Abstract
Recent studies have focused on the identification and manipulation of memory traces in rodent models. The two main mouse models utilized are either a CreERT2/loxP tamoxifen (TAM)- or a tetracycline transactivator (tTA)/tetracycline-response element (TRE) doxycycline (DOX)-inducible system. These systems, however, could be improved to label a more specific population of activated neurons corresponding to behavior. Here, we sought to identify an improved selective estrogen receptor (ER) modulator (SERM) in which we could label an individual memory trace in ArcCreERT2 mice. We found that 4-hydroxytamoxifen (4-OHT) is the most selective SERM in the ArcCreERT2 x ROSA26-CAG-stopflox-channelrhodospin (ChR2)-enhanced yellow fluorescent protein (eYFP) mice. The half-life of 4-OHT is also shorter than TAM, allowing for more specificity of memory trace labeling. Furthermore, 4-OHT allowed for context-specific labeling in the dentate gyrus (DG) and CA3. In summary, we believe 4-OHT improves the specificity of memory trace labeling and will allow for refined memory trace studies in the future.
Introduction
Memory traces or engrams, as first coined by Richard Semon in 1921, are believed to be ensembles of neurons used to store particular memories (Schacter et al., 2001; Josselyn et al., 2010). Although the search for the engram has been ongoing for almost 100 years, there has been significant progress recently in definitively showing that particular neurons are indeed engrams. These studies have utilized several increasingly sophisticated methods: 1) electrophysiology (Smith and Mizumori et al., 2006; Leutgeb et al., 2007), 2) compartmental analysis of temporal activation by fluorescence in situ hybridization (catFISH) (Guzowski et al., 1999); 3) novel mouse models to tag active neurons (Reijmers et al., 2007); 4) selective ablation of activated neurons for memory erasure (Han et al., 2009); 5) optogenetic excitation to show sufficiency to evoke learned behavior (Liu and Ramirez et al., 2012); and 6) optogenetic inhibition to show necessity for behavioral expression (Denny et al., 2014). A number of brain regions have been shown to contain engrams, such as: the basolateral amygdala (BLA) (Reijmers et al., 2007; Tayler et al., 2013), lateral amygdala (LA) (Han et al., 2009), DG (Liu and Ramirez et al., 2012; Tayler et al., 2013; Denny et al., 2014), CA3 (Denny et al., 2014), CA1 (Deng et al., 2013; Tayler et al., 2013), cortical amygdala (Root et al., 2014); and cochlear nucleus (Guenthner et al., 2013). These data broadly show that specific neurons are involved in memory encoding, memory retrieval, and memory expression.
A number of murine lines have been created to label activated neurons during a behavioral experience. Many of these lines have utilized immediate early gene (IEG) expression, such as Arc/Arg3.1 or c-fos, as a proxy for neuronal activation (Reijmers et al., 2007; Guenthner et al., 2013; Denny et al., 2014). We created the ArcCreERT2 bacterial artificial chromosome (BAC) transgenic mice in order to visualize engrams not just on a short timescale, such as minutes (Guzowski et al., 1999), or days (Reijmers et al., 2007), but indefinitely (Denny et al., 2014). We utilized Arc/Arg3.1, which has been widely implicated in synaptic plasticity and hippocampus-dependent memory (Link et al., 1995; Lyford et al., 1995), and found that distinct memory traces are located in the DG and in CA3, but that the strength of the memory is related to reactivation in CA3. This mouse line has allowed for the assessment of how and where a memory is formed and expressed under a number of conditions that include stress or ablation of adult hippocampal neurogenesis. Finally, we showed the necessity of this population for memory expression by optogenetically inhibiting the population of cells active during the initial memory encoding.
In the CreERT2 system, TAM must be administered in order for indelible labeling to occur in the activated cells. TAM allows CreERT2 to enter the nucleus of an activated neuron so that it may remove the STOP codon and allow for expression of a reporter gene (Hayashi and McMahon et al., 2002). In the tTA/TetO system, mice are raised on food containing DOX in order to inhibit TetO activation (Reijmers et al., 2007). In order to induce reporter expression, DOX is removed from the diet, which allows activation of the transcriptional feedback loop. The ArcCreERT2 mice were designed with the hypothesis that the CreERT2 system was superior to the tTA/TetO system because of the shortened window of activation. However, TAM has a half-life of more than 24 h (Hayashi and McMahon et al., 2002). Therefore, as with the tTA/TetO system, which has a relatively large window for reporter expression to occur, TAM’s effectiveness for labeling a specific memory trace may be diluted by labeling non-specific behavioral neurons, as it remains in the mouse’s system for such an extended period of time.
In this study, we sought to identify improved conditions in which we could label memory traces in the ArcCreERT2 mice with increased specificity for activated neurons corresponding to a behavioral experience. We determined that 4-OHT was the superior drug for recombination. We show that the half-life of 4-OHT is also significantly shorter than the half-life of TAM. Finally, 4-OHT allowed for context-specific memory trace labeling. We believe that future utilization of 4-OHT will improve the specificity of memory trace labeling studies.
Results
Characterization of the ArcCreERT2 x ROSA26-CAG-stopflox-ChR2-eYFP mice
We generated ArcCrERT2(+) x ROSA26-CAG-stopflox-ChR2-eYFP heterozygous (f/+) mice (Fig. 1A). In these mice, a single injection of TAM produces a robust ChR2-eYFP label (Fig. 1B), specifically in the DG. However, the TAM injection often produces a background haze staining in the DG (Fig. 1C), which was not previously seen in the ArcCreERT2 x eYFP line (Denny et al., 2014). eYFP+/c-fos+ cells are still identifiable (Fig. 1D–1F), as ChR2-eYFP labeling produces a halo around c-fos+ cells due to the membrane bound ChR2-eYFP. However, the ChR2-eYFP labeling is unlike the eYFP labeling (Denny et al., 2014), which produces distinctly yellow co-labeled cells due to cytoplasmic and membrane bound eYFP. Furthermore, we do not suggest breeding these mice as ChR2-eYFP homozygous (f/f) mice, as this produces an extremely dense label and results in seizure activity following optogenetic stimulation (Fig. S01).
Figure 1.

Characterization of ArcCreERT2 x ROSA26-CAG-stopflox-ChR2-eYFP mice. (A) Breeding strategy. Administration of TAM results in an indelible ChR2-eYFP label in the initially activated Arc+ cells. (B) Representative image. A single injection of TAM produces a robust ChR2-eYFP in the HPC. (C) The eYFP label is robust, yet there is a background labeling in this line. (D) eYFP+ cells corresponding to CFC encoding. (E) c-fos+ DG cells corresponding to memory retrieval. (F) Representative co-labeled eYFP+/c-fos+ cell. Scale bars represent 100 mm.
Comparison of SERMs for eYFP labeling
To improve the ChR2-eYFP labeling, we first manipulated the drug administration protocol. Firstly, we sought to identify a SERM with either more potent activity or a shorter half-life, and therefore, one that would produce a more specific eYFP label to the CFC experience. We identified 4-OHT, which is a more potent ER agonist/antagonist than TAM and is a metabolite of TAM (Klinge et al., 1998). Furthermore, 4-OHT has a serum half-life of 6 h, whereas TAM has a serum half-life of 11.9 h (Robinson et al., 1991). Mice were injected with either TAM or 4-OHT and 5 h later administered 3-shock CFC (Fig. 2A). Five days later mice were re-exposed to the CFC training context and sacrificed 1 h later. When compared with TAM (Fig. 2B–2E), 4-OHT produced a less intense, yet distinct eYFP signal (Fig. 2F–2I).
Figure 2.
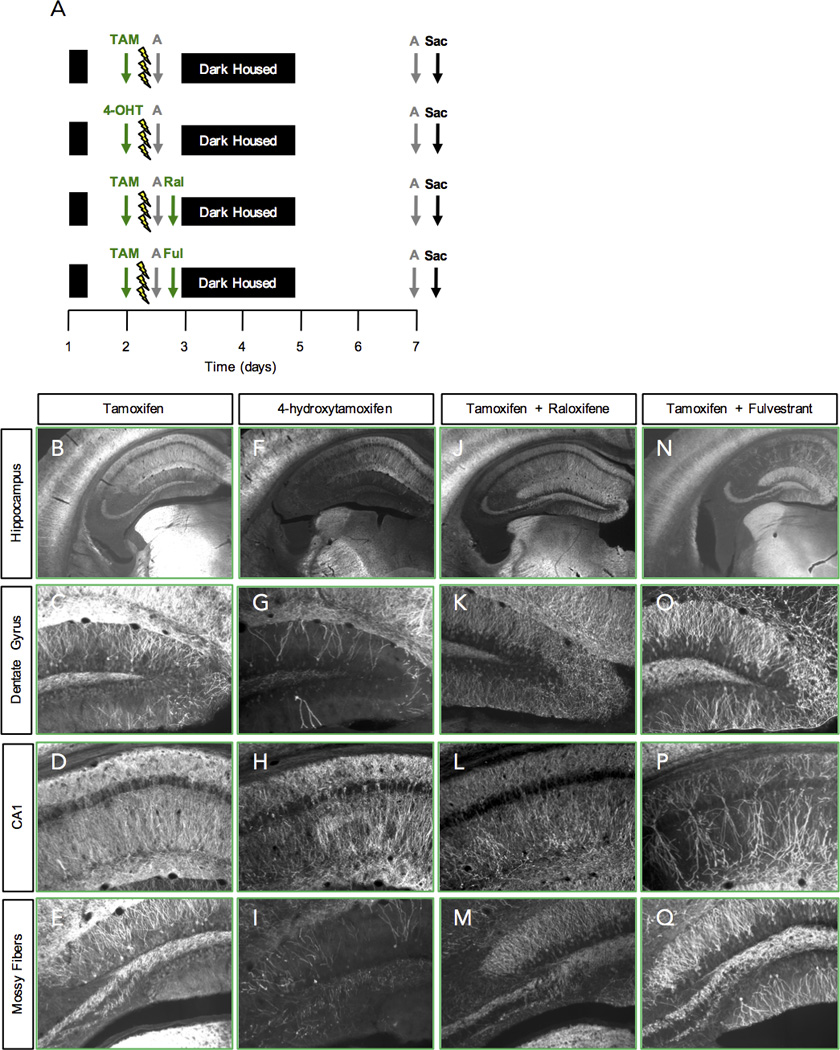
Comparison of SERMs for eYFP labeling. (A) Experimental design. (B-E) TAM-injected mice have a dense eYFP label. (F-I) 4-OHT-injected mice have a less intense, yet distinct eYFP signal. (J-M) Ral–injected and (N-Q) Ful-injected mice have an extremely dense eYFP label. n = 3–6 mice per group.
Secondly, we sought to identify a SERM with opposing actions to TAM and therefore, a drug to potentially block future recombination. We identified two compounds: raloxifene (Ral), an ER agonist in bone and an ER antagonist in the breast and uterus; and fulvestrant (Ful), an ER antagonist with no agonist effects, which works by down regulating and degrading the ER. We injected mice with TAM 5 h before CFC training, and then injected either of these compounds immediately after CFC (Fig. 2A). However, Ral (Fig. 2J–2M) and Ful (Fig. 2N–2Q) were not effective in decreasing eYFP expression from the CFC experience.
Tamoxifen and 4-hydroxytamoxifen produced comparable eYFP labeling
To examine whether 4-OHT impacts behavior and eYFP expression in a comparable manner to TAM, we quantified freezing behavior and the number of eYFP+ DG cells. Mice were administered TAM or 4-OHT and 5 h later administered 3-shock CFC. Five days later, mice were re-exposed to the CFC training context and sacrificed 1 h later. Mice administered TAM or 4-OHT exhibited similar levels of freezing during the retrieval test (Fig. 3A). Mice administered TAM and 4-OHT also had comparable levels of eYFP+ DG cells (Fig. 3B). However, as shown in Fig. 2, mice administered TAM (Fig. 3C) had much more background haze staining when compared with mice administered 4-OHT (Fig. 3D). This staining did not reach the threshold of eYFP labeling, but was more of a diffuse haze that was strikingly apparent in the DG (Fig. 3E–3F). Interestingly, 4-OHT produced a much more specific cortical labeling and was also less dense when compared with TAM (Fig. 3G–3H). As expected, 4-OHT did not produce eYFP labeling in ArcCreERT2(−) x ROSA26-CAG-stopflox-ChR2-eYFPf/+ mice (Fig. S02). Moreover, a vehicle (Veh) injection produced very little eYFP DG and CA3 labeling, suggesting there is a low level of leak in this line (Fig. S03).
Figure 3.

4-OHT produces distinct eYFP labeling. (A) Mice administered TAM or 4-OHT exhibited similar levels of freezing during the retrieval test in the CFC chamber. (B) Mice administered TAM and 4-OHT also had comparable number of eYFP+ DG cells. (C and E) Mice administered TAM had a background haze staining when compared with (D and F) mice administered 4-OHT. Arrows indicate areas of background haze staining. (G–H) eYFP labeling in the retrosplenial granular cortex following a 4-OHT injection is noticeably less than following a TAM injection. n = 3–9 mice per group. Error bars represent ± SEM.
4-hydroxytamoxifen’s recombination efficiency decreases between 12–18 h post injection
To determine the half-life of 4-OHT and the best time to inject 4-OHT relative to the CFC experience, we varied the time of the 4-OHT injections before CFC (Fig. 4A). Mice were injected with 4-OHT 36 h, 24 h, 18 h, 12 h, 9 h, or 5 h before CFC. The number of eYFP+ DG cells was then quantified to determine the most effective time at which 4-OHT should be administered relative to CFC encoding (Fig. 4B). The number of eYFP+ DG cells was significantly lower at 24 and 36 h when compared to the 5 and 9 h groups. Furthermore, the number of eYFP+ DG cells was significantly lower at 18 h when compared with the 9 h group. These data suggest that 4-OHT becomes significantly less active between 12 and 18 h and therefore, the rate of recombination decreases.
Figure 4.

4-OHT’s recombination efficiency decreases between 12–18 h post injection. (A) Experimental design. (B) The number of eYFP+ cells in the DG was significantly lower at 24 and 36 h when compared to the 5 and 9 h time points. The number of eYFP+ cells in the DG was significantly lower at 18 h when compared with the 9 h group. n = 3–7 mice per group. Error bars represent + SEM. * p < 0.05; ** p < 0.01, *** p < 0.001.
4-hydroxytamoxifen is effective in labeling engrams
Although 4-OHT produces a clear eYFP labeled population, we wanted to confirm that that the 4-OHT labeling was specific to the behavioral experience. Therefore, we quantified reactivation in the HPC of ArcCreERT2 mice under a number of conditions (Fig. 5A). Mice were placed into one of the following groups: 1) Home cage (HC): injected with 4-OHT in the HC and sacrificed from the HC; 2) Context exposure: injected with 4-OHT, exposed to context A, and re-exposed to context A; 3) CFC in A, re-exposure to A: injected with 4-OHT, administered 3-shock CFC, and re-exposed to context A; or 4) CFC in A, exposure to B: injected with 4-OHT, administered 3-shock CFC, and exposed to a novel context B. All mice explored context A in a similar manner before shocks were administered (RMANOVA, Context: p = 0.12) (Fig. 5B). However, after the shocks were administered, mice froze significantly more than mice given context exposure (RMANOVA, Context x Time: p = 0.0002). During the retrieval test, mice administered CFC exhibited markedly higher levels of freezing than mice exposed to the context without footshock or mice that were administered CFC but then placed into a novel context B (RMANOVA, Context x Time: p = 0.0097) (Fig. 5C).
Figure 5.

4-OHT is effective in labeling memory traces. (A) Experimental design. (B) All mice explored context A in a similar manner before shocks were administered. After the shocks were administered, mice froze significantly more than mice given context exposure. (C) During the retrieval test, mice administered CFC exhibited markedly higher levels of freezing than mice exposed to the context without footshock or mice that were administered CFC but then placed into a novel context B. (D) CFC resulted in an increase in the number of eYFP+ cells in the DG when compared to mice injected with 4-OHT in their HC. (E) Mice exposed to a context (A or B) had significantly more Arc+ cells in the DG than mice left in their HC. (F) The percent of co-labeled eYFP+/Arc+ cells in the DG was specific to the context experience. (G) CFC resulted in an increase in the number of eYFP+ cells when compared to mice injected with 4-OHT in their HC or to mice injected with 4-OHT and given context A exposure. (H) Mice exposed to a context (A or B) had significantly more c-fos+ cells in CA3 than mice left in their HC. (I) The percent of co-labeled eYFP+/c-fos+ cells in CA3 was specific to the context experience. n = 5 mice per group. Error bars represent ± SEM. * p < 0.05; ** p < 0.01, *** p < 0.001.
In the DG, CFC resulted in an increase in the number of eYFP+ cells when compared to mice injected with 4-OHT in their HC (Fig. 5D). Mice exposed to a context (A or B) had significantly more Arc+ DG cells than mice left in their HC (Fig. 5E). As previously shown with TAM (Denny et al., 2014), the percent of co-labeled eYFP+/Arc+ DG cells was specific to the context experience (Fig. 5F). Mice administered 3-shock CFC and then re-exposed to the training context A had the greatest percent of co-labeled eYFP+/Arc+ DG cells (4.5%). Mice administered a 3-shock CFC paradigm and then re-exposed to a novel context B (2.0%), or mice exposed to context A twice (3.0%) had significantly less co-labeled eYFP+/Arc+ DG cells. Finally, mice that remained in the HC has the lowest level of eYFP+/Arc+ DG cells (1.5%). These data indicate that the majority of eYFP+ DG cells are specific for the CFC experience. To ensure the specificity of 4-OHT, we performed a simple regression analysis with the co-labeled populations. In the DG, the percent of co-labeled eYFP+/Arc+ cells correlates with the freezing percentage (Fig. S04), suggesting that we are indeed labeling a neuronal population that represents a memory trace.
In CA3, CFC resulted in an increase in the number of eYFP+ cells when compared to mice injected with 4-OHT in their HC or to mice injected with 4-OHT and given context A exposure (Fig. 5G). Mice exposed to a context (A or B) had significantly more c-fos+ cells than mice left in their HC (Fig. 5H). As previously shown with TAM (Denny et al., 2014), the percent of co-labeled eYFP+/Arc+ CA3 cells was specific to the context experience (Fig. 5I). Mice administered a 3-shock CFC paradigm and then re-exposed to the training context A had the greatest percent of co-labeled eYFP+/c-fos+ CA3 cells (5.6%). Mice administered a 3-shock CFC paradigm and then re-exposed to a novel context B (2.8%), or mice exposed to context A twice (3.3%) had significantly less co-labeled eYFP+/c-fos+ CA3 cells. Finally, mice that remained in the HC had the lowest level of eYFP+/Arc+ DG cells (0.6%). These data indicate that the majority of eYFP+ CA3 cells are specific for the CFC experience.
In CA1, all groups of mice had similar levels of eYFP+ cells, suggesting that the level of recombination is not efficient in this region (Fig. S05A). However, there was a significant correlation between freezing percent during the retrieval test with the number of eYFP+ CA1 cells (Fig. S05C), suggesting that with refined parameters or an increased number of mice, memory trace studies in CA1 are possible.
4-hydroxytamoxifen is effective when administered following CFC
If 4-OHT becomes bioactive more readily than TAM, we hypothesized that injection following CFC may also produce a reliable eYFP+ label from CFC encoding. Mice were administered 3-shock CFC and immediately following CFC, were injected with 4-OHT (Fig. 6A). The retrieval test was administered 5 d later. Four-OHT labeling post-CFC also produced distinct eYFP labeling (Fig. 6B). Moreover, the number of eYFP+ cells (Fig. 6C), Arc+ cells (Fig. 6D), and percent co-labeled eYFP+/Arc+ cells (Fig. 6E) (5.3%) in the DG was similar to when mice were injected with 4-OHT before CFC, suggesting that 4-OHT has a rapid onset.
Figure 6.
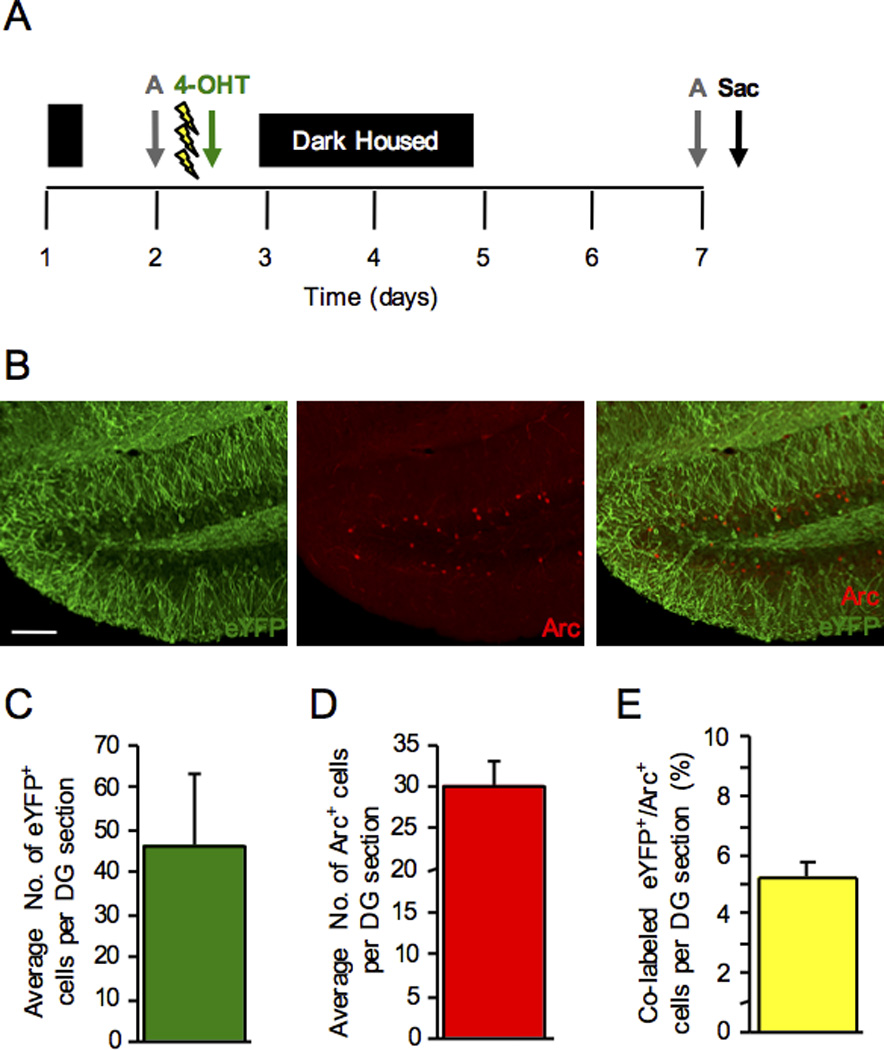
4-OHT is effective for eYFP labeling when administered following CFC. (A) Mice were administered a 3-shock CFC paradigm and immediately following the training procedure, were injected with 4-OHT. The retrieval test was administered 5 d later. (B) 4-OHT labeling post-CFC produced distinct eYFP labeling in the DG. (C) The number of eYFP+ cells per DG section. (D) The number of Arc+ cells per DG section. (E) The percent co-labeled eYFP+/Arc+ cells per DG section. n = 3 mice per group. Error bars represent ± SEM. Scale bar represents 100 µm.
Discussion
In this study, we identified a more effective SERM than TAM in order to label neuronal populations activated during learning. We found that 4-OHT produced a more specific neuronal label, had a shorter half-life, and had a rapid onset of activity. Specificity is essential for memory trace studies because if neurons activated after encoding are labeled with a reporter, the population of neurons corresponding to encoding becomes contaminated. Our data is in accord with our previous report on TAM-injected ArcCreERT2(+) x ROSA26-stopflox-eYFPf/+ mice (Denny et al., 2014) and with a previous study on TAM- and 4-OHT-injected TRAP mice (Guenthner et al., 2013). The authors report that 4-OHT produced more consistent results than TAM in the FosTRAP and ArcTRAP mice. We believe that moving forward 4-OHT should be the drug of choice for recombination in CreERT2 memory trace studies.
A single injection of TAM in ArcCreERT2(+) x ROSA26-CAG-stopflox-ChR2-eYFPf/+ mice produced a distinctive haze in the DG that was not previously seen in the eYFP reporter mice (Denny et al., 2014). ArcCreERT2(+) and (−) x ROSA26-CAG-stopflox-ChR2-eYFPf/+ mice administered TAM have this background haze, which is most likely an artifact of the TAM injection in combination with the ChR2-eYFP line. Mice administered 4-OHT did not have this distinctive background haze, making confocal and multi-photon imaging of these brain slices was much clearer. A recent report suggested that TAM may continue to label a significant number of cells for weeks after the TAM treatment, possibly contaminating the interpretation of TAM-dependent models (Reinert et al., 2012). Therefore, it is possible that this haze represents cells that have recently undergone recombination and are beginning to express eYFP. This haze artifact may not be present in the mice administered 4-OHT, as cells may not be continuing to undergo recombination.
Raloxifene and fulvestrant were injected following CFC with the goal of partially blocking some of TAM’s recombination activity. We identified these drugs because they either had an opposing action to TAM or were a complete antagonist with no agonist activity. Although these experiments did not prove fruitful for inhibiting the activity of TAM, we believe that it highlights the need for drug development of a class of inhibitors to limit the activity of SERM labeling during learning. Ideally, a SERM could be administered immediately before a learning experiment and a blocker could be administered immediately following training. Therefore, the extraneous cells that are labeled from contextual cues, handling, or home cage social interactions could be removed from the encoding population, allowing for more refined studies.
Similarly, in our initial characterization, we developed a procedure of dark housing the mice before and after CFC for 3 days in order to decrease the number of cells being labeled following a learning experience (Denny et al., 2014). Dark housing allowed for a more refined population of activated neurons from a CFC experience. Our findings following dark housing were consistent with previous studies showing that after a single injection of TAM, there is a significant nuclear accumulation of CreER within 24 h and this nuclear signal disappears approximately by 48 h, indicating that TAM is active 24 h post injection. In these experiments, the appearance of the reporter is detected at approximately 12 h after the injection and these cells continue to accumulate for about 36 h (Hayashi and McMahon et al., 2002). A single injection of 4-OHT seems to be superior to TAM mainly because of its shorter half-life. In mice, the rate of elimination from the serum following a single large oral dose of TAM was 11.9 h, whereas following a single large oral dose of 4-OHT was 6 h (Robinson et al., 1991). We are currently still combining dark housing with 4-OHT, but future studies may allow for a decrease in the amount of dark housing if 4-OHT is no longer active past 12–18 h.
Although 4-OHT did not produce the background haze as TAM did, TAM and 4-OHT did produce a similar number of eYFP+ cells in the DG following 3-shock CFC (approximately 50 eYFP+ cells per section). This is less than previously reported in the eYFP line (approximately 100 eYFP+ cells per section). It is promising though that the ChR2-eYFP line produces a more representative snapshot of initially active Arc+ cells, as there are typically 25–35 Arc+ cells per section in the DG following CFC. We initially hypothesized that TAM in the eYFP line labeled the initially active Arc+ cells, the consolidation Arc+ cells, and some HC Arc+ cells, resulting in the approximately 100 eYFP+ cells per section. We believe that the ChR2-eYFP line may have increased specificity for future memory trace studies.
Context-specific eYFP+ labeling was found in the DG and CA3, but not in CA1. The number of CA1 eYFP+ neurons was not previously quantified in the eYFP line and therefore, we do not have levels to compare to. However, it is troubling that only 6–10 eYFP+ neurons are labeled in CA1 per section, which may be underrepresenting the number of Arc+ cells active during encoding. It remains to be seen though if CA1 is an efficient region for engram studies in the ArcCreERT2 x ROSA26-CAG-stopflox-ChR2-eYFP mice. It highlights the issue of underrepresenting active neuronal populations, such as is commonly seen with viral injections, which do not always sufficiently label or reach entire brain structures. It is important to point out that perhaps it is not that the 4-OHT is inefficient in CA1 for recombination, but that Arc is differentially expressed in CA1 versus the DG or CA3. There may be reduced tagging in CA1 if cells expressing Arc protein make the protein from previous stores of RNA, rather than de novo transcription, which would tag the cells.
Most promising, memory traces were localized in the DG and in CA3, as we have previously reported (Denny et al., 2014). Interestingly, although the number of eYFP+ cells was reduced in the DG and in CA3 of the ChR2-eYFP line, we found comparable percentages of reactivated cells (2–6%). We believe that comparable percentages were found, as we quantified the number of Arc+ or c-fos+ cells that are co-labeled with eYFP, rather than the number of eYFP+ cells that are co-labeled with Arc or c-fos. We also show that context exposure produces more co-labeled cells than home cage exposure, but not to the extent that context exposure plus fear learning. We believe that Arc expression is sensitive enough to detect fear learning is occurring as opposed to just context exposure. Although the number of Arc cells is not altered following context exposure, the behavioral profiles and co-labeled eYFP+/Arc+ cells do differ. These data give further validation to memory trace studies, as we have now replicated our previous findings in a separate reporter line. Furthermore, this line is extremely valuable, as optogenetic manipulations in concert with whole-brain engram labeling can be now be studied using methods such as iDISCO (Renier et al., 2014) or CLARITY (Chung et al., 2013).
We believe that this characterization of memory traces and of the time course of 4-OHT will be useful for future studies labeling distinct populations of Arc+ neurons. For example, IEG (Arc or c-fos) expression peaks at 1–1.5 h following a behavioral or learning experience (Lonergan et al., 2010), but there is a residual wave (Katche et al., 2010) or peaks of expression following learning (e.g. a trend for an increase at 24 h in Lonergan et al., 2010). Studies labeling individual consolidation or waves of activity will be possibly by timing the 4-OHT injection in concert with behavioral training. Analyzing the different waves of Arc activity may provide valuable information on the role of these neurons in memory trace studies.
In summary, we characterized hippocampal memory traces in the ArcCreERT2 x ROSA26-CAG-stopflox-ChR2-eYFP mice. We determined that the half-life of 4-OHT is shorter than TAM and allows for improved eYFP labeling when compared with TAM. Furthermore, 4-OHT allowed for context-specific memory trace labeling in the DG and CA3. In summary, we believe the use of 4-OHT will allow for refined memory trace studies in the future.
Materials and Methods
Mice
ArcCreERT2(+) female mice (Denny et al., 2014) were bred with ROSA26-CAG-stopflox-ChR2(H134R)-eYFP (Ai32) male mice (Madisen et al., 2012). All experimental mice were heterozygous for the reporter, with the exception of when noted in Figure S01. Mice (8 to 12 weeks of age) were housed 4 to 5 per cage in a 12-h (06:00–18:00) light-dark colony room at 22°C. Food and water were provided ad libitum. Behavioral testing was performed during the light phase. The procedures described herein were conducted in accordance with National Institutes of Health regulations and approved by the Institutional Animal Care and Use Committees of Columbia University and the New York State Psychiatric Institute.
For all experiments, cages were changed and mice were placed into a separate housing room the night before the TAM/4-OHT injection (day 1). The next day, mice were injected with TAM/4-OHT and administered a behavioral task (e.g. CFC) 5 h later (day 2). Following the behavioral task, mice were placed into the dark for that night and the following 3 d (days 3–5). Mice were taken out of the dark, cages were changed, and they were returned to the normal colony room. All precautions to prevent disturbances to the ArcCreERT2 mice were taken.
Genotyping
Genotyping was performed as previously described (Denny et al., 2014).
Drugs
Tamoxifen (TAM) and 4-hydroxytamoxifen (4-OHT)
Recombination was induced using TAM (Sigma, St. Louis, MO, T5648), or 4-OHT (Sigma, St. Louis, MO, H7904). Both drugs were dissolved by sonication in 10% EtOH / 90% corn oil at a concentration of 10 mg/ml. One injection of 200 µl (2 mg) was injected intraperitoneally (i.p.) into adult mice.
Raloxifene (Ral)
Raloxifene (Sigma, St. Louis, MO, R1402) was dissolved by sonication in a 1:3 (v:v) of DMSO/100% EtOH. One injection of 200 µl (2 mg) was injected i.p. into mice.
Fulvestrant (Ful)
Fulvestrant (AdooQ BioScience, Irvine, CA, A10410) was dissolved by sonication in 10% EtOH / 90% corn oil at a concentration of 10 mg/ml. One injection of 200 µl (2 mg) was injected i.p. into mice.
Contextual Fear Conditioning (CFC)
The 3-shock CFC procedure was based on that of Drew et al. (2010). Context information is included in Table S01.
Immunohistochemistry
Mice were deeply anesthetized and brains were processed as previously described in (Denny et al., 2014). EYFP, Arc, and c-fos immunohistochemistry was performed as previously described, with the exception that a new c-fos antibody was used (rabbit anti-c-fos, 1:1000, Santa Cruz Biotechnology, Inc., Dallas, TX).
Cell Quantification
An investigator blind to treatment used a Zeiss Axioplan-2 upright microscope (Oberkochen, Germany) to count eYFP+, Arc+, and c-fos+ immunoreactive cells bilaterally in the granule cell layer of the DG, in CA3, or in CA1 throughout the entire rostro-caudal axis of the HPC (Denny et al., 2014). Cells were counted bilaterally using a 20X or 40X objective. The average eYFP+, Arc+, and c-fos+ cells per section are presented throughout the text.
Confocal Microscopy
For co-labeled cells, fluorescent confocal micrographs were captured with Leica TCS SP8 MP confocal microscope with the aid of LAS X software. Individual tiled images were acquired at a z-increment of 1.41 µm. Split-panel and z stack analysis was performed as previously described with the exception that the LAS X software was utilized.
Statistical Analysis
All data were analyzed using StatView (v. 5.0) software (SAS Institute). Alpha was set to 0.05 for all analyses. Data are expressed as means ± SEM. * p < 0.05, ** p < 0.01, *** p < 0.001. All statistical tests are included in Table S02.
Supplementary Material
Acknowledgments
A.S.C. was supported by a NIH DP5 OD017908. R.M. was supported by the BRAINYAC program at Columbia University. N.K.T. was supported by a NIH DP5 OD017908. C.A.D. was supported by a NIH DP5 OD017908-01, a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation, and a NYSTEM N13S-006. We thank Isuri Poththewala, Stephanie A. Cajigas, and Sean C. Lim for assistance. We thank members of the Denny and Hen laboratories for comments.
Footnotes
Competing interests
The authors declare that they have no competing interests.
References
- 1.Chung K, Wallace J, Kim S-Y, Kalyanasundaram S, Andalman AS, Davidson TJ, Mirzabekov JJ, Zalocusky KA, Mattis J, Denisin AK, Pak S, Bernstein H, Ramakrishnan C, Grosenick L, Gradinaru V, Deisseroth K. Structural and molecular interrogation of intact biological systems. Nature. 2013;497:332–337. doi: 10.1038/nature12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deng W, Mayford M, Gage FH. Selection of distinct populations of dentate granule cells in response to inputs as a mechanism for pattern separation in mice. eLife. 2013;2:e00312. doi: 10.7554/eLife.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denny CA, Burghardt NS, Schachter DM, Hen R, Drew MR. 4- to 6-week old adult born hippocampal neurons influence novelty-evoked exploration and contextual fear conditioning. Hippocampus. 2012;22:1188–1201. doi: 10.1002/hipo.20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denny CA, Kheirbek MA, Alba EL, Tanaka KF, Brachman RA, Laughman KB, Tomm NK, Turi GF, Losonczy A, Hen R. Hippocampal Memory Traces Are Differentially Modulated by Experience, Time, and Adult Neurogenesis. Neuron. 2014;83:189–201. doi: 10.1016/j.neuron.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drew MR, Denny CA, Hen R. Arrest of adult hippocampal neurogenesis in mice impairs single- but not multiple-trial contextual fear conditioning. Behavioral Neuroscience. 2010;124:446–454. doi: 10.1037/a0020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guenthner CJ, Miyamichi K, Yang HH, Heller HC, Luo L. Permanent genetic access to transiently active neurons via TRAP: targeted recombination in active populations. Neuron. 2013;78:773–784. doi: 10.1016/j.neuron.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate early gene Arc in hippocampal neuronal ensembles. Nature. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- 8.Han J, Kushner SA, Yiu AP, Hsiang H, Buch T, Waisman A, Bontempi B, Neve RL, Frankland PW, Josselyn SA. Selective Erasure of a Fear Memory. Science. 2009;323:1492–1495. doi: 10.1126/science.1164139. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen inducible form of cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Bio. 2002;244:305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- 10.Josselyn SA. Continuing the search for the engram: examining the mechanism of fear memories. Journal of Psychiatry and Neuroscience. 2010;35:221–228. doi: 10.1503/jpn.100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katche C, Bekinschtein P, Slipczuk L, Goldin A, Izquierdo IA, Cammarota M, Medina JH. Delayed wave of c-Fos expression in the dorsal hippocampus involved specifically in persistence of long-term memory storage. Proc Natl Acad Sci USA. 2010;107:349–354. doi: 10.1073/pnas.0912931107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klinge CM, Studinski-Jones AL, Julakosky PC, Bambara RA, Hilf R. Comparison of tamoxifen ligands on estrogen receptor interaction with estrogen response elements. Molecular and Cellular Endocrinology. 1998;143:79–90. doi: 10.1016/s0303-7207(98)00130-0. [DOI] [PubMed] [Google Scholar]
- 13.Leutgeb JK, Leutgeb S, Moser M-B, Moser EI. Pattern Separation in the Dentate Gyrus and CA3 of the Hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- 14.Link W, Konietzko U, Kauselmann G, Krug M, Schwanke B, Frey U, Kuhl D. Somatodendritic expression of an immediate early gene is regulated by synaptic activity. P Natl Acad Sci USA. 1995;92:5734–5738. doi: 10.1073/pnas.92.12.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K, Tonegawa S. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature. 2012;484:381–385. doi: 10.1038/nature11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lonergan ME, Gafford GM, Jarome TJ, Helmstetter FJ. Time-Dependent Expression of Arc and Zif268 after Acquisition of Fear Conditioning. Neural Plast. 2010;139891:1–12. doi: 10.1155/2010/139891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, Gilbert DJ, Jenkins NA, Lanahan AA, Worley PF. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14:433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- 18.Madisen L, Mao T, Koch H, Zhuo J-M, Berenyi A, Fujisawa S, Hsu Y-WA, Garcia AJ, III, Gu X, Zanella S, Kidney J, Gu H, Mao Y, Hooks BM, Boyden ES, Buzsáki G, Ramirez JM, Jones AR, Svoboda K, Han X, Turner EE, Zeng H. A toobox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat Neurosci. 2012;5:793–802. doi: 10.1038/nn.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reijmers LG, Perkins BL, Matsuo N, Mayford M. Localization of a Stable Neural Correlate of Associative Memory. Science. 2007;317:1230–1233. doi: 10.1126/science.1143839. [DOI] [PubMed] [Google Scholar]
- 20.Reinert RB, Kantz J, Misfeldt AA, Poffenberger G, Gannon M, Brissova M, Powers AC. Tamoxifen-Induced Cre-loxP Recombination Is Prolonged in Pancreatic Islets of Adult Mice. PLoS ONE. 2012;7:e33529. doi: 10.1371/journal.pone.0033529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Renier N, Wu Z, Simon DJ, Yang J, Ariel B, Tessier-Lavigne M. iDISCO: A Simple, Rapid Method to Immunolabel Large Tissue Samples for Volume Imaging. Cell. 2014;159:896–910. doi: 10.1016/j.cell.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Robinson SP, Langan-Fahey SM, Johnson DA, Jordon VC. Metabolites, pharmacodynamics, and pharmacokinetics of tamoxifen in rats and mice compared to the breast cancer patient. Drug Metabolism & Disposition. 1991;19:36–43. [PubMed] [Google Scholar]
- 23.Root CM, Denny CA, Hen R, Axel RA. The participation of cortical amygdala in innate, odour-driven behaviour. Nature. 2014;515:269–273. doi: 10.1038/nature13897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schacter DL. Forgotten, neglected pioneers; Richard Semon and the story of memory. Philadelphia (PA): Psychology Press; 2001. [Google Scholar]
- 25.Smith DM, Mizumori SJY. Hippocampal Place Cells, Context, and Episodic Memory. Hippocampus. 2006;16:716–729. doi: 10.1002/hipo.20208. [DOI] [PubMed] [Google Scholar]
- 26.Tayler KK, Tanaka KZ, Reijmers LG, Wiltgen BJ. Reactivation of Neural Ensembles during the Retrieval of Recent and Remote Memory. Current Biology. 2013;23:1–8. doi: 10.1016/j.cub.2012.11.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


