Abstract
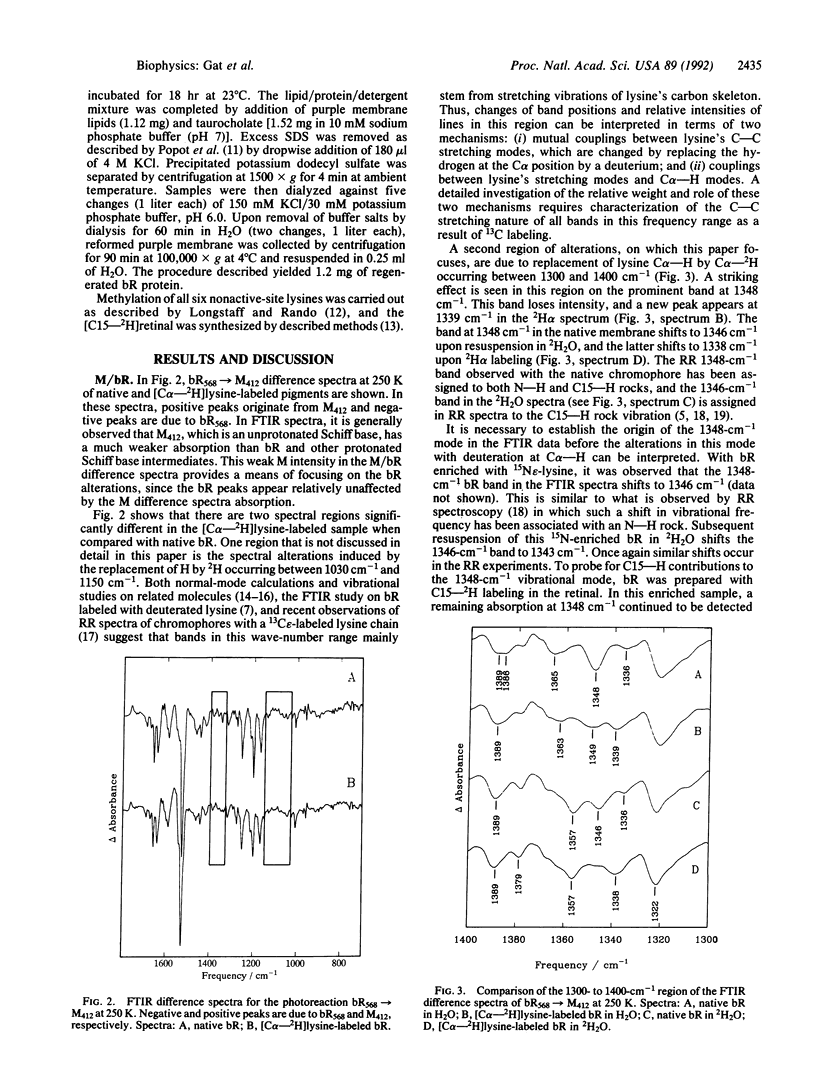
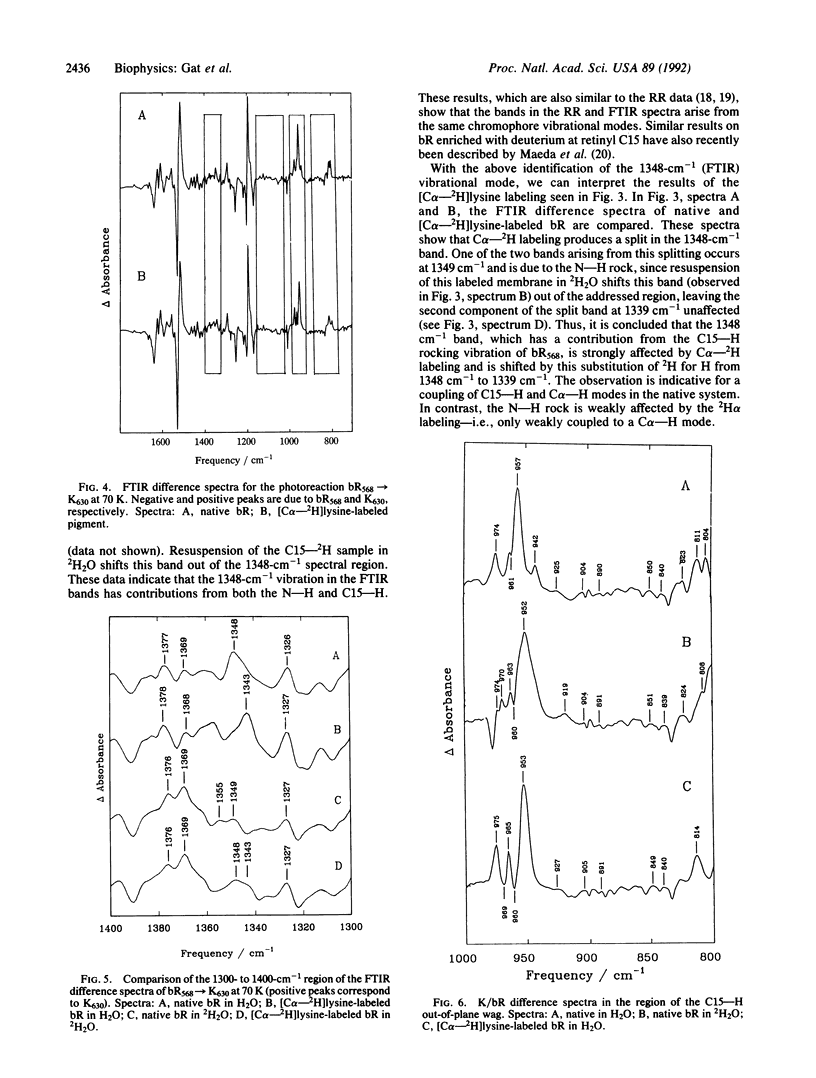
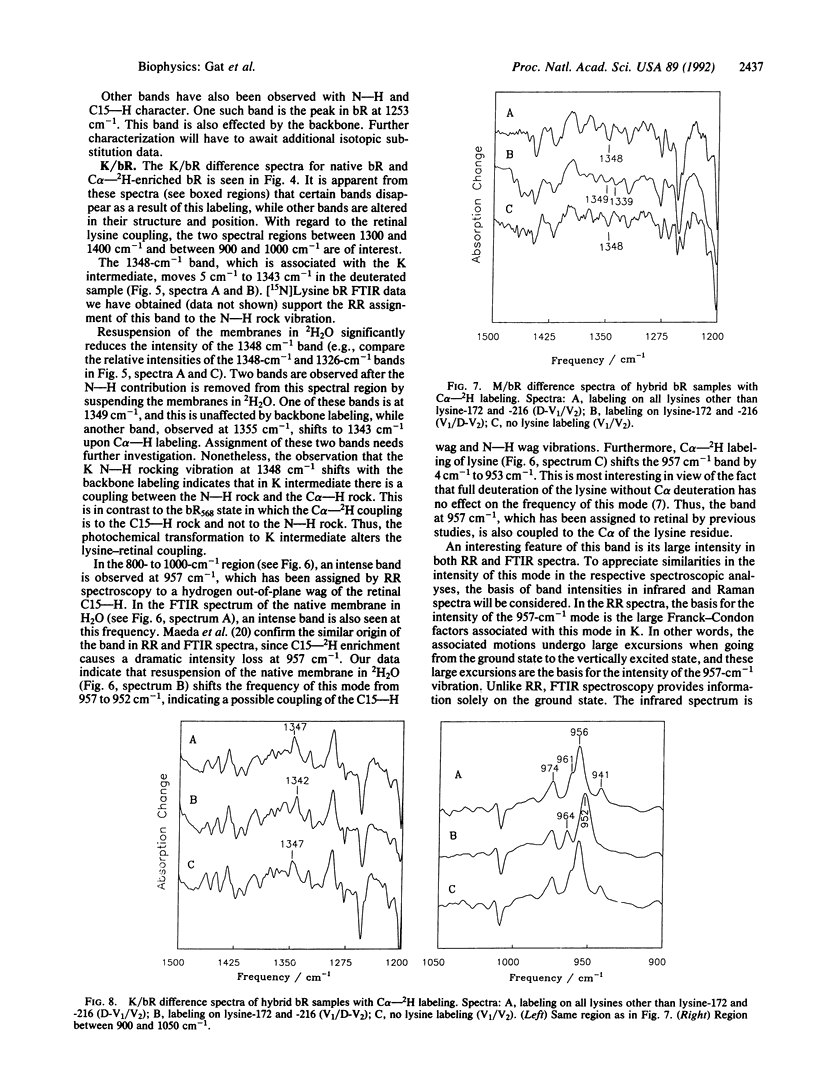
Bacteriorhodopsin (bR) has been biosynthetically prepared with lysine deuterated at its alpha carbon (C alpha--H). The labeled membranes containing bR were investigated by difference Fourier transform infrared (FTIR) spectroscopy. It has been derived from K/bR and M/bR difference spectra (K and M are photocycle intermediates) that several bands previously assigned to the retinal chromophore are coupled to the C alpha--H. The vibrational modes that exhibit this coupling are principally associated with C15--H and N--H vibrations. [C alpha--2H]Lysine-labeled bR was fragmented enzymatically, and bR structures were regenerated with the C alpha--2H label either on lysine-216 and -172 or on the remaining five lysine residues of the protein. FTIR studies of the regenerated bR system, together with methylation of all lysines except the active-site lysine, reveal that the changes observed due to backbone labeling arise from the active-site lysine. The intensity of the C15--H out-of-plane wag is interpreted as a possible indication of a twist around the C15 = N bond.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames J. B., Fodor S. P., Gebhard R., Raap J., van den Berg E. M., Lugtenburg J., Mathies R. A. Bacteriorhodopsin's M412 intermediate contains a 13-cis, 14-s-trans, 15-anti-retinal Schiff base chromophore. Biochemistry. 1989 May 2;28(9):3681–3687. doi: 10.1021/bi00435a009. [DOI] [PubMed] [Google Scholar]
- Argade P. V., Rothschild K. J., Kawamoto A. H., Herzfeld J., Herlihy W. C. Resonance Raman spectroscopy of specifically [epsilon-15N]lysine-labeled bacteriorhodopsin. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1643–1646. doi: 10.1073/pnas.78.3.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braiman M. S., Rothschild K. J. Fourier transform infrared techniques for probing membrane protein structure. Annu Rev Biophys Biophys Chem. 1988;17:541–570. doi: 10.1146/annurev.bb.17.060188.002545. [DOI] [PubMed] [Google Scholar]
- Lewis A., Spoonhower J., Bogomolni R. A., Lozier R. H., Stoeckenius W. Tunable laser resonance raman spectroscopy of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4462–4466. doi: 10.1073/pnas.71.11.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longstaff C., Rando R. R. Deprotonation of the Schiff base of bacteriorhodopsin is obligate in light-induced proton pumping. Biochemistry. 1987 Sep 22;26(19):6107–6113. doi: 10.1021/bi00393a024. [DOI] [PubMed] [Google Scholar]
- McMaster E., Lewis A. Evidence for light-induced lysine conformational changes during the primary event of the bacteriorhodopsin photocycle. Biochem Biophys Res Commun. 1988 Oct 14;156(1):86–91. doi: 10.1016/s0006-291x(88)80808-8. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol. 1971 Sep 29;233(39):149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- Pande C., Callender R. H., Chang C. H., Ebrey T. G. Resonance Raman spectra of the "blue" and the regenerated "purple" membranes of Halobacterium halobium. Photochem Photobiol. 1985 Nov;42(5):549–552. doi: 10.1111/j.1751-1097.1985.tb01608.x. [DOI] [PubMed] [Google Scholar]
- Popot J. L., Trewhella J., Engelman D. M. Reformation of crystalline purple membrane from purified bacteriorhodopsin fragments. EMBO J. 1986 Nov;5(11):3039–3044. doi: 10.1002/j.1460-2075.1986.tb04603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigrist H., Wenger R. H., Kislig E., Wüthrich M. Refolding of bacteriorhodopsin. Protease V8 fragmentation and chromophore reconstitution from proteolytic V8 fragments. Eur J Biochem. 1988 Oct 15;177(1):125–133. doi: 10.1111/j.1432-1033.1988.tb14352.x. [DOI] [PubMed] [Google Scholar]
- Smith S. O., Mathies R. A. Resonance Raman spectra of the acidified and deionized forms of bacteriorhodopsin. Biophys J. 1985 Feb;47(2 Pt 1):251–254. doi: 10.1016/s0006-3495(85)83899-6. [DOI] [PMC free article] [PubMed] [Google Scholar]



