Abstract
Research has implicated low 25-hydroxyvitamin D (25(OH)D) level as a risk factor for infection; however, results have not been consistent. To further determine the nature of this relationship, we conducted a cohort study using Medicare beneficiaries participating in the 2001–2002 and 2003–2004 cycles of the National Health and Nutrition Examination Survey with data individually linked to hospital records from the Centers for Medicare and Medicaid Services. The primary exposure was a 25(OH)D level of <15 ng/mL versus ≥15 ng/mL. The outcomes were a hospitalization with or without an infection within 1 year of participation in the National Health and Nutrition Examination Survey, as determined from the final hospital discharge codes (International Classification of Diseases, Ninth Revision, Clinical Modification). Of 1,713 individuals, 348 had a baseline serum 25(OH)D level of <15 ng/mL, 77 experienced a hospitalization with an infection, and 287 experienced a hospitalization without an infection. In multivariable analyses, a serum 25(OH)D level of <15 ng/mL was associated with a higher risk of hospitalization with an infection (risk ratio = 2.8, 95% confidence interval: 1.3, 5.9, P < 0.01) but not of hospitalization without an infection (risk ratio = 1.4, 95% confidence interval: 0.9, 2.1, P = 0.1). In this study, we found an association between a serum 25(OH)D concentration of <15 ng/mL and a higher subsequent risk for hospitalization with an infection among Medicare beneficiaries.
Keywords: hospitalization, 25-hydroxyvitamin D, infection, sepsis, vitamin D
The discovery of vitamin D receptors and 1-α-hydroxylase, the enzyme that converts circulating 25-hydroxyvitamin D (25(OH)D) into its biologically active form, in several types of immune cells has led to research elucidating the role of vitamin D in immune function (1). Vitamin D is integral to the innate immune system's production of antimicrobial peptides in response to pathogens (2). One such peptide, cathelicidin, has direct microbicidal activity on various bacterial pathogens, disrupts bacterial biofilms, promotes phagocytosis and the production of reactive oxygen metabolites, and induces chemotaxis of other immune cells to sites of infection (3–6).
While the basic science describing the role of vitamin D in the immune response is compelling, the results of clinical studies have been mixed regarding an association between vitamin D and clinically apparent infections (7–25). Some observational studies have demonstrated an association between low circulating 25(OH)D levels and risk of respiratory infections, while others have not demonstrated this finding (7, 8, 11–15). Furthermore, observational studies have demonstrated associations between low circulating 25(OH)D concentrations and postsurgical infections, methicillin-resistant Staphylococcus aureus nasal carriage, bloodstream infections, and hospital admission with sepsis (22–24, 26). Although the results from these observational studies have been informative, they are consistently limited by the lack of large, generalizable cohorts with 25(OH)D concentrations drawn during a time free from acute illness (22–24, 26). Furthermore, while the few controlled intervention trials that have been published did not demonstrate a protective or therapeutic effect of vitamin D supplementation, these trials have been criticized for including study populations that might not have been truly vitamin D–deficient (16, 17, 27, 28).
Recent developments at the National Center for Health Statistics (NCHS) have allowed for the creation of large cohort studies with rich baseline data through linkage of individual records from several NCHS national surveys to outcomes in Centers for Medicare and Medicaid Services (CMS) records. Pertinent to this study, the NCHS carries out the continuous National Health and Nutrition Examination Survey (NHANES), which has measured serum 25(OH)D concentrations in nationally representative samples of the US population since 2000. Using the NHANES-CMS linkage, in the present study, we examined the relationship between serum 25(OH)D concentrations in Medicare beneficiaries and the 1-year risk of hospitalization with an infection. We hypothesized that persons with serum 25(OH)D concentrations of <15 ng/mL would be at higher risk for a hospitalization with an infection.
METHODS
We conducted a cohort study of Medicare beneficiaries who participated in the 2001–2002 and 2003–2004 cycles of NHANES; these were the only available years with 25(OH)D measurements that were linked to CMS records at the time of our study. The continuous NHANES started in 1999. National surveys are conducted on a 2-year cycle using a complex, multistage, probability-sampling design to select participants representative of the civilian, noninstitutionalized US population. The NHANES survey is conducted with a standardized in-home questionnaire followed by further questionnaires, a physical examination, and various laboratory tests in a mobile examination unit (29). The Medicare Provider Analysis and Review file from CMS contains hospital admission and discharge dates, discharge diagnosis codes, procedure codes, and billing information.
We included those participants who had a serum 25(OH)D concentration measured during the NHANES medical examination and agreed to have their data linked to CMS records. We excluded those who were enrolled in an additional health insurance program at any time during the year of and the year following the NHANES survey, because they might have received services not submitted to Medicare, and thus CMS records would not have captured those outcome data.
The primary exposure of interest was serum 25(OH)D concentration, defined as low if the concentration was <15 ng/mL and normal if the concentration was ≥15 ng/mL. We chose this cutpoint given its precedence in the literature and our prior investigation in this field (23, 30–32). However, because the optimal 25(OH)D concentration for immune function has not been determined, we also explored 25(OH)D as a continuous variable, at different cutpoints, and as an ordinal variable in the analyses. Other covariate data collected from the NHANES included demographic information, self-reported medical history, body mass index, family income, date of examination, and latitude of residence. A 4-category season variable was created using the equinoxes and solstices of the corresponding years. A poverty-index ratio was calculated by dividing reported family income by the national poverty threshold of the given year (33). Because the latitudes of the continental United States are between 24°N and 49°N, the original latitude of the NHANES examination variable was categorized as <32°N, 32°N–38°N, 39°N–45°N, or ≥46°N; however, the 2 northernmost categories were collapsed into 1 because of the low sample size in the northernmost category (34–36). Dates of hospital admission and discharge were obtained from the CMS records.
The primary outcome of the study was a 3-category variable: no hospitalization, hospitalization with an infection (HWI), and hospitalization without an infection (HWOI). An HWI was defined as having an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), diagnosis code for an infection listed as the primary diagnosis or among any of the 10 additional final discharge codes during the first hospitalization within 1 year after the NHANES examination. Alternatively, a subject was defined as having an HWOI if the first hospitalization within 1 year of the NHANES examination did not have an ICD-9-CM code for infection as the primary diagnosis or among any of the final discharge diagnoses. (For a list of the ICD-9-CM codes used, see Web Table 1, available at http://aje.oxfordjournals.org/.)
The planned secondary outcome of interest was all-cause mortality within 1 year of the NHANES examination. However, because there were no deaths during this time period, we did not report on this outcome.
Public-use data from NHANES were obtained from files available on the NCHS website (http://www.cdc.gov/nchs/index.htm), and data on restricted NHANES variables and all CMS variables were accessed on-site at the NCHS Research Data Center at the Centers for Disease Control and Prevention (Atlanta, Georgia). The data were merged by a Research Data Center analyst using the unique sequence number given to each NHANES participant. To protect the confidentiality and security of CMS data, all analyses were performed on-site at the Research Data Center. In compliance with Research Data Center information security policies, raw frequencies are publicly released only for the major study population subgroups and not for individual cells in data tables. Additionally, any proportion or mean calculated from a raw frequency less than 5 is censored. Finally, if an omitted statistic's percentage can be calculated using other values in a given table column, additional statistics are also censored. (See Web Tables 2 and 3 for expanded data tables.) This study was approved by the Research Data Center of the Centers for Disease Control and Prevention.
All statistical analyses were performed with SAS, version 9.3 (SAS Institute, Inc., Cary, North Carolina), using the PROC SURVEY procedures to account for the complex NHANES sampling design. The NHANES sample weights were adjusted for the combination of multiple NHANES survey years and used in every analysis. We applied the Taylor series linearization method and handled missing data as not missing completely at random for variance estimation (37). Domain analysis was used to analyze our study group within the entire NHANES cohort to allow for more accurate variance estimation.
For all analyses, “no hospitalization” was used as the reference category for calculating the risk ratios for each category of hospitalization. We used a logistic regression model that was developed a priori based on explanatory factors shown to be related to 25(OH)D concentration and the risk of infection. These factors included race/ethnicity, poverty-index ratio, body mass index, latitude of residence, and history of renal disease and cancer, with age and sex added later at the request of scientific peer reviewers (38–42). For bivariate analyses, because we were comparing means and proportions across 3 categories of outcome and because of the constraints of the SAS SURVEY procedures, which do not perform analysis of variance, we defined significance for these analyses as nonoverlapping 95% confidence intervals. For all model estimates, significance was defined as P < 0.05.
RESULTS
Study population
There were 2,868 participants in the 2001–2002 and 2003–2004 NHANES surveys who reported enrollment in Medicare, with 2,179 (76.0%) having their file linked to CMS records and 1,806 (63.0%) having 24 months of continuous enrollment in Medicare with no additional health insurance enrollment. Of this group, 1,713 (94.9%) had a serum 25(OH)D concentration measured during the NHANES examination (Figure 1).
Figure 1.
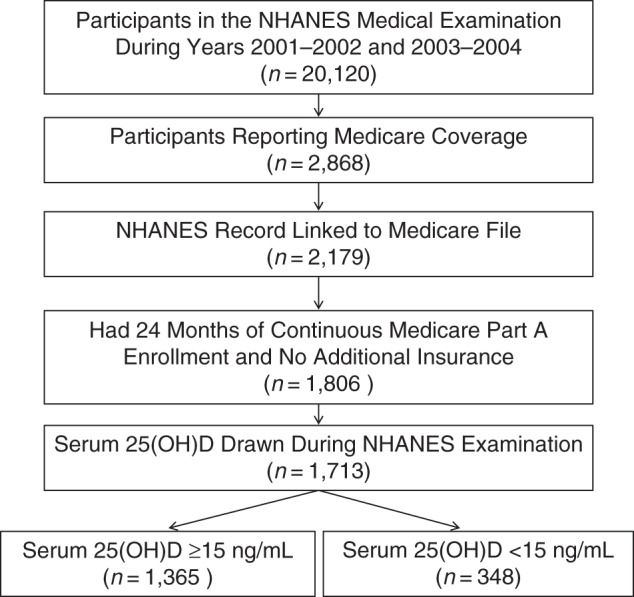
Selection of study participants from a national cohort of Medicare beneficiaries with serum 25-hydroxyvitamin D (25(OH)D) concentrations measured during medical examination for the National Health and Nutrition Examination Survey (NHANES), 2001–2004.
Characteristics by 25(OH)D status
Twenty percent (n = 348) of the study population had a serum 25(OH)D concentration of <15 ng/mL (Table 1). Those with 25(OH)D <15 ng/mL were significantly more likely to be female (64.2% (95% confidence interval (CI): 57.9, 70.5) vs. 51.2% (95% CI: 48.7, 53.6)), to be black (20.3% (95% CI: 12.8, 27.8) vs. 4.9% (95% CI: 3.5, 6.4)), to have a higher mean body mass index (weight(kg)/height (m)2; 29.7 (95% CI: 28.9, 30.5) vs. 27.6 (95% CI: 27.3, 27.9)), to have a poverty-index ratio less than 2.0 (59.3% (95% CI: 51.3, 67.3) vs. 40.4% (95% CI: 34.3, 46.6)), and to have a history of renal disease (11.6% (95% CI: 7.4, 15.8) vs. 4.8% (95% CI: 3.7, 6.0)) or diabetes mellitus (31.3% (95% CI: 25.4, 37.2) vs. 18.9% (95% CI: 15.8, 21.9)). Compared with subjects with a 25(OH)D level of ≥15 ng/mL, those with a 25(OH)D level of <15 ng/mL were more likely to have an HWI (10.3% (95% CI: 5.3, 15.3) vs. 3.8% (95% CI: 2.8, 4.8)).
Table 1.
Demographic and Clinical Characteristics of a Cohort of Medicare Beneficiaries by Serum 25-Hydroxyvitamin D Level (n = 1,713), United States, 2001–2004a
| Variable | Serum 25(OH)D Concentration |
|||
|---|---|---|---|---|
| <15 ng/mL |
≥15 ng/mL |
|||
| % | 95% CI | % | 95% CI | |
| Demographic Factors | ||||
| Age, yearsb | 69.5 | 68.2, 70.7 | 69.9 | 68.5, 71.2 |
| Age group, yearsc | ||||
| 45–54 | 6.1 | 2.6, 9.6 | 5.1 | 3.1, 7.2 |
| 55–64 | 9.7 | 5.2, 14.2 | 6.7 | 4.2, 9.1 |
| 65–74 | 41.5 | 35.1, 47.9 | 46.6 | 41.8, 51.4 |
| 75–84 | 27.0 | 21.4, 32.5 | 29.4 | 25.8, 32.9 |
| ≥85 | 9.2 | 5.7, 12.7 | 6.9 | 4.9, 8.8 |
| Female sexd | 64.2 | 57.9, 70.5 | 51.2 | 48.7, 53.6 |
| Body mass indexb,d,e | 29.7 | 28.9, 30.5 | 27.6 | 27.3, 27.9 |
| Body mass index category | ||||
| <25 | 30.4 | 24.7, 36.2 | 34.9 | 31.8, 38.1 |
| 25–29 | 28.6 | 19.6, 37.6 | 38.4 | 34.4, 42.4 |
| ≥30d | 41.0 | 31.7, 50.3 | 26.7 | 24.3, 29.0 |
| Poverty-index ratio <2.0d | 59.3 | 51.3, 67.3 | 40.4 | 34.3, 46.6 |
| Race/ethnicity | ||||
| Non-Hispanic whited | 65.9 | 54.4, 77.4 | 87.3 | 82.4, 92.2 |
| Non-Hispanic blackd | 20.3 | 12.8, 27.8 | 4.9 | 3.5, 6.4 |
| Mexican-American | 5.8 | 0.2, 11.5 | 2.6 | 0.4, 4.8 |
| Other Hispanic | 3.7 | 0.0, 8.3 | 2.5 | 0.1, 4.9 |
| Other race/ethnicity | 4.3 | 0.6, 8.0 | 2.6 | 1.3, 4.0 |
| Latitude | ||||
| <32°N | 27.1 | 12.7, 41.5 | 17.6 | 10.1, 25.1 |
| 32°N–38°N | 37.2 | 20.5, 53.9 | 40.1 | 25.3, 55.0 |
| ≥39°N | 35.7 | 22.5, 48.9 | 42.3 | 29.8, 54.8 |
| Medical History | ||||
| Liver disease | 6.4 | 1.6, 11.2 | 4.1 | 2.6, 5.6 |
| Asthma | 8.0 | 5.4, 10.7 | 11.1 | 8.7, 13.6 |
| Emphysema | 6.2 | 2.5, 10.0 | 5.3 | 3.4, 7.2 |
| Chronic bronchitis | 13.0 | 7.5, 18.5 | 9.5 | 6.5, 12.4 |
| Congestive heart failure | 11.5 | 7.0, 16.1 | 7.8 | 6.7, 9.0 |
| Coronary artery disease | 13.4 | 9.3, 17.5 | 12.8 | 10.9, 14.7 |
| Renal diseased | 11.6 | 7.4, 15.8 | 4.8 | 3.7, 6.0 |
| Diabetes mellitusd | 31.3 | 25.4, 37.2 | 18.9 | 15.8, 21.9 |
| Hypertension | 63.3 | 55.5, 71.1 | 54.9 | 51.6, 58.2 |
| Stroke | 10.5 | 7.5, 13.5 | 7.5 | 5.7, 9.3 |
| Cancerf | 17.7 | 12.0, 23.4 | 21.0 | 18.0, 24.0 |
| Hospital Data | ||||
| No hospitalizationd | 69.8 | 62.4, 77.1 | 81.0 | 78.4, 83.6 |
| Hospitalization without infection | 19.9 | 14.8, 25.1 | 15.2 | 12.9, 17.5 |
| Hospitalization with infectiond | 10.3 | 5.3, 15.3 | 3.8 | 2.8, 4.8 |
| Primary admission diagnosis | ||||
| Infection | 19.3 | 8.0, 30.6 | 10.5 | 4.9, 16.2 |
| Cardiovascular condition | 24.2 | 11.6, 36.7 | 29.4 | 22.2, 36.6 |
| Dermatological or musculoskeletal condition | 13.9 | 5.0, 22.9 | 12.0 | 7.2, 16.9 |
| Endocrinological condition | 10.0 | 2.3, 17.6 | 4.4 | 0.2, 8.5 |
| Gastroenterological condition | NAc | NAc | 14.1 | 8.8, 19.4 |
| Season of hospital admission | ||||
| Winter | 56.4 | 40.3, 72.5 | 47.1 | 34.5, 59.7 |
| Spring | 15.1 | 7.7, 22.5 | 17.0 | 9.0, 25.0 |
| Summer | 16.3 | 3.8, 28.7 | 13.1 | 8.3, 17.9 |
| Fall | 12.3 | 4.0, 20.5 | 22.8 | 16.2, 29.4 |
| Season of vitamin D testing | ||||
| Winter | 23.5 | 2.6, 44.5 | 14.0 | 2.9, 25.2 |
| Spring | 32.2 | 11.8, 52.6 | 26.0 | 7.2, 44.9 |
| Summer | 18.3 | 5.5, 31.1 | 22.8 | 9.3, 36.2 |
| Fall | 26.0 | 5.8, 46.1 | 37.2 | 15.4, 58.9 |
Abbreviations: CI, confidence interval; NA, not available; 25(OH)D, 25-hydroxyvitamin D.
a Data from the 2001–2002 and 2003–2004 cycles of the National Health and Nutrition Examination Survey.
b Continuous variable, presented as a mean value.
c The National Research Data Center does not permit any statistic based on a frequency of <5 to be reported.
d Statistically significant; 95% confidence intervals do not overlap.
e Body mass index was calculated as weight (kg)/height (m)2.
f Excluding nonmelanoma skin cancer.
Characteristics by hospitalization status
Within 1 year after serum 25(OH)D testing, 77 (4.5%) participants had an HWI, and 287 (16.8%) participants had an HWOI (Table 2). Compared with the nonhospitalized group, those with an HWI had lower mean baseline serum 25(OH)D concentrations (20.0 ng/mL (95% CI: 17.6, 22.5) vs. 24.0 ng/mL (95% CI: 23.2, 24.7)) and a higher proportion with 25(OH)D levels of <15 ng/mL (34.7% (95% CI: 20.2, 49.3) vs. 14.6% (95% CI: 11.4, 17.7)).
Table 2.
Demographic, Clinical, and Vitamin D Characteristics of a Cohort of Medicare Beneficiaries According to Hospitalization Status Within 1 Year of Vitamin D Testing (n = 1,713), United States, 2001–2004a
| Variable | Hospitalized With an Infection |
Hospitalized Without an Infection |
Not Hospitalized |
|||
|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | % | 95% CI | |
| Serum 25(OH)D concentration, ng/mLb | 20.0b,c | 17.6, 22.5 | 22.1b | 20.5, 23.6 | 24.0b | 23.2, 24.7 |
| Categories | ||||||
| 0–9 | 12.3 | 2.8, 21.7 | 4.4 | 1.7, 7.2 | 3.9 | 2.7, 5.1 |
| 10–19 | 33.3 | 21.9, 44.7 | 33.6 | 25.2, 42.0 | 27.5 | 24.0, 31.0 |
| 20–29 | 38.1 | 27.5, 48.6 | 46.3 | 38.6, 54.2 | 43.7 | 40.2, 47.1 |
| ≥30 | 16.3 | 8.6–24.1 | 15.6 | 10.0, 21.2 | 25.0 | 21.8, 28.2 |
| Dichotomous | ||||||
| <10 | 12.3 | 2.8, 21.7 | 4.4 | 1.7, 7.2 | 3.9 | 2.7, 5.1 |
| <15 | 34.7c | 20.2, 49.3 | 20.6 | 13.6, 27.6 | 14.6 | 11.4, 17.7 |
| <20 | 45.6 | 33.9, 57.2 | 38.0 | 28.9, 47.2 | 31.4 | 27.2, 35.5 |
| Demographic Factors | ||||||
| Age, yearsb | 71.7b | 68.7, 74.8 | 69.7b | 67.5, 71.8 | 69.6b | 68.5, 70.8 |
| Age group, years | ||||||
| 45–54 | 8.4 | 0.0, 18.4 | NAd | NAd | 5.2 | 3.5, 6.8 |
| 55–64 | 6.6 | 1.4, 11.8 | 9.9 | 3.7, 16.2 | 6.6 | 3.9, 9.3 |
| 65–74 | 42.3 | 28.5, 56.1 | 36.0 | 27.3, 44.7 | 47.9 | 43.8, 52.0 |
| 75–84 | 30.9 | 18.9, 42.9 | 30.7 | 24.7, 36.9 | 28.5 | 24.7, 32.2 |
| ≥85 | 10.7 | 3.2, 18.1 | 10.5 | 6.9, 13.9 | 6.4 | 4.5, 8.3 |
| Female sex | 61.3 | 44.4, 78.1 | 47.7 | 41.8, 53.4 | 54.0 | 51.2, 56.8 |
| Body mass indexb,e | 28.4b | 26.2, 30.6 | 28.6b | 27.7, 29.4 | 28.2b | 28.0, 28.4 |
| Body mass indexe category | ||||||
| <25 | 38.2 | 23.3, 53.1 | 33.4 | 25.7, 41.1 | 34.1 | 31.3, 36.9 |
| 25–29 | 32.8 | 18.6, 46.9 | 31.0 | 24.4, 37.7 | 38.2 | 34.7, 41.7 |
| ≥30 | 29.0 | 16.3, 41.7 | 35.6 | 28.1, 43.0 | 27.7 | 25.5, 30.0 |
| Poverty index ratio <2.0 | 50.9 | 36.2, 65.7 | 50.6 | 41.7, 59.6 | 41.6 | 35.3, 47.8 |
| Race/ethnicity | ||||||
| Non-Hispanic white | 87.7 | 80.5, 94.9 | 83.7 | 76.3, 91.2 | 83.5 | 77.7, 89.3 |
| Non-Hispanic black | 5.1 | 0.5, 9.6 | 8.2 | 4.5, 11.9 | 7.5 | 5.1, 9.9 |
| Mexican-American | 3.5 | 0.0, 7.4 | 2.8 | 0.0, 5.9 | 3.2 | 0.5, 5.8 |
| Latitude | ||||||
| <32°N | 24.7 | 11.6, 37.8 | 26.9 | 14.7, 39.0 | 17.2 | 9.6, 24.9 |
| 32°N–38°N | 37.2 | 19.1, 55.4 | 35.4 | 20.7, 50.0 | 40.7 | 25.8, 55.5 |
| ≥39°N | 38.0 | 22.6, 53.5 | 37.7 | 23.8, 51.7 | 42.1 | 30.1, 54.1 |
| Medical History | ||||||
| Liver disease | NAd | NAd | 5.3 | 1.8, 8.8 | 4.3 | 2.8, 5.8 |
| Asthma | 17.3 | 7.6, 26.9 | 14.0 | 8.5, 19.5 | 9.5 | 7.4, 11.7 |
| Emphysema | 24.1 | 1.08, 37.3 | 6.9 | 2.1, 11.6 | 4.0 | 2.6, 5.5 |
| Chronic bronchitis | 13.4 | 1.8, 25.0 | 13.4 | 6.6, 20.2 | 9.2 | 7.1, 11.3 |
| Congestive heart failure | 11.5 | 2.6, 20.3 | 17.2 | 12.1, 22.2 | 6.5 | 5.2, 7.8 |
| Coronary artery disease | 21.0 | 9.3, 32.7 | 19.5 | 12.8, 26.2 | 11.1 | 8.8, 13.3 |
| Renal disease | 14.8b | 7.1, 22.6 | 10.2 | 5.3, 15.1 | 4.6 | 3.3, 5.8 |
| Diabetes mellitus | 26.8 | 8.9, 44.7 | 32.0b | 25.7, 38.2 | 18.3 | 15.4, 21.3 |
| Hypertension | 67.9 | 57.1, 78.6 | 55.2 | 47.0, 63.5 | 55.8 | 52.1, 59.5 |
| Stroke | 11.8 | 3.0, 20.6 | 11.5 | 8.0, 14.9 | 7.1 | 5.6, 8.6 |
| Cancerf | 25.4 | 13.5, 37.2 | 24.4 | 18.5, 30.3 | 19.3 | 16.8, 21.9 |
| Hospital Data | ||||||
| Season of hospital admission | ||||||
| Winter | 45.5 | 28.9, 62.1 | 50.5 | 37.3, 63.7 | ||
| Spring | 23.0 | 13.8, 32.2 | 14.6 | 7.1, 22.0 | ||
| Summer | 18.2 | 6.2, 30.3 | 12.5 | 7.1, 17.9 | ||
| Fall | 13.3 | 3.8, 22.8 | 22.4 | 6.1, 28.7 | ||
Abbreviations: CI, confidence interval; NA, not available; 25(OH)D, 25-hydroxyvitamin D.
a Data from the 2001–2002 and 2003–2004 cycles of the National Health and Nutrition Examination Survey.
b Continuous variable, presented as a mean value.
c Statistically significant; 95% confidence intervals do not overlap.
d The National Research Data Center does not permit any statistic based on a frequency of <5 to be reported.
e Body mass index was calculated as weight (kg)/height (m)2.
f Excluding nonmelanoma skin cancer.
Compared with the nonhospitalized group, those with an HWOI had a higher prevalence of diabetes mellitus (32.0% (95% CI: 25.7, 38.2) vs. 18.3% (95% CI: 15.4, 21.3)). In addition, those with an HWOI had a higher proportion of individuals with 25(OH)D levels of <15 ng/mL (20.6% (95% CI: 13.6, 27.6) vs. 14.6% (95% CI: 11.4, 17.7)), but these proportions were not significantly different.
Associations of 25(OH)D concentrations with hospitalization and mortality
In the unadjusted analyses, a baseline serum 25(OH)D level of <15 ng/mL was associated with higher risks of both HWI and HWOI (risk ratio (RR) = 3.1, 95% CI: 1.6, 6.0 (P < 0.01) for HWI and RR = 1.5, 95% CI: 1.1, 2.2 (P = 0.02) for HWOI) (Web Table 4). Furthermore, when compared with subjects with a serum 25(OH)D level of ≥30 ng/mL, the risk of an HWI increased progressively as 25(OH)D concentrations decreased. This concentration-dependent relationship was not detected for the risk of an HWOI (Table 3).
Table 3.
Unadjusted Risk Ratios for Hospitalization With an Infection and Hospitalization Without an Infection in a Cohort of Medicare Beneficiaries According to Serum 25-Hydroxyvitamin D Concentration as an Ordinal Variable (n = 1,713), United States, 2001–2004a,b
| Serum 25(OH) D Concentration, ng/mL | Hospitalization With Infection vs. No Hospitalization |
Hospitalization Without Infection vs. No Hospitalization |
||
|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | |
| <10 | 4.8 | 1.7, 13.4 | 1.8 | 0.8, 4.2 |
| 10–19 | 1.9 | 1.0, 3.5 | 2.0 | 1.2, 3.2 |
| 20–29 | 1.3 | 0.7, 2.4 | 1.7 | 1.1, 2.6 |
| ≥30 | 1.0 | Referent | 1.0 | Referent |
Abbreviations: CI, confidence interval; 25(OH)D, 25-hydroxyvitamin D; RR, risk ratio.
a Data from the 2001–2002 and 2003–2004 cycles of the National Health and Nutrition Examination Survey.
b Global test of the null hypothesis—likelihood ratio test: P < 0.01; Wald test: P < 0.01.
In the multivariable analyses (Table 4)—when adjusting for sex, age, race/ethnicity, poverty-index ratio, body mass index, latitude, and history cancer, renal disease, and diabetes mellitus—a baseline serum 25(OH)D level of <15 ng/mL was associated with a higher risk of an HWI but not of an HWOI (RR = 2.8, 95% CI: 1.3, 5.9 (P < 0.01) for HWI and RR = 1.4, 95% CI: 0.9, 2.1 (P = 0.10) for HWOI). When comparing participants in different categories of 25(OH)D concentration with those who had a concentration of >30 ng/mL, those with the lowest 25(OH)D concentrations had the highest risk of an HWI (RR = 5.3, 95% CI: 1.5, 19.2; P = 0.01), and the association was concentration-dependent (Web Table 5).
Table 4.
Adjusted Risk Ratios for Hospitalization With an Infection and Hospitalization Without an Infection Among a Cohort of Medicare Beneficiaries (n = 1,512), United States, 2001–2004a,b
| Variable | Hospitalization With Infection vs. No Hospitalization |
P Value | Hospitalization Without Infection vs. No Hospitalization |
P Value | ||
|---|---|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | |||
| Serum 25(OH)D concentration <15 ng/mL | 2.8 | 1.3, 5.9 | <0.01 | 1.4 | 0.9, 2.1 | 0.1 |
| Female sex | 1.3 | 0.7, 2.6 | 0.4 | 0.8 | 0.6, 1.0 | 0.1 |
| Age, per 1-year increase | 1.0 | 1.0, 1.1 | 0.2 | 1.0 | 1.0, 1.0 | 0.4 |
| Race/ethnicity | ||||||
| Non-Hispanic white | 1.0 | Referent | 1.0 | Referent | ||
| Non-Hispanic black | 0.4 | 0.1, 1.2 | 0.1 | 0.8 | 0.5, 1.1 | 0.1 |
| Mexican-American | 0.6 | 0.3, 1.2 | 0.1 | 0.6 | 0.3, 0.9 | 0.02 |
| Other Hispanic | 0.4 | 0.0, 4.2 | 0.5 | 1.1 | 0.6, 2.0 | 0.8 |
| Other | 0.6 | 0.1, 2.7 | 0.5 | 0.7 | 0.2, 2.3 | 0.6 |
| Poverty-index ratio <2.0 | 1.2 | 0.7, 2.3 | 0.5 | 1.4 | 0.9, 2.1 | 0.1 |
| Body mass indexc category | ||||||
| <25 | 1.0 | Referent | 1.0 | Referent | ||
| 25–29 | 0.7 | 0.3, 1.6 | 0.5 | 0.9 | 0.6, 1.4 | 0.6 |
| ≥30 | 0.8 | 0.4, 1.7 | 0.6 | 1.2 | 0.8, 1.9 | 0.4 |
| Latitude | ||||||
| <32°N | 1.0 | Referent | 1.0 | Referent | ||
| 32°N–38°N | 0.7 | 0.4, 1.3 | 0.2 | 0.5 | 0.3, 0.8 | <0.01 |
| ≥39°N | 0.7 | 0.4, 1.4 | 0.3 | 0.6 | 0.4, 0.9 | 0.02 |
| Cancerd | 1.2 | 0.6, 2.3 | 0.6 | 1.3 | 1.0, 1.7 | 0.1 |
| Renal disease | 3.5 | 1.5, 8.2 | <0.01 | 1.9 | 1.1, 3.4 | 0.02 |
| Diabetes mellitus | 1.4 | 0.6, 3.5 | 0.4 | 2.0 | 1.4, 2.7 | <0.01 |
Abbreviations: CI, confidence interval; 25(OH)D, 25-hydroxyvitamin D; RR, risk ratio.
a Data from the 2001–2002 and 2003–2004 cycles of the National Health and Nutrition Examination Survey.
b Global test of the null hypothesis—likelihood ratio test: P < 0.01; Wald test: P < 0.01.
c Body mass index was calculated as weight (kg)/height (m)2.
d Excluding nonmelanoma skin cancers.
DISCUSSION
In the present study of Medicare beneficiaries participating in NHANES in 2001–2002 and 2003–2004, we found that a baseline serum 25(OH)D concentration of <15 ng/mL was associated with an approximately 3-fold higher risk of an HWI. We did not find a statistically significant association between low 25(OH)D concentrations and an HWOI. To our knowledge, this is the first study to have found an association between low 25(OH)D concentration and a higher 1-year risk of an HWI.
These results are consistent with our a priori hypothesis that low circulating 25(OH)D concentration is both a potential marker of poor health status, portending a higher risk of hospitalization, and a potential risk factor for immunological vulnerability, portending a larger and independent risk for infection. In line with this hypothesis, the association between low 25(OH)D concentrations and an HWOI was suggestive of an association but not statistically significant after adjustment for other chronic health conditions and socioeconomic factors, whereas the association between low 25(OH)D concentrations and an HWI remained significant. Strengthening these findings is the fact that we also observed a concentration-dependent relationship only for HWI, with the lowest concentrations of serum 25(OH)D being associated with the highest risk of HWI (Table 3 and Web Table 5).
The strengths of this study are that it was based on data from a representative sample of noninstitutionalized Medicare beneficiaries and followed them prospectively to capture important infection outcomes. The serum 25(OH)D testing was done as part of the NHANES national survey, therefore preventing the potential bias of physician ordering and testing. The study took advantage of the highly standardized survey techniques and 25(OH)D measurements used in NHANES, allowing comprehensive characterization of the study population and extensive confounding assessment. The large, nationally derived sample and complex sampling design of NHANES increased the study's generalizability to a large proportion of persons living in the United States.
This study had several limitations. First, the observational design did not allow us to exclude the possibility that residual confounding might account for the association between low 25(OH)D concentrations and HWI risk. Other potential and plausible confounders that were not accounted for in this study include overall comorbidity, disability or nutritional status, outdoor activity, and other health-related behaviors. Second, the outcome was defined by ICD-9-CM diagnosis codes used for billing rather than actual clinical chart data, allowing for potential misclassification of infection. Other researchers have assessed similar methods for identifying cases of sepsis using administrative data, finding the method to have a sensitivity of 50% and a specificity and 96% (43). Extrapolating from these findings, our study's reliance on administrative coding most likely resulted in some nondifferential misclassification of the infection designation of the hospitalization outcome. Because an HWOI was not associated with a low 25(OH)D concentration, nondifferential misclassification of HWIs would have tended to bias the results towards a null association. Another limitation of our study is that we were not able to report the types of infections present in our cohort; the infections were spread out over 10 variables, and the Research Data Center information security rules required censoring of data cells calculated from small frequencies. Although this does not bias the findings, it does give a less detailed picture of the association.
There is ample research from the basic sciences to lend biological plausibility to our findings. Several excellent reviews on this topic have summarized the findings to date that 25(OH)D is involved in the innate immune system's production of antimicrobial peptides in response to pathogens (44–47). However, the results from clinical studies have been inconsistent, and controlled trials of vitamin D supplementation for preventing infections have largely been negative (16, 17, 28). Several explanations could account for this lack of a consistent observable effect in clinical trials. First, the baseline serum 25(OH)D concentrations of the populations in all 3 negative trials were greater than 20 ng/mL and therefore might have not been low enough to give the placebo groups a sufficiently higher risk of infection. Our findings, in concurrence with those of others, have demonstrated a concentration-dependent association, with a serum 25(OH)D level of <10 ng/mL being associated with the highest risk of infection (22, 48). In addition to not having a true placebo group, the trials likely did not have sufficient sample size to detect differences.
In conclusion, we found a significant association between a low baseline serum 25(OH)D concentration and the subsequent risk of hospitalization with an infection among Medicare beneficiaries. The finding provides further evidence for the continued study of vitamin D as a correctable risk factor for infections in persons with severely depleted vitamin D levels. However, further work is needed to demonstrate the causal nature of the observed association. A large clinical trial with correct dosing in a population that had substantially low 25(OH)D levels and was at risk for infections, serially monitored the effects of treatment across the entire vitamin D axis, assessed the interaction of treatment with vitamin D receptor polymorphisms, and followed the cohort for an extended period of time would be an ideal (albeit resource-intensive) study design for further analysis of this association.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Division of Pulmonary, Allergy, Critical Care and Sleep Medicine, School of Medicine, Emory University, Atlanta, Georgia (Jordan A. Kempker, Greg S. Martin); Division of Epidemiology and Biostatistics, School of Public Health, Georgia State University, Atlanta, Georgia (Matthew J. Magee); and Division of Tuberculosis Elimination, Centers for Disease Control and Prevention, Atlanta, Georgia (J. Peter Cegielski).
This work was supported in part by National Institutes of Health grants T32 AA013528 and KL2 TR000455 (J.A.K.), R21 HL110044 (G.S.M.), and P50 AA013757 (G.S.M.) and by the Atlanta Clinical and Translational Science Institute (grant UL1 TR000454).
We thank Ajay Yesupriya, our assigned Research Data Center analyst, who helped facilitate this study.
These data were presented in part as a scientific poster at the American Thoracic Society 2015 International Conference, May 15–20, 2015, Denver, Colorado.
The conclusions and interpretations of data presented in this report are solely those of the authors and do not necessarily represent an official position of the Centers for Disease Control and Prevention or the US government.
Conflict of interest: none declared.
REFERENCES
- 1.Baeke F, Takiishi T, Korf H et al. . Vitamin D: modulator of the immune system. Curr Opin Pharmacol. 2010;104:482–496. [DOI] [PubMed] [Google Scholar]
- 2.Hewison M. Antibacterial effects of vitamin D. Nat Rev Endocrinol. 2011;76:337–345. [DOI] [PubMed] [Google Scholar]
- 3.Yim S, Dhawan P, Ragunath C et al. . Induction of cathelicidin in normal and CF bronchial epithelial cells by 1,25-dihydroxyvitamin D(3). J Cyst Fibros. 2007;66:403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turner J, Cho Y, Dinh NN et al. . Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob Agents Chemother. 1998;429:2206–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nijnik A, Hancock RE. The roles of cathelicidin LL-37 in immune defences and novel clinical applications. Curr Opin Hematol. 2009;161:41–47. [DOI] [PubMed] [Google Scholar]
- 6.Hertting O, Holm Å, Lüthje P et al. . Vitamin D induction of the human antimicrobial peptide cathelicidin in the urinary bladder. PLoS One. 2010;512:e15580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ginde AA, Mansbach JM, Camargo CA Jr. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2009;1694:384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabetta JR, DePetrillo P, Cipriani RJ et al. . Serum 25-hydroxyvitamin D and the incidence of acute viral respiratory tract infections in healthy adults. PLoS One. 2010;56:e11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Remmelts HH, van de Garde EM, Meijvis SC et al. . Addition of vitamin D status to prognostic scores improves the prediction of outcome in community-acquired pneumonia. Clin Infect Dis. 2012;5511:1488–1494. [DOI] [PubMed] [Google Scholar]
- 10.Leow L, Simpson T, Cursons R et al. . Vitamin D, innate immunity and outcomes in community acquired pneumonia. Respirology. 2011;164:611–616. [DOI] [PubMed] [Google Scholar]
- 11.Karatekin G, Kaya A, Salihoğlu O et al. . Association of subclinical vitamin D deficiency in newborns with acute lower respiratory infection and their mothers. Eur J Clin Nutr. 2009;634:473–477. [DOI] [PubMed] [Google Scholar]
- 12.McNally JD, Leis K, Matheson LA et al. . Vitamin D deficiency in young children with severe acute lower respiratory infection. Pediatr Pulmonol. 2009;4410:981–988. [DOI] [PubMed] [Google Scholar]
- 13.Roth DE, Jones AB, Prosser C et al. . Vitamin D status is not associated with the risk of hospitalization for acute bronchiolitis in early childhood. Eur J Clin Nutr. 2009;632:297–299. [DOI] [PubMed] [Google Scholar]
- 14.Roth DE, Shah R, Black RE et al. . Vitamin D status and acute lower respiratory infection in early childhood in Sylhet, Bangladesh. Acta Paediatr. 2010;993:389–393. [DOI] [PubMed] [Google Scholar]
- 15.Wayse V, Yousafzai A, Mogale K et al. . Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. Eur J Clin Nutr. 2004;584:563–567. [DOI] [PubMed] [Google Scholar]
- 16.Laaksi I, Ruohola JP, Mattila V et al. . Vitamin D supplementation for the prevention of acute respiratory tract infection: a randomized, double-blinded trial among young Finnish men. J Infect Dis. 2010;2025:809–814. [DOI] [PubMed] [Google Scholar]
- 17.Li-Ng M, Aloia JF, Pollack S et al. . A randomized controlled trial of vitamin D3 supplementation for the prevention of symptomatic upper respiratory tract infections. Epidemiol Infect. 2009;13710:1396–1404. [DOI] [PubMed] [Google Scholar]
- 18.Manaseki-Holland S, Maroof Z, Bruce J et al. . Effect on the incidence of pneumonia of vitamin D supplementation by quarterly bolus dose to infants in Kabul: a randomised controlled superiority trial. Lancet. 2012;3799824:1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manaseki-Holland S, Qader G, Isaq Masher M et al. . Effects of vitamin D supplementation to children diagnosed with pneumonia in Kabul: a randomised controlled trial. Trop Med Int Health. 2010;1510:1148–1155. [DOI] [PubMed] [Google Scholar]
- 20.Urashima M, Segawa T, Okazaki M et al. . Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr. 2010;915:1255–1260. [DOI] [PubMed] [Google Scholar]
- 21.Su LX, Jiang ZX, Cao LC et al. . Significance of low serum vitamin D for infection risk, disease severity and mortality in critically ill patients. Chin Med J (Engl). 2013;12614:2725–2730. [PubMed] [Google Scholar]
- 22.Quraishi SA, Litonjua AA, Moromizato T et al. . Association between prehospital vitamin D status and hospital-acquired Clostridium difficile infections. JPEN J Parenter Enteral Nutr. 2015;391:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moromizato T, Litonjua AA, Braun AB et al. . Association of low serum 25-hydroxyvitamin D levels and sepsis in the critically ill. Crit Care Med. 2014;421:97–107. [DOI] [PubMed] [Google Scholar]
- 24.Lange N, Litonjua AA, Gibbons FK et al. . Pre-hospital vitamin D concentration, mortality, and bloodstream infection in a hospitalized patient population. Am J Med. 2013;1267:640.e19–640.e27. [DOI] [PubMed] [Google Scholar]
- 25.Amrein K, Zajic P, Schnedl C et al. . Vitamin D status and its association with season, hospital and sepsis mortality in critical illness. Crit Care. 2014;182:R47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quraishi SA, Bittner EA, Blum L et al. . Association between preoperative 25-hydroxyvitamin D level and hospital-acquired infections following Roux-en-Y gastric bypass surgery. JAMA Surg. 2014;1492:112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amrein K, Schnedl C, Holl A et al. . Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: the VITdAL-ICU randomized clinical trial. J Am Med Assoc. 2014;31215:1520–1530. [DOI] [PubMed] [Google Scholar]
- 28.Murdoch DR, Slow S, Chambers ST et al. . Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: the VIDARIS randomized controlled trial. J Am Med Assoc. 2012;30813:1333–1339. [DOI] [PubMed] [Google Scholar]
- 29.Office of Information Services, National Center for Health Statistics. About the National Health and Nutrition Examination Survey. http://www.cdc.gov/nchs/nhanes/about_nhanes.htm Updated February 3, 2014. Accessed July 1, 2015.
- 30.Braun A, Chang D, Mahadevappa K et al. . Association of low serum 25-hydroxyvitamin D levels and mortality in the critically ill. Crit Care Med. 2011;394:671–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ross AC, Taylor CL, Yaktine AL et al. eds. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academies Press; 2011:1132. [PubMed] [Google Scholar]
- 32.Kempker JA, West KG, Kempker RR et al. . Vitamin D status and the risk for hospital-acquired infections in critically ill adults: a prospective cohort study. PLoS One. 2015;104:e0122136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bureau of the Census, US Department of Commerce. Poverty thresholds. http://www.census.gov/hhes/www/poverty/data/threshld/index.html Updated February 3, 2016. Accessed March 2013.
- 34.Kempker JA, Martin GS. Vitamin D and sepsis: from associations to causal connections. Inflamm Allergy Drug Targets. 2013;124:246–252. [DOI] [PubMed] [Google Scholar]
- 35.Pettifor JM, Moodley GP, Hough FS et al. . The effect of season and latitude on in vitro vitamin D formation by sunlight in South Africa. S Afr Med J. 1996;8610:1270–1272. [PubMed] [Google Scholar]
- 36.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;672:373–378. [DOI] [PubMed] [Google Scholar]
- 37.Wolter KM. Introduction to Variance Estimation. New York, NY: Springer-Verlag; 1985. [Google Scholar]
- 38.Looker AC, Dawson-Hughes B, Calvo MS et al. . Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;305:771–777. [DOI] [PubMed] [Google Scholar]
- 39.McQuillan GM, Kruszon-Moran D, Kottiri BJ et al. . Racial and ethnic differences in the seroprevalence of 6 infectious diseases in the United States: data from NHANES III, 1988–1994. Am J Public Health. 2004;9411:1952–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Powe CE, Evans MK, Wenger J et al. . Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013;36921:1991–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sarnak MJ, Jaber BL. Mortality caused by sepsis in patients with end-stage renal disease compared with the general population. Kidney Int. 2000;584:1758–1764. [DOI] [PubMed] [Google Scholar]
- 42.Esper AM, Moss M, Lewis CA et al. . The role of infection and comorbidity: factors that influence disparities in sepsis. Crit Care Med. 2006;3410:2576–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iwashyna TJ, Odden A, Rohde J et al. . Identifying patients with severe sepsis using administrative claims: patient-level validation of the Angus implementation of the international consensus conference definition of severe sepsis. Med Care. 2014;526:e39–e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bartley J. Vitamin D: emerging roles in infection and immunity. Expert Rev Anti Infect Ther. 2010;812:1359–1369. [DOI] [PubMed] [Google Scholar]
- 45.Youssef DA, Miller CW, El-Abbassi AM et al. . Antimicrobial implications of vitamin D. Dermatoendocrinol. 2011;34:220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hewison M. Antibacterial effects of vitamin D. Nat Rev Endocrinol. 2011;76:337–345. [DOI] [PubMed] [Google Scholar]
- 47.Kempker JA, Tangpricha V, Ziegler TR et al. . Vitamin D in sepsis: from basic science to clinical impact. Crit Care. 2012;164:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quraishi SA, Litonjua AA, Moromizato T et al. . Association between prehospital vitamin D status and hospital-acquired bloodstream infections. Am J Clin Nutr. 2013;984:952–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


