TO THE EDITOR
Multiple acyl–coenzyme A dehydrogenation deficiency is an inborn error of metabolism with frequent muscle involvement. This deficiency is due to defects in the electron-transfer flavoprotein genes ETFA and ETFB1 or in the electron-transfer flavoprotein ubiquinone oxidoreductase gene ETFDH.2 In patients with this deficiency, all the mitochondrial flavoprotein dehydrogenases are defective with a specific biochemical phenotype for multiple acyl–coenzyme A dehydrogenation deficiency. Yet, in a few patients who have a deficiency that is similar to multiple acyl–coenzyme A dehydrogenation deficiency, no mutations are identified in ETFA, ETFB, or ETFDH.3
We report on a 14-year-old girl who presented with recurrent exercise intolerance. She had biochemical features of multiple acyl–coenzyme A dehydrogenation deficiency, but she did not have mutations in ETFA, ETFB, or ETFDH. Oral supplementation with riboflavin led to dramatic improvement in the clinical and biologic abnormalities.
Riboflavin is the precursor of flavin adenine dinucleotide (FAD), the cofactor for electron-transfer flavoprotein and electron-transfer flavoprotein ubiquinone oxidoreductase. We thus suspected a defect in FAD biosynthesis or transport and studied genes involved in riboflavin metabolism.
Two heterozygous mutations, c.425G→A (p.Trp142*) and c.440G→A (p.Arg147His), were identified in solute carrier family 25, member 32 (SLC25A32). SLC25A32 encodes the mitochondrial FAD transporter, an inner mitochondrial membrane carrier that imports FAD from the cytosol into the mitochondria.3,4 Allelic segregation confirmed autosomal recessive transmission. At the complementary DNA level, the missense mutation appeared homozygous, but the substitution responsible for the nonsense mutation was not identified. Consequently, we speculate that the messenger RNA (mRNA) harboring the nonsense mutation is degraded by the nonsense-mediated mRNA decay machinery.
We performed studies in yeast that showed that the missense mutation introduced in FLX1, the homologue of human SLC25A32 in Saccharomyces cerevisiae, resulted in a severe growth defect that was rescued by exogenous expression of wild-type FLX1 and also by SLC25A32. These findings show the deleterious effect of this mutation (Fig. S1A, S1B, and S1C in the Supplementary Appendix, available with the full text of this letter at NEJM.org).
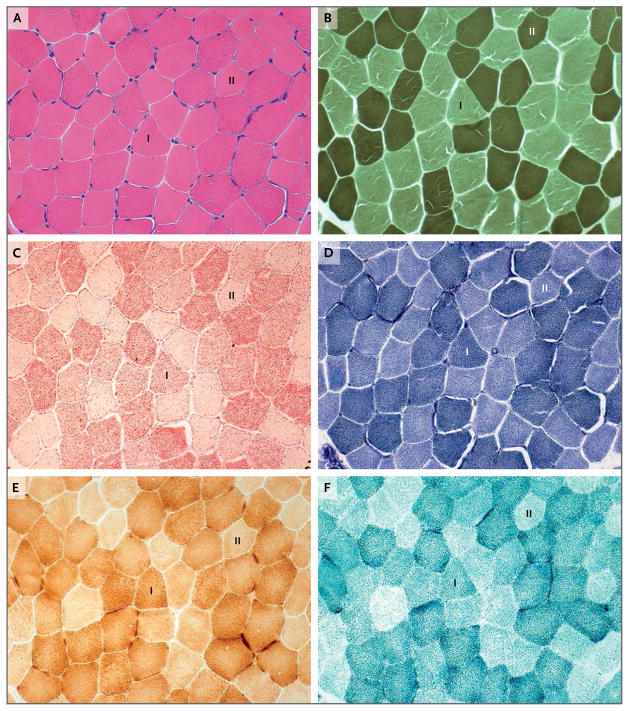
We hypothesized that impaired mitochondrial FAD transport in our patient affected the activities of mitochondrial flavoproteins involved in fatty acid oxidation, as indicated by the biochemical profile of multiple acyl–coenzyme A dehydrogenation deficiency and by impaired fatty acid oxidation flux in lymphocytes (not shown). We also hypothesized that impaired mitochondrial FAD transport affected the activities of mitochondrial respiratory-chain flavoproteins.3,5 Our hypothesis was supported by faint succinate dehydrogenase staining observed in a skeletal-muscle–biopsy specimen obtained from the patient (Fig. 1) and by a modest effect of SLC25A32 haploinsufficiency on FAD-dependent mitochondrial enzymes observed in the patient’s fibroblasts (Fig. S1D in the Supplementary Appendix).
Figure 1. Histochemical Images of Skeletal-Muscle–Biopsy Specimens Obtained from the Patient.
Cross sections of skeletal-muscle–biopsy specimens stained with hematoxylin and eosin (Panel A) show some ragged-red fibers observed mainly in type I fibers (I), which have a predominantly oxidative metabolism, rather than in type II fibers (II), which have a predominantly glycolytic metabolism. ATPase staining at pH 9.4 (Panel B) shows type II fibers (dark), which are slightly smaller than type I fibers (clear). Lipid storage was detected, particularly in type I fibers (Panel C). Oxidative enzyme reactions showed the presence of ragged-red fibers in some muscle fibers with NADH–tetrazolium reductase staining (Panel D) and with cytochrome c oxidase staining (Panel E). In contrast, the large majority of the fibers (Panel F) stained poorly for succinate dehydrogenase (flavin adenine dinucleotide–dependent mitochondrial respiratory-chain complex II), and some did not appear to stain at all.
In conclusion, in our patient with haploinsufficiency of SLC25A32, a deficiency in FAD mitochondrial transport caused late-onset exercise intolerance associated with abnormalities in the acylcarnitine profile. The patient’s symptoms appeared to be highly responsive to oral supplementation with riboflavin. Our findings underscore the importance of studying genes involved in riboflavin metabolism in such patients and expand knowledge of the spectrum of inborn errors of riboflavin transport.
Supplementary Material
Acknowledgments
Supported in part by a grant (HL22174) from the National Institutes of Health.
Footnotes
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
Contributor Information
Manuel Schiff, Robert-Debré University Hospital, Paris, France
Alice Veauville-Merllié, Lyon University Hospital, Lyon, France
Chen Hsien Su, Columbia University, New York, NY
Alexander Tzagoloff, Columbia University, New York, NY
Malgorzata Rak, Robert-Debré University Hospital, Paris, France
Hélène Ogier de Baulny, Robert-Debré University Hospital, Paris, France
Audrey Boutron, Kremlin-Bicêtre University Hospital, Paris, France
Hélène Smedts-Walters, University of California, San Francisco, Benioff Children’s Hospital Oakland, San Francisco, CA
Norma B. Romero, Pitié–Salpétrière University Hospital, Paris, France
Odile Rigal, Robert-Debré University Hospital, Paris, France
Pierre Rustin, Robert-Debré University Hospital, Paris, France
Christine Vianey-Saban, Lyon University Hospital, Lyon, France
Cécile Acquaviva-Bourdain, Email: cecile.acquaviva-bourdain@chu-lyon.fr, Lyon University Hospital, Lyon, France
References
- 1.Schiff M, Froissart R, Olsen RK, Acquaviva C, Vianey-Saban C. Electron transfer flavoprotein deficiency: functional and molecular aspects. Mol Genet Metab. 2006;88:153–8. doi: 10.1016/j.ymgme.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Goodman SI, Binard RJ, Woontner MR, Frerman FE. Glutaric acidemia type II: gene structure and mutations of the electron transfer flavoprotein:ubiquinone oxidoreductase (ETF:QO) gene. Mol Genet Metab. 2002;77:86–90. doi: 10.1016/s1096-7192(02)00138-5. [DOI] [PubMed] [Google Scholar]
- 3.Spaan AN, Ijlst L, van Roermund CW, Wijburg FA, Wanders RJ, Waterham HR. Identification of the human mitochondrial FAD transporter and its potential role in multiple acyl-CoA dehydrogenase deficiency. Mol Genet Metab. 2005;86:441–7. doi: 10.1016/j.ymgme.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 4.Tzagoloff A, Jang J, Glerum DM, Wu M. FLX1 codes for a carrier protein involved in maintaining a proper balance of flavin nucleotides in yeast mitochondria. J Biol Chem. 1996;271:7392–7. doi: 10.1074/jbc.271.13.7392. [DOI] [PubMed] [Google Scholar]
- 5.Bafunno V, Giancaspero TA, Brizio C, et al. Riboflavin uptake and FAD synthesis in Saccharomyces cerevisiae mitochondria: involvement of the Flx1p carrier in FAD export. J Biol Chem. 2004;279:95–102. doi: 10.1074/jbc.M308230200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.