP3 content in caspase-1 complex to 533%, 288% and 844% (Fig. 4B). It is known that activated caspase-1 cleaves precursor IL-1β (37kDa) and produce an activated IL-1β (17kDa). We assessed IL-1β cleavage and found that precursor IL-1β was reduced to 55% by Hcy treatment, which was further potentiated by LPS treatment to 46%, while activated IL-1β was increased to 324% by Hcy and 473% by LPS. Caspase-1 inhibitor largely reversed Hcy and Hcy+LPS induced IL-1β activation from 324% and 473% to 147% and 164% (Fig. 4C).
Figure 4. Hcy induces inflammasome assembly, leading to casp1 activation, and IL-1β cleavage in the presence/absence of LPS.
Celle were treated with L-Hcy (500µM)/LPS (10 µg/mL) as described in Fig. 1. Precursor and substrate of casp1, component of inflammasome and IL-1β were detected by IP and WB, respectively. A, Schematic sketch for Inflammasome complex assembly and cleavage. B, Inflammasome complex analysis. After Hcy/LPS treatment, cell lysate was used for IP and WB. C, IL-1β expression and activation. Cells were pretreated with Casp1 inhibitor for 30m prior to Hcy/LPS treatment. Protein levels were normalized by β-actin density. Numbers above on each bar is the percentage normalized by the mean of control. Arrows indicate the direction of significant changes. *, p < 0.05 vs control in same group; £, p < 0.05 vs Hcy treatment in same group; #, p < 0.05 vs same treatment in vehicle. LRRs: leucine-rich repeat; NACHT, acronym standing for NAIP (neuronal apoptosis inhibitor protein); PYD, pyrin domain; CARD, caspase activation and recruitment domain; P20, protein 20; P10, protein 10; Act-IL-1β, activated interleukin-1β. Values are Mean±SEM; n=4.
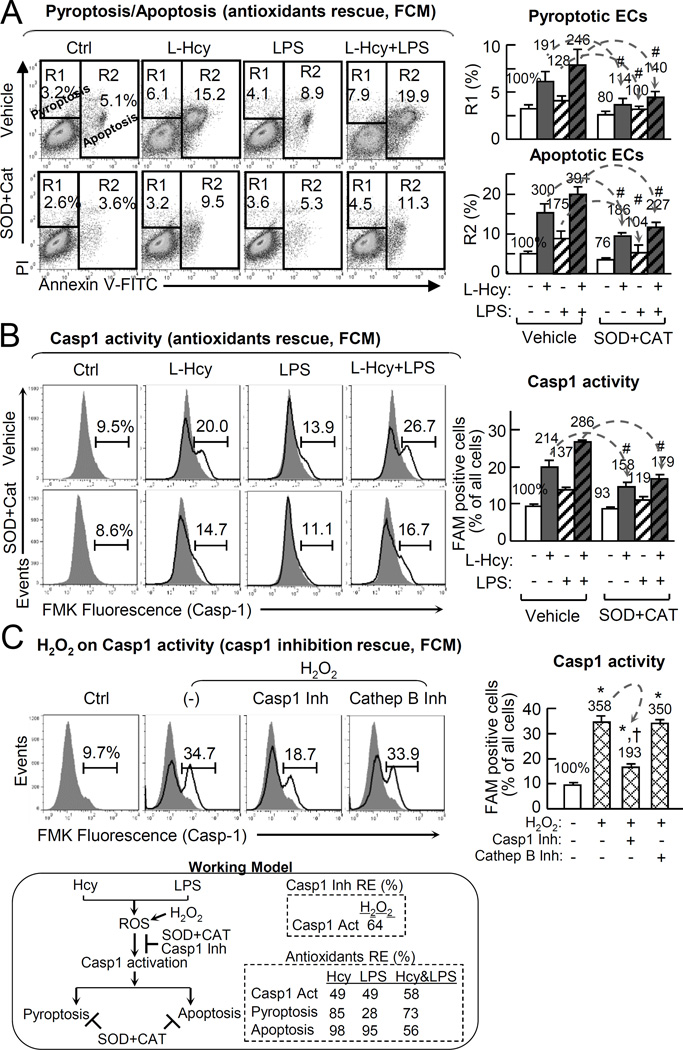
Antioxidants prevents Hcy/LPS-induced pyroptosis/apoptosis and caspase-1 activation in EC
It was suggested that caspase-1 can be activated by danger signals including intracellular reactive oxygen species (ROS),18, 41 We examine the role of ROS in inducing Hcy-induced PCD by the combination of two antioxidants, adenoviral transduced ec-SOD (online Figure IX) and PEG-catalase. Pretreatment of antioxidants reversed Hcy-induced pyroptosis from 191% to 114%, LPS pyroptosis from 128% to 100%, and Hcy+LPS pyroptosis from 246% to 140%. Antioxidants inhibited Hcy-induced apoptosis from 300% to 186%, LPS apoptosis from 175% to 104%, and Hcy+LPS apoptosis from 391% to 227% (Fig. 5A). Similarly, antioxidants reversed Hcy-induced caspase-1 activation from 214% to 158%, LPS-induced that from 137% to 119%, and Hcy+LPS-induced that from 286% to 179% (Fig. 5B). The role of ROS in caspase-1 activation was further validated by using H2O2, which induced cells with activated caspase-1 from 9.7% (100%) to 34.7% (358%). H2O2-induced caspase-1 activation was reversed by caspase-1 inhibitor from 34.7% to 18.1% (193%). Cathepsin B inhibitor had no effect on H2O2-induced caspase-1 activation (Fig. 5C).
Figure 5. Hcy derived ROS trigger endothelial casp1 activation.
HUVECs were cultured in 6cm dishes as in Fig.1. A&B, Cells were transduced by adenoviral ec-SOD (1 MOI) for 48hr, treated with PEG-catalase (25mg/mL) for 30m with refreshed medium, and then with L-Hcy (500µM) and/or LPS (10 µg/mL) for additional 24hr before subjected for cell death and casp1 activity analysis. A, Pyroptosis/apoptosis (anti-oxidants rescue, FCM). B, Casp1 activity (anti-oxidants rescue, FCM). C, H2O2 on Casp1 activity (casp1 inhibition rescue, FCM). Cells were pretreated with casp1 inhibitor or inhibitor of cathepsin B (a lysosomal cysteine protease) for 30m, and then treated with H2O2 (500 µM) for additional 24 hours before subjected for casp1 activity analysis. Numbers above on each bar is the percentage normalized by the mean of control. Data are representative of 4 separated experiments and presented as Mean±SEM. #, P<0.05 vs vehicle in A, B; *, P<0.05 vs non-treatment control; †, P<0.05 vs H2O2 treatment alone in C. Arrows indicate the direction of significant changes. Values are Mean±SEM; n=4.
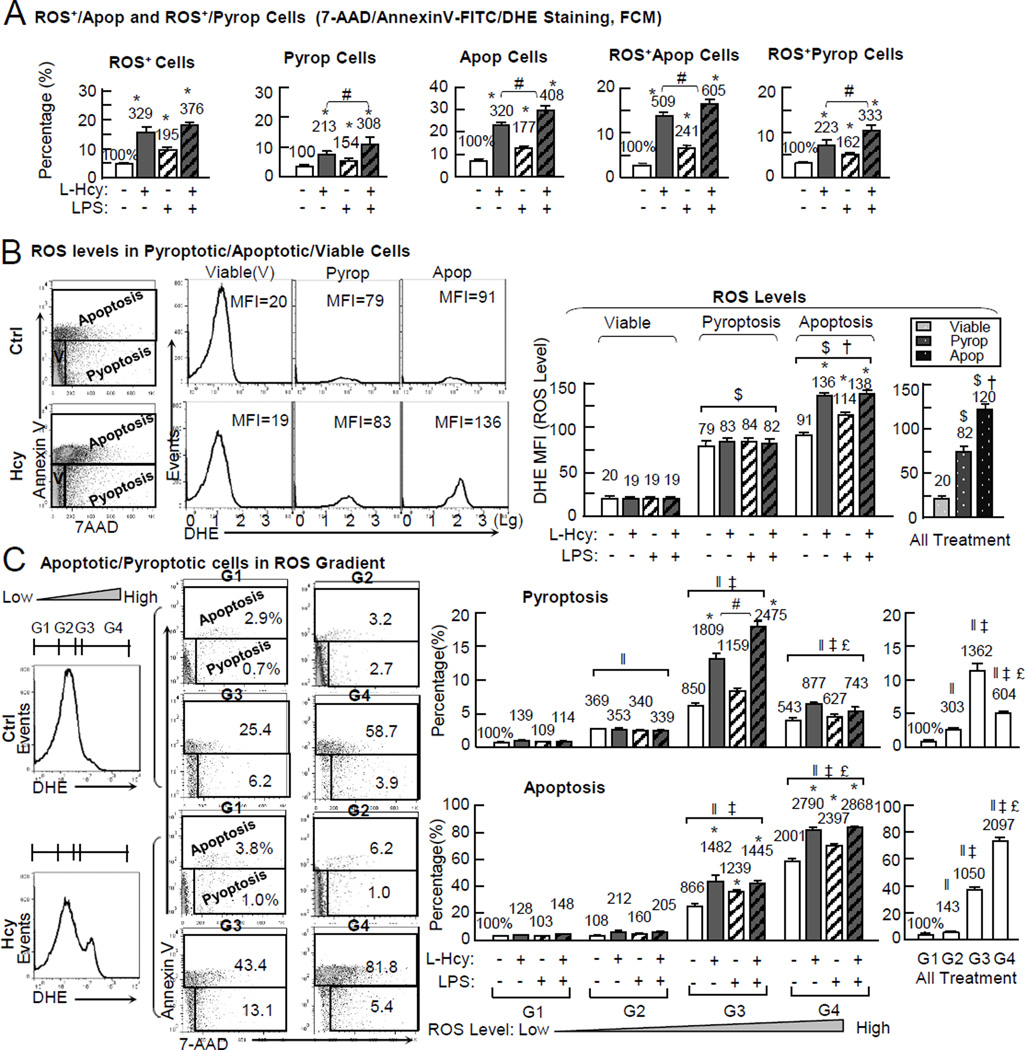
Intracellular ROS levels determines Hcy/LPS-induced death destiny in ECs
To define the association between the intracellular ROS levels and Hcy- and LPS-induced pyroptosis/apoptosis, we quantified intracellular superoxide and cell death markers by triple staining using flow cytometric analysis (histogram and dot plots shown in online Figure VIII). As shown in Fig. 6A, Hcy increased ROS+ cells from 4.8% in the control to 15.8% (329%). LPS induced it to 9.4% (195%). Hcy+LPS induced it to 18.1% (376%). ROS+/Apoptotic cells with elevated superoxide production (DHE+/Annexin-V+) was increased by Hcy from 2.7% to 13.7% (509%), by LPS to 6.5% (241%), and by Hcy+LPS to 16.3% (605%). ROS+/Pyroptotic cells (DHE+/7-AAD+) was increased by Hcy from 3.1% to 11.4% (363%), by LPS to 6.1% (194%), by Hcy+LPS to 13.3% (422%), and by H2O2 to 15.1% (481%).
Figure 6. Hcy/LPS-induced intracellular ROS levels determines cell destiny (pyroptosis/apoptosis/viable cell) in ECs.
HUVECs were cultured and treated with L-Hcy (500µM), LPS (10 µg/mL) or H2O2 (500µM) for 24hr as described in Fig. 1. Triple staining was applied to detect intracellular ROS (DHE) and apoptosis/pyroptosis simultaneously (Annexin-V-FITC and 7-AAD). A, ROS+/apoptosis and ROS+/pyroptosis cells (FCM). The ROS+/apoptosis and ROS+/pyroptosis cells were defined and quantified (dot blot and histogram shown in online Figure VIII). B, ROS level quantification in viable, pyroptotic, apoptotic cell population. ROS level was quantified by mean fluorescence intensity (MFI) of DHE in apoptotic (Annexin V+), pyroptotic (7-AAD+/Annexin V−), and viable (7-AAD−/Annexin V−) cells. C, ROS gradient and apoptosis/pyroptosis. Four regions of ROS level (low, intermediate, high, and extreme high) were defined by their DHE fluorescence intensities. Apoptosis/pyroptosis was quantified in each region. Numbers above each bar is the percentage normalized by the mean of control. *, p < 0.05 vs control in same group or same ROS gradient; #, p < 0.05 vs Hcy in same group or same ROS gradient; †, p < 0.05 vs same treatment in pyroptosis; $, p < 0.05 vs same treatment in viable cells; ‖, p < 0.05 vs same treatment in G1; ‡, p < 0.05 vs same treatment in G2; £, p < 0.05 vs same treatment in G3.) Values are Mean±SEM; n=4.
We further evaluated ROS levels by measuring mean fluorescence intensity (MFI) of DHE staining. We found that ROS levels were unchanged in viable cells in all 4 groups (MFI 19–20), increased to 79/83/84/82 MFI in pyroptosis in the control/Hcy/LPS/Hcy+LPS groups, and raised to 91/136/114/138 in apoptosis (Fig. 6B). Hcy and LPS did not further elevated ROS levels in pyroptotic population, but increased it in apoptosis from 91 MFI in the control group to 136/114/138 in Hcy/LPS/Hcy+LPS groups. Combining all treatment groups, apoptotic cells had the highest ROS levels (120 MFI), compared with 82 in pyroptotic, and 20 in viable populations.
Next, we divided cells into four ROS gradient groups (G1, G2, G3, and G4), and determined their pyroptosis/apoptosis occupation. As shown in Fig. 6C, G1 cells had very low events of pyroptosis/apoptosis (0.7%/2.9% in the control) and were not changed by all treatments. G2 cells had increased pyroptosis (2.5% in control, 303% of that in G1 of all treatments), no changes in apoptosis. G3 cells had the highest pyroptosis population (1362% of that in G1 of all treatment) and increased apoptosis (1050% of that in G1 of all treatment). Hcy and Hcy+LPS induced G3 pyroptosis from 6.2% in the control to 13.1% and 18.0% (1809% and 2475% induction) and G3 apoptosis from 25.4% to 43.4% and 42.3% (1482% and 1445%). G4 cells were predominantly apoptotic (73.7% in the control, 2097% in all treatments). Hcy and Hcy+LPS induced G4 apoptosis from 58.7% in the control to 81.8% and 84.1% (2790% and 2868%). G4 pyroptosis cells (5.1%) were reduced from G3 (11.4%), but still higher than that in G1 and G2 (0.8% and 2.5%). Hcy and Hcy+LPS induced G4 pyroptosis to 5.4% and 5.3% (877% and 743%).
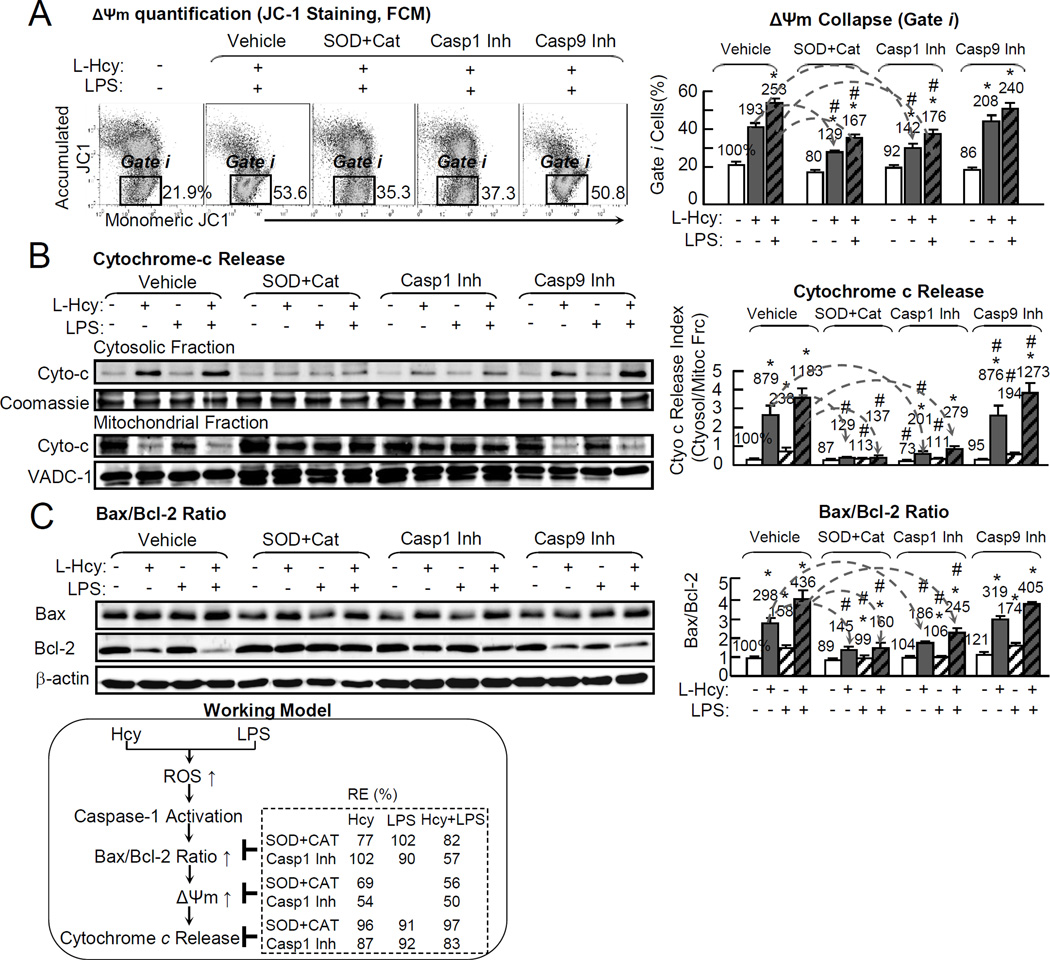
Hcy induces mitochondrial membrane potential (Δψm) collapse and cytochrome-c release, and increases Bax/Bcl-2 Ratio via oxidative stress, caspase-1 activation in ECs
Δψm defines mitochondrial function because Δψm collapse regulates the cell life/death transition, and is the earliest and irreversible events in both pyroptotic and apoptotic processes.42 We examined Δψm by JC-1 immunochemistry staining (images shown in online Figure X) and by flow cytometry. Δψm collapse cells are shown in green staining (online Figure X) in gate i (Fig. 7A). Hcy/Hcy+LPS induced Δψm collapse by 193%/253%, which was reduced to 129%/167% and 142%/176% by antioxidants and caspase-1 inhibitor, respectively (Fig. 7A).
Figure 7. Hcy induces Δψm collapse and cytochrome-c release, and increase Bax/Bcl-2 Ratio via oxidative stress, Casp1 activation in ECs.
HUVECs were cultured in 6cm dish and treated with L-Hcy (500µM) and/or LPS (10 µg/mL) as described in Fig. 1. Cells were pretreated with antioxidants-adenoviral ec-SOD (1MOI) (WB shown in online Figure IX) for 48hr and PEG-catalase (25mg/mL) for 30min, or Casp1, 9 inhibitors for 30min, prior to Hcy/LPS treatment. Mitochondrial function was accessed by JC-1 staining to determine Δψm. Apoptosis signaling was examined by WB for Bax (pro-apoptotic protein) to Bcl-2(anti-apoptotic) ratio. A, Δψm quantification (JC-1 staining, FCM). Δψm collapse cells were quantitated as percentage in gate i. Δψm detection by JC-I staining by fluorescent microscope images shown in online Figure X. B, Cytochrome-c (WB). Cell homogenates were separated to cytosolic and mitochondrial fraction for cytochrome-c protein content by WB. Cytochrome-c ratio (cytosolic fraction/mitochondrial fraction content) was calculated to reflect its leakage from mitochondrial to cytosol. Coomassie blue staining and VADC-1 (mitochondrial protein) were used as loading controls. C, Bax/Bcl-2 (WB). Bax/Bcl-2 ratio was examined by WB. Bax/Bcl-2 ratio is used as apoptosis/pyroptosis index. Arrows indicate the direction of significant changes. Values are Mean±SEM; n=4. Numbers above each bar is the percentage normalized by the mean of control. *, P<0.05 vs control in same group; #, P<0.05 vs same treatment in vehicle. Δψm, mitochondrial potential.
Cytochrome-c release from mitochondrial to cytosol reflects mitochondrial dysfunction and is the key process for PCD initiation. We found that Hcy/LPS/Hcy+LPS increased cytochrome-c release index by 879%/238%/1183% (Fig. 7B), which were rescued by antioxidants to 120%/113%/137% in each group. Similarly, caspase-1 inhibitor rescued cytochrome-c release to 201%, 111% and 279%, respectively.
Bax/Bcl-2 ratio is considered as an indicator of mitochondrion-dependent cell death and an upstream event of mitochondrail dysfunction.43 We found that Hcy/LPS/Hcy+LPS increased Bax/Bcl-2 ratio by 298%/158%/436%, respectively (Fig. 7C). Antioxidants reduced Bax/Bcl-2 ratio to 145%/99%/160%. Similarly, caspase-1 inhibitor rescued Bax/Bcl-2 ratio to 186%/106%/245%, respectively.
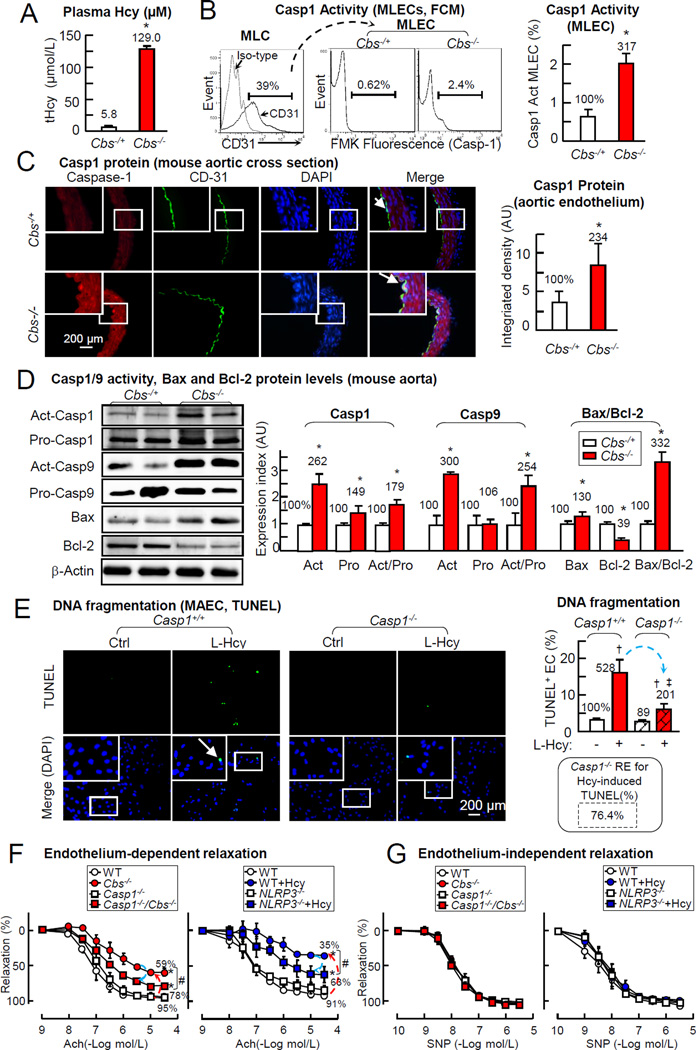
HHcy induces endothelial caspas-1, aortic caspase-1/9 activation and Bax/Bcl-2 ratio in Cbs−/− mice
We characterized HHcy related caspase-1 and cell death signaling in the aorta of Cbs−/− mice (plasma Hcy 129µM) (Fig. 8A). Caspase-1 activity was increased by 317% in lung ECs (MLECs) from Cbs−/− mice (Fig. 8B). Caspase-1 expression was detected in the aorta, co-localized with EC maker CD31 and elevated by 234% in Cbs−/− mice by aortic cross section immunohistochemical staining (Fig. 8C). HHcy increased activated and pro-caspase-1 by 262% and 149%, and activated/pro-caspase-1 179%, induced activated caspase-9 and activated/pro-caspase-9 by 300% and 254%, augmented the pro-apoptotic protein-Bax by 130%, reduced anti-apoptotic protein-Bcl-2 to 39%, and increased Bax/Bcl-2 ratio, an indicator of cell death, by 332% in the aorta of Cbs−/− mice (Fig. 8D). These results is consistent with our observation in HUVECs (Fig. 2/3/7) and support the notion that HHcy induces endothelial caspase-1/9 activation which contributes to increased cell death.
Figure 8. HHcy-induced Casp1 activation mediates EC death in MAEC and impaired endothelial-dependent vessel relaxation in mouse aorta.
Plasma Hcy level, Casp1/9 activity, and Bax and Bcl-2 protein expression were measured in Cbs−/− and control mice. Vascular reactivity were determined in aorta from Casp1−/−, Cbs−/−, Casp1−/−/Cbs−/− and NLRP3−/− mice. A, Plasma Hcy levels in mice. Hcy levels were measured in plasma by LC-ESI-MS/MS. B, Casp1 activation in mouse MLECs. Lung ECs (CD31+, MLECs) were isolated from Cbs−/− mice and assayed for Casp1 activity by FCM. C, Casp1 protein levels (aorta). Mouse aortas were isolated. Aortic cross sections were double-stained with antibodies against Casp1 (Red) and endothelial maker-CD31 (Green). D, Casp1,9 activities and Bax/Bcl-2 ratio (mouse aorta). Whole aortae were isolated from hCBS/Cbs−/− and control mice (n=5), homogenized for WB, and quantified. Casp1/9 activities are expressed as Act/Pro, apoptosis index as Bax/Bcl-2 ratio. E, DNA fragmentation (MAEC, TUNEL). Aortic EC were isolated from Casp1−/− mice, cultured till 80% confluence and then treated with L-Hcy (500µM) for 24hr as described in Fig. 1. Cell death was examined by TUNEL staining. F, Endothelial-dependent vessel relaxation. Aortic rings were pre-constricted with PE (1uM) and tested for endothelial-dependent relaxation to cumulative addition of Ach. Vessels from NLRP3−/− mice were incubated with Hcy (500µM, 48Hr) prior to relaxation assessment. G. Endothelial-independent vessel relaxation. Aortic rings were pre-constricted (PE) and tested for endothelial-independent relaxation to cumulative addition of SNP. Numbers above each bar are the percentage normalized by the mean of control. *, P<0.05 vs control mice; #, P<0.05 vs. mouse with Casp1−/− or NLRP3−/− mice; †, P<0.05 vs Cbs+/+ MAEC control; ‡, P<0.05 vs Cbs+/+ MAEC treated with Hcy. Arrows indicate the direction of significant changes. Values are Mean±SEM; n=4. Ach, acetylcholine. SNP, sodium nitroprusside; PE, phenylephrine; MLEC, mouse lung endothelial cells.
Caspase-1 deficiency prevents Hcy-induced cell death in Caspase-1−/− MAECs
We examined the role of caspase-1 in Hcy-induced PCD by TUNEL assay, a terminal and definitive feature shared by late stage apoptosis and caspase-1 dependent pyroptosis.44 Hcy increased TUNEL+ cells by 528% in MAECs from control mice, which was attenuated to 201% in Casp1−/− MAECs (RE: 76.4%, Fig. 8E). This data confirmed that caspase-1 mediated HHcy induced cell death in EC.
Caspase-1 and NLRP3 deficiency largely rescued HHcy-impaired endothelial dependent vessel relaxation in mouse aorta
Consistent with our previous findings,8, 28, 34 HHcy impaired endothelium-dependent vascular relaxation response to Ach, also termed as endothelial function, in the aorta of Cbs−/− and wild type mice treated with Hcy (500µM, 48Hr) from 95% and 91% to 59% and 35% (Fig. 8F & 8G). HHcy-impaired endothelial function was recovered from 59% and 35% to 78% and 63% in aorta from Casp1−/−/Cbs−/− mice and in NLRP3−/− aorta treated with Hcy.
DISCUSSION
We and others reported that HHcy inhibits EC growth and endothelial apoptosis,4,6,45 leading to impaired post-injury endothelial repair,4–7 and endothelial dysfunction.8, 28 We also demonstrated that HHcy promoted systemic and vessel wall inflammation by inducing inflammatory monocyte differentiation.9–11 This manuscript, for the first time, describes Hcy-induced endothelial pyroptosis, an inflammatory cell death form, and the role of inflammasome activation in HHcy-induced cell death and endothelial dysfunction.
We proposed a novel gating method and defined pyroptotic cells as PI+/Annexin V− cells (Q4, Fig. 1A), which is justified by caspase activities. It was long-recognized that all Annexin V+ cells are apoptosis. Recent studies considered Annexin V+/PI+ cells as pyropotosis,46–48 which overlaps with the previous apoptosis gating. Our novel strategy excludes Annexin V+/PI+ cells (Q3, Fig. 1A/1C) from pyroptosis population and considered all Annexin V+ cells, including Annexin V+/PI+ cells (Q3) and Annexin V+/PI− cells (Q2), as apoptosis based on their common feature of high levels of caspase-9 activation, an established marker for apoptosis and core component of apoptosome.37 We consider caspase-1 activation as a characteristic for both pyroptosis and apoptosis. Our study is the first to characterize PCD by using functional assessment, caspase activities, in addition to the traditional chemical AV/PI paired staining.
We found that Hcy induced pyroptosis and apoptosis in a dose dependent manner and had synergistic effect with proinflammatory endotoxin-LPS. This finding is supported by increased DNA fragmentation, a feature for both pyroptosis and apoptosis.39 We report, for the first time, that Hcy induces pyroptosis/apoptosis in EC. We believe that Hcy-induced endothelial apoptosis is related with impaired vascular repair and that Hcy-induced endothelial pyroptosis contributes to vascular inflammation. The synergistic effect of Hcy and LPS on EC PCD and inflammsome activation suggests that HHcy-induced vascular inflammation can be exacerbated by proinflammatory endotoxin-LPS, a condition caused by bacterial infection and high-fat diet-induced microbiota changes.49
Considering that apoptosis is featured by caspase-3/9 activation17,19,37 and that pyroptosis is marked by inflammasome formation and requires caspase-1 activation,39 we characterized the caspase cascade and found that Hcy, LPS, and the combination of both activated caspase-1, -8, -9 and -3 in a sequential order. We found that caspase-1 activation is the earliest event with higher hierarchy in caspase cascade in EC (Fig. 2). These results suggest that caspase-1 activation maybe primarily responsible for Hcy-induced pyroptosis/apoptosis in ECs.
Our data supported the conclusion that caspase-3 activation is a key downstream event for Hcy/LPS-induced pyroptosis/apoptosis because that caspase-3 inhibitor largely reversed Hcy/LPS-induced pyroptosis and apoptosis by 79%/86% and 71%/46% (Fig. 3C). Caspases-9 inhibitor prevented Hcy-induced pyroptosis/apoptosis (RE 75/62%) and LPS-induced pyroptosis (RE 118%) but not LPS-induced apoptosis (RE 12.8%). Caspase-1 inhibitor largely reversed Hcy-induced caspases-9/3 activation (RE 57/53%), pyroptosis (RE 70%) and apoptosis (RE 52%, Fig. 3C). We conclude that caspase-1 activation is at the top of caspase cascade and mostly responsible for caspases-9/3 activation and pyroptosis/apoptosis. The partial rescue effect of caspase-1 inhibitor maybe related with its incomplete suppression on caspase-1 activity (RE 59%-63%, Fig. 3D). It is possible that caspases-9/3 activation partially via a caspase-1 independent pathway. Further, we found that Hcy-caspase activation, predominantly leads to pyroptosis, and, to a lesser extent, contributes to apoptosis in ECs.
We provided evidence showing that Hcy/LPS increased the formation of inflammasome complex and increased content of pro-/activated-caspase-1 and NLRP3, which binds to caspase-1 via an adaptor protein ASC (Fig. 4 A/B).21 We found that caspase-1 inhibitor largely reversed Hcy/LPS-induced IL-1β cleavage/activation (Fig. 4C). Our data indicates that Hcy and/or LPS promote inflammasome assembly and caspase-1 activation which is responsible for IL-1β activation and endothelial inflammation.
Consistent with the notion that increased ROS mediate pyroptosis and apoptosis,41,50 we demonstrated that Hcy/LPS increased ROS levels in pyroptotic/apoptotic cells (Fig. 6A). Antioxidants largely reversed Hcy-induced pyroptosis/apoptosis (RE 85/98%) and caspase-1 activation (RE 49%) (Fig. 5), suggesting that other redox pathway may play partial role in Hcy-induced pyroptosis/apoptosis. Importantly, we found that apoptotic cells had the highest ROS levels (120 MFI) and the pyroptotic cells had relative lower ROS (82 MFI) compared to 20 MFI in viable cells (Fig. 6B). We demonstrated here, for the first time, that intracellular oxidative gradient determines cell death destiny in ECs. Pyroptotic cells are featured as PI+/Annexin V−, highly dependent on caspase-1 activation and have moderate intracellular oxidative gradient. In contrast, apoptotic cells are marked as Annexin V+, moderately dependent on caspase-1 activation and have high intracellular oxdative gradient. Interestingly, Hcy predominantly induces pyroptosis with intermediate ROS levels. Whereas, LPS is more potent to induce apoptosis with high ROS levels. Our data indicates that HHcy preferentially induces endothelial inflammation which might be a major mechanism underlying HHcy-related vascular diseases.
It is agreed that mitochondrial dysfunction and Δψm collapse are the major causes involved in the mechanism of caspase-9/3 responsive cell death.51 We demonstrated that Hcy induces Δψm collapse and cytochrome-c release, and increases Bax/Bcl-2 ratio via oxidative stress, caspase-1 activation in ECs (Fig. 6C). It is possible that Hcy-induced intracellular oxidative gradient change is resulted from mitochondrial ROS leakage due to membrane permeabilization and Δψm collapse,52 leading to cytochrome-c release. We also confirmed the role of caspase-1 activation in controlling mitochondrial dysfunction and Δψm collapse, since caspase-1 inhibitor reversed Hcy/LPS-induced cytochrome-c release and Δψm collapse (Fig.7 B/C). We assume that Hcy/LPS cause mitochondrial damage via Bcl-2 suppression and Bax induction as that caspase-1 inhibitor reversed Hcy/LPS-induced Bax/Bcl-2 ratio (Fig. 7D). It is known that anti-apoptotic protein Bcl-2 inhibits apoptosis via binding to Bax and sequester it from its role governing mitochondrial permeability and Δψm collapse. We suspect that caspase-1 may cleaves Bid, a Bcl-2 family protein containing domain of homology (BH)-3, to form truncated Bid (tBid), which binds to Bcl-2,53, 54 leading to Bax activation and apoptosis.
We validated HHcy-induced caspase-1 activation in EC from HHcy mice (hCBS/Cbs−/−, Fig. 8A/C) and confirmed caspase-1/-9 activation and Bax/Bcl-2 induction in HHcy mouse aorta (Fig. 8B). We also found that caspase-1−/− MAEC prevented Hcy-induced DNA fragmentation (Fig. 8E) with a similar rescue efficacy of caspase-1 inhibitor on Hcy-induced pyroptosis/apoptosis (RE 76.4% vs 70/52%). This could be partially explained by potential limited sensitivity of TUNEL staining on pyroptosis. Notably, gene deficiency of inflammasome components in mouse model (caspase-1−/−, NLRP3−/−) prevented HHcy or Hcy induced endothelial dysfunction (Fig. 8F, 8G). Studies in hyperlipidemia mice have reported contradictory results for the implication of NLRP3 during the process of atherosclerosis.23, 55 Our study is the first to provide evidence supporting the notion that caspase-1 activation is the key upstream event mediating HHcy-induced EC death and endothelial dysfunction.
Taken together, we reported here, for the first time, that Hcy is a sterile stimuli which triggers the danger signal and induces pyroptosis inflammatory cell death. We characterized Hcy-induced caspase-1 dependent cell death signal pathway and presented a working model (online Figure XI). We propose that Hcy/LPS induce inflammasome assembly via ROS elevation, leading to caspase-1 activation and endothelial inflammation by IL-1β activation. Caspase-1 activation causes mitochondrial dysfunction through Bax/Bcl-2 change, leading to caspase-9/3 cascade activation and pyroptosis and apoptosis in EC.
We propose two major conclusions. Firstly, HHcy is an endogenous metabolic stress stimuli and able to activate inflammatory pyroptosis and non-inflammatory apoptosis in EC, both mediated by the signaling of caspase-1 activation/mitochondrial dysfunction/caspase-9/3 activation. We termed caspase-1 responsive pyroptosis/apoptosis as pyrop-apoptosis. Secondly, HHcy preferentially induces endothelial pyroptosis via caspase-1-dependent inflammasome activation, which might be a major mechanism underlying HHcy-related vascular inflammation and atherosclerosis.
Supplementary Material
Novelty and Significance.
What Is Known?
Homocysteinemia (HHcy) is an independent risk factor for cardiovascular disease.
Homocysteine (Hcy)-lowering therapy prevented stroke in primary and secondary prevention trails.
HHcy inhibits endothelial cells growth and impairs endothelial function and vascular repair.
HHcy induces inflammatory monocyte differentiation.
What New Information Does This Article Contribute?
Hcy acts as a sterile stimulus that triggers danger signals and induces pyroptosis inflammatory cell death.
Hcy induces inflammatory pyroptotic and non-inflammatory apoptotic cell death by activating the caspase-1, -8, -9, -3 cascade.
Caspase-1 triggered cell death is dependent upon Hcy/NALP3-containing inflammasome assembly/caspase-1 activation/IL-1β cleavage-activation/Bax:Bcl-2 induction/mitochondrial membrane potential collapse/cytochrome c release/caspase9/3 activation.
NLRP3 activation in Hcy-impaired endothelial dysfunction is mediated by caspase-1.
Oxidative gradient determines cell death destiny for pyroptosis, apoptosis or viable cell.
In this study, we investigated the effect of Hcy on inflammatory cell death in cultured primary human/mouse endothelial cells, and endothelium in HHcy mice. We established a novel flow cytometric gating method to characterize pyrotosis cells and discovered that Hcy induced inflammatory pyroptotic and non-inflammatory apoptotic cell death via caspase-1, -8, -9, -3 cascade activation. We delineate the signaling of Hcy-induced cell death as Hcy/NALP3-containing inflammasome assembly/caspase-1 activation/IL-1β cleavage-activation/Bax:Bcl-2 induction/mitochondrial membrane potential collapse/cytochrome c release/caspase9/3 activation. We demonstrate that oxidative gradient determines cell death destiny for pyroptosis, apoptosis or viable cell. Taken together, these observations suggest that HHcy preferentially induces EC pyroptosis and inflammation via caspase-1-dependent inflammasome activation.
Acknowledgments
The authors acknowledge the contributions of Jonathan Yi from Temple University, for linguistic inspection of this manuscript.
SOURCES OF FUNDING
This work was supported in part by National Institutes of Health (NIH) Grants number: HL67033, HL77288, HL82774, HL110764, HL117654, DK104116 and HL131460 (HW); HL9445, HL108910 and HL116917 (XFY), and by the National Science Foundation of China 81330004 (YJ).
Nonstandard Abbreviations and Acronyms
- Ach
Acetylcholine
- Bax
Bcl-2-associated X protein
- Bcl-2
B-cell lymphoma 2
- Bid
BH3 interacting-domain death agonist
- tBid
Truncated Bid
- Cbs
Cystathionine β-synthase
- CVD
Cardiovascular disease
- DAMPs
Danger associated molecular patterns
- DHE
Dihydroethidium
- EC
Endothelial cell
- ER
Endoplasmic reticulum
- FAM
Carboxyfluorescein
- FCM
Flow cytometry
- FMK
Fluoromethyl ketone
- Hcy
Homocysteine
- HHcy
Hyperhomocysteinemia
- IP
Immunoprecipitation
- MFI
Mean fluorescence intensity
- NLRP3
Nucleotide-binding oligomerization domain, and Leucine rich Repeat and Pyrin domain containing Protein 3
- P20
Protein 20
- PAMPs
Pyroptosis responses to pathogen associated molecular patterns
- PI
Propidium iodide
- ROS
Reactive oxidative species
- SNP
Sodium nitroprusside
- SOD
Superoxide dismutase
- TUNEL
Terminal deoxynucleotidyl transferase dUTP nick end labeling
- Δψm
Mitochondrial potential
Footnotes
DISCLOSURE
None.
REFERENCES
- 1.Eikelboom JW, Lonn E, Genest J, Jr, Hankey G, Yusuf S. Homocyst(e)ine and cardiovascular disease: A critical review of the epidemiologic evidence. Annals of internal medicine. 1999;131:363–375. doi: 10.7326/0003-4819-131-5-199909070-00008. [DOI] [PubMed] [Google Scholar]
- 2.van den Berg M, Stehouwer CD, Bierdrager E, Rauwerda JA. Plasma homocysteine and severity of atherosclerosis in young patients with lower-limb atherosclerotic disease. Arteriosclerosis, thrombosis, and vascular biology. 1996;16:165–171. doi: 10.1161/01.atv.16.1.165. [DOI] [PubMed] [Google Scholar]
- 3.McCully KS. Homocysteine and vascular disease. Nature medicine. 1996;2:386–389. doi: 10.1038/nm0496-386. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Jiang X, Yang F, Gaubatz JW, Ma L, Magera MJ, Yang X, Berger PB, Durante W, Pownall HJ, Schafer AI. Hyperhomocysteinemia accelerates atherosclerosis in cystathionine beta-synthase and apolipoprotein e double knock-out mice with and without dietary perturbation. Blood. 2003;101:3901–3907. doi: 10.1182/blood-2002-08-2606. [DOI] [PubMed] [Google Scholar]
- 5.Wang H, Yoshizumi M, Lai K, Tsai JC, Perrella MA, Haber E, Lee ME. Inhibition of growth and p21ras methylation in vascular endothelial cells by homocysteine but not cysteine. The Journal of biological chemistry. 1997;272:25380–25385. doi: 10.1074/jbc.272.40.25380. [DOI] [PubMed] [Google Scholar]
- 6.Tan H, Jiang X, Yang F, Li Z, Liao D, Trial J, Magera MJ, Durante W, Yang X, Wang H. Hyperhomocysteinemia inhibits post-injury reendothelialization in mice. Cardiovascular research. 2006;69:253–262. doi: 10.1016/j.cardiores.2005.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jamaluddin MD, Chen I, Yang F, Jiang X, Jan M, Liu X, Schafer AI, Durante W, Yang X, Wang H. Homocysteine inhibits endothelial cell growth via DNA hypomethylation of the cyclin a gene. Blood. 2007;110:3648–3655. doi: 10.1182/blood-2007-06-096701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng Z, Jiang X, Pansuria M, Fang P, Mai J, Mallilankaraman K, Gandhirajan RK, Eguchi S, Scalia R, Madesh M, Yang X, Wang H. Hyperhomocysteinemia and hyperglycemia induce and potentiate endothelial dysfunction via mu-calpain activation. Diabetes. 2015;64:947–959. doi: 10.2337/db14-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang D, Jiang X, Fang P, Yan Y, Song J, Gupta S, Schafer AI, Durante W, Kruger WD, Yang X, Wang H. Hyperhomocysteinemia promotes inflammatory monocyte generation and accelerates atherosclerosis in transgenic cystathionine beta-synthase-deficient mice. Circulation. 2009;120:1893–1902. doi: 10.1161/CIRCULATIONAHA.109.866889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang D, Fang P, Jiang X, Nelson J, Moore JK, Kruger WD, Berretta RM, Houser SR, Yang X, Wang H. Severe hyperhomocysteinemia promotes bone marrow-derived and resident inflammatory monocyte differentiation and atherosclerosis in ldlr/cbs-deficient mice. Circulation research. 2012;111:37–49. doi: 10.1161/CIRCRESAHA.112.269472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang P, Zhang D, Cheng Z, Yan C, Jiang X, Kruger WD, Meng S, Arning E, Bottiglieri T, Choi ET, Han Y, Yang XF, Wang H. Hyperhomocysteinemia potentiates hyperglycemia-induced inflammatory monocyte differentiation and atherosclerosis. Diabetes. 2014;63:4275–4290. doi: 10.2337/db14-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: Testing and clinical relevance. Circulation. 2007;115:1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 13.Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS, Golstein P, Green DR, Hengartner M, Knight RA, Kumar S, Lipton SA, Malorni W, Nunez G, Peter ME, Tschopp J, Yuan J, Piacentini M, Zhivotovsky B, Melino G. Classification of cell death: Recommendations of the nomenclature committee on cell death 2009. Cell death and differentiation. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang C, Cai Y, Adachi MT, Oshiro S, Aso T, Kaufman RJ, Kitajima S. Homocysteine induces programmed cell death in human vascular endothelial cells through activation of the unfolded protein response. The Journal of biological chemistry. 2001;276:35867–35874. doi: 10.1074/jbc.M100747200. [DOI] [PubMed] [Google Scholar]
- 15.Kumar S. Caspase function in programmed cell death. Cell death and differentiation. 2007;14:32–43. doi: 10.1038/sj.cdd.4402060. [DOI] [PubMed] [Google Scholar]
- 16.Elmore S. Apoptosis: A review of programmed cell death. Toxicologic pathology. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horke S, Witte I, Wilgenbus P, Kruger M, Strand D, Forstermann U. Paraoxonase-2 reduces oxidative stress in vascular cells and decreases endoplasmic reticulum stress-induced caspase activation. Circulation. 2007;115:2055–2064. doi: 10.1161/CIRCULATIONAHA.106.681700. [DOI] [PubMed] [Google Scholar]
- 18.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through nalp3 inflammasome sensing of asbestos and silica. Science (New York, N.Y. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamkanfi M. Emerging inflammasome effector mechanisms. Nature reviews. Immunology. 2011;11:213–220. doi: 10.1038/nri2936. [DOI] [PubMed] [Google Scholar]
- 20.Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT. The nalp3 inflammasome is involved in the innate immune response to amyloid-beta. Nature immunology. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 22.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nature immunology. 11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 23.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. Nlrp3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katsnelson MA, Rucker LG, Russo HM, Dubyak GR. K+ efflux agonists induce nlrp3 inflammasome activation independently of ca2+ signaling. J Immunol. 2015;194:3937–3952. doi: 10.4049/jimmunol.1402658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hari A, Zhang Y, Tu Z, Detampel P, Stenner M, Ganguly A, Shi Y. Activation of nlrp3 inflammasome by crystalline structures via cell surface contact. Scientific reports. 2014;4:7281. doi: 10.1038/srep07281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee SJ, Kim KM, Namkoong S, Kim CK, Kang YC, Lee H, Ha KS, Han JA, Chung HT, Kwon YG, Kim YM. Nitric oxide inhibition of homocysteine-induced human endothelial cell apoptosis by down-regulation of p53-dependent noxa expression through the formation of s-nitrosohomocysteine. The Journal of biological chemistry. 2005;280:5781–5788. doi: 10.1074/jbc.M411224200. [DOI] [PubMed] [Google Scholar]
- 27.Nelson J, Wu Y, Jiang X, Berretta R, Houser S, Choi E, Wang J, Huang J, Yang X, Wang H. Hyperhomocysteinemia suppresses bone marrow cd34+/vegf receptor 2+ cells and inhibits progenitor cell mobilization and homing to injured vasculature-a role of beta1-integrin in progenitor cell migration and adhesion. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2015;29:3085–3099. doi: 10.1096/fj.14-267989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng Z, Jiang X, Kruger WD, Pratico D, Gupta S, Mallilankaraman K, Madesh M, Schafer AI, Durante W, Yang X, Wang H. Hyperhomocysteinemia impairs endothelium-derived hyperpolarizing factor-mediated vasorelaxation in transgenic cystathionine beta synthase-deficient mice. Blood. 2011;118:1998–2006. doi: 10.1182/blood-2011-01-333310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xi H, Akishita M, Nagai K, Yu W, Hasegawa H, Eto M, Kozaki K, Toba K. Potent free radical scavenger, edaravone, suppresses oxidative stress-induced endothelial damage and early atherosclerosis. Atherosclerosis. 2007;191:281–289. doi: 10.1016/j.atherosclerosis.2006.05.040. [DOI] [PubMed] [Google Scholar]
- 30.Yang Y, Yang F, Xiong Z, Yan Y, Wang X, Nishino M, Mirkovic D, Nguyen J, Wang H, Yang XF. An n-terminal region of translationally controlled tumor protein is required for its antiapoptotic activity. Oncogene. 2005;24:4778–4788. doi: 10.1038/sj.onc.1208666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fisher PW, Salloum F, Das A, Hyder H, Kukreja RC. Phosphodiesterase-5 inhibition with sildenafil attenuates cardiomyocyte apoptosis and left ventricular dysfunction in a chronic model of doxorubicin cardiotoxicity. Circulation. 2005;111:1601–1610. doi: 10.1161/01.CIR.0000160359.49478.C2. [DOI] [PubMed] [Google Scholar]
- 32.Monick MM, Powers LS, Barrett CW, Hinde S, Ashare A, Groskreutz DJ, Nyunoya T, Coleman M, Spitz DR, Hunninghake GW. Constitutive erk mapk activity regulates macrophage atp production and mitochondrial integrity. J Immunol. 2008;180:7485–7496. doi: 10.4049/jimmunol.180.11.7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ekert PG, Silke J, Vaux DL. Caspase inhibitors. Cell death and differentiation. 1999;6:1081–1086. doi: 10.1038/sj.cdd.4400594. [DOI] [PubMed] [Google Scholar]
- 34.Jiang X, Yang F, Tan H, Liao D, Bryan RM, Jr, Randhawa JK, Rumbaut RE, Durante W, Schafer AI, Yang X, Wang H. Hyperhomocystinemia impairs endothelial function and enos activity via pkc activation. Arteriosclerosis, thrombosis, and vascular biology. 2005;25:2515–2521. doi: 10.1161/01.ATV.0000189559.87328.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim KY, Shin HK, Choi JM, Hong KW. Inhibition of lipopolysaccharide-induced apoptosis by cilostazol in human umbilical vein endothelial cells. J Pharmacol Exp Ther. 2002;300:709–715. doi: 10.1124/jpet.300.2.709. [DOI] [PubMed] [Google Scholar]
- 36.Riccardi C, Nicoletti I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nature protocols. 2006;1:1458–1461. doi: 10.1038/nprot.2006.238. [DOI] [PubMed] [Google Scholar]
- 37.Riedl SJ, Salvesen GS. The apoptosome: Signalling platform of cell death. Nature reviews. Molecular cell biology. 2007;8:405–413. doi: 10.1038/nrm2153. [DOI] [PubMed] [Google Scholar]
- 38.Miao EA, Rajan JV, Aderem A. Caspase-1-induced pyroptotic cell death. Immunological reviews. 243:206–214. doi: 10.1111/j.1600-065X.2011.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Labbe K, Saleh M. Cell death in the host response to infection. Cell death and differentiation. 2008;15:1339–1349. doi: 10.1038/cdd.2008.91. [DOI] [PubMed] [Google Scholar]
- 40.Smolewski P, Bedner E, Du L, Hsieh TC, Wu JM, Phelps DJ, Darzynkiewicz Z. Detection of caspases activation by fluorochrome-labeled inhibitors: Multiparameter analysis by laser scanning cytometry. Cytometry. 2001;44:73–82. doi: 10.1002/1097-0320(20010501)44:1<73::aid-cyto1084>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 41.Harijith A, Ebenezer DL, Natarajan V. Reactive oxygen species at the crossroads of inflammasome and inflammation. Frontiers in physiology. 2014;5:352. doi: 10.3389/fphys.2014.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duprez L, Wirawan E, Vanden Berghe T, Vandenabeele P. Major cell death pathways at a glance. Microbes and infection / Institut Pasteur. 2009;11:1050–1062. doi: 10.1016/j.micinf.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 43.Chresta CM, Masters JR, Hickman JA. Hypersensitivity of human testicular tumors to etoposide-induced apoptosis is associated with functional p53 and a high bax:Bcl-2 ratio. Cancer research. 1996;56:1834–1841. [PubMed] [Google Scholar]
- 44.Chudakov DM, Matz MV, Lukyanov S, Lukyanov KA. Fluorescent proteins and their applications in imaging living cells and tissues. Physiological reviews. 90:1103–1163. doi: 10.1152/physrev.00038.2009. [DOI] [PubMed] [Google Scholar]
- 45.Sipkens JA, Krijnen PA, Meischl C, Cillessen SA, Smulders YM, Smith DE, Giroth CP, Spreeuwenberg MD, Musters RJ, Muller A, Jakobs C, Roos D, Stehouwer CD, Rauwerda JA, van Hinsbergh VW, Niessen HW. Homocysteine affects cardiomyocyte viability: Concentration-dependent effects on reversible flip-flop, apoptosis and necrosis. Apoptosis : an international journal on programmed cell death. 2007;12:1407–1418. doi: 10.1007/s10495-007-0077-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sagulenko V, Thygesen SJ, Sester DP, Idris A, Cridland JA, Vajjhala PR, Roberts TL, Schroder K, Vince JE, Hill JM, Silke J, Stacey KJ. Aim2 and nlrp3 inflammasomes activate both apoptotic and pyroptotic death pathways via asc. Cell death and differentiation. 2013;20:1149–1160. doi: 10.1038/cdd.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu J, Nagasu H, Murakami T, Hoang H, Broderick L, Hoffman HM, Horng T. Inflammasome activation leads to caspase-1-dependent mitochondrial damage and block of mitophagy. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:15514–15519. doi: 10.1073/pnas.1414859111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, Warren SE, Wewers MD, Aderem A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nature immunology. 11:1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanz Y, Santacruz A, Gauffin P. Gut microbiota in obesity and metabolic disorders. The Proceedings of the Nutrition Society. 2010;69:434–441. doi: 10.1017/S0029665110001813. [DOI] [PubMed] [Google Scholar]
- 50.Irani K. Oxidant signaling in vascular cell growth, death, and survival : A review of the roles of reactive oxygen species in smooth muscle and endothelial cell mitogenic and apoptotic signaling. Circulation research. 2000;87:179–183. doi: 10.1161/01.res.87.3.179. [DOI] [PubMed] [Google Scholar]
- 51.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 52.Scherz-Shouval R, Elazar Z. Ros, mitochondria and the regulation of autophagy. Trends in cell biology. 2007;17:422–427. doi: 10.1016/j.tcb.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 53.Zhang WH, Wang X, Narayanan M, Zhang Y, Huo C, Reed JC, Friedlander RM. Fundamental role of the rip2/caspase-1 pathway in hypoxia and ischemia-induced neuronal cell death. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:16012–16017. doi: 10.1073/pnas.2534856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adams JM, Cory S. The bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Menu P, Pellegrin M, Aubert JF, Bouzourene K, Tardivel A, Mazzolai L, Tschopp J. Atherosclerosis in apoe-deficient mice progresses independently of the nlrp3 inflammasome. Cell death & disease. 2011;2:e137. doi: 10.1038/cddis.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.