Abstract
Objective
Freezing of gait (FOG) is a locomotor disturbance in Parkinson disease (PD) related to impaired motor automaticity. In this study, we investigated the impact of freezing on automaticity in the oculomotor system using an anti-saccade paradigm.
Methods
Subjects with PD with (PD-FOG, n=13) and without (PD-NON, n=13) FOG, and healthy age-matched controls (CTRL, n=12) completed automatic pro-saccades and non-automatic anti-saccades. Primary outcomes were saccade latency, velocity, and gain.
Results
PD-FOG (pro-saccade latency = 271 ms, anti-saccade latency = 412 ms) were slower to execute both types of saccades compared to PD-NON (253 ms, 330 ms) and CTRL (246 ms, 327 ms). Saccade velocity and gain variability was also increased in PD-FOG.
Conclusions
Saccade performance was affected in PD-FOG for both types of saccades, indicating differences in automaticity and control in the oculomotor system related to freezing.
Significance
These results and others show that FOG impacts non-gait motor functions, suggesting global motor impairment in PD-FOG.
Keywords: Freezing of gait, saccades, Parkinson’s disease, motor automaticity
1. Introduction
Among the many gait difficulties in people with Parkinson’s disease (PD), freezing of gait (FOG) is one of the most common, affecting over half of the PD population (Forsaa et al., 2015). FOG manifests as episodic interruptions of the gait cycle during normal walking and other complex gait tasks like turning (Bloem et al., 2004, Nutt et al., 2011). Additional research into the mechanisms of FOG showed that freezing is not limited to gait, but can also be observed in other motor tasks, such as upper limb movements and speech (Moreau et al., 2007, Williams et al., 2013, Vercruysse et al., 2014a). Altogether, these studies indicate that freezing may be a global phenomenon impacting not just gait but the entire motor system.
Many hypotheses explaining FOG phenomenology have been proposed (Nieuwboer et al., 2013), and two specifically relate FOG to impairments in cognitive-motor function. The interference model suggests excessive overlap of activity in sensorimotor, associative, and limbic circuits of the basal ganglia leads to abnormal inhibition from the globus pallidus, leading to freezing episodes. (Lewis et al., 2009). Additionally, the cognitive model proposes freezers have impaired conflict resolution and response automaticity in challenging environments, resulting in an increased reliance on cortical resources (Vandenbossche et al., 2012). Evidence for this is seen in dual-task experiments, commonly used to assess automaticity, during which people with PD and FOG (PD-FOG) have poorer gait performance during dual-task tests compared to those who do not have FOG (PD-NON) (Spildooren et al., 2010). Recent neuroimaging data also support the cognitive model, showing increased activation and connectivity of cortical regions in PD-FOG (Fling et al., 2014, Vercruysse et al., 2014b). Tying back into the interference model, increased activity may lead to resource “overloads”, particularly during cognitively demanding tasks, inducing motor arrests observed during a freezing episode (Shine et al., 2013). Given these hypotheses, it is reasonable to predict that impaired automaticity is a common feature of freezing that would affect all motor output.
Saccades are fast eye movements that allow us to quickly foveate objects of interest, and are mediated by both cortical (DLPFC, FEF, SEF) and subcortical (thalamus, basal ganglia, superior colliculus) circuits as well as oculomotor neurons in cranial nerves (Moschovakis et al., 1996, Munoz et al., 2004). Saccadic output follows highly stereotyped patterns and is well-described in both healthy (Bahill et al., 1975, Peltsch et al., 2011) adults and PD. These studies show that people with PD are generally slower to respond (i.e. increased latency) and make slower (i.e. decreased velocity) volitional saccades (Crawford et al., 1989, Briand et al., 1999), supporting the traditional view that slowed voluntary movement is a result of increased inhibition of the basal ganglia (Terao et al., 2013).
The anti-saccade task is a common way to study a different aspect of oculomotor control (Hallett, 1978). In this task, participants make saccades either toward a visual target (the automatic pro-saccade) or to a mirrored position of a visual target (non-automatic anti-saccade). Anti-saccades require inhibition of a visually-guided response as well as initiation of a non-visually guided saccade. As such, anti-saccade tasks are useful to assess both the cognitive and motor aspects of oculomotor control and have been used in both healthy individuals and patients with neurological conditions (Guitton et al., 1985, Kristjansson et al., 2001, Chan et al., 2013). In addition, anti-saccade performance correlates well with other measures of executive function in adults (Klein et al., 2010, Mirsky et al., 2011). Altogether, anti-saccades likely involve parallel processing of cognitive and motor commands mediated by the basal ganglia, and are a suitable approach to study cognitive-motor processing and its relationship to freezing. However to our knowledge only one recent study directly examined the impact of FOG on saccades. This study noted that PD-FOG made more anti-saccade errors, which were related to grey-matter loss in visual, frontal, and parietal regions (Walton et al., 2015) Interestingly, no differences in pro- or anti-saccade latency were noted between freezer subgroups, suggesting the oculomotor impairment was specific to response inhibition and not selection. Since freezing is associated with a maladaptive response to increased cognitive-motor demand and impaired automaticity, the link between freezing and oculomotor function merits further investigation.
In this study, we investigated automaticity and control using an anti-saccade task in PD-NON and PD-FOG relative to healthy adult controls. We hypothesized that PD-FOG would demonstrate impaired saccade automaticity, as evidenced by slowness of movements and prolonged response latency during both pro- and anti-saccades compared to PD-NON and controls. In contrast, we predicted that PD-NON would be slower and more variable during volitional anti-saccades compared only to controls. This work aimed to increase our knowledge of the oculomotor system in PD-NON and PD-FOG in an effort to better understand the impact of freezing as a potential global motor disturbance and inform the development of treatment approaches to address freezing.
2. Materials and Methods
2.1 Participants
A sample of twenty six people with PD (13 PD-NON and 13 PD-FOG) and twelve age-matched neurologically healthy older adults took part in the study. PD participants were recruited from the Movement Disorders Center at Washington University School of Medicine and had a diagnosis of idiopathic PD as defined by previous criteria (Calne et al., 1992). Healthy older adults were recruited from a volunteer database managed by the Department of Psychological & Brain Sciences at Washington University. All subjects were free of other neurological conditions including dementia (Montreal cognitive assessment (MOCA) > 21 (Dalrymple-Alford et al., 2010)), and were able to walk independently with or without an assistive device. Additionally, PD participants were excluded if they were unable to tolerate medication withdrawal or had previous deep brain stimulation surgery. Given our sample size, the effect size was calculated to be 0.48, assuming 80% power and Type I error rate of 5%.
We classified the group of PD participants as freezers (PD-FOG) and non-freezers (PD-NON) based on self-report of freezing episodes over the past month using the New Freezing of Gait Questionnaire (NFOGQ), a reliable instrument which uses both written and video descriptions of FOG to determine FOG severity (Nieuwboer et al., 2009). If the participant reports s/he has not experienced any freezing episodes over the past month, s/he is classified as PD-NON and given a score of zero. If the participant responds that s/he has experienced freezing over the past month, s/he is asked additional questions about the duration and frequency of episodes and a composite NFOGQ score ranging from 1–28 is determined. PD participants were evaluated in the off state, defined as at least a 12-hour withdrawal from any anti-Parkinson medication, and clinically evaluated for descriptive purposes using the Movement Disorder Society version of the Unified Parkinson Disease Rating Scale (MDS-UPDRS). Sub-sections I (non-motor symptoms), II (motor aspects of daily living), and III (motor sign severity) were administered and scored by a trained physical therapist. This protocol was approved by the Human Research Protection Office at Washington University School of Medicine. Participants provided informed consent before participating and were compensated for their time.
2.2 Saccade Tasks
We used a modified anti-saccade paradigm to study saccadic eye movements (Hallett, 1978, Antoniades et al., 2013). The task parameters were chosen based on previously published best practices for saccade testing in people with neurological conditions (Antoniades et al., 2013). The tasks required participants to either make saccades toward (pro-saccade) or to a symmetrically-opposite location away from (anti-saccade) a visually presented target. Stimuli were presented on a 22″ LCD monitor and controlled by E-Prime v2.0 (Psychology Software Tools, Sharpsburg, PA) on a Dell E6440 Latitude laptop computer. Participants sat approximately 50 cm from the display, which was adjusted to eye level. A chin rest was used to minimize head movement. Participants performed one block of 50 pro-saccades and another block of 50 anti-saccades, the order of which was counter-balanced across participants. The number of trials was chosen both to minimize fatigue and to get reliable estimations of saccade parameters for each participant (Antoniades et al., 2013).
Each trial began with a blue or red fixation cross (2.6°) centered on a white background (see Figure 1). A blue cross indicated a pro-saccade should be made; a red cross indicated an anti-saccade should be made. Following a random delay period (750–2000 ms), the fixation cross was extinguished and a black circular target (diameter = 1.2°) was displayed randomly to the right or left at 15° eccentricity. Participants were instructed to make the appropriate eye movement as soon as the target appeared. After 1000 ms, the target was extinguished, leaving a white screen for 2500 ms (inter-trial interval). Participants completed 5–10 practice trials of each type before beginning the experiment.
Figure 1.
Anti-saccade paradigm. A blue fixation cross indicated a pro-saccade trial while a red cross indicated an anti-saccade trial. Fixation was maintained for a variable period (750–2000 ms, blue/red bar), and the target (black bar) appeared immediately after the fixation cross was removed. A correct saccade is shown in the gray trace.
2.3 Cognitive Tasks
Two neuropsychological tests, the Go-NoGo (GNG) and Trail-making tests (TMT), were administered to assess general cognitive function. The GNG task tests processing speed as well as response inhibition, and consisted of a string of letters or the number “5” presented individually for 750 ms (stimulus inter-stimulus interval = 1250 ms, total trials = 150). The GNG was administered with EPrime v2.0 on the same laptop computer as used during the saccade tasks. Participants were instructed to press the spacebar key as quickly as possible whenever a letter (target) appeared on the screen, but to not press the key when the number “5” (foil) appeared. Up to 10 practice trials were performed for familiarization. False alarm rate (number of responses to foils/total number of trials), miss rate (number of non-responses to targets/total number of trials), and reaction time (RT, correct responses only) were calculated. The TMT requires the participants to connect a series of numbers (TMT A) or alternate between numbers and letters (TMT B). To account for differences in visuomotor speed and to address task-set switching, the difference in completion time between TMT B and A was reported.
2.4 Data analysis
Eye movement data were collected using a binocular head-mounted videooculography system (Eye-Trac 6, Applied Science Laboratories, Bedford, MA). This system detects eye position using both pupil and corneal reflection and is accurate to < 1°. For each participant, the system was calibrated using a 9-point display and an array of 5 targets at known eccentricities (to convert voltage signal to angular position). Raw eye position from both eyes was measured at 120 Hz for 1000ms, beginning at target onset. All analyses were performed using custom written Matlab scripts and built-in functions (R2011b, The Mathworks Inc., Natick, MA). Raw position data were low-pass filtered at 30 Hz, and velocity and acceleration profiles were calculated based on position-time and velocity-time differentiation, respectively. Movement onset was determined when the first saccade following target onset exceeded 30°/s and 8000 °/s2 (DeSimone et al., 2014). Trials were labeled as invalid and excluded if no saccade was detected or if excessive blinking or eyelid drooping contaminated the signal. Saccade errors were defined as a measured saccade made in the incorrect direction; these trials were marked as errors and excluded from further analysis (Chan et al., 2005). Our primary outcome variables were saccade latency, gain (saccade amplitude normalized to target amplitude), and peak velocity, which were calculated for all remaining trials (non-error valid trials). In addition, we calculated saccade error rate as the ratio of error trials to valid trials. There were no valid trials with latencies less than 100 ms, which represents the threshold for preparatory or anticipatory saccades (Cameron et al., 2010), thus we did not exclude any saccades based on latency from our results.
2.5 Statistical analyses
Statistical procedures were carried out using SPSS v23 (IBM Corp, Armonk, NY) and Matlab v2011b. Baseline demographic and cognitive data were compared using one-way ANOVA (comparing all groups) and independent samples t-tests (comparing PD-NON and PD-FOG) for continuous and normally distributed variables and Mann-Whitney U tests for categorical or non-normally distributed variables.
We examined saccade latency and velocity across all trials in each group (fixed-effects analysis) and at the group level (mixed-effects analysis). In the fixed-effects analysis, we computed cumulative distribution functions of latency, velocity, and gain and compared them using 2-sample Kolmogirov-Smirnov (K-S) tests. Since three comparisons were needed, we accounted for multiple comparisons by adjusting α as: α/n, where n is the number of K-S tests performed. In the mixed-effects analysis, an individual measure of central tendency and variability was calculated for each participant. Because of non-normal distributions, median and interquartile range (IQR) were used for latency while mean and standard deviation (SD) were used for velocity and gain. Then, a mixed-effects ANOVA model was used to compare the between-subjects effect of group (CTRL/PD-NON/PD-FOG) and within-subjects effect of task (pro-saccade/anti-saccade) in the block condition. Finally, we used bivariate Pearson correlations to explore the relationship between clinical and oculomotor variables separately in the two PD groups. We chose to examine clinical characteristics of PD (disease severity and levodopa equivalent daily dose (LEDD)) and cognitive function (MOCA) given their associations with oculomotor function (Peltsch et al., 2011, Perneczky et al., 2011). For all tests, unless otherwise stated, the level of significance was set at a = 0.05.
3. Results
Demographic and clinical characteristics of participants are shown in Table 1. Groups did not differ by age (p= 0.20) or cognitive function (MOCA score, p=0.68). PD-FOG participants had greater disease duration, took greater doses of dopaminergic medication and had worse non-motor disease severity (MDS-UPDRS I, p = 0.012). Motor aspects of daily living (MDS-UPDRS II, p = 0.085) and motor sign severity (MDS-UPDRS III, p = 0.35) did not significantly differ between the two PD groups. In the blocked condition, there was a floor effect such that median error rate for pro-saccade was zero in all groups. Thus, we analyzed error rate for just the anti-saccade task. PD-FOG groups committed more errors compared to CTRL (Table 1), however rates were similar between PD-FOG and PD-NON (post-hoc, p=0.83) and PD-FOG and CTRL (post-hoc, p = 0.08). There were no significant differences in GNG RT, false alarm rate, miss rate, or TMT completion time.
Table 1.
Subject Demographics and Cognitive Task Data
| Demographics
|
CTRL (n= 12)
|
PD-NON (n=13)
|
PD-FOG (n= 13)
|
|---|---|---|---|
| Age (yr) | 72.3 ± 5.28 | 68.1 ± 7.04 | 68.7 ± 5.84 |
| Sex (# male) | 4 | 5 | 7 |
| MOCA | 26.4 ± 2.43 | 26.3 ± 2.78 | 25.6 ± 2.18 |
| Years since diagnosis | 4.73 ± 3.93 | 8.73 ± 5.93 | |
| LEDD (mg) | 691 ± 734 | 1043 ± 684 | |
| MDS-UPDRS-I | 7.23 ± 3.00 | 13.2 ± 7.06* | |
| MDS-UPDRS-II | 8.08 ± 5.35 | 11.9 ± 5.09 | |
| MDS-UPDRS-III | 36.3 ± 13.3 | 40.4 ± 7.72 | |
| NFOGQ | 10.9 ± 5.54 | ||
| a Anti-saccade error rate (%) | 15.8 ± 18.6 | 34.6 ± 37.8^ | 25.4 ± 42.6 |
|
Cognitive Tasks
|
|||
| a GNG RT (ms) | 400.0 ± 44.0 | 414.0 ± 43.5 | 392.0 ± 70.5 |
| GNG False Alarm (%) | 4.33 ± 3.86 | 3.49 ± 2.08 | 3.90 ± 2.69 |
| GNG Misses (%) | 0.72 ± 1.15 | 0.46 ± 0.69 | 1.23 ± 0.64 |
| TMT B-A Completion Time (s) | 46.4 ± 20.7 | 45.9 ± 19.7 | 68.6 ± 47.1 |
Values represent Mean ± SD;
Median ± IQR. MOCA: Montreal Cognitive Assessment; LEDD: Levodopa equivalent daily dose; MDS-UPRDS: Movement Disorder Society Unified Parkinson Disease Rating Scale I (Non-motor) II (Motor Aspects of Daily Living III (Motor Assessment), NFOGQ: New Freezing of Gait Questionnaire; GNG: Go-NoGo; RT: Reaction Time; TMT: Trail-making task.
Significantly greater than PD-NON (p<0.05);
Significantly greater than CTRL (p<0.05)
3.1 Saccade latency
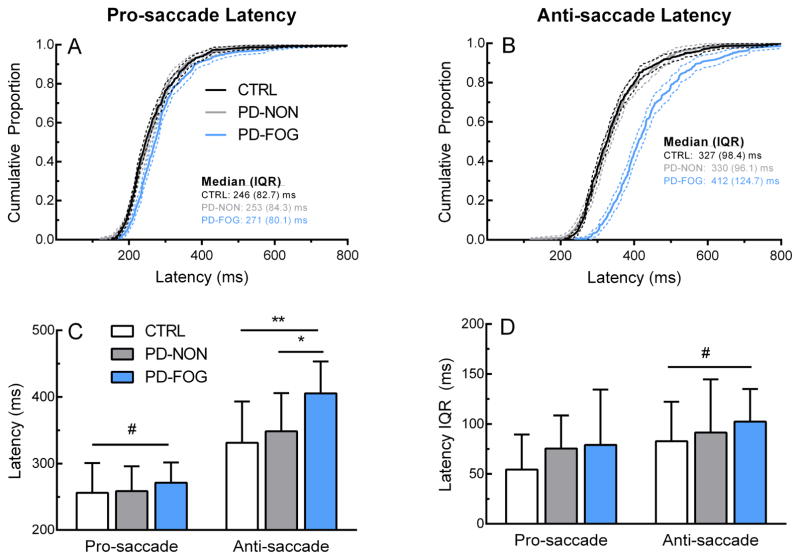
Group distributions and average data for block saccade latency are shown in Figure 2. PD-FOG distributions differed from both PD-NON and CTRL for pro-saccades (2-sample K-S test; PD-FOG vs. PD-NON: p <0.01, PD-FOG vs. CTRL: p <0.01) and anti-saccades (PD-FOG vs. PD-NON: p <0.001, PD-FOG vs. CTRL: p <0.001), while the PD-NON distribution was not different than CTRL (pro-saccade: p = 0.60, anti-saccade: p = 0.81). Group average of individual median latencies showed significant main effects as expected for task (Figure 2C; anti-saccade > pro-saccade, F(35,1) = 145, p <0.001) as well as group (F(35,2) = 4.30, p= 0.02). Post-hoc t-tests revealed anti-saccade latency was greater in PD-FOG compared to CTRL (t = −3.36, p <0.01) and PD-NON (t = −2.75, p = 0.01). No differences were noted between PD-NON and CTRL. Across all trials, anti-saccade latency variability as measured by IQR was largest in PD-FOG (Figure 2B). Group mean of individual variability revealed only a significant task effect, confirming that latency variability increased during anti-saccades (Figure 2D; F(33,1) = 8.27, p = 0.01).
Figure 2.
Saccade latency. Top row: Latency distributions for pro-saccade (A) and anti-saccade (B). Bottom row: Group mean (C) and variability (D) of pro-saccade and anti-saccade latency across groups. Dotted lines in (A) and (B) represent 95% confidence bands. Error bars in (C) and (D) represent ± 1 SD. *p<0.05, **p<0.01 (between-subjects effect), #p<0.05 (within-subjects effect)
3.3 Saccade velocity
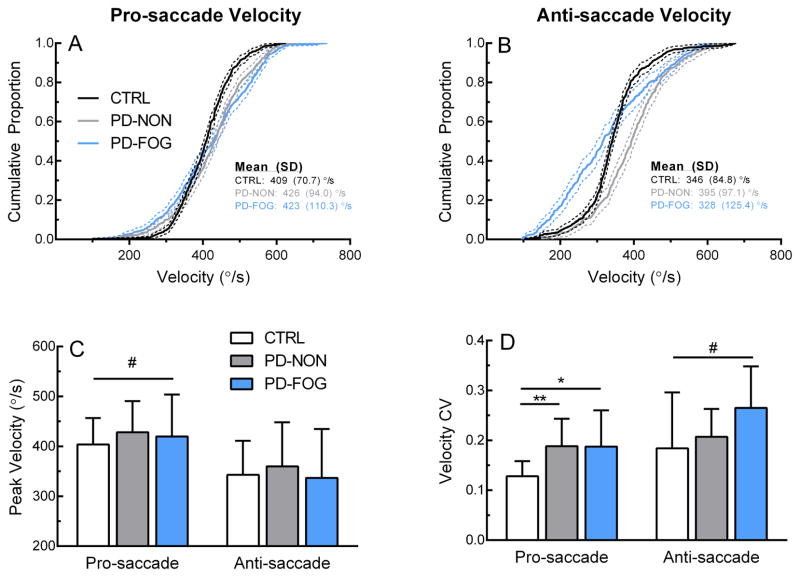
The group distribution of saccade velocity indicated that PD-FOG group made more frequent low-velocity saccades, particularly during the anti-saccade task (Figure 3A and B). When comparing both pro- and anti-saccade velocity distributions, significant differences were noted for each pairwise comparison of the three groups (2-sample K-S test, ps<0.001). However, average group data of peak velocity showed only a significant main task effect (Figure 3C; pro-saccade>anti-saccade, F(35,1) = 45.8, p<0.001). Analysis of individual velocity variability (SD) showed both a main task (Figure 3D; F = 12.8, p = 0.001) and group (F(33,2) = 4.48, p = 0.02) effect. Post-hoc testing showed that both PD groups had greater velocity variability for pro-saccades (PD-NON vs. CTRL: t = −3.38, p < 0.01; PD-FOG vs. CTRL: t = −2.623, p= 0.02). PD-FOG was also more variable compared to CTRL (t = −2.02, p = 0.06) and PD-NON (t = −2.01, p = 0.06), but these comparisons failed to reach significance.
Figure 3.
Saccade velocity. Top row: Velocity distributions for pro-saccades (A) and anti-saccades (B). Bottom row: Group mean (C) and variability (D) of pro-saccade and anti-saccade velocity across groups. Dotted lines in (A) and (B) represent 95% confidence bands. Error bars in (C) and (D) represent ± 1 SD. CV: Coefficient of variation. *p<0.05, **p<0.01 (between-subjects effect), #p<0.05 (within-subjects effect).
3.4 Saccade gain
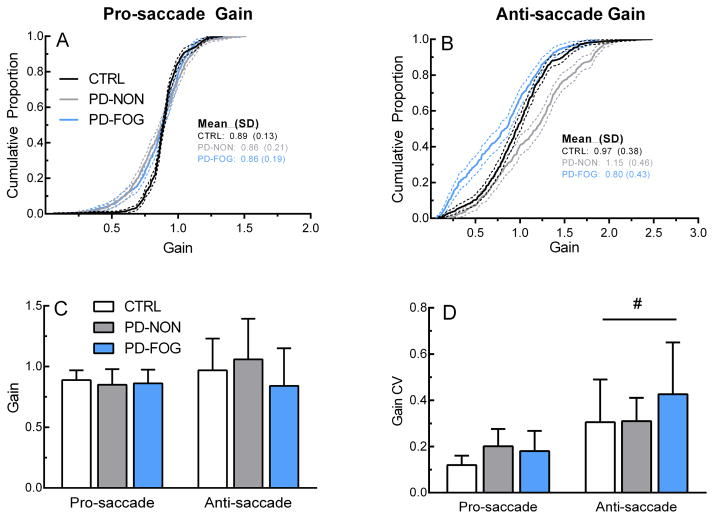
As expected based on saccade main sequence relationships, saccade gain showed similar patterns to the velocity data. Figures 4A and 4B show the group distribution of saccade gain across groups. For pro-saccades, the CTRL distribution was significantly different than the PD-NON (p<0.001) and PD-FOG (p<0.001), however there was no difference between the PD groups (p = 0.35). There were significant differences in gain distribution between all three groups for anti-saccades (ps<0.001). Mean individual gain depicted in Figure 4C showed non-significant task (F(33,2) = 3.85, p = .06) and group (F(33,1) = 1.21, p = 0.31) effects. There was a significant task effect for gain variability, as measured by the coefficient of variation (F(33,2) = 36.6, p < 0.001), indicating variability was greater during anti-saccades (Figure 4D). No group effect for gain variability was noted (F(33,1) = 2.28, p = 0.12).
Figure 4.
Saccade gain. Top row: Gain distributions for pro-saccades (A) and anti-saccades (B). Bottom row: Group mean (C) and variability (D) of pro-saccade and anti-saccade gain across groups. Dotted lines in (A) and (B) represent 95% confidence bands. Error bars in (C) and (D) represent ± 1 SD. CV: Coefficient of variation. #p<0.05 (within-subjects effect).
3.5 Relationship of saccade parameters to clinical features
Disease severity (MDS-UPDRS III) was significantly related to pro-saccade velocity (r = −0.55, p = 0.05) and gain (r = −0.62, p = 0.02), anti-saccade latency (r = 0.69, p = 0.01) and error rate in PD-NON (r = 0.58, p = 0.04). In addition, MOCA score was significantly related to anti-saccade error rate only in PD-NON (r = −0.59, p = 0.04). The only significant correlation in PD-FOG was between LEDD and pro-saccade velocity (r = −0.55, p = 0.05).
4. Discussion
In this study, we investigated the automaticity of the oculomotor system in people with PD with and without FOG. Overall, PD-FOG were slower to initiate both automatic pro-saccades and non-automatic anti-saccades. Saccade velocity and gain were also impacted, as PD-FOG made more frequent slow, low amplitude saccades during both conditions compared to PD-NON. This is the first study to our knowledge to demonstrate differences in timing and execution of saccadic eye movements between PD-NON and PD-FOG.
Saccade performance and response automaticity
Several previous studies consistently show that PD participants commit more errors and are slower, both in terms of velocity and latency, during anti-saccades compared to pro-saccades (Briand et al., 1999, Chan et al., 2005, Cameron et al., 2010). This observation fits with general deficits in reflexive response inhibition and slowing of internally-generated motor responses in PD (Obeso et al., 2011). However, the association between freezing and cognitive-motor function of saccades has been less examined. We noted that PD-FOG were slower to respond during both saccade tasks when compared to PD-NON and CTRL. It is noteworthy that we detected differences during pro-saccades, for which evidence regarding the effect of PD is mixed (White et al., 1983, Briand et al., 1999, Michell et al., 2006, Cameron et al., 2010). In prior studies, subgroups of freezers and non-freezers were not considered, which may have masked any differences and perhaps contributed to the varied results. Visually-elicited pro-saccades are thought to be automatic movements because they require little control from the frontal cortex and basal ganglia, (Munoz et al., 2004). The increased latency in pro-saccades for the PD-FOG group may be related to increased cortical input needed to execute the movement. Despite the differences between gait and saccades, these data support the idea that there is a common deficit in automaticity unique to FOG, where performance of automatic movement requires additional control via the cerebral cortex (Vandenbossche et al., 2012).
Increased variability is also associated with less automatized movement and is characteristic of movement in PD (Nanhoe-Mahabier et al., 2011) and PD-FOG (Barbe et al., 2014). There were clear differences in velocity and gain variability for PD-FOG, as seen in the elevated SD and near-linear shape of the distribution functions in Figures 3A and B and 4A and B. At the group level, both PD groups showed greater pro-saccade velocity variability relative to CTRL, while anti-saccade variability was also larger in PD-FOG. Together, these results show that the range of saccade velocities is wider both within and across PD-FOG participants. Surprisingly, average velocity and gain were not different across groups, primarily because PD groups made hypermetric and high velocity saccades that shifted the mean closer to that of the CTRL group. In general, variability may be associated with the target amplitude of each saccade (15°), which was relatively large compared to other studies (Briand et al., 1999, Cameron et al., 2010). As such, participants may have produced large high-velocity saccades to compensate, thereby increasing variability. Another factor that may influence variability is fatigue, which is likely to be worse in the PD groups. We did not formally assess fatigue but required participants to complete a manageable number of consecutive trials and provided rest breaks in between sets to minimize fatigue. While these factors may contribute to some of the observed differences, velocity and gain variability was overall pronounced in PD-FOG, supporting further that automaticity of saccades may be impaired in this group.
Interestingly, PD-FOG anti-saccade error rates were similar if not slightly lower than PD-NON (Table 1). This is in contrast to a recent study showing that error rates were elevated in PD-FOG during a similar anti-saccade task and were associated with grey matter loss in many cortical regions (Walton et al., 2015). At minimum, there are two processes that need to occur for a successful anti-saccade: 1) inhibition of a pro-saccade and 2) execution of a non-visually guided saccade (DeSouza et al., 2003, Munoz et al., 2004). The Walton et al. study suggests that saccade inhibition is impaired in PD-FOG, related to a problem with cognitive control. It is unclear at the moment why we also did not see increased error rates in the PD-FOG group. The results from our GNG task also show that there were no significant differences in response inhibition between groups, which agrees with previous work (Cohen et al., 2014). Therefore, there is a distinction between the inhibitory control required for the cognitive and saccade tasks. One major difference between these tasks is that the GNG does not require an alternate motor response following inhibition of the automatic response. While there is likely an inhibitory control problem associated with PD, our data show that the difficulty unique to PD-FOG involves executing the anti-saccade, as seen in the large differences in anti-saccade latency compared to PD-NON and CTRL (Figure 2B and 2C). The saccade velocity and gain data further support this idea given that the proportion of low velocity, low gain saccades was greater in PD-FOG compared to PD-NON and CTRL. The deficit of anti-saccade execution in PD-FOG may be due to an inability to release inhibition on the oculomotor circuit. Other studies examining the role of deep brain stimulation (DBS) on oculomotor function indicated that subthalamic nucleus DBS (STN-DBS) improves anti-saccade latency but does not improve error rates (Yugeta et al., 2010, Nilsson et al., 2013, Antoniades et al., 2015). DBS stimulation may normalize the inhibitory drive of the substantia nigra pars reticulata on the oculomotor circuit, thereby allowing for more efficient saccade performance. Overall, additional research using saccade tasks that isolate response inhibition and execution in conjunction with neurophysiological techniques is needed to fully explore the impact of both PD and FOG on oculomotor and cognitive function.
Two potential confounds when examining the effects of FOG are disease severity and medication usage. Typically, PD-FOG occurs later in disease progression and thus is associated with greater disease severity (Nutt et al., 2011). Therefore, any FOG-specific differences may simply be due to worsened motor signs. Our PD groups were well matched for disease severity as measured by the MDS-UPDRS-III (Table 1). The correlation analysis also showed that latency, velocity, gain and error rates were significantly related to disease severity, but only in PD-NON. This suggests that saccade performance may be less dependent on overall motor function in PD-FOG, further supporting the link between FOG phenotype and impaired oculomotor function. Participants were also tested off dopaminergic medication, thus controlling for effects of medication use on saccade output. Still, it is unclear how saccade performance would change if participants were then tested in a medicated state. Previous work showed dopaminergic medication led to increases in latency variability ((Michell et al., 2006). It is possible, then, that saccade variability would be increased in PD-FOG when on medication, as PD-FOG were on higher doses of medication compared to PD-NON. In some cases, medication will alleviate gait freezing duration and frequency, while in other cases FOG episodes are worsened with medication (Giladi, 2008). The relationships between non-gait freezing and medication use remain to be explored.
FOG represents a global motor dysfunction
Our results contribute to the growing body of evidence that freezing affects gait and non-gait movements alike (Vercruysse et al., 2012a, Vercruysse et al., 2012b, Williams et al., 2013, Barbe et al., 2014). Together, these studies suggest that the pathophysiology underlying FOG may be a common contributor to motor dysfunction. While festination or freezing of the upper limb and speech have been documented, pure oculomotor freezing has yet to be reliably reported. One study noted that during a rhythmic saccade task, some subjects “froze” between consecutive saccades (Bronstein et al., 1985). In our data, some saccade traces showed features similar to gait freezing, such as increased frequency and small amplitude. However, it is difficult to directly compare saccades and gait freezing, given the differences in movement amplitude, rhythmicity, and velocity. While the current evidence shows a general deficit of cognitive-motor processing may underpin freezing, the actual manifestation of freezing may differ across various effectors. For instance, one recent experiment showed that upper-limb and lower-limb freezing co-occur in PD, but are not correlated (Barbe et al., 2014). Future studies that manipulate the timing of stimuli (e.g. rhythmic saccades) or the cognitive demand (e.g. difficult dual-tasks), may be helpful in directly comparing motor behavior across body parts or movement types. This may lead us toward approaching freezing as a global motor phenotype that reflects impairment not just of gait but of the entire motor system.
5. Conclusion
Latency, velocity, and gain of automatic and non-automatic saccades were different across groups of people with PD with and without FOG. Additional deficits in saccade automaticity were evidenced by increased velocity and gain variability across and within participants. Overall, our results support the idea that FOG is a distinct phenotype in PD with an underlying pathophysiology related to impaired cognitive-motor control. Furthermore, this deficit impacts multiple effectors and it not limited to gait alone. Additional work is needed to fully elucidate how freezing impacts automaticity across motor systems.
Highlights.
PD patients with freezing of gait (PD-FOG) had longer pro- and anti-saccade latencies than non-freezers (PD-NON).
PD-FOG showed greater variability of saccade velocity and gain than PD-NON.
Findings were unrelated to saccade error rate, disease severity, or cognition, and suggest freezing is related to a global disturbance in motor automaticity.
Acknowledgments
The authors thank Dr. Ryan Duncan for assistance with participant evaluations. S.N. is supported by the Washington University Institute of Clinical and Translational Sciences grant UL1TR000448, sub-award TL1TR000449 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH). Additional support was provided by the Washington University Program in Physical Therapy, the Greater St. Louis American Parkinson Disease Association (APDA) and the APDA Center for Advanced PD Research at Washington University.
Footnotes
Conflicts of interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antoniades C, Ettinger U, Gaymard B, Gilchrist I, Kristjansson A, Kennard C, et al. An internationally standardised antisaccade protocol. Vision Res. 2013;84:1–5. doi: 10.1016/j.visres.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Antoniades CA, Rebelo P, Kennard C, Aziz TZ, Green AL, FitzGerald JJ. Pallidal Deep Brain Stimulation Improves Higher Control of the Oculomotor System in Parkinson’s Disease. J Neurosci. 2015;35:13043–52. doi: 10.1523/JNEUROSCI.2317-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahill AT, Clark MR, Stark L. The main sequence, a tool for studying human eye movements. Mathematical Biosciences. 1975;24:191–204. [Google Scholar]
- Barbe MT, Amarell M, Snijders AH, Florin E, Quatuor EL, Schonau E, et al. Gait and upper limb variability in Parkinson’s disease patients with and without freezing of gait. J Neurol. 2014;261:330–42. doi: 10.1007/s00415-013-7199-1. [DOI] [PubMed] [Google Scholar]
- Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and freezing of gait in Parkinson’s disease: a review of two interconnected, episodic phenomena. Mov Disord. 2004;19:871–84. doi: 10.1002/mds.20115. [DOI] [PubMed] [Google Scholar]
- Briand KA, Strallow D, Hening W, Poizner H, Sereno AB. Control of voluntary and reflexive saccades in Parkinson’s disease. Exp Brain Res. 1999;129:38–48. doi: 10.1007/s002210050934. [DOI] [PubMed] [Google Scholar]
- Bronstein AM, Kennard C. Predictive ocular motor control in Parkinson’s disease. Brain. 1985;108:925–40. doi: 10.1093/brain/108.4.925. [DOI] [PubMed] [Google Scholar]
- Calne DB, Snow BJ, Lee C. Criteria for diagnosing Parkinson’s disease. Ann Neurol. 1992;32(Suppl):S125–7. doi: 10.1002/ana.410320721. [DOI] [PubMed] [Google Scholar]
- Cameron IG, Watanabe M, Pari G, Munoz DP. Executive impairment in Parkinson’s disease: response automaticity and task switching. Neuropsychologia. 2010;48:1948–57. doi: 10.1016/j.neuropsychologia.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Chan F, Armstrong IT, Pari G, Riopelle RJ, Munoz DP. Deficits in saccadic eye-movement control in Parkinson’s disease. Neuropsychologia. 2005;43:784–96. doi: 10.1016/j.neuropsychologia.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Chan JL, DeSouza JF. The effects of attentional load on saccadic task switching. Exp Brain Res. 2013;227:301–9. doi: 10.1007/s00221-013-3452-1. [DOI] [PubMed] [Google Scholar]
- Cohen RG, Klein KA, Nomura M, Fleming M, Mancini M, Giladi N, et al. Inhibition, executive function, and freezing of gait. J Parkinsons Dis. 2014;4:111–22. doi: 10.3233/JPD-130221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford TJ, Henderson L, Kennard C. Abnormalities of nonvisually-guided eye movements in Parkinson’s disease. Brain. 1989;112:1573–86. doi: 10.1093/brain/112.6.1573. [DOI] [PubMed] [Google Scholar]
- Dalrymple-Alford JC, MacAskill MR, Nakas CT, Livingston L, Graham C, Crucian GP, et al. The MoCA: Well-suited screen for cognitive impairment in Parkinson disease. Neurology. 2010;75:1717–25. doi: 10.1212/WNL.0b013e3181fc29c9. [DOI] [PubMed] [Google Scholar]
- DeSimone JC, Weiler J, Aber GS, Heath M. The unidirectional prosaccade switch-cost: Correct and error antisaccades differentially influence the planning times for subsequent prosaccades. Vision Research. 2014;96:17–24. doi: 10.1016/j.visres.2013.12.005. [DOI] [PubMed] [Google Scholar]
- DeSouza JF, Menon RS, Everling S. Preparatory set associated with pro-saccades and anti-saccades in humans investigated with event-related FMRI. J Neurophysiol. 2003;89:1016–23. doi: 10.1152/jn.00562.2002. [DOI] [PubMed] [Google Scholar]
- Fling BW, Cohen RG, Mancini M, Carpenter SD, Fair DA, Nutt JG, et al. Functional reorganization of the locomotor network in Parkinson patients with freezing of gait. PLoS One. 2014;9:e100291. doi: 10.1371/journal.pone.0100291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsaa EB, Larsen JP, Wentzel-Larsen T, Alves G. A 12-year population-based study of freezing of gait in Parkinson’s disease. Parkinsonism Relat Disord. 2015;21:254–8. doi: 10.1016/j.parkreldis.2014.12.020. [DOI] [PubMed] [Google Scholar]
- Giladi N. Medical treatment of freezing of gait. Mov Disord. 2008;23(Suppl 2):S482–8. doi: 10.1002/mds.21914. [DOI] [PubMed] [Google Scholar]
- Guitton D, Buchtel HA, Douglas RM. Frontal lobe lesions in man cause difficulties in suppressing reflexive glances and in generating goal-directed saccades. Exp Brain Res. 1985;58:455–72. doi: 10.1007/BF00235863. [DOI] [PubMed] [Google Scholar]
- Hallett PE. Primary and secondary saccades to goals defined by instructions. Vision Res. 1978;18:1279–96. doi: 10.1016/0042-6989(78)90218-3. [DOI] [PubMed] [Google Scholar]
- Klein C, Rauh R, Biscaldi M. Cognitive correlates of anti-saccade task performance. Exp Brain Res. 2010;203:759–64. doi: 10.1007/s00221-010-2276-5. [DOI] [PubMed] [Google Scholar]
- Kristjansson A, Chen Y, Nakayama K. Less attention is more in the preparation of antisaccades, but not prosaccades. Nat Neurosci. 2001;4:1037–42. doi: 10.1038/nn723. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Barker RA. A pathophysiological model of freezing of gait in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15:333–8. doi: 10.1016/j.parkreldis.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Michell AW, Xu Z, Fritz D, Lewis SJ, Foltynie T, Williams-Gray CH, et al. Saccadic latency distributions in Parkinson’s disease and the effects of L-dopa. Exp Brain Res. 2006;174:7–18. doi: 10.1007/s00221-006-0412-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirsky JB, Heuer HW, Jafari A, Kramer JH, Schenk AK, Viskontas IV, et al. Anti-saccade performance predicts executive function and brain structure in normal elders. Cogn Behav Neurol. 2011;24:50–8. doi: 10.1097/WNN.0b013e318223f6c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau C, Ozsancak C, Blatt JL, Derambure P, Destee A, Defebvre L. Oral festination in Parkinson’s disease: biomechanical analysis and correlation with festination and freezing of gait. Mov Disord. 2007;22:1503–6. doi: 10.1002/mds.21549. [DOI] [PubMed] [Google Scholar]
- Moschovakis AK, Scudder CA, Highstein SM. The microscopic anatomy and physiology of the mammalian saccadic system. Prog Neurobiol. 1996;50:133–254. doi: 10.1016/s0301-0082(96)00034-2. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci. 2004;5:218–28. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- Nanhoe-Mahabier W, Snijders AH, Delval A, Weerdesteyn V, Duysens J, Overeem S, et al. Walking patterns in Parkinson’s disease with and without freezing of gait. Neuroscience. 2011;182:217–24. doi: 10.1016/j.neuroscience.2011.02.061. [DOI] [PubMed] [Google Scholar]
- Nieuwboer A, Giladi N. Characterizing freezing of gait in Parkinson’s disease: models of an episodic phenomenon. Mov Disord. 2013;28:1509–19. doi: 10.1002/mds.25683. [DOI] [PubMed] [Google Scholar]
- Nieuwboer A, Rochester L, Herman T, Vandenberghe W, Emil GE, Thomaes T, et al. Reliability of the new freezing of gait questionnaire: agreement between patients with Parkinson’s disease and their carers. Gait Posture. 2009;30:459–63. doi: 10.1016/j.gaitpost.2009.07.108. [DOI] [PubMed] [Google Scholar]
- Nilsson MH, Patel M, Rehncrona S, Magnusson M, Fransson PA. Subthalamic deep brain stimulation improves smooth pursuit and saccade performance in patients with Parkinson’s disease. J Neuroeng Rehabil. 2013;10:33. doi: 10.1186/1743-0003-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A. Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol. 2011;10:734–44. doi: 10.1016/S1474-4422(11)70143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso I, Wilkinson L, Casabona E, Bringas ML, Alvarez M, Alvarez L, et al. Deficits in inhibitory control and conflict resolution on cognitive and motor tasks in Parkinson’s disease. Exp Brain Res. 2011;212:371–84. doi: 10.1007/s00221-011-2736-6. [DOI] [PubMed] [Google Scholar]
- Peltsch A, Hemraj A, Garcia A, Munoz DP. Age-related trends in saccade characteristics among the elderly. Neurobiol Aging. 2011;32:669–79. doi: 10.1016/j.neurobiolaging.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Perneczky R, Ghosh BC, Hughes L, Carpenter RH, Barker RA, Rowe JB. Saccadic latency in Parkinson’s disease correlates with executive function and brain atrophy, but not motor severity. Neurobiol Dis. 2011;43:79–85. doi: 10.1016/j.nbd.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine JM, Matar E, Ward PB, Frank MJ, Moustafa AA, Pearson M, et al. Freezing of gait in Parkinson’s disease is associated with functional decoupling between the cognitive control network and the basal ganglia. Brain. 2013;136:3671–81. doi: 10.1093/brain/awt272. [DOI] [PubMed] [Google Scholar]
- Spildooren J, Vercruysse S, Desloovere K, Vandenberghe W, Kerckhofs E, Nieuwboer A. Freezing of gait in Parkinson’s disease: the impact of dual-tasking and turning. Mov Disord. 2010;25:2563–70. doi: 10.1002/mds.23327. [DOI] [PubMed] [Google Scholar]
- Terao Y, Fukuda H, Ugawa Y, Hikosaka O. New perspectives on the pathophysiology of Parkinson’s disease as assessed by saccade performance: a clinical review. Clin Neurophysiol. 2013;124:1491–506. doi: 10.1016/j.clinph.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbossche J, Deroost N, Soetens E, Coomans D, Spildooren J, Vercruysse S, et al. Freezing of gait in Parkinson’s disease: disturbances in automaticity and control. Front Hum Neurosci. 2012;6:356. doi: 10.3389/fnhum.2012.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercruysse S, Gilat M, Shine JM, Heremans E, Lewis S, Nieuwboer A. Freezing beyond gait in Parkinson’s disease: a review of current neurobehavioral evidence. Neurosci Biobehav Rev. 2014a;43:213–27. doi: 10.1016/j.neubiorev.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Vercruysse S, Spildooren J, Heremans E, Vandenbossche J, Levin O, Wenderoth N, et al. Freezing in Parkinson’s disease: a spatiotemporal motor disorder beyond gait. Mov Disord. 2012a;27:254–63. doi: 10.1002/mds.24015. [DOI] [PubMed] [Google Scholar]
- Vercruysse S, Spildooren J, Heremans E, Vandenbossche J, Wenderoth N, Swinnen SP, et al. Abnormalities and cue dependence of rhythmical upper-limb movements in Parkinson patients with freezing of gait. Neurorehabil Neural Repair. 2012b;26:636–45. doi: 10.1177/1545968311431964. [DOI] [PubMed] [Google Scholar]
- Vercruysse S, Spildooren J, Heremans E, Wenderoth N, Swinnen SP, Vandenberghe W, et al. The neural correlates of upper limb motor blocks in Parkinson’s disease and their relation to freezing of gait. Cereb Cortex. 2014b;24:3154–66. doi: 10.1093/cercor/bht170. [DOI] [PubMed] [Google Scholar]
- Walton CC, O’Callaghan C, Hall JM, Gilat M, Mowszowski L, Naismith SL, et al. Antisaccade errors reveal cognitive control deficits in Parkinson’s disease with freezing of gait. J Neurol. 2015;262:2745–54. doi: 10.1007/s00415-015-7910-5. [DOI] [PubMed] [Google Scholar]
- White OB, Saint-Cyr JA, Tomlinson RD, Sharpe JA. Ocular motor deficits in Parkinson’s disease. II. Control of the saccadic and smooth pursuit systems. Brain. 1983;106:571–87. doi: 10.1093/brain/106.3.571. [DOI] [PubMed] [Google Scholar]
- Williams AJ, Peterson DS, Ionno M, Pickett KA, Earhart GM. Upper extremity freezing and dyscoordination in Parkinson’s disease: effects of amplitude and cadence manipulations. Parkinsons Dis. 2013;2013:595378. doi: 10.1155/2013/595378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yugeta A, Terao Y, Fukuda H, Hikosaka O, Yokochi F, Okiyama R, et al. Effects of STN stimulation on the initiation and inhibition of saccade in Parkinson disease. Neurology. 2010;74:743–8. doi: 10.1212/WNL.0b013e3181d31e0b. [DOI] [PubMed] [Google Scholar]