Abstract
Over the past several years, a number of cytokines and growth factors including transforming growth factor β1, tumor necrosis factor α, and angiotensin II have been shown to play a crucial role in renal fibrosis. The Janus kinase family (JAK) and signal transducers and activators of transcription (STATs) constitute one of the primary signaling pathways that regulate cytokine expression, and the JAK/STAT signaling pathway has increasingly been implicated in the pathophysiology of renal disease. This review examines the role of the JAK/STAT signaling pathway in fibrotic renal disease. The JAK/STAT signaling pathway is activated in a variety of renal diseases and has been implicated in the pathophysiology of renal fibrosis. Experimental evidence suggests that inhibition of the JAK/STAT signaling pathway, in particular JAK2 and STAT3, may suppress renal fibrosis and protect renal function. However, it is incompletely understood which cells activate the JAK/STAT signaling pathway and which JAK/STAT signaling pathway is activated in each renal disease. Research regarding JAK/STAT signaling and its contribution to renal disease is still ongoing in humans. Future studies are required to elucidate the potential role of JAK/STAT signaling inhibition as a therapeutic strategy in the attenuation of renal fibrosis.
Keywords: Kidney, Fibrosis, STAT, Janus kinases, Cytokines
1. Introduction
Regardless of the primary disease of the kidney, renal fibrosis is the final common pathway leading to end-stage renal disease (ESRD) [1]. The histologic changes associated with renal fibrosis include tubulointerstitial fibrosis, inflammatory infiltration, loss of renal parenchyma characterized by tubular atrophy, peritubular capillary loss, and podocyte depletion [1,2]. The pathophysiological changes associated with renal fibrosis include macrophage infiltration and activation with the subsequent elaboration of a number of cytokines and growth factors. These mediators, in turn, stimulate downstream cellular changes including mesangial cell activation, fibroblast proliferation and activation with the elaboration of extracellular matrix (ECM), and progressive apoptotic cell death [3,4].
Over the past several years, a number of cytokines and growth factors have been implicated in the pathophysiology of renal fibrosis [5]. Most notable among these is transforming growth factor β1 (TGF-β1). Upregulation of TGF-β1 expression has been demonstrated in nearly every type of fibrotic renal disease in both animals and humans [4,6]. TGF-β1 is a major regulator of fibrosis via stimulation of epithelial–mesenchymal transition ((EMT), a process by which tubular epithelial cells (TECs) are transformed into matrix-producing fibroblasts), fibroblast proliferation, ECM synthesis (i.e., collagen types I, III, and IV, proteoglycans, laminin, and fibronectin), and the simultaneous inhibition of collagenase and degradative matrix metalloproteinases [7]. In addition, tumor necrosis factor α (TNF-α), angiotensin II (ANG II), and, more recently, interleukin 18 (IL-18) have been implicated in the pathophysiology of renal fibrosis [8,9].
The Janus kinase family (JAK)/signal transducers and activators of transcription (STAT) signaling pathway constitutes one of the primary regulatory pathways for cytokine expression [10], and JAK/STAT signaling has increasingly been implicated in the pathophysiology of renal disease. This review will examine the available evidence regarding the JAK/STAT signaling pathway and its role in fibrotic renal disease.
2. JAK/STAT signaling pathway
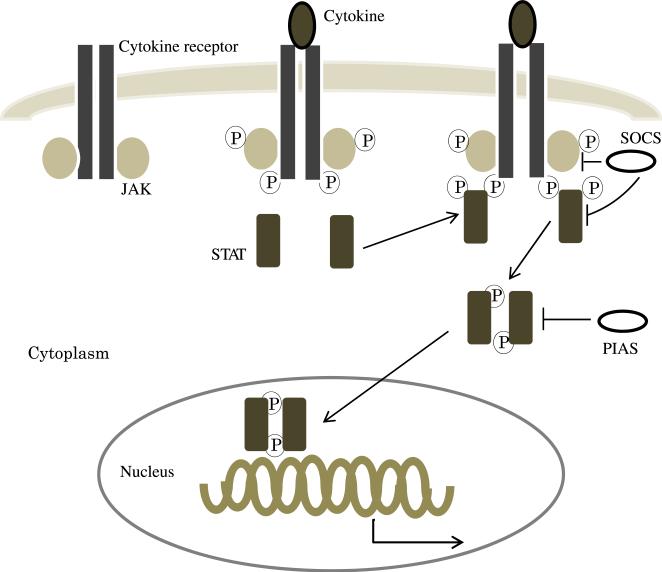
The JAK/STAT signaling pathway is an important cascade for signal transduction for a wide variety of growth factors and cytokines [11] (Fig. 1). This pathway regulates gene expression, as well as cellular activation, proliferation, and differentiation [12,13]. Most of the STAT-activating cytokine receptors (i.e., type I and type II cytokine receptors) do not have tyrosine kinase activity and instead require JAKs to initiate intercellular signaling. The JAK family of proteins are tyrosine kinases and constitute four members (JAK1, JAK2, JAK3, and TYK2) in mammals [11,14]. JAKs are constitutively associated with a proline-rich, membrane proximal domain of the cytokine receptors [15]. After ligand binding to the cytokine receptor, the receptor sets up conformational change that brings JAKs into proximal binding sites on the receptor. JAKs then phosphorylate tyrosine residues on the cytoplasmic domain of the cytokine receptor. STATs possessing src homology 2 domains capable of binding these phosphotyrosine residues are then recruited to the receptors. After STAT phosphorylation and activation, STATs form homo- or heterodimers that can translocate into the nucleus and activate the transcription of target genes [16].
Fig. 1.
The JAK/STAT signaling pathway. After cytokine binding to the cytokine receptor, JAK becomes activated and phosphorylates tyrosine residues of the cytokine receptor to create docking sites for STAT. After STAT phosphorylation and activation, STATs form dimers and translocate into the nucleus where they activate the transcription of target genes. STATs are negatively regulated by several mechanisms such as SOCS and PIAS. SOCS = suppressors of cytokine signaling; PIAS = protein inhibitors of activated STAT. (Color version of figure is available online.)
STAT proteins are composed of seven members (STAT1–STAT4, STAT5A, STAT5B, and STAT6) in mammals. JAK/STAT signalingcombinations are specific to each ligandand cytokine receptor, although cell type is also a factor in determining STAT specificity (Table) [17–19]. The JAK/STAT signaling pathway is activated by numerous growth factors and cytokines, including the interferon (IFN) family (IFN-α, β, γ, κ, ω, ε, or λ, IL-10, IL-19, IL-20, IL-22, IL-24, and IL-26), gp130 family (IL-6, IL-11, oncostatin M, leukemia inhibitory factor, ciliary neurotrophic factor, novel neurotrophin 1/B cell–stimulating factor 3, cardiotropin 1, granulocyte colony–stimulating factor, leptin, IL-12, and IL-23), γC family (IL-2, IL-3, IL-4, IL-5, IL-7, IL-9, IL-15, IL-21, and thymic stromal–derived lymphopoietin), and the single chain family (erythropoietin, growth hormone, prolactin, and thrombopoietin) [15–19]. For example, IFN-α or IFN-β and their receptor stimulate STAT1, STAT2, STAT3, and STAT5 via JAK1 and TYK2, whereas IFN-γ stimulates STAT1, STAT3, and STAT5 via JAK1 and JAK2. The gp130 family stimulates STAT1, STAT3, and STAT5 via JAK1, JAK2, and TYK2, except IL-12 and IL-23, which stimulate STAT3 and STAT4 via JAK2 and TYK2 [15–21]. STATs can also be activated via JAK-independent pathways, including epidermal growth factor (EGF), platelet-derived growth factor (PDGF), extracellular signal–regulated kinase, protein kinase C, and mitogen-activated protein kinase [22]. Individual growth factors also appear to be linked to specific STATs, for example, colony-stimulating factor 1 and EGF stimulate STAT1, STAT3, and STAT5, whereas PDGF stimulates STAT1 and STAT3. Growth hormone and prolactin stimulate exclusively STAT5 [15,17]. Among the JAK/STAT signaling pathways, the JAK2/STAT3 combination appears to be central and is best correlated with renal disease.
Table.
JAK/STAT signaling in fibrotic renal disease.
| Disease model (animal) | Expression cell | Related JAK | Related STAT | References |
|---|---|---|---|---|
| UUO | ||||
| Rat | TEC and tubulointerstitial cell | STAT3 | [35] | |
| Mouse | Tubulointerstitial cell | STAT3 | [36] | |
| I/R | ||||
| Rat | TEC | JAK2 | STAT1 and STAT3 | [42] |
| Mouse | TEC | JAK2 | STAT3 | [43,44] |
| Mouse | Not shown | STAT4 and STAT6 | [45] | |
| Oxidative stress and cyclosporin A (mouse) | Endothelial cell and TEC | JAK2 | STAT3 | [44] |
| Type II DN (human) | Glomerular cell and tubulointerstitial cell | JAK1, JAK2, and JAK3 | STAT1, STAT3, STAT4, and STAT5B | [51] |
| STZ-induced DN | ||||
| Rat | Mesangial cell | JAK2 | STAT1, STAT3, STAT5A, and STAT5B | [52,55] |
| Mouse | Podocyte | STAT3 | [53] | |
| Lupus nephritis (human)a | Glomerular cell (a, b, and c) and tubulointerstitial cell (b and c) | STAT3 | [57] | |
| IgA nephropathyb | ||||
| Vasculitisc | ||||
| Immune complex GN (rat) | Glomerular cell, TEC, tubulointerstitial cell, and macrophage | STAT3 | [58] | |
| Anti-Thy 1.1 GN (mouse) | Mesangial cell | STAT3 | [59,60] | |
| Lupus nephritis (mouse) | Glomerular cell, TEC, and tubulointerstitial cell | JAK2 | STAT1 | [61] |
UUO = unilateral ureteral obstruction; I/R = ischemia/reperfusion; DN = diabetic nephropathy; IgA = immunoglobulin A; GN = glomerulonephritis; STZ = streptozotocin.
The JAK/STAT signaling pathway is negatively regulated through a variety of mechanisms. The suppressors of cytokine signaling (SOCS) family of proteins inhibit STAT phosphorylation by either binding to the kinase inhibitory region and directly inhibiting JAK tyrosine kinase activity or competing with STATs for phosphotyrosine-binding sites on cytokine receptors [23]. STATs are also negatively regulated by protein inhibitors of activated STAT, which inhibit the transcriptional activity of STATs through several mechanisms, including blocking the DNA-binding activity of STAT, recruitment of other coregulators, such as histone deacetylases, to STAT-binding sites, and promotion of STAT protein sumoylation [24]. Finally, protein tyrosine phosphatases remove phosphates from cytokine receptors and activated STATs, thereby negatively regulating JAK/STAT signaling [25].
3. Obstructive nephropathy
Obstructive nephropathy is a major cause of renal failure and ESRD in both adults and children. The histologic derangements associated with obstructive renal injury include tubular dilatation, apoptotic cell death, and progressive tubulointerstitial fibrosis [26]. A profound interstitial inflammatory infiltrate occurs early after the onset of renal obstruction and results in the elaboration of a number of cytokines and growth factors, including TGF-β1, TNF-α, ANG II, and IL-18 [27]. ANG II, in turn, stimulates STAT1 and STAT3 activation in both proximal TECs and mesangial cells in various models of disease [28,29].
α-Smooth muscle actin–positive myofibroblasts become activated early in the course of obstruction and are considered the principal effector cells responsible for ECM deposition. Accumulating evidence suggests that a large proportion of myofibroblasts are derived from renal TECs during pathologic states via the process of EMT [30,31]. Using fibroblast-specific protein (Fsp1), a marker that is normally expressed by fibroblasts but not in epithelial cells, Strutz et al. [32] showed that TECs could express Fsp1 in a mouse model of antitubular basement membrane disease. This was the first study to demonstrate the capability of epithelial cells to transform into fibroblast-like cells. A subsequent study by Iwano et al. revealed that a substantial number of interstitial fibroblasts originate from tubular epithelium (EMT) during renal fibrosis. Of the Fsp1-positive fibroblast pool in the fibrotic kidney, 36% coexpressed LacZ that had been tagged to renal proximal tubules, providing proof that fibroblasts can form locally by EMT during the pathologic stress of tissue fibrosis [31].
TGF-β1 is a major regulator of tubulointerstitial fibrosis [2,6]. TGF-β1 can independently initiate and complete the entire course of EMT in vitro. In addition, TGF-β1 stimulates ECM synthesis while simultaneously inhibiting collagenase and degradative matrix metalloproteinases in vivo [3]. TNF-α has also been implicated in fibrotic renal injury, stimulating ECM accumulation, inhibiting ECM degradation, and upregulating a number of cytokines and transcription factors involved in tubulointerstitial fibrosis [8,33,34]. Recently, IL-18 has been identified as an important mediator of obstruction-induced renal fibrosis, ECM deposition, and EMT, independent of TGF-β1 or TNF-α activity [9].
Despite the clear role of cytokine signaling in obstructive renal injury, few studies have investigated the potential contribution of JAK/STAT signaling to obstructive nephropathy [35,36]. Kuratsune et al. have examined STAT3 activation in a rat model of unilateral ureteral obstruction. The authors demonstrated STAT3 activation in renal TECs and myofibroblasts in response to 3 or 7 days of obstruction, with peak pSTAT3 expression occurring 7 days after the onset of obstruction [35]. Pang et al. have subsequently demonstrated that S3I-201, a specific STAT3 inhibitor, attenuates ECM deposition and inflammatory cell infiltration in mice subjected to 7 days of unilateral ureteral obstruction. Treatment with S3I-201 reduces the expression of profibrotic markers, such as fibronectin, α-smooth muscle actin, and type I collagen, and a number of proinflammatory mediators, such as TGF-β1, TNF-α, IL-1β, and intercellular adhesion molecule 1 (ICAM-1) [36]. Although preliminary evidence suggests that STAT3 has an important role in the pathophysiology of obstructive nephropathy, the contribution of JAK to obstruction-induced renal injury remains unknown.
4. Ischemia and reperfusion injury
Ischemic acute renal failure is associated with significant morbidity and mortality in humans [37]. A significant inflammatory response is generated in response to renal ischemia and reperfusion (I/R) injury, resulting inendothelial injury, apoptotic cell death, enhanced endothelial cell–leukocyte adhesion, leukocyte entrapment, and a compromise in microvascular blood flow [38,39]. If the initial insult is severe, this series of eventsculminatesinthe development of acute renal failure. The pathophysiological mechanism of renal I/R injury includes the elaboration of proinflammatory cytokines from TECs, such as TNF-α, TGF-β1, IL-6, and IL-1β [40]. Furthermore, infiltrating leukocytes produce TNF-α, IL-1, reactive oxygen species, and eicosanoids,inaddition to chemoattractants such as IL-8, ICAM-1, and monocyte chemotactic protein 1 (MCP-1) [39,41]. These cytokines and chemokines serve as effectors for a positive feedback pathway that stimulates inflammation and further cellular injury in response to ischemia.
Recently, Yang et al. [42] have observed that phosphorylation of JAK2, STAT1, and STAT3 is induced in the rat kidney in response to I/R injury. Treatment with AG490, a selective JAK2 inhibitor, before and immediately after I/R significantly inhibits pJAK2, pSTAT1, and pSTAT3 expression. AG490 pretreatment improves renal function and attenuates necrosis and apoptosis in TECs and reduces macrophage infiltration into interstitial tissues. In contrast, delaying AG490 treatment until 3 h after I/R fails to improve renal function, suggesting that the JAK/STAT signaling develops early in the course of renal I/R injury. AG490 treatment also inhibits the renal expression of MCP-1 and ICAM-1 in response to ischemia and thereby ameliorates the ischemia-induced inflammatory response [42].
Arany et al. [43] have also demonstrated that severe oxidant stress leads to phosphorylation of STAT3 via the activation of the EGF receptor and JAK2 kinase in mice subjected to renal I/R injury. Inhibition of JAK2 or STAT3, on the other hand, leads to the activation of extracellular signal–regulated protein kinase and promotes cell survival during severe oxidative stress [43,44]. In contrast, Yokota et al. have demonstrated that STAT6 has a primarily protective role during renal I/R injury. STAT6 –/– mice exhibit a significant deterioration in renal function compared with wild-type mice in response to renal I/R injury, with cytokine staining of T cells harvested from STAT6 –/– mice demonstrating increased IFN-γ production and minimal IL-4 production compared with wild-type mice [45]. STAT4 –/– mice demonstrate a slight improvement in renal function in response to ischemic renal injury, with T cells demonstrating decreased production of IFN-γ and active IL-4 production. These studies suggest that STAT6 has a protective role in renal function after I/R injury and that IL-4 deficiency may be a major mechanism through which STAT6 –/– mice develop a marked deterioration in renal function in response to ischemic injury [45]. Although the activation of the JAK/STAT signaling pathway has been clearly demonstrated in response to renal I/R injury, the exact role of the JAK/STAT signaling in renal I/R injury remains unknown.
5. Diabetic nephropathy
Diabetes has become the most common cause of ESRD in the United States. The early histologic features of diabetic nephropathy in humans are thickening of the glomerular basement membrane and mesangial expansion associated with the accumulation of ECM by glomerular mesangial cells. As albuminuria progresses, glomerulosclerosis and tubulointerstitial fibrosis develop and lead to ESRD [46]. High glucose levels stimulate a number of proinflammatory and profibrotic factors [47]. TGF-β1 and vascular endothelial growth factor contribute to cellular hypertrophy, collagen synthesis, and vascular changes [48,49]. The JAK/STAT signaling pathway has been implicated in the pathophysiology of diabetic nephropathy and has been most widely studied in this model of renal disease [50].
Berthier et al. [51] have described the JAK/STAT signaling pathway in renal tissue specimens obtained from patients with both early and progressive diabetic nephropathy using a transcriptomic approach. In the tubulointerstitial compartment of the kidney, microarray analysis identified several JAK/STAT family members that were downregulated in patients with early diabetic nephropathy, whereas most JAK/STAT family members, that is, JAK1, JAK2, JAK3, STAT1, STAT3, STAT4, and STAT5B were upregulated in patients with progressive diabetic nephropathy compared with control subjects. Conversely, in the glomerular compartment of the kidney, most JAK/STAT family members were upregulated in patients with early diabetic nephropathy and downregulated in patients with progressive diabetic nephropathy. In contrast to the microarray results, JAK1, JAK2, JAK3, STAT1, and STAT3 messenger RNA (mRNA) expression were increased in the glomerular compartment of the kidney in patients with both early and progressive diabetic nephropathy, whereas mRNA expression was only increased in the tubulointerstitial compartment of the kidney in patients with progressive diabetic nephropathy. Finally, the estimated glomerular filtration rate among patients with both early and progressive diabetic nephropathy has been found to be inversely correlated with JAK1, JAK2, JAK3, and STAT1 mRNA expression in the tubulointerstitial compartment of the kidney, whereas no correlation between glomerular filtration rate and JAK/STAT activation was identified in the glomerular compartment of the kidney [51]. These findings suggest that increased JAK/STAT signaling may have an important role in diabetic nephropathy and is inversely correlated with renal function.
In a rat model of streptozotocin-induced diabetes, Banes et al. [52] demonstrated that high glucose levels induce activation of JAK2, STAT1, STAT3, and STAT5 via an ANG II–dependent mechanism, and furthermore, that AG490 reduces urinary protein excretion in these animals. Similarly, Lu et al. [53] have examined the role of STAT3 in streptozotocin-induced diabetes in mice with reduced STAT3 activity. Mice with 25% STAT3 activity exhibited significantly less proteinuria, mesangial expansion, glomerular cell proliferation, and macrophage infiltration compared with those with 75% STAT3 activity. The mRNA expression of IL-6, MCP-1, nuclear factor κB, collagen type IV, TGF-β1, and ICAM-1 and protein levels of type IV collagen and TGF-β1 were also significantly reduced in mice with 25% STAT3 activity compared with those with 75% STAT3 activity [53]. These findings cumulatively suggest that STAT3 has a crucial role in the progression of interstitial fibrosis, inflammatory cell infiltration, and abnormal matrix synthesis in the early stages of diabetic nephropathy.
High glucose levels have also been shown to enhance ANG II–induced JAK2, STAT1, STAT3, and STAT5A or STAT5B phosphorylation in glomerular mesangial cells in vitro. Under high glucose conditions, ANG II induced glomerular mesangial cell growth (hyperplasia and hypertrophy) and stimulated collagen IV synthesis and production of TGF-β1 and fibronectin [29,54]. AG490 reduced JAK2, STAT1, and STAT3 tyrosine phosphorylation and prevented the glucose-induced production of TGF-β1 and fibronectin in glomerular mesangial cells [54].
Further evidence of the importance of JAK/STAT signaling in diabetic nephropathy is presented in studies examining SOCS proteins. In rats with streptozotocin-induced diabetes, SOCS1 and SOCS3 gene overexpression via adenovirus injection have been shown to decrease the activation of STAT1 and STAT3 in the kidney and expression of proinflammatory and profibrotic cytokines [55]. SOCS gene overexpression also improves creatinine clearance and reduces urinary albumin levels in this model of diabetic nephropathy, and finally, it improves the pathologic changes associated with diabetic nephropathy, including glomerular hypertrophy, mesangial cell expansion, fibrosis, and macrophage infiltration [55]. These findings provide significant evidence of the role of JAK/STAT signaling in the pathophysiology of diabetic nephropathy.
6. Glomerulonephritis
Acute glomerulonephritis is one of the major causes of renal injury. Rapidly progressive glomerulonephritis is a small subgroup of glomerulonephritis that leads to renal failure within a few days to months and is characterized by the presence of extensive extra capillary proliferation [56]. Increased JAK/STAT signaling has been implicated in various forms of glomerulonephritis.
Arakawa et al. have demonstrated that pSTAT3-positive glomerular cells are increased in patients with lupus nephritis (immune complex glomerulonephritis), IgA nephropathy, and vasculitis compared with normal kidneys, and pSTAT3-positive tubulointerstitial cells are increased in patients with IgA nephropathy and vasculitis. Renal function is inversely correlated with the number of pSTAT3-positive glomerular and tubulointerstitial cells in all forms of glomerulonephritis [57].
STAT3 is activated in the glomerular regions of the kidney in rats with immune complex glomerulonephritis. Zhang et al. [58] have demonstrated that STAT3, pSTAT3, and tissue inhibitor of metalloproteinase 1 (TIMP-1) expression are all increased in the kidneys of rats with immune complex glomerulonephritis. Immunohistochemical studies have demonstrated ED-1 (surface marker for monocytes or macrophages)–positive cells in glomeruli that were pSTAT3 positive. Based on these results, the authors surmised that macrophages may play an important role in the activation of STAT3 during immune complex glomerulonephritis. The expression of TIMP-1 was also increased in kidneys with immune complex glomerulonephritis and appeared to correlate with pSTAT3 expression in the glomeruli [58]. The angiotensin-converting enzyme inhibitor, fosinopril, decreased the expression of STAT3, pSTAT3, and TIMP-1 and reduced ED-1–positive cells within the glomeruli and tubulointerstitial compartments of the kidney in rats with immune complex glomerulonephritis, suggesting that ANG II has a role in JAK/STAT signaling [58].
The expression of pSTAT3 is also increased in proliferating mesangial cells in a rat model of anti-Thy 1.1 glomerulonephritis (mesangial proliferative glomerulonephritis) [59,60]. Contrary to Zhang et al., Hirai et al. were unable to demonstrate ED-1 and pSTAT3 dual localization in this model of injury and concluded that macrophages may be not a signifi-cant source of pSTAT3 in anti-Thy 1.1 glomerulonephritis [59]. Signal transduction inhibitor 571, a PDGF receptor tyrosine kinase inhibitor, inhibits pSTAT3 signaling in mesangial cells and is found to suppress mesangial cell proliferation during anti-Thy 1.1 glomerulonephritis [59]. Although pSTAT3 appears to have a role in anti-Thy 1.1 glomerulonephritis, these studies suggest that the cellular localization of pSTAT3 may vary among the different experimental models of glomerulonephritis.
Finally, in a mouse model of lupus nephritis, which is manifested by immune complex deposition, the proliferation of glomerular cells, and inflammatory infiltration, the expression of pJAK2 and pSTAT1 was significantly increased compared with normal control mice, and pSTAT1 expression localized to the TECs, interstitial cells, and glomerular cells in these mice. Treatment of the mice with AG490 significantly reduced activation of JAK2 and STAT1, decreased expression of MCP-1, IFN-γ, and class II major histocompatibility complex, improved renal function, and reduced proteinuria [61]. These findings suggest that the JAK/STAT signaling pathway contributes to various forms of glomerulonephritis, and the inhibition of JAK/STAT signaling may protect against deterioration in renal function. It remains unclear, however, which cells activate JAK/STAT signaling and what cellular events improve renal function during JAK/STAT inhibition in each form of glomerulonephritis.
7. Conclusions
The JAK/STAT signaling pathway has been implicated in the progression of a variety of renal diseases. Activation of JAK/STAT signaling has been shown to play an important role in inflammatory infiltration, the accumulation of ECM, and the development of tubulointerstitial fibrosis. In experimental models, inhibition of the JAK/STAT signaling pathway, particularly JAK2 and STAT3, reduces renal fibrosis and protects against deterioration in renal function. Future studies are required to elucidate the specific JAK/STAT family members involved in each model of fibrotic renal disease and determine their signaling relationship and contribution to renal injury. Inhibition of the JAK/STAT signaling pathway, however, holds promise as a novel therapeutic approach to the treatment of fibrotic renal disease.
REFERENCES
- 1.Brenner BM. Remission of renal disease: recounting the challenge, acquiring the goal. J Clin Invest. 2002;110:1753. doi: 10.1172/JCI200217351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 2006;69:213. doi: 10.1038/sj.ki.5000054. [DOI] [PubMed] [Google Scholar]
- 3.Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol. 2004;15:1. doi: 10.1097/01.asn.0000106015.29070.e7. [DOI] [PubMed] [Google Scholar]
- 4.Zeisberg M, Strutz F, Müller GA. Renal fibrosis: an update. Curr Opin Nephrol Hypertens. 2001;10:315. doi: 10.1097/00041552-200105000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Eddy AA. Molecular basis of renal fibrosis. Pediatr Nephrol. 2000;15:290. doi: 10.1007/s004670000461. [DOI] [PubMed] [Google Scholar]
- 6.Böttinger EP, Bitzer M. TGF-beta signaling in renal disease. J Am Soc Nephrol. 2002;13:2600. doi: 10.1097/01.asn.0000033611.79556.ae. [DOI] [PubMed] [Google Scholar]
- 7.Bani-Hani AH, Campbell MT, Meldrum DR, et al. Cytokines in epithelial-mesenchymal transition: a new insight into obstructive nephropathy. J Urol. 2008;180:461. doi: 10.1016/j.juro.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Misseri R, Meldrum DR, Dinarello CA, et al. TNF-alpha mediates obstruction-induced renal tubular cell apoptosis and proapoptotic signaling. Am J Physiol Renal Physiol. 2005;288:406. doi: 10.1152/ajprenal.00099.2004. [DOI] [PubMed] [Google Scholar]
- 9.Bani-Hani AH, Leslie JA, Asanuma H, et al. IL-18 neutralization ameliorates obstruction-induced epithelial-mesenchymal transition and renal fibrosis. Kidney Int. 2009;76:500. doi: 10.1038/ki.2009.216. [DOI] [PubMed] [Google Scholar]
- 10.Schindler CW. Series introduction. JAK-STAT signaling in human disease. J Clin Invest. 2002;109:1133. doi: 10.1172/JCI15644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 12.Bromberg J, Darnell JE., Jr The role of STATs in transcriptional control and their impact on cellular function. Oncogene. 2000;19:2468. doi: 10.1038/sj.onc.1203476. [DOI] [PubMed] [Google Scholar]
- 13.Bromberg JF, Wrzeszczynska MH, Devgan GZ, et al. Stat3 as an oncogene. Cell. 1999;98:295. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 14.Schindler C, Darnell JE., Jr Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Annu Rev Biochem. 1995;64:621. doi: 10.1146/annurev.bi.64.070195.003201. [DOI] [PubMed] [Google Scholar]
- 15.Kisseleva T, Bhattacharya S, Braunstein J, et al. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene. 2002;285:1. doi: 10.1016/s0378-1119(02)00398-0. [DOI] [PubMed] [Google Scholar]
- 16.Murray PJ. The JAK-STAT signaling pathway: input and output integration. J Immunol. 2007;178:2623. doi: 10.4049/jimmunol.178.5.2623. [DOI] [PubMed] [Google Scholar]
- 17.O'Sullivan LA, Liongue C, Lewis RS, et al. Cytokine receptor signaling through the Jak-Stat-Socs pathway in disease. Mol Immunol. 2007;44:2497. doi: 10.1016/j.molimm.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 18.Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signaling and immune regulation. Nat Rev Immunol. 2007;7:454. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- 19.Aaronson DS, Horvath CM. A road map for those who don't know JAK-STAT. Science. 2002;296:1653. doi: 10.1126/science.1071545. [DOI] [PubMed] [Google Scholar]
- 20.Ozaki K, Leonard WJ. Cytokine and cytokine receptor pleiotropy and redundancy. J Biol Chem. 2002;277:29355. doi: 10.1074/jbc.R200003200. [DOI] [PubMed] [Google Scholar]
- 21.Kotenko SV, Langer JA. Full house: 12 receptors for 27 cytokines. Int Immunopharmacol. 2004;4:593. doi: 10.1016/j.intimp.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Aggarwal BB, Kunnumakkara AB, Harikumar KB, et al. Signal transducer and activator of transcription-3, inflammation, and cancer: how intimate is the relationship? Ann N Y Acad Sci. 2009;1171:59. doi: 10.1111/j.1749-6632.2009.04911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krebs DL, Hilton DJ. SOCS proteins: negative regulators of cytokine signaling. Stem Cells. 2001;19:378. doi: 10.1634/stemcells.19-5-378. [DOI] [PubMed] [Google Scholar]
- 24.Shuai K. Regulation of cytokine signaling pathways by PIAS proteins. Cell Res. 2006;16:196. doi: 10.1038/sj.cr.7310027. [DOI] [PubMed] [Google Scholar]
- 25.Stahl N, Farruggella TJ, Boulton TG, et al. Choice of STATs and other substrates specified by modular tyrosine-based motifs in cytokine receptors. Science. 1995;267:1349. doi: 10.1126/science.7871433. [DOI] [PubMed] [Google Scholar]
- 26.Chevalier RL, Thornhill BA, Forbes MS, et al. Mechanisms of renal injury and progression of renal disease in congenital obstructive nephropathy. Pediatr Nephrol. 2010;25:687. doi: 10.1007/s00467-009-1316-5. [DOI] [PubMed] [Google Scholar]
- 27.Misseri R, Rink RC, Meldrum DR, et al. Inflammatory mediators and growth factors in obstructive renal injury. J Surg Res. 2004;119:149. doi: 10.1016/j.jss.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 28.Chen X, Wang J, Zhou F, et al. STAT proteins mediate angiotensin II-induced production of TIMP-1 in human proximal tubular epithelial cells. Kidney Int. 2003;64:459. doi: 10.1046/j.1523-1755.2003.00133.x. [DOI] [PubMed] [Google Scholar]
- 29.Amiri F, Shaw S, Wang X, et al. Angiotensin II activation of the JAK/STAT pathway in mesangial cells is altered by high glucose. Kidney Int. 2002;61:1605. doi: 10.1046/j.1523-1755.2002.00311.x. [DOI] [PubMed] [Google Scholar]
- 30.Yang J, Liu Y. Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am J Pathol. 2001;159:1465. doi: 10.1016/S0002-9440(10)62533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iwano M, Plieth D, Danoff TM, et al. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strutz F, Okada H, Lo CW, et al. Identification and characterization of a fibroblast marker: FSP1. J Cell Biol. 1995;130:393. doi: 10.1083/jcb.130.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meldrum KK, Misseri R, Metcalfe P, et al. TNF-alpha neutralization ameliorates obstruction-induced renal fibrosis and dysfunction. Am J Physiol Regul Integr Comp Physiol. 2007;292:1456. doi: 10.1152/ajpregu.00620.2005. [DOI] [PubMed] [Google Scholar]
- 34.Guo G, Morrissey J, McCracken R, et al. Role of TNFR1 and TNFR2 receptors in tubulointerstitial fibrosis of obstructive nephropathy. Am J Physiol. 1999;277:766. doi: 10.1152/ajprenal.1999.277.5.F766. [DOI] [PubMed] [Google Scholar]
- 35.Kuratsune M, Masaki T, Hirai T, et al. Signal transducer and activator of transcription 3 involvement in the development of renal interstitial fibrosis after unilateral ureteral obstruction. Nephrology. 2007;12:565. doi: 10.1111/j.1440-1797.2007.00881.x. [DOI] [PubMed] [Google Scholar]
- 36.Pang M, Ma L, Gong R, et al. A novel STAT3 inhibitor, S3I-201, attenuates renal interstitial fibroblast activation and interstitial fibrosis in obstructive nephropathy. Kidney Int. 2010;78:257. doi: 10.1038/ki.2010.154. [DOI] [PubMed] [Google Scholar]
- 37.Bonventre JV. Mechanisms of ischemic acute renal failure. Kidney Int. 1993;43:1160. doi: 10.1038/ki.1993.163. [DOI] [PubMed] [Google Scholar]
- 38.Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol. 2003;14:2199. doi: 10.1097/01.asn.0000079785.13922.f6. [DOI] [PubMed] [Google Scholar]
- 39.Bonventre JV, Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int. 2004;66:480. doi: 10.1111/j.1523-1755.2004.761_2.x. [DOI] [PubMed] [Google Scholar]
- 40.Daha MR, van Kooten C. Is the proximal tubular cell a proinflammatory cell? Nephrol Dial Transplant. 2000;15:41. doi: 10.1093/ndt/15.suppl_6.41. [DOI] [PubMed] [Google Scholar]
- 41.Donnahoo KK, Meng X, Ayala A, et al. Early kidney TNF-alpha expression mediates neutrophil infiltration and injury after renal ischemia-reperfusion. Am J Physiol. 1999;277:922. doi: 10.1152/ajpregu.1999.277.3.R922. [DOI] [PubMed] [Google Scholar]
- 42.Yang N, Luo M, Li R, et al. Blockage of JAK/STAT signalling attenuates renal ischaemia-reperfusion injury in rat. Nephrol Dial Transplant. 2008;23:91. doi: 10.1093/ndt/gfm509. [DOI] [PubMed] [Google Scholar]
- 43.Arany I, Megyesi JK, Nelkin BD, et al. STAT3 attenuates EGFR-mediated ERK activation and cell survival during oxidant stress in mouse proximal tubular cells. Kidney Int. 2006;70:669. doi: 10.1038/sj.ki.5001604. [DOI] [PubMed] [Google Scholar]
- 44.Neria F, Castilla MA, Sanchez RF, et al. Inhibition of JAK2 protects renal endothelial and epithelial cells from oxidative stress and cyclosporin A toxicity. Kidney Int. 2009;75:227. doi: 10.1038/ki.2008.487. [DOI] [PubMed] [Google Scholar]
- 45.Yokota N, Burne-Taney M, Racusen L, et al. Contrasting roles for STAT4 and STAT6 signal transduction pathways in murine renal ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2003;285:319. doi: 10.1152/ajprenal.00432.2002. [DOI] [PubMed] [Google Scholar]
- 46.Mauer SM, Steffes MW, Ellis EN, et al. Structural-functional relationships in diabetic nephropathy. J Clin Invest. 1984;74:1143. doi: 10.1172/JCI111523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schrijvers BF, De Vriese AS, Flyvbjerg A. From hyperglycemia to diabetic kidney disease: the role of metabolic, hemodynamic, intracellular factors and growth factors/cytokines. Endocr Rev. 2004;25:971. doi: 10.1210/er.2003-0018. [DOI] [PubMed] [Google Scholar]
- 48.Chiarelli F, Gaspari S, Marcovecchio ML. Role of growth factors in diabetic kidney disease. Horm Metab Res. 2009;41:585. doi: 10.1055/s-0029-1220752. [DOI] [PubMed] [Google Scholar]
- 49.Rask-Madsen C, King GL. Kidney complications: factors that protect the diabetic vasculature. Nat Med. 2010;16:40. doi: 10.1038/nm0110-40. [DOI] [PubMed] [Google Scholar]
- 50.Marrero MB, Banes-Berceli AK, Stern DM, et al. Role of the JAK/STAT signaling pathway in diabetic nephropathy. Am J Physiol Renal Physiol. 2006;290:762. doi: 10.1152/ajprenal.00181.2005. [DOI] [PubMed] [Google Scholar]
- 51.Berthier CC, Zhang H, Schin M, et al. Enhanced expression of Janus kinase-signal transducer and activator of transcription pathway members in human diabetic nephropathy. Diabetes. 2009;58:469. doi: 10.2337/db08-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Banes AK, Shaw S, Jenkins J, et al. Angiotensin II blockade prevents hyperglycemia-induced activation of JAK and STAT proteins in diabetic rat kidney glomeruli. Am J Physiol Renal Physiol. 2004;286:653. doi: 10.1152/ajprenal.00163.2003. [DOI] [PubMed] [Google Scholar]
- 53.Lu TC, Wang ZH, Feng X, et al. Knockdown of Stat3 activity in vivo preventsdiabetic glomerulopathy. Kidney Int. 2009;76:63. doi: 10.1038/ki.2009.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang X, Shaw S, Amiri F, et al. Inhibition of the Jak/STAT signaling pathway prevents the high glucose-induced increase in tgf-beta and fibronectin synthesis in mesangial cells. Diabetes. 2002;51:3505. doi: 10.2337/diabetes.51.12.3505. [DOI] [PubMed] [Google Scholar]
- 55.Ortiz-Muñoz G, Lopez-Parra V, Lopez-Franco O, et al. Suppressors of cytokine signaling abrogate diabetic nephropathy. J Am Soc Nephrol. 2010;21:763. doi: 10.1681/ASN.2009060625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Little MA, Pusey CD. Rapidly progressive glomerulonephritis: current and evolving treatment strategies. J Nephrol. 2004;17:10. [PubMed] [Google Scholar]
- 57.Arakawa T, Masaki T, Hirai T, et al. Activation of signal transducer and activator of transcription 3 correlates with cell proliferation and renal injury in human glomerulonephritis. Nephrol Dial Transplant. 2008;23:3418. doi: 10.1093/ndt/gfn314. [DOI] [PubMed] [Google Scholar]
- 58.Zhang W, Chen X, Shi S, et al. Expression and activation of STAT3 in chronic proliferative immune complex glomerulonephritis and the effect of fosinopril. Nephrol Dial Transplant. 2005;20:892. doi: 10.1093/ndt/gfh652. [DOI] [PubMed] [Google Scholar]
- 59.Hirai T, Masaki T, Kuratsune M, et al. PDGF receptor tyrosine kinase inhibitor suppresses mesangial cell proliferation involving STAT3 activation. Clin Exp Immunol. 2006;144:353. doi: 10.1111/j.1365-2249.2006.03073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yanagita M, Arai H, Nakano T, et al. Gas6 induces mesangial cell proliferation via latent transcription factor STAT3. J Biol Chem. 2001;276:42364. doi: 10.1074/jbc.M107488200. [DOI] [PubMed] [Google Scholar]
- 61.Wang S, Yang N, Zhang L, et al. Jak/STAT signaling is involved in the inflammatory infiltration of the kidneys in MRL/lpr mice. Lupus. 2010;19:1171. doi: 10.1177/0961203310367660. [DOI] [PubMed] [Google Scholar]