Supplemental Digital Content is Available in the Text.
Key Words: HIV, mortality, inflammation, monocyte activation, coagulation
Abstract
Background:
HIV infection and biomarkers of inflammation [measured by interleukin-6 (IL-6)], monocyte activation [soluble CD14 (sCD14)], and coagulation (D-dimer) are associated with morbidity and mortality. We hypothesized that these immunologic processes mediate (explain) some of the excess risk of mortality among HIV infected (HIV+) versus uninfected people independently of comorbid diseases.
Methods:
Among 2350 (1521 HIV+) participants from the Veterans Aging Cohort Study Biomarker Cohort (VACS BC), we investigated whether the association between HIV and mortality was altered by adjustment for IL-6, sCD14, and D-dimer, accounting for confounders. Participants were followed from date of blood draw for biomarker assays (baseline) until death or July 25, 2013. Analyses included ordered logistic regression and Cox Proportional Hazards regression.
Results:
During 6.9 years (median), 414 deaths occurred. The proportional odds of being in a higher quartile of IL-6, sCD14, or D-dimer were 2–3 fold higher for viremic HIV+ versus uninfected people. Mortality rates were higher among HIV+ compared with uninfected people [incidence rate ratio (95% CI): 1.31 (1.06 to 1.62)]. Mortality risk increased with increasing quartiles of IL-6, sCD14, and D-dimer regardless of HIV status. Adjustment for IL-6, sCD14, and D-dimer partially attenuated mortality risk among HIV+ people with unsuppressed viremia (HIV-1 RNA ≥10,000 copies per milliliter) compared with uninfected people—hazard ratio (95% CI) decreased from 2.18 (1.60 to 2.99) to 2.00 (1.45 to 2.76).
Conclusions:
HIV infection is associated with elevated IL-6, sCD14, and D-dimer, which are in turn associated with mortality. Baseline measures of these biomarkers partially mediate excess mortality risk among HIV+ versus uninfected people.
INTRODUCTION
Biomarkers of inflammation [measured by interleukin-(6 IL-6)], monocyte activation [soluble CD14 (sCD14)], and coagulation (D-dimer) are associated with morbidity and increased mortality among HIV infected (HIV+) and uninfected people.1–5 However, it is unclear whether these immunologic processes explain the excess risk of mortality among HIV+ versus uninfected people6,7 independently of comorbid diseases. There are sparse data comparing HIV+ with uninfected people who have similar demographic and behavior characteristics (ie, prevalence of smoking and alcohol consumption) while also accounting for HIV-specific biomarkers, co-morbidities, substance use, biomarkers of inflammation, monocyte activation and coagulation, and complete capture of mortality events.
Our objective, therefore, was to determine whether increased inflammation, monocyte activation, and coagulation explain the excess mortality risk among HIV+ compared with uninfected people. Data for these analyses were from the Veterans Aging Cohort Study Biomarker Cohort (VACS BC), an observational, longitudinal cohort of HIV+ and uninfected Veterans in care with detailed phenotypic data, biomarkers of immune function, and thorough capture of mortality outcomes. We assessed whether the association between HIV status and mortality persisted after adjusting for multiple potentially confounding comorbid conditions alone and when combined with IL-6, sCD14, and D-dimer.
METHODS
Cohort
The VACS BC is a subset of the VACS Survey,8 a prospectively enrolled observational longitudinal study of HIV+ and uninfected veterans matched on age, race-ethnicity, sex, and geographic region.8 In 2005–2007, 1525 HIV+ and 843 uninfected VACS Survey participants consented to provide blood samples forming the VACS BC as previously described.9 These specimens were collected using serum separator and EDTA blood collection tubes and shipped to a central repository at the Massachusetts Veterans Epidemiology Research and Information Center in Boston, MA. The date of the blood draw was used as the baseline date for each participant in the VACS BC. Those with available measurements of IL-6, sCD14, D-dimer, and HIV-1 RNA (for HIV+) were included in analyses. Participants were followed from their baseline date until death or censored on July 25, 2013.
Independent, Dependent, and Potentially Mediating Variables
HIV status was the primary independent variable. We collected data on HIV-1 RNA, CD4+ T-cell (CD4) count, and antiretroviral therapy use at baseline. We used HIV-1 RNA measurements obtained as part of clinical care at baseline (±180 days). Death was the primary outcome. It was determined from the VHA vital status file, which uses inputs from the Social Security Administration death master file, the Beneficiary Identification and Records Locator Subsystem, and the VHA Medical Statistical Analysis Systems inpatient datasets. We assessed whether biomarkers of inflammation (IL-6), monocyte activation (sCD14), and altered coagulation (D-dimer) altered the association between HIV and mortality (see description of mediation in statistical analysis below). IL-6, sCD14, and D-dimer were assessed as categorical values (quartiles) or as a composite inflammatory burden score (number of elevated biomarkers ie, ≥75th percentile threshold among those who died).10 Measurement of these biomarkers has been previously described.9
Covariates
Covariate data were obtained closest to baseline date and have been previously described.9 Briefly, sociodemographic data included age, sex, and race-ethnicity. Cardiovascular disease was defined by myocardial infarction11,12 and diagnostic or procedural codes for congestive heart failure, coronary artery bypass graft, percutaneous coronary intervention, or ischemic stroke. Cancer was determined using VA Central Cancer Registry data.13 Chronic obstructive pulmonary disease was defined by ICD-9 code.14 Hypertension was categorized as no hypertension (untreated BP < 120/80 mm Hg); prehypertension (untreated BP 120–139/80–89 mm Hg); controlled hypertension (treated BP < 140/90 mm Hg); or uncontrolled hypertension (BP ≥ 140/90 mm Hg).15 Diabetes was diagnosed using a combination of glucose measurements, use of insulin or oral hypoglycemic agents, and/or ICD-9 codes.16 Smoking was self-reported and obesity was defined as body mass index (BMI) >30 kg/m2. Cholesterol lowering medication use [HMG CoA reductase inhibitor (statins) or gemfibrozil] was assessed using patient pharmacy data. Total cholesterol measurements were obtained from the VA Decision Support System and categorized as <200 mg/dL untreated, <200 mg/dL treated, or ≥200 mg/dL.17 Medication data were from the VA Pharmacy Benefits Management database.
Cocaine and alcohol use at baseline were determined by self-report. We categorized alcohol use with data from the Alcohol Use Disorders Identification Test (AUDIT-C) and alcohol abuse and dependence diagnoses using ICD-9 codes based on prior work in VACS as: (1) Low risk current drinking, (2) no current drinking, (3) at-risk or heavy current drinking, and (4) alcohol abuse or dependence diagnosis and current drinking. Current drinking was defined as any drinking reported in the prior 12 months. VACS index was calculated as previously described.18,19 Hepatitis C virus infection was defined as a positive Hepatitis C virus antibody test or at least 1 inpatient and/or 2 outpatient ICD-9 codes.20 Liver fibrosis was estimated using FIB-4 scores.21 Hemoglobin was dichotomized at 12 g/dL. Renal disease was defined as an estimated glomerular filtration rate less than 60 mL·min−1·1.73 m−2.22
Statistical Analysis
We compared continuous variables (t test or median test) and categorical variables (χ2 test) by HIV status overall and among participants who died. Kaplan–Meier curves were used to describe time to death by HIV status and/or elevations in IL-6, D-dimer, sCD14, and inflammatory burden (number of elevated biomarkers ie, ≥75th percentile threshold among those who died).
We adapted the method described by Baron and Kearny23 and MacKinnon et al24 to assess whether these immunological biomarkers mediate (explain) the relationship between HIV and mortality. This approach requires fulfillment of 4 conditions: (1) a significant relation between the independent and dependent variables, (2) a significant relation between the independent and mediating variables, (3) a significant relation between the mediating and dependent variables after adjustment for the independent variable, (4) given 1–3 hold, an attenuation (in absolute value) of the association between the independent and dependent variables following adjustment for the mediating variable.
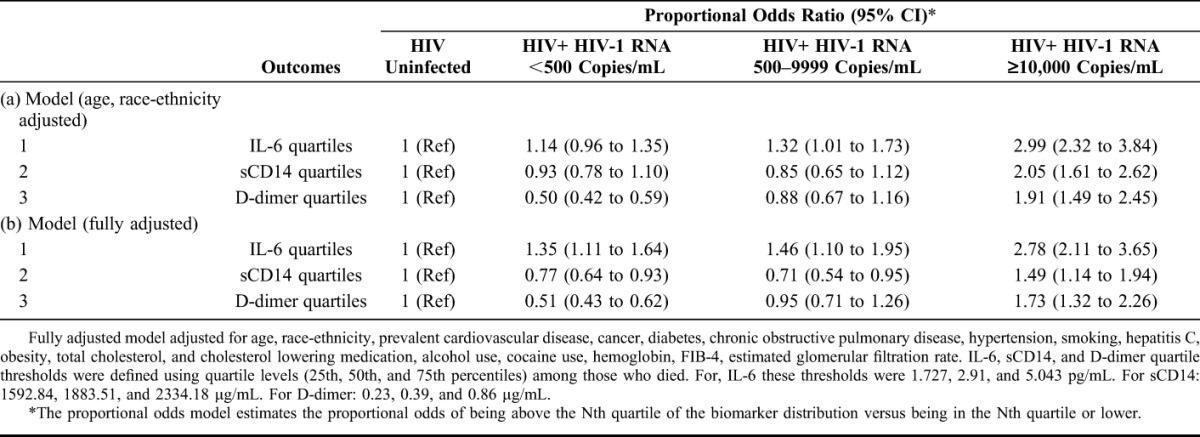
Proportional odds models were used to estimate the association between HIV (stratified by HIV-1 RNA <500, 500–9999, ≥10,000 copies per milliliter) and elevated IL-6, sCD14, and D-dimer. The proportional odds model estimates the proportional odds of being above the Nth quartile of the biomarker distribution versus being in the Nth quartile or lower based on an assumption of proportional odds. To illustrate: the model assumes that coefficients that describe the relationship between the third and fourth quartiles versus first and second quartiles of IL-6 are the same as those that describe the relationship between the second, third, and fourth quartiles versus the first quartile. We selected this model because it is more parsimonious than a set of logistic regression models for each pair of quartiles while still incorporating all levels of the different outcome variables. This assumption was assessed using the Brant Test (Stata Spost package)25 and found to be valid for all final models except sCD14. Sensitivity analyses using multinomial logistic regression for sCD14 showed consistent results.
Cox proportional hazards models were used to estimate the associations between HIV (stratified by HIV-1 RNA) and mortality adjusting for multiple confounders. All analyses were performed using Stata 13 (StataCorp 2013. Stata Statistical Software: Release 13; StataCorp LP, College Station, TX). P values <0.05 were considered statistically significant.
RESULTS
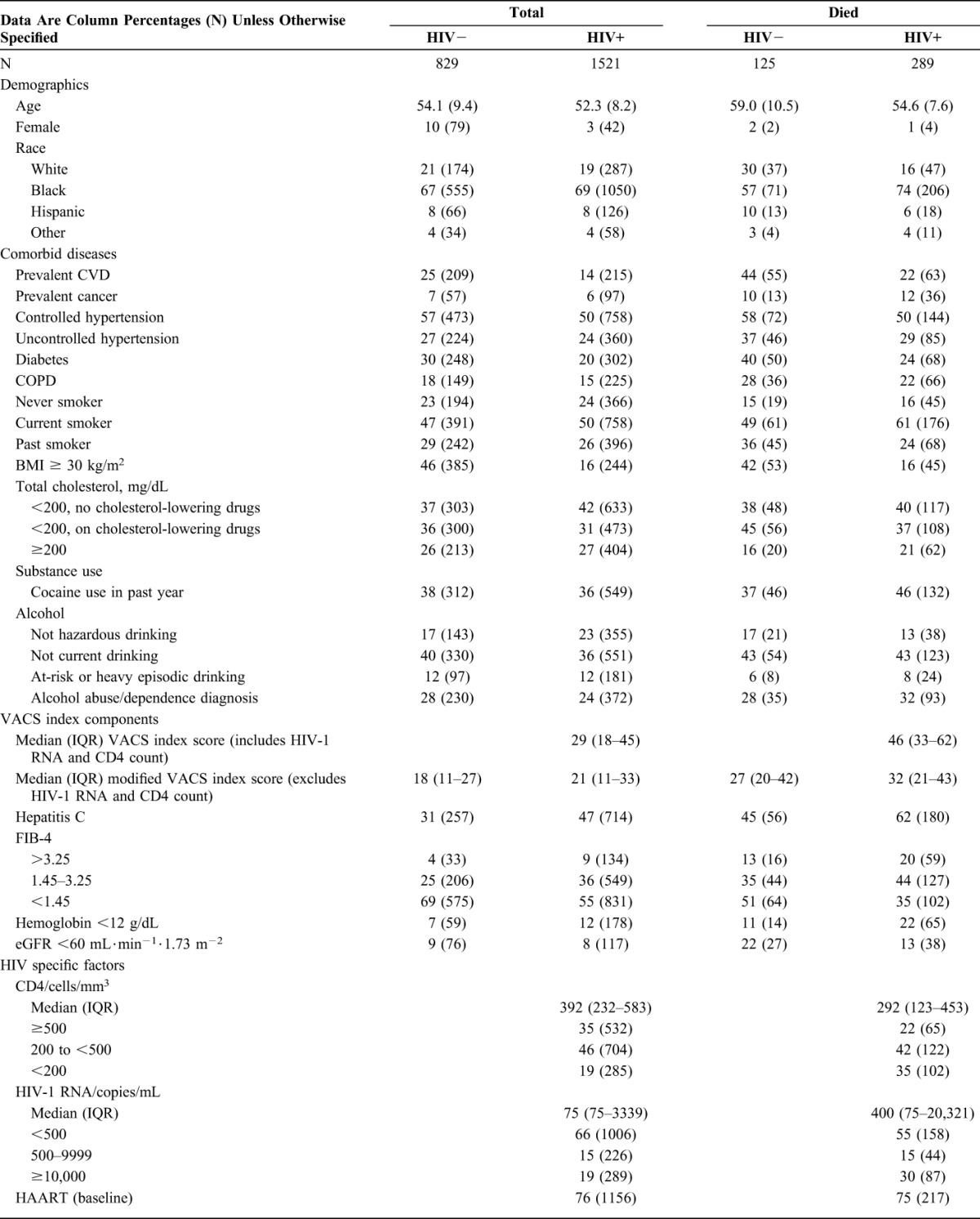
Of 2389 participants who provided blood specimens, 35 did not have IL-6, sCD14, and D-dimer measured, 4 HIV+ participants had missing HIV-1 RNA, and 1 patient subsequently withdrew consent. Of the remainder, 829 were HIV uninfected and 1521 were HIV+. During a median of 6.9 (interquartile range 6.2–7.4) years from baseline (ie, date of blood drawn), 414 deaths occurred (15% of uninfected and 19% of HIV+). Compared with uninfected participants, HIV+ participants were younger and less likely to be female (Table 1). They also had less prevalent cardiovascular disease (14 versus 25%), diabetes (20 versus 30%), BMI ≥ 30 kg/m2 (16 versus 46) and alcohol abuse/dependence (28 versus 24%), and more hepatitis C (47 versus 31%), FIB-4 greater than 3.25, ie, suggestive of advanced fibrosis (9 versus 4%) and hemoglobin <12g/dL (12 versus 7%) at baseline (Table 1).
TABLE 1.
Characteristics of Study Population at Baseline
HIV and Mortality
Mortality rates per 100 person years were higher among HIV+ versus uninfected people [incidence rate ratio (95% CI): 1.31 (1.06 to 1.62)]. Compared with uninfected participants, HIV infection with HIV-1 RNA ≥500–9999 and ≥10,000 copies per milliliter was associated with a higher risk of mortality in age and race-ethnicity adjusted models (Hazard ratio (95% CI): 1.55 (1.09 to 2.19) and 2.94 (2.22 to 3.91), respectively). This increased risk remained for both HIV groups after further adjusting for comorbid diseases, substance use, and VACS Index components but was only statistically significant among those with HIV-1 RNA ≥10,000 copies per milliliter (1.34 (0.93 to 1.92) and 2.18 (1.60 to 2.99), respectively). HIV infected people with CD4+ T-cell count below 350 cells per cubic millimeter had increased mortality risk relative to uninfected people [1.67 (1.28–2.18)]. The association of HIV status stratified by ART receipt at baseline and mortality did not reach statistical significance (data not shown).
HIV and IL-6, sCD14, and D-dimer
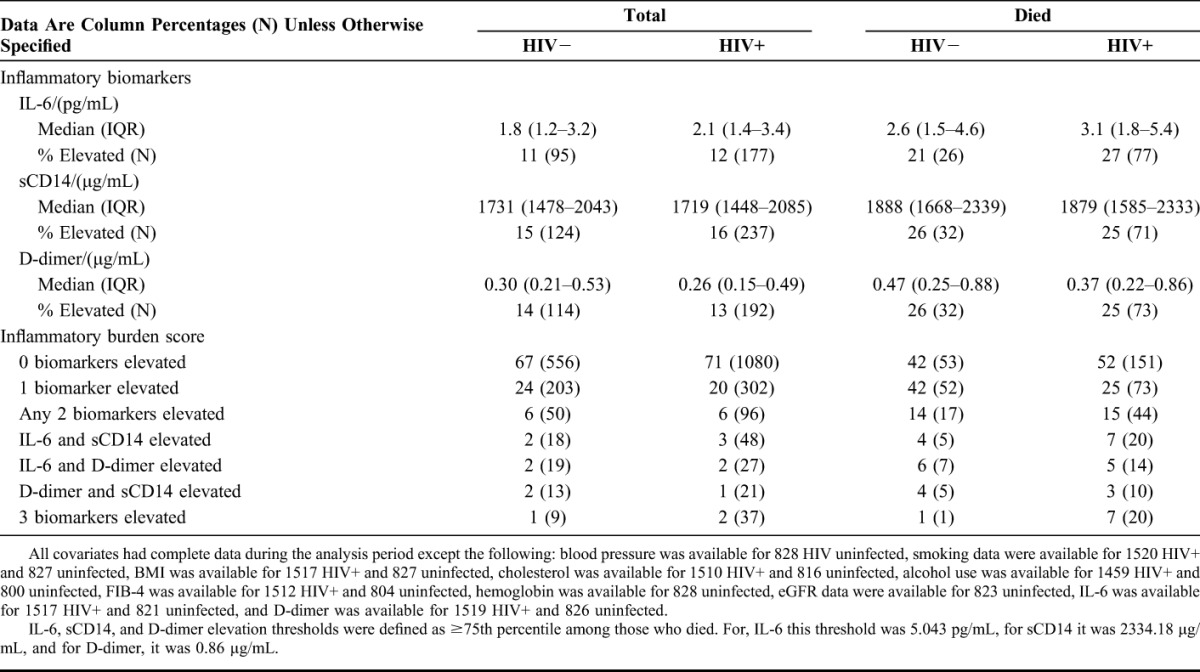
After adjustment for demographics, comorbidities, and substance use, HIV+ participants with viremia ≥10,000 copies per milliliter had greater proportional odds of elevated IL-6, sCD14, and D-dimer relative to uninfected participants (Table 2). The proportional odds (95% CI) of being in a higher quartile of IL-6, sCD14, or D-dimer was 2–3 folds higher for HIV+ (HIV-1 RNA ≥10,000 copies per milliliter) versus uninfected people (Table 2).
TABLE 2.
Association Between HIV Infection and IL-6, sCD14, and D-dimer Adjusted for (a) Age and Race-Ethnicity and (b) All Covariates
IL-6, sCD14, D-dimer, and Mortality
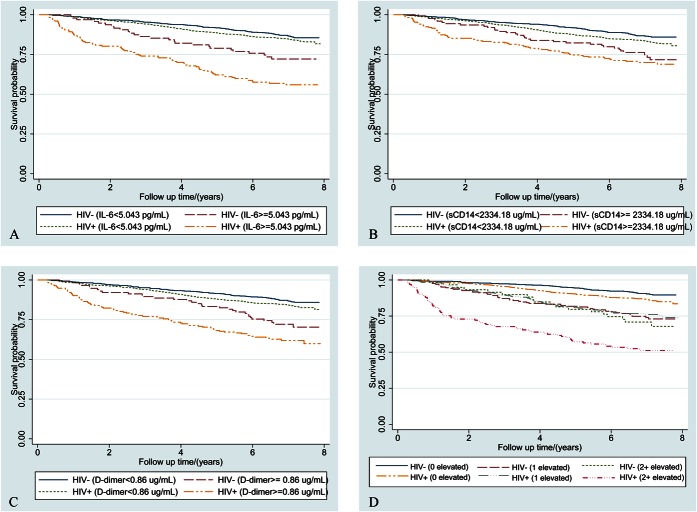
Mortality rates increased with elevations in IL-6, sCD14, and D-dimer (Table 3) and inflammatory burden (Fig. 1) among HIV+ and uninfected participants. In Cox proportional hazards models, IL-6, sCD14, and D-dimer elevations (highest quartile) were significantly associated with mortality risk independently of HIV infection or viral suppression (Table 3). These associations were attenuated but persisted after adjustment for comorbid disease, substance use and VACS Index components and the remaining 2 inflammatory biomarkers (Table 3). The association of elevated sCD14 and mortality persisted after comorbidity adjustment (see Table S1, Supplemental Digital Content, http://links.lww.com/QAI/A793) but was no longer statistically significant after adjustment for IL-6 and D-dimer (Table 3). These results were consistent in analyses excluding HIV infected people with unsuppressed viral replication (HIV-1 RNA ≥500 copies per milliliter; see Table S2, Supplemental Digital Content, http://links.lww.com/QAI/A793).
TABLE 3.
Mortality Rates, Rate Ratios and Risks by IL-6, sCD14 and D-dimer Quartile Mutually Adjusted for Each Other and Adjusted for HIV Status and Comorbid Conditions
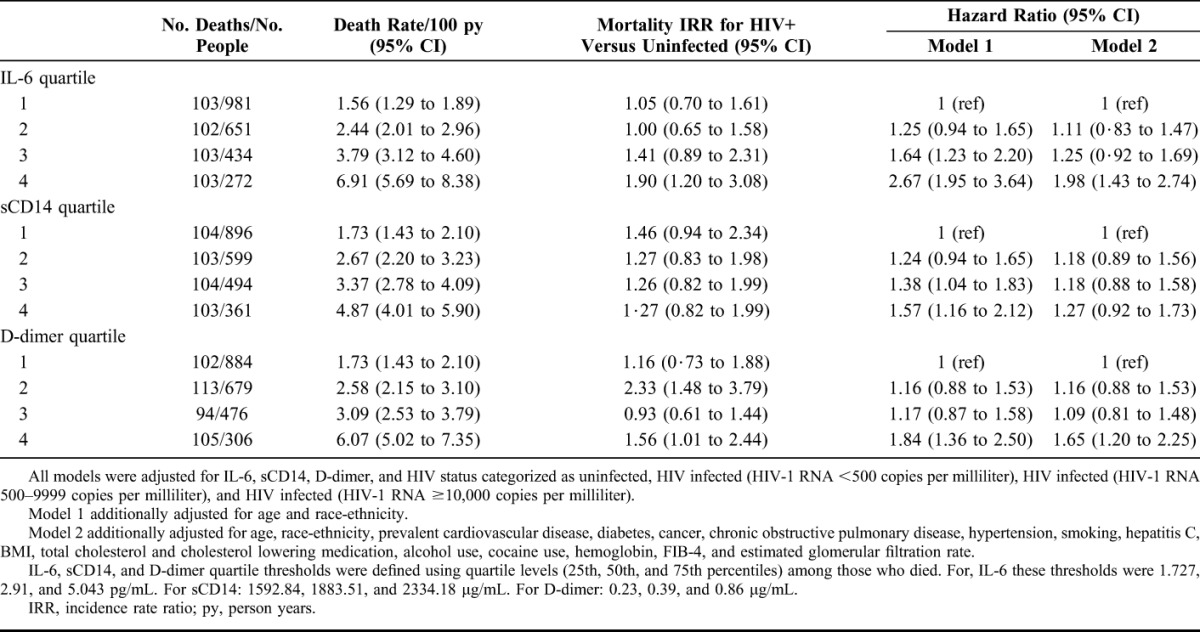
FIGURE 1.
Kaplan–Meier survival curves describing mortality by HIV status and (A) IL-6, (B) sCD14, (C) D-dimer and (D) inflammatory burden [number of inflammatory biomarkers (0, 1, or 2+) elevated >75th percentile]. IL-6, sCD14, and D-dimer elevation thresholds were defined as ≥75th percentile among those who died. For, IL-6 this threshold was 5.043 pg/mL, for sCD14 it was 2356·12 μg/mL, and for D-dimer, it was 0.88 μg/mL.
Association of HIV and Mortality Adjusting for IL-6, sCD14, and D-dimer
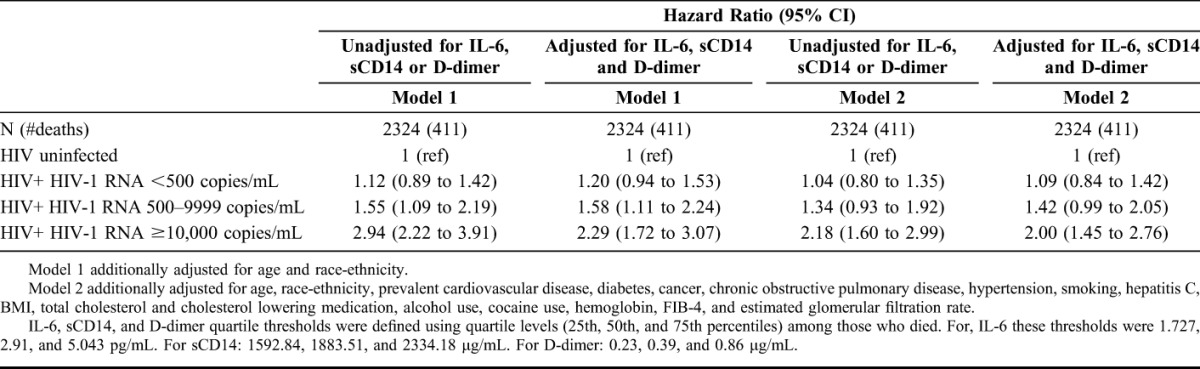
The association between HIV (with HIV-1 RNA ≥10,000 copies per milliliter or CD4+ T-cell count <350 cells per cubic millimeter) and mortality was partially attenuated after further adjusting the Cox models for IL-6, sCD14, and D-dimer (Table 4). The degree of attenuation was greatest when all 3 biomarkers were considered simultaneously as quartiles within a single model (Table 4). The risk of death among those with HIV-1 RNA ≥10,000 copies per milliliter went from 2.18 (1.60–2.99) to 2.00 (1.45–2.76) when IL-6, sCD14, and D-dimer were included in the model (Table 4). Similar attenuation was not observed among those with HIV-1 RNA <10,000 copies per milliliter. Relative to uninfected people, the risk of death for those with CD4+ T-cell counts <350 cells per cubic millimeter went from 1.67 (1.28–2.18) to 1.63 (1.25–2.14) after adjustment for IL-6, sCD14, and D-dimer.
TABLE 4.
Assessing Whether Addition of Inflammatory Biomarkers to Cox Regression Models attenuates the Association Between HIV Infection (Stratified by HIV-1 RNA at Baseline) and Mortality
We did not find significant interactions between HIV status and biomarker elevations on mortality risk (P ≥ 0.1).
DISCUSSION
We report that HIV infection is associated with biomarkers of inflammation, monocyte activation, and altered coagulation and an increased risk of death compared with those without HIV infection. These biomarkers are also associated with an increased risk of mortality, independently of HIV status or viremia. After adjustment for comorbid diseases and substance use, biomarkers of inflammation, monocyte activation, and altered coagulation partially explain the excess risk of mortality among viremic HIV infected people compared with uninfected people.
Our results are consistent with prior work linking elevated biomarkers of immune function to an increased risk of mortality among HIV infected people.4,5 The lack of uninfected comparators in prior work makes it challenging to assess if these biomarkers contribute to excess mortality among HIV infected people. With our cohort of HIV infected and demographically and behaviorally similar uninfected participants, we have extended these findings. Our results show that some of the excess risk of mortality among viremic HIV infected people is explained by biomarkers of inflammation, monocyte activation, and altered coagulation.
This study brings together a number of important findings within a single, well-phenotyped cohort of HIV infected and uninfected people with thorough capture of mortality outcomes. The fact that (1) mortality decreases with viral suppression (HIV-1 RNA <500 copies per milliliter in this study), (2) these biomarkers do not attenuate the association between HIV infection and mortality among those with lower HIV-1 RNA, and (3) HIV viremia is associated with higher levels of these biomarkers, all support the hypothesis that HIV viremia increases the levels of inflammation, monocyte activation, and altered coagulation, which drive increased mortality. Importantly, our results also demonstrate that the 3 biomarkers we studied do not explain the majority of the excess risk of mortality associated with HIV infection in our cohort. If immune system activation drives this excess risk, our finding may be explained by the fact that 3 biomarkers, when measured only at baseline, cannot fully capture the complexity of immune system activation. Additionally, mechanisms beyond inflammation, monocyte activation, and altered coagulation may contribute to the excess risk of mortality associated with viremic HIV infection. Furthermore, these biomarkers can change for reasons unrelated to HIV, eg, comorbid conditions. Finally, although these specific biomarkers do not explain most of the excess total mortality risk associated with HIV infection, they may explain more cause-specific mortality.
This study has limitations that warrant discussion. First, as the majority of our cohort is men, our results may not be generalizable to women. Second, our analysis did not have multiple longitudinal measures of inflammatory biomarkers to assess the impact of changes in the biomarkers on mortality risk. Third, while all 3 of our selected biomarkers are associated with HIV infection and increased risk of mortality among those with and without HIV, there are other potentially important biomarkers (eg, CD163, TNF alpha) and immunologic processes that were not included in our analysis. Finally, like all observational studies, we cannot eliminate the possibility of unmeasured or residual confounding.
In conclusion, increased HIV viral loads are associated with higher levels of biomarkers of inflammation (IL6), monocyte activation (sCD14), and altered coagulation (D-dimer). Elevated levels of these biomarkers are associated with mortality among HIV infected and uninfected people and in combination, these biomarkers partially explain the excess risk of mortality among viremic HIV infected compared with uninfected people.
Supplementary Material
ACKNOWLEDGMENTS
The authors acknowledge the veterans who participate in the Veterans Aging Cohort Study and the study coordinators and staff at each of our sites and at the West Haven Coordinating Center. Without the commitment and care of these individuals, this research would not be possible.
Footnotes
Supported by National Institute on Alcohol Abuse and Alcoholism and National Heart Lung and Blood Institute at the National Institutes of Health (Grant Number R01HC095136-04 5R01HC095126-04 to M.F; R01AI110259-01A1 and 1I01BX002358-01A1 to MMS). Veterans Aging Cohort Study funded by: National Institute on Alcohol Abuse and Alcoholism (U10 AA 13566). The authors would also like to acknowledge the substantial in-kind support we receive from the Veterans Affairs Healthcare System. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. And also supported by Gilead and AbbVie (Adeel Butt).
Some of these data were presented at Conference on Retroviruses and Opportunistic Infections, February 23–26, 2015, Seattle, WA.
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
REFERENCES
- 1.Reiner AP, Lange EM, Jenny NS, et al. Soluble CD14: genomewide association analysis and relationship to cardiovascular risk and mortality in older adults. Arterioscler Thromb Vasc Biol. 2013;33:158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Castelnuovo A, de Curtis A, Costanzo S, et al. Association of D-dimer levels with all-cause mortality in a healthy adult population: findings from the MOLI-SANI study. Haematologica. 2013;98:1476–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baune BT, Rothermundt M, Ladwig KH, et al. Systemic inflammation (Interleukin 6) predicts all-cause mortality in men: results from a 9-year follow-up of the MEMO Study. Age (Dordr). 2011;33:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandler NG, Wand H, Roque A, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203:780–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhaskaran K, Hamouda O, Sannes M, et al. Changes in the risk of death after HIV seroconversion compared with mortality in the general population. JAMA. 2008;300:51–59. [DOI] [PubMed] [Google Scholar]
- 7.Zhu H, Napravnik S, Eron JJ, et al. Decreasing excess mortality of HIV-infected patients initiating antiretroviral therapy: comparison with mortality in general population in China, 2003-2009. J Acquir Immune Defic Syndr. 2013;63:e150–e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Justice AC, Dombrowski E, Conigliaro J, et al. Veterans Aging Cohort Study (VACS): overview and description. Med Care. 2006;44(8 suppl 2):S13–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armah KA, McGinnis K, Baker J, et al. HIV status, burden of comorbid disease, and biomarkers of inflammation, altered coagulation, and monocyte activation. Clin Infect Dis. 2012;55:126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armah KA, Quinn EK, Cheng DM, et al. Human immunodeficiency virus, hepatitis C, and inflammatory biomarkers in individuals with alcohol problems: a cross-sectional study. BMC Infect Dis. 2013;13:399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events. The cardiovascular health study. Ann Epidemiol. 1995;5:278–285. [DOI] [PubMed] [Google Scholar]
- 12.Every NR, Fihn SD, Sales AE, et al. Quality enhancement research initiative in ischemic heart disease: a quality initiative from the department of veterans affairs. Queri IHD executive committee. Med Care. 2000;38(6 suppl 1):I49–I59. [PubMed] [Google Scholar]
- 13.Park LS, Tate JP, Rodriguez-Barradas MC, et al. Cancer incidence in HIV-infected versus uninfected veterans: comparison of cancer registry and ICD-9 code diagnoses. J AIDS Clin Res. 2014;5:1000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crothers K, Huang L, Goulet JL, et al. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med. 2011;183:388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 16.Butt AA, McGinnis K, Rodriguez-Barradas MC, et al. HIV infection and the risk of diabetes mellitus. AIDS. 2009;23:1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA. 2001;285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 18.Justice AC, Modur SP, Tate JP, et al. Predictive accuracy of the Veterans Aging Cohort Study index for mortality with HIV infection: a North American cross cohort analysis. J Acquir Immune Defic Syndr. 2013;62:149–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tate JP, Justice AC, Hughes MD, et al. An internationally generalizable risk index for mortality after one year of antiretroviral therapy. AIDS. 2013;27:563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butt AA, Xiaoqiang W, Budoff M, et al. Hepatitis C virus infection and the risk of coronary disease. Clin Infect Dis. 2009;49:225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. [DOI] [PubMed] [Google Scholar]
- 22.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 suppl 1):S1–S266. [PubMed] [Google Scholar]
- 23.Baron RM, Kenny DA. The moderator–mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. [DOI] [PubMed] [Google Scholar]
- 24.MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2007;58:593–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Long JS, Freese J. Regression Models for Categorical Dependent Variables. 3rd ed College Station, TX: Stata Press; 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.