Abstract
Hispanic and non-Hispanic black breast cancer patients are more likely than non-Hispanic white patients to be diagnosed with breast cancer that is negative for estrogen and progesterone receptors (ER/PR-negative). This disparity might be transmitted through socioeconomic and reproductive factors. Data on 746 recently diagnosed breast cancer patients (300 non-Hispanic white, 303 non-Hispanic black, 143 Hispanic) were obtained from the population-based Breast Cancer Care in Chicago Study (Chicago, Illinois, 2005–2008). Income, educational level, and census tract measures of concentrated disadvantage and affluence were combined into a single measure of socioeconomic position (SEP). Parity and age at first birth were combined into a single measure of reproductive factors (RPF). We constructed path models to estimate direct and indirect associations of SEP and RPF, and we estimated average marginal controlled direct associations. Compared with non-Hispanic white patients, non-Hispanic black patients and Hispanic patients were more likely to have ER/PR-negative disease (28% and 20% for non-Hispanic black patients and Hispanic patients, respectively, vs. 12% for non-Hispanic white patients; P ≤ 0.001). The ethnic disparity in ER/PR-negative breast cancer (prevalence difference = 0.13, 95% confidence interval: 0.07, 0.18) was reduced by approximately 60% (prevalence difference = 0.05, 95% confidence interval: −0.04, 0.13) after control for SEP and RPF. At least part of the ethnic disparity in the aggressiveness of breast tumors might be transmitted through social influences on tumor biology.
Keywords: breast cancer, health status disparities, mediation, reproductive factors, socioeconomic position
Since the early 1980s, non-Hispanic black women in the United States have consistently experienced higher death rates from breast cancer than have non-Hispanic white women despite having lower incidence of the disease (1). By contrast, available data suggest that Hispanic women have experienced lower incidence rates as well as lower mortality rates than white women. However, lower apparent mortality for Hispanics contrasts with studies revealing later stage at diagnosis, markers of more aggressive tumors at diagnosis, and shorter survival for Hispanic women than for non-Hispanic white women (2, 3).
Many breast cancers rely on estrogen and/or progesterone for growth, and this effect is mediated through the presence of estrogen receptors (ER) and progesterone receptors (PR). Binding of hormone to receptor results in unmasking of the DNA-binding sites on the receptor, migration into the nucleus, and binding to specific estrogen-response elements near the genes responsible for the physiological actions of the hormone (4). The presence of estrogen and progesterone receptors has both predictive value and prognostic value in breast cancer management. It has been estimated that 75%–85% of ER-positive patients are likely to respond to hormone therapy, whereas only 5%–10% of those with ER-negative tumors are likely to respond (5). ER status also predicts benefit from second-line and subsequent hormone therapy (6–8). ER/PR status therefore represents an important intermediate endpoint that predicts both prognosis and response to treatment.
It is well established that Hispanic and non-Hispanic black breast cancer patients are more likely than their non-Hispanic white counterparts to be diagnosed with tumors that are negative for estrogen and progesterone receptors, and ER/PR-negative tumors tend to be diagnosed at later stages and show aggressive pathological features (e.g., high nuclear grade, poor histological differentiation, and high proliferative index) (9–11). The higher rate of biologically aggressive tumors in minority women contributes not only to disparities in stage at diagnosis but also to disparities in prognosis more generally. In addition, associations of hormonal and reproductive factors with breast cancer risk appear to vary by subtype, including by ER and PR status (9, 12–14). For example, early age at menarche, nulliparity, and late age at first birth appear to increase the risk of ER-positive disease but not of ER-negative disease (13, 15).
Higher individual and neighborhood socioeconomic position (SEP) have been associated with a higher risk of ER-positive breast cancer; in a study by Palmer et al. (16), associations were attenuated after adjustment for parity and age at first birth, and SEP associations with ER-positive breast cancer were eliminated after control for additional risk factors. The authors concluded that their findings suggested that associations with SEP might be largely due to associations of SEP with reproductive factors (16). In the present study, a population-based study of urban breast cancer patients, we sought to investigate the extent to which the ethnic disparity in ER/PR-negative disease might be transmitted through SEP and through hormonal and reproductive factors, and whether mediation by SEP could be accounted for by socioeconomic differences in hormonal and reproductive factors.
METHODS
This study, the Breast Cancer Care in Chicago Study, was approved by the Institutional Review Board at the University of Illinois at Chicago. Eligible patients were women between 30 and 79 years of age at diagnosis who resided in Chicago and were diagnosed with a first primary breast cancer (in situ or invasive) between 2005 and 2008. Participants identified themselves as non-Hispanic white, non-Hispanic black, or Hispanic. All diagnosing facilities in the greater Chicago area (n = 56) were visited on a monthly basis, and all eligible newly diagnosed cases were ascertained by certified tumor registrars employed by the Illinois State Cancer Registry; registrars reviewed pathology reports, hospital tumor registry records, or both, depending on the protocol at the individual hospital. Information defining patient race and ethnicity was sought from the patient's medical record when not available in the hospital tumor registry.
A letter describing the present study and a recruitment brochure were mailed to each eligible patient within 3 months of initial diagnosis (in both Spanish and English if ethnicity was unknown or was known to be Hispanic). If a patient did not respond either by mail or by telephone within 10 days, a second contact was attempted by mail, telephone, or both. Patients who expressed interest in participating were placed in contact with the Survey Research Laboratory at the University of Illinois at Chicago, where staff screened interested women for eligibility and scheduled eligible women for an in-person interview. If a patient said she was not interested, her record was flagged for contact again 2 months later, allowing the patient more time to adjust to her diagnosis. The interviewer obtained written informed consent before the interview was administered. As part of the consent process, patients were informed that they would receive a gift of $100 for their participation. The 90-minute interview was administered in English or Spanish as appropriate, using computer-assisted personal interview procedures. The final interview response rate was 56% (989 completed interviews among eligible patients). Of patients who were interviewed, 86% (n = 849) consented to medical record reviews to obtain information regarding pathological stage, histological grade, ER and PR status, and other aspects of diagnosis and treatment.
Race/ethnicity
Patients were categorized as white, non-Hispanic; black, non-Hispanic; or Hispanic or Latino. Ethnicity was defined through separate self-identification of Hispanic ethnicity and white or black race. Ethnicity was defined as Hispanic if the patient identified as Hispanic, reported a Latin American country of origin, or reported a Latin American country of origin for both biological parents.
Socioeconomic position
Four variables were defined in order to assess SEP. Level of education was reported in years completed, and annual household income was reported in 12 categories that were collapsed for some analyses to create a binary variable for low income, defined as below $20,000/year. Two measures of census tract advantage or disadvantage were defined using data from the 2000 US Census (17). Concentrated disadvantage was defined by the percentage of families in the census tract with incomes below the poverty line, the percentage of families receiving public assistance, the percentage of persons who were unemployed, and the percentage of female-headed households with children. Concentrated affluence was measured by percentage of families with incomes of $75,000 or more, percentage of adults with a college education or more, and percentage of the civilian labor force in professional and managerial occupations. Both measures were defined by creating an equally weighted sum across the relevant variables and standardizing the sum to have a mean of zero and a standard deviation of 1. In order to reduce the dimensionality of our mediation models and to reduce multiple comparisons, we combined the 4 socioeconomic measures into a single variable as follows. We first standardized income and educational level and then summed the 4 standardized socioeconomic measures together into a single measure of SEP with a high internal reliability (Cronbach's α = 0.74).
Hormonal and reproductive factors
Parity was defined as 0 (nulliparous), 1, 2, or 3 or more live births and was collapsed into parous versus nulliparous for some analyses. Age at first birth was reported at the interview. In order to be able to model both parity and age at first birth together, we examined the predicted probabilities of ER/PR-negative disease by parity and age at first birth. Because the probability of ER/PR-negative disease was approximately that of patients with the latest age at first birth, we chose to assign nulliparous women a value corresponding to an age at first birth equal to 40 years (Figure 1). There were only 11 women with an age at first birth of 40 years or older in our sample. This combined variable is referred to as “reproductive factors” (RPF) in our analyses. Breastfeeding was defined as a binary variable (ever vs. never) and as never, 1–12 months, and greater than 12 months. Body mass index (weight (kg)/height (m)2) was defined using the Centers for Disease Control and Prevention categories and combining the few patients categorized as underweight with the normal weight category. Oral contraceptive use and use of hormone replacement therapy were assessed separately; ever versus never use was reported, and duration of use in years was reported for patients with a history of use.
Figure 1.
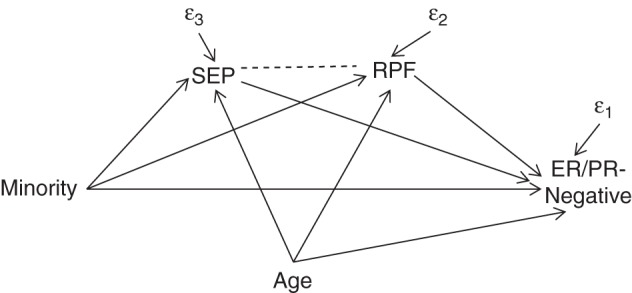
Path diagram corresponding to a structural equation model used to examine the contributions of socioeconomic position (SEP) and reproductive factors (RPF) to the racial/ethnic disparities in the prevalence of estrogen/progesterone receptor (ER/PR)–negative breast cancer, Breast Cancer Care in Chicago Study, 2005–2008. RPF is a single variable that combines parity and age at first birth. SEP is a single variable that combines income, educational level, census tract disadvantage, and census tract affluence.
Tumor characteristics
Stage at diagnosis, hormone receptor status, and histological grade were defined as abstracted from patient medical records. Tumor stage at diagnosis was categorized using the American Joint Commission on Cancer categories of 0, 1, 2, and 3 and 4 combined (18). Hormone receptor status was defined as positive if the tumor contained either estrogen or progesterone receptors and as negative if the tumor contained neither type of receptor. Histological grade was defined as low, intermediate, or high.
Statistical analysis
Ordinal and continuous variables were categorized into a small number of categories for descriptive analyses of predictors of hormone receptor–negative tumors and racial/ethnic differences. A series of logistic regression models of ER/PR-negative disease were fitted, including the following predictors: 1) race/ethnicity and age; 2) race/ethnicity, age, and SEP; 3) race/ethnicity, age, and RPF; 4) race/ethnicity, age, SEP, and RPF; and 5) race/ethnicity, age, SEP, RPF, body mass index, oral contraceptive use, and breastfeeding history. Likelihood ratio tests were conducted in order to compare models. Based on these likelihood ratio tests, mediation analyses focused on the potential mediating roles of SEP and RPF.
We used 3 approaches in order to examine the potential mediation of the disparity in ER/PR-negative breast cancer by RPF and SEP. First, we estimated structural equation models using the program Mplus, version 7.31 (Muthén and Muthén, Los Angeles, California (https://www.statmodel.com/)), to estimate direct and indirect associations of SEP and RPF (Figure 1) (19, 20). Direct and indirect associations have been called “direct and indirect effects” elsewhere in the literature. However, given the observational and cross-sectional nature of the present study, we refer to these as direct and indirect associations because they represent associations from which causal conclusions cannot be drawn. The following binary and ordered probit models were conducted simultaneously using full information maximum likelihood to account for missing data, and specifying a correlation between RFP and SEP. A path corresponding to a correlation between age and minority ethnicity was removed (P = 0.8).
All coefficients were fully (XY) standardized in order to place them on roughly equivalent scales. The direct association of minority ethnicity was estimated from β3. The indirect association of minority ethnicity via SEP was estimated from the product of γ1 and β2. The indirect association of minority ethnicity via RPF was estimated from the product of θ1 and β1. The total association was estimated as β3 + γ1 × β2 + θ1 × β1. The extent to which SEP or RPF appeared to mediate the ethnic disparity in ER/PR-negative disease was estimated as the corresponding indirect association(s) divided by the total association, based on fully standardized coefficients.
We also conducted logistic regression with model-based standardization (predictive margins) in order to estimate the age-adjusted prevalence difference and 95% confidence interval in ER/PR-negative disease by ethnicity (21, 22) and a series of average controlled direct associations (ACDA) controlling for SEP, RPF, and both SEP and RPF (all models adjusted for age). ACDA can be used to estimate what the prevalence difference for the ethnic disparity in ER/PR-negative disease might be if the distributions of SEP and RPF were equalized within ethnic groups and equal to the total sample distribution. ACDA estimation enabled us to easily account for interdependencies in our mediating variables by including product terms between SEP, RFP, ethnicity, and covariates as necessary. We chose to include any 2-way or 3-way interactions with a P value below our preset α of 0.20. This approach enabled us to express total and direct associations as prevalence differences; this was preferable to standardized coefficients, which are on a scale that is arbitrary with respect to public health meaning. We estimated bias-corrected 95% confidence intervals for these measures.
Finally, we used a method of rescaled coefficients in order to estimate the proportion of the ethnic disparity in ER/PR-negative breast cancer statistically modified by SEP and RPF, respectively (23). Analyses were performed using Stata, version 12 (StataCorp LP, College Station, Texas). All P values are 2-sided.
RESULTS
Compared with non-Hispanic white patients, non-Hispanic black patients and Hispanic patients were more likely to have hormone receptor–negative tumors (28% and 20% for non-Hispanic black and Hispanic patients, respectively, vs. 12% for non-Hispanic white patients; P ≤ 0.001), more likely to have less income and education, more likely to live in more disadvantaged and less affluent neighborhoods, more likely to be parous, and more likely to have an earlier age at first birth (P < 0.001 for all) (Table 1). Parity, age at first birth, and 3 of 4 measures of SEP were strongly associated with ER/PR-negative status (P = 0.01 or less), whereas concentrated affluence was marginally associated (Table 2).
Table 1.
Racial/Ethnic Differences in the Prevalence (%) of Socioeconomic and Reproductive Factors (n = 989), Breast Cancer Care in Chicago Study, 2005–2008
| Characteristic | No. of Womena | Race/Ethnicityb |
||
|---|---|---|---|---|
| Non-Hispanic White (n = 397) | Non-Hispanic Black (n = 411) | Hispanic (n = 181) | ||
| Age at diagnosis, years | ||||
| <50 | 302 | 31 | 28 | 34 |
| 50–59 | 307 | 31 | 31 | 32 |
| 60–79 | 380 | 38 | 41 | 34 |
| Educational level, yearsc | ||||
| <12 | 176 | 5 | 19 | 44 |
| 12 | 193 | 13 | 25 | 22 |
| >12 | 617 | 82 | 56 | 35 |
| Annual income, dollarsc | ||||
| <20,000 | 262 | 11 | 38 | 38 |
| 20,000–75,000 | 439 | 40 | 50 | 50 |
| >75,000 | 259 | 49 | 13 | 12 |
| Concentrated affluencec | ||||
| <1 SD below mean | 65 | 1 | 10 | 11 |
| Within 1 SD of mean | 729 | 59 | 83 | 85 |
| >1 SD above mean | 193 | 40 | 7 | 4 |
| Concentrated disadvantagec | ||||
| <1 SD below mean | 142 | 31 | 1 | 7 |
| Within 1 SD of mean | 652 | 67 | 56 | 87 |
| >1 SD above mean | 193 | 1 | 43 | 6 |
| Parityc | ||||
| Parous | 773 | 61 | 90 | 91 |
| Nulliparous | 215 | 39 | 10 | 9 |
| No. of live birthsc,d | ||||
| 0 | 215 | 39 | 10 | 9 |
| 1 | 163 | 14 | 22 | 11 |
| 2 | 251 | 27 | 23 | 28 |
| >3 | 359 | 20 | 45 | 51 |
| Age at first birth, yearsc,d | ||||
| 13–19 | 256 | 8 | 43 | 27 |
| 20–29 | 388 | 33 | 41 | 48 |
| 30–49 | 128 | 19 | 6 | 16 |
| Nulliparous | 215 | 39 | 10 | 9 |
| Breastfeedingc,d | ||||
| No (nulliparous) | 215 | 39 | 10 | 9 |
| No (parous) | 383 | 23 | 56 | 34 |
| Yes | 389 | 37 | 34 | 56 |
| Duration of oral contraceptive use, yearsc | ||||
| 0 | 406 | 40 | 36 | 55 |
| 1–5 | 284 | 27 | 31 | 27 |
| 6–10 | 159 | 16 | 19 | 10 |
| >10 | 140 | 18 | 14 | 8 |
| Hormone replacement therapyc | ||||
| No (premenopausal) | 192 | 18 | 18 | 27 |
| No | 559 | 49 | 65 | 54 |
| Yes | 238 | 33 | 18 | 19 |
| Body mass indexc,e | ||||
| Underweight or normal weight | 321 | 51 | 19 | 23 |
| Overweight | 307 | 24 | 32 | 44 |
| Obese | 355 | 25 | 48 | 33 |
| Family history of breast cancerc,f | ||||
| None | 758 | 75 | 77 | 84 |
| Weak | 166 | 18 | 18 | 13 |
| Strong | 57 | 7 | 6 | 3 |
Abbreviation: SD, standard deviation.
a A total of 989 women completed the final interview for inclusion. Due to missing data, not all numbers in the groups in this table add up to 989.
b Due to rounding, percentages range from 99 to 101.
c P < 0.0001; referent group comprised non-Hispanic white women.
d P value for a comparison that excluded nulliparous women.
e Body mass index was calculated as weight (kg)/height (m)2, where underweight/normal was defined as ≤24.9, overweight as 25.0–29.9, and obese as ≥30.0.
f Family history of breast cancer was defined as weak when the patient had 1 affected relative diagnosed on or after age 50 years or strong when the patient had multiple affected relatives or at least 1 relative diagnosed before age 50 years).
Table 2.
Associations of Socioeconomic and Reproductive Factors With the Prevalence of ER/PR-Negative Breast Cancer (n = 746), Breast Cancer Care in Chicago, 2005–2008
| Characteristic | No. of Cases of Breast Cancera | % of Cases ER/PR-Negative | P Value |
|---|---|---|---|
| Race/ethnicity | 0.004 | ||
| Non-Hispanic white | 300 | 12 | |
| Non-Hispanic black | 303 | 28 | |
| Hispanic | 143 | 20 | |
| Age at diagnosis, years | |||
| <50 | 230 | 23 | |
| 50–59 | 228 | 19 | |
| 60–79 | 288 | 19 | |
| Educational level, years | 0.001 | ||
| <12 | 127 | 29 | |
| 12 | 158 | 23 | |
| >12 | 459 | 17 | |
| Annual income, dollars | <0.0001 | ||
| <20,000 | 190 | 29 | |
| 20,000–75,000 | 346 | 20 | |
| >75,000 | 193 | 11 | |
| Concentrated affluence | 0.11 | ||
| <1 SD below mean | 47 | 19 | |
| Within 1 SD of mean | 547 | 22 | |
| >1 SD above mean | 151 | 14 | |
| Concentrated disadvantage | 0.004 | ||
| <1 SD below mean | 111 | 11 | |
| Within 1 SD of mean | 494 | 20 | |
| >1 SD above mean | 140 | 26 | |
| Parity | 0.007 | ||
| Parous | 585 | 22 | |
| Nulliparous | 161 | 12 | |
| No. of live births | 0.02 | ||
| 0 | 161 | 12 | |
| 1 | 119 | 24 | |
| 2 | 187 | 20 | |
| >3 | 279 | 23 | |
| Age at first birth, years | 0.007b | ||
| 13–19 | 192 | 29 | |
| 20–29 | 292 | 20 | |
| 30–49 | 100 | 14 | |
| Nulliparous | 161 | 12 | |
| Breastfeeding | 0.09b | ||
| No (nulliparous) | 161 | 12 | |
| No (parous) | 279 | 25 | |
| Yes | 305 | 19 | |
| Duration of oral contraceptive use, years | 0.07 | ||
| 0 | 311 | 17 | |
| 1–5 | 225 | 21 | |
| 6–10 | 110 | 22 | |
| >10 | 100 | 25 | |
| Hormone replacement therapy | 0.61 | ||
| No (premenopausal) | 152 | 20 | |
| No | 408 | 21 | |
| Yes | 186 | 18 | |
| Body mass indexc | 0.02 | ||
| Underweight or normal weight | 231 | 16 | |
| Overweight | 229 | 18 | |
| Obese | 283 | 24 | |
| Family history of breast cancerd | 0.61 | ||
| None | 580 | 21 | |
| Weak | 114 | 18 | |
| Strong | 45 | 20 |
Abbreviations: ER, estrogen receptor; PR, progesterone receptor; SD, standard deviation.
a Due to missing data, not all numbers in the groups in this table add up to the total sample size of 746.
b P value for a comparison that excluded nulliparous women.
c Body mass index was calculated as weight (kg)/height (m)2, where underweight/normal was defined as ≤24.9, overweight as 25.0–29.9, and obese as ≥30.0.
d Family history of breast cancer was defined as weak when the patient had 1 affected relative diagnosed on or after age 50 years or strong when the patient had multiple affected relatives or at least 1 relative diagnosed before age 50 years).
Likelihood ratio tests were performed on nested logistic regression models (Table 3). These models revealed that other potentially important covariates did not contribute to model fit beyond the fit provided by including SEP, RFP, age, and ethnicity (P = 0.4307; Table 4).
Table 3.
Nested Logistic Regression Models of the Association of Socioeconomic and Reproductive Factors With ER/PR-Negative Breast Cancer, Breast Cancer Care in Chicago Study, 2005–2008
| Predictor | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 |
|---|---|---|---|---|---|
| Minority ethnicity | X | X | X | X | X |
| Age at diagnosis | X | X | X | X | X |
| SEPa | X | X | X | ||
| RPFb | X | X | X | ||
| Body mass index | X | ||||
| Breastfeeding | X | ||||
| Oral contraceptives | X |
Abbreviations: ER, estrogen receptor; PR, progesterone receptor; RPF, reproductive factors; SEP, socioeconomic position.
a A single variable representing RPF that combined parity and age at first birth.
b A single variable representing SEP that combined income, educational level, census tract disadvantage, and census tract affluence.
Table 4.
P Values From Likelihood Ratio Tests Conducted on Nested Logistic Regression Models for the Association of Socioeconomic and Reproductive Factors With ER/PR-Negative Breast Cancer, Breast Cancer Care in Chicago Study, 2005–2008
| Modela | Model 2b | Model 3c | Model 4d | Model 5e |
|---|---|---|---|---|
| Model 1 | 0.0087 | 0.0024 | 0.002 | 0.0285 |
| Model 2 | —f | 0.0185 | 0.1466 | |
| Model 3 | 0.0728 | 0.2661 | ||
| Model 4 | 0.4307 |
Abbreviations: ER, estrogen receptor; PR, progesterone receptor; RPF, reproductive factors; SEP, socioeconomic position.
a Model 1 was a logistic regression model of ER/PR-negative breast cancer as a function of age and minority ethnicity.
b Model 2 consisted of model 1 with the addition of the composite variable for SEP.
c Model 3 consisted of model 1 with the addition of the composite variable for RPF.
d Model 4 consisted of model 1 with the addition of the composite variables for both SEP and RPF.
e Model 5 consisted of model 4 with the addition of the variables for body mass index, breastfeeding, and oral contraceptive use.
f Corresponds to nonnested models.
Structural equation models
In structural equation models, SEP (P = 0.06), RPF (P = 0.018), and age (P = 0.005) predicted ER/PR-negative disease, but ethnicity was no longer a significant predictor (Table 5). The fully standardized indirect association with ER/PR status was slightly greater for SEP (0.0871, equal to −0.660 × −0.132) than for RPF (0.0719, equal to −0.461 × −0.156), and both were approximately equal in magnitude to the nonsignificant direct association with minority ethnicity (β3XY = 0.081). SEP and RPF accounted for approximately 36% and 30% of the disparity in ER/PR-negative breast cancer, respectively (Table 5).
Table 5.
Structural Equation Model of the Association of Socioeconomic and Reproductive Factors With ER/PR-Negative Breast Cancer Among Non-Hispanic Black and Hispanic Women Versus Non-Hispanic White Women, Breast Cancer Care in Chicago Study, 2005–2008
| Estimated Path | Coefficienta | SE | P Value | StdXY Coefficientb | % of Totalc |
|---|---|---|---|---|---|
| ER/PR-negative status on: | |||||
| RPF | −0.143 | 0.060 | 0.018 | −0.156 | |
| SEP | −0.104 | 0.056 | 0.062 | −0.132 | |
| Minorityd | 0.175 | 0.150 | 0.244 | 0.081 | |
| Age | −0.014 | 0.005 | 0.005 | −0.148 | |
| RPFe on: | |||||
| Minority | −1.086 | 0.073 | 0.000 | −0.461 | |
| Age | −0.019 | 0.003 | 0.000 | −0.190 | |
| SEPf on: | |||||
| Minority | −1.807 | 0.079 | 0.000 | −0.660 | |
| Age | −0.011 | 0.003 | 0.000 | −0.092 | |
| SEP with: | |||||
| RPF | 0.315 | 0.031 | 0.000 | 0.315 | |
| Total association | 0.240 | ||||
| Indirect (via SEP) | 0.087 | 36 | |||
| Indirect (via RPF) | 0.072 | 30 |
Abbreviations: ER, estrogen receptor; PR, progesterone receptor; RPF, reproductive factors; SE, standard error; SEP, socioeconomic position.
a Unstandardized coefficient from a probit regression model of ER/PR-negative status.
b Fully standardized probit coefficient.
c Proportion of the total association between minority ethnicity and ER/PR-negative status that is represented by the estimated path.
d Minority defined as non-Hispanic black or Hispanic versus non-Hispanic white (referent).
e A single variable representing RPF that combined parity and age at first birth.
f A single variable representing SEP that combined income, educational level, census tract disadvantage, and census tract affluence.
Model-based standardization
In age-adjusted models, minority patients were 13 percentage points more likely than their non-Hispanic white counterparts to have ER/PR-negative tumors (prevalence difference = 0.133, 95% confidence interval: 0.081, 0.191) (Table 6). The average marginal controlled direct association of ethnicity with ER/PR status was reduced to 7 percentage points after adjustment for SEP, 9 percentage points after adjustment for RPF, and 5 percentage points after adjustment for both SEP and RPF (Table 6). Based on the method of rescaled coefficients, SEP and RPF accounted for approximately 35% and 26% of the disparity in ER/PR-negative breast cancer when modeled together, accounting in combination for 61% of the disparity (P = 0.002) (Table 6).
Table 6.
Potential Mediation of Racial/Ethnic Disparities Among Women Diagnosed with ER/PR-Negative Breast Cancer, Breast Cancer Care in Chicago Study, 2005–2008
| Characteristic | No. of Women | Average Controlled Direct Associations |
Rescaled Coefficients |
|||
|---|---|---|---|---|---|---|
| RDa | 95% CIb | % Reductionc | % Reductiond | P Valuee | ||
| Minority Women Versus Non-Hispanic White Women | ||||||
| Age (baseline model) | 746 | 0.133 | 0.081, 0.191 | |||
| Modeled separately | ||||||
| SEP | 746 | 0.072 | −0.020, 0.145 | 46 | ||
| RPF | 746 | 0.091 | 0.032, 0.161 | 32 | ||
| Modeled together | 746 | 0.053 | −0.039, 0.125 | 60 | 61 | 0.002 |
| SEP | 35 | |||||
| RPF | 26 | |||||
| Non-Hispanic Black Women Versus Non-Hispanic White Women | ||||||
| Age (baseline model) | 603 | 0.163 | 0.100, 0.225 | |||
| Modeled separately | ||||||
| SEP | 603 | 0.102 | 0.008, 0.193 | 37 | ||
| RPF | 603 | 0.122 | 0.050, 0.193 | 25 | ||
| Modeled together | 603 | 0.087 | −0.010, 0.189 | 47 | 46 | 0.002 |
| SEP | 26 | |||||
| RPF | 20 | |||||
| Hispanic Women Versus Non-Hispanic White Women | ||||||
| Age (baseline model) | 443 | 0.075 | −0.004, 0.150 | |||
| Modeled separately | ||||||
| SEP | 443 | 0.001 | −0.091, 0.097 | 99 | ||
| RPF | 443 | 0.040 | −0.030, 0.112 | 47 | ||
| Modeled together | 443 | −0.013 | −0.095, 0.091 | 117 | 119 | 0.007 |
| SEP | 82 | |||||
| RPF | 36 | |||||
Abbreviations: CI, confidence interval; ER, estrogen receptor; PR, progesterone receptor; RD, risk difference; RPF, reproductive factors; SEP, socioeconomic position.
a RD calculated using logistic regression with marginal standardization.
b 95% CI calculated using bias-corrected bootstrap methods.
c Percent reduction in the risk difference when compared with the baseline model.
d Percent reduction based on the method of rescaled coefficients.
e P value from a test of the difference in coefficients between the reduced model (without the mediators) and the full model (which included the mediators).
DISCUSSION
In the present study, we found that, compared with non-Hispanic white patients, non-Hispanic black patients and Hispanic patients were more likely to have hormone receptor–negative tumors and that the disparity in ER/PR-negative disease was explained largely by racial/ethnic differences in SEP and RPF. To our knowledge, this is the first study to attempt to tease out the separate roles of RPF and SEP in this disparity, and we found that the 2 domains appeared to exert separate mediating effects. In other words, not only did racial/ethnic disparities in ER/PR-negative breast cancer appear to be transmitted by RPF that correlate with SEP but other mechanisms related to SEP appeared to exert their own separate mediating influences. This was a cross-sectional study, and our outcome measure was prevalence of ER/PR-negative breast cancer. For this reason we could not determine whether an association of a risk factor with prevalence of ER/PR-negative disease was the result of an association with risk of ER/PR-positive breast cancer, an association with risk of ER/PR-negative breast cancer, or perhaps both. For example, we found that nulliparity was associated with reduced prevalence of ER/PR-negative breast cancer, which might represent an association of nulliparity with a lower risk of ER/PR-negative breast cancer, a higher risk of ER/PR-positive breast cancer, or both.
Greater parity and earlier timing of offspring are associated with a lower risk of hormone receptor–positive breast cancer (13, 15, 24, 25). Risk of ER/PR-positive breast cancer might be reduced by higher parity through several potential mechanisms, including changing profiles of circulating reproductive hormones and terminal differentiation of mammary epithelium, rendering the breast less susceptible to carcinogenic influences. Other hormonal and reproductive factors, including ages at menarche and menopause and a history of breastfeeding, are also associated with specific subtypes of breast cancer (13, 15), but these factors were not associated with ER/PR-negative breast cancer in the present study after parity and age at first birth were included in our analyses. Associations of reproductive factors with triple-negative breast cancer and basal-like breast cancer (both of which are subsets of ER/PR-negative breast cancer) have been reported to be similar to those of ER/PR-negative breast cancer, including higher risk with parity (15, 26) and younger age at first full-term pregnancy (15). Breastfeeding appears to attenuate the higher risk of ER/PR-negative breast cancer associated with multiparity (14, 27), and breastfeeding was associated with a lower risk of basal-like breast cancer (15). Incomplete study participation might also have produced a selection bias that could have affected our results in a manner that is difficult to anticipate.
We did not find an association of body mass index with prevalence of ER/PR-negative breast cancer in the present study. Obesity has been found to be associated with ER/PR-negative breast cancer in younger women (<50 years of age) but with ER/PR-positive breast cancer in older women (28). We did not have a sufficient sample size to examine this potential heterogeneity of association with obesity.
Because of the cross-sectional nature of the present study, we were limited to examining disparities in the prevalence ER/PR-negative breast cancer as a proportion of all breast cancers rather than examining the role of SEP and RPF in incidence of ER/PR-negative breast cancer. Nonetheless, we found strong associations of ER/PR-negative breast cancer with SEP, parity, and age at first birth, which were consistent with the literature regarding incidence. These factors together accounted for approximately two thirds of the disproportionate prevalence of ER/PR-negative breast cancer among non-Hispanic black and Hispanic patients. While hormonal and reproductive factors accounted for a substantial proportion of the disparity, SEP accounted for an equal or greater proportion of the disparity, and the apparent mediation by SEP was not due to its association with parity, age at first birth, or other hormonal and reproductive factors that tend to differ by SEP. Results suggest that other potential causes of the disparity in the prevalence of ER/PR-negative breast cancer exist in addition to hormonal and reproductive factors.
ACKNOWLEDGMENTS
Author affiliations: Division of Epidemiology and Biostatistics, School of Public Health, University of Illinois at Chicago, Chicago, Illinois (Garth H. Rauscher, Richard T. Campbell); Department of Pathology, College of Medicine, University of Illinois at Chicago, Chicago, Illinois (Elizabeth L. Wiley); Department of Medicine, College of Medicine, University of Illinois at Chicago, Chicago, Illinois (Kent Hoskins); and Institute for Health Research and Policy, University of Illinois at Chicago, Chicago, Illinois (Garth H. Rauscher, Richard T. Campbell, Melinda R. Stolley, Richard B. Warnecke).
This work was supported by 2 grants to the University of Illinois at Chicago from the National Cancer Institute (grants 1P50CA106743 and 2P50CA106743).
We thank the Illinois State Cancer Registry for providing the data.
Conflict of interest: none declared.
REFERENCES
- 1.DeSantis C, Ma J, Bryan L et al. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;641:52–62. [DOI] [PubMed] [Google Scholar]
- 2.Karliner LS, Kerlikowske K. Ethnic disparities in breast cancer. Womens Health (Lond Engl). 2007;36:679–688. [DOI] [PubMed] [Google Scholar]
- 3.Maskarinec G, Sen C, Koga K et al. Ethnic differences in breast cancer survival: status and determinants. Womens Health (Lond Engl). 2011;76:677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deroo BJ, Korach KS. Estrogen receptors and human disease. J Clin Invest. 2006;1163:561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gruvberger-Saal SK, Bendahl PO, Saal LH et al. Estrogen receptor beta expression is associated with tamoxifen response in ERalpha-negative breast carcinoma. Clin Cancer Res. 2007;137:1987–1994. [DOI] [PubMed] [Google Scholar]
- 6.Bezwoda WR, Esser JD, Dansey R et al. The value of estrogen and progesterone receptor determinations in advanced breast cancer. Estrogen receptor level but not progesterone receptor level correlates with response to tamoxifen. Cancer. 1991;684:867–872. [DOI] [PubMed] [Google Scholar]
- 7.Ravdin PM, Green S, Dorr TM et al. Prognostic significance of progesterone receptor levels in estrogen receptor-positive patients with metastatic breast cancer treated with tamoxifen: results of a prospective Southwest Oncology Group study. J Clin Oncol. 1992;108:1284–1291. [DOI] [PubMed] [Google Scholar]
- 8.Gao Q, Patani N, Dunbier AK et al. Effect of aromatase inhibition on functional gene modules in estrogen receptor-positive breast cancer and their relationship with antiproliferative response. Clin Cancer Res. 2014;209:2485–2494. [DOI] [PubMed] [Google Scholar]
- 9.Miller BA, Hankey BF, Thomas TL. Impact of sociodemographic factors, hormone receptor status, and tumor grade on ethnic differences in tumor stage and size for breast cancer in US women. Am J Epidemiol. 2002;1556:534–545. [DOI] [PubMed] [Google Scholar]
- 10.Chlebowski RT, Chen Z, Anderson GL et al. Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J Natl Cancer Inst. 2005;976:439–448. [DOI] [PubMed] [Google Scholar]
- 11.Joslyn SA. Hormone receptors in breast cancer: racial differences in distribution and survival. Breast Cancer Res Treat. 2002;731:45–59. [DOI] [PubMed] [Google Scholar]
- 12.Ballard-Barbash R, Griffin MR, Fisher LD et al. Estrogen receptors in breast cancer. Association with epidemiologic risk factors. Am J Epidemiol. 1986;1241:77–84. [DOI] [PubMed] [Google Scholar]
- 13.Huang WY, Newman B, Millikan RC et al. Hormone-related factors and risk of breast cancer in relation to estrogen receptor and progesterone receptor status. Am J Epidemiol. 2000;1517:703–714. [DOI] [PubMed] [Google Scholar]
- 14.Largent JA, Ziogas A, Anton-Culver H. Effect of reproductive factors on stage, grade and hormone receptor status in early-onset breast cancer. Breast Cancer Res. 2005;74:R541–R554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Millikan RC, Newman B, Tse CK et al. Epidemiology of basal-like breast cancer [Erratum appears in Breast Cancer Res Treat. 2008;109(1):141 Note: Dressler, Lynn G [added]] Breast Cancer Res Treat. 2008;1091:123–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palmer JR, Boggs DA, Wise LA et al. Individual and neighborhood socioeconomic status in relation to breast cancer incidence in African-American women. Am J Epidemiol. 2012;17612:1141–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US Census Bureau. American Fact Finder. http://factfinder.census.gov/faces/nav/jsf/pages/index.xhtml. Accessed March 20, 2015. [Google Scholar]
- 18.American Joint Committee on Cancer. Cancer Staging References. https://cancerstaging.org/references-tools/Pages/What-is-Cancer-Staging.aspx. Accessed March 15, 2016. [Google Scholar]
- 19.Ditlevsen S, Keiding N, Christensen U et al. Mediation proportion. Epidemiology. 2005;164:592. [DOI] [PubMed] [Google Scholar]
- 20.Ditlevsen S, Christensen U, Lynch J et al. The mediation proportion: a structural equation approach for estimating the proportion of exposure effect on outcome explained by an intermediate variable. Epidemiology. 2005;161:114–120. [DOI] [PubMed] [Google Scholar]
- 21.Ahern J, Hubbard A, Galea S. Estimating the effects of potential public health interventions on population disease burden: a step-by-step illustration of causal inference methods. Am J Epidemiol. 2009;1699:1140–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenland S. Model-based estimation of relative risks and other epidemiologic measures in studies of common outcomes and in case-control studies. Am J Epidemiol. 2004;1604:301–305. [DOI] [PubMed] [Google Scholar]
- 23.Karlson KB, Holm A. Decomposing primary and secondary effects: a new decomposition method. Res Soc Stratif Mobil. 2011;292:221–237. [Google Scholar]
- 24.Ma H, Bernstein L, Pike MC et al. Reproductive factors and breast cancer risk according to joint estrogen and progesterone receptor status: a meta-analysis of epidemiological studies. Breast Cancer Res. 2006;84:R43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Althuis MD, Fergenbaum JH, Garcia-Closas M et al. Etiology of hormone receptor-defined breast cancer: a systematic review of the literature. Cancer Epidemiol Biomarkers Prev. 2004;1310:1558–1568. [PubMed] [Google Scholar]
- 26.Phipps AI, Chlebowski RT, Prentice R et al. Reproductive history and oral contraceptive use in relation to risk of triple-negative breast cancer. J Natl Cancer Inst. 2011;1036:470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Work ME, John EM, Andrulis IL et al. Reproductive risk factors and oestrogen/progesterone receptor-negative breast cancer in the Breast Cancer Family Registry. Br J Cancer. 2014;1105:1367–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang XR, Chang-Claude J, Goode EL et al. Associations of breast cancer risk factors with tumor subtypes: a pooled analysis from the Breast Cancer Association Consortium studies. J Natl Cancer Inst. 2011;1033:250–263. [DOI] [PMC free article] [PubMed] [Google Scholar]


