Abstract
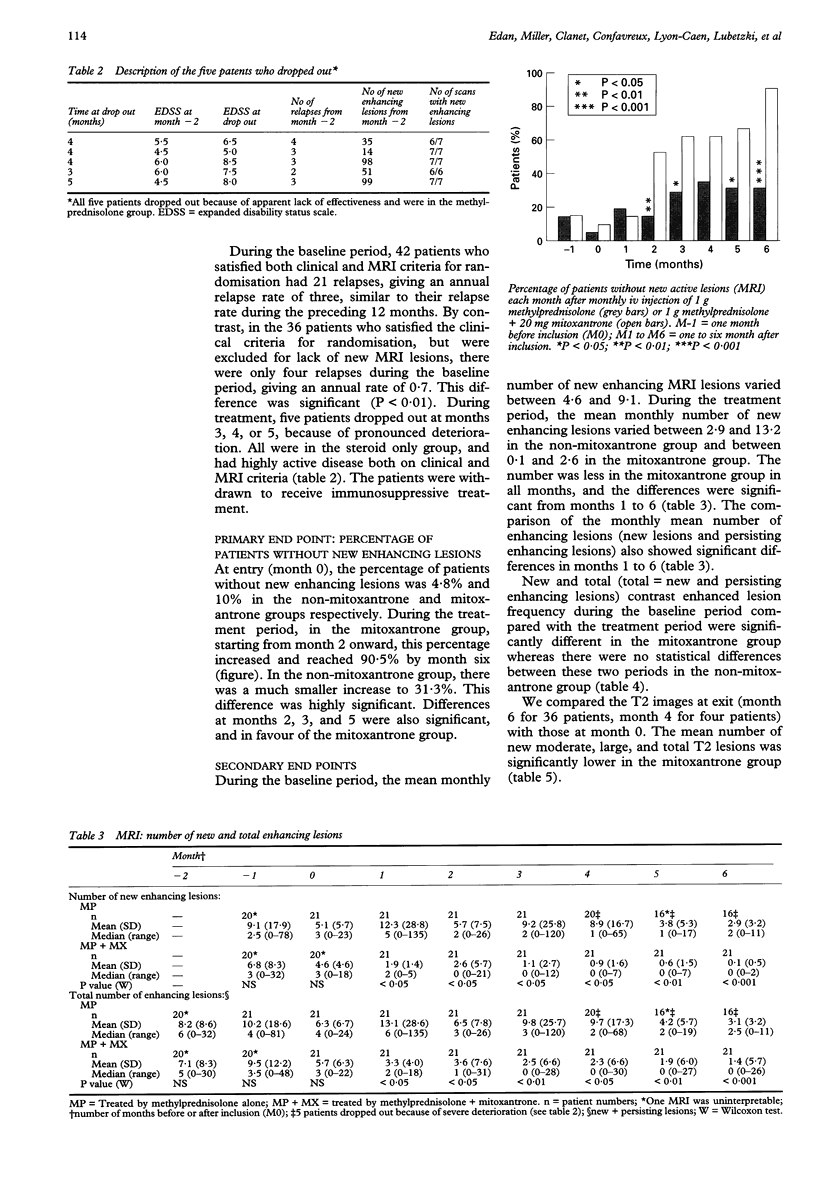
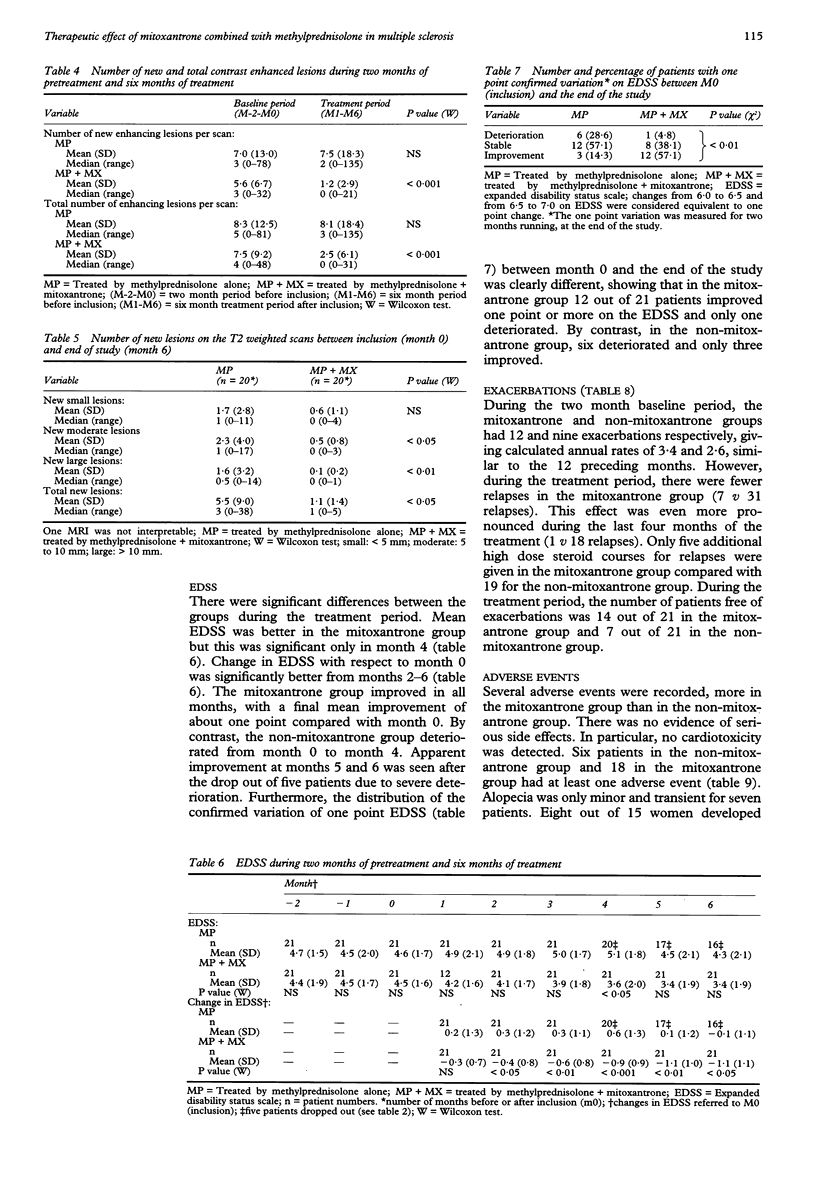
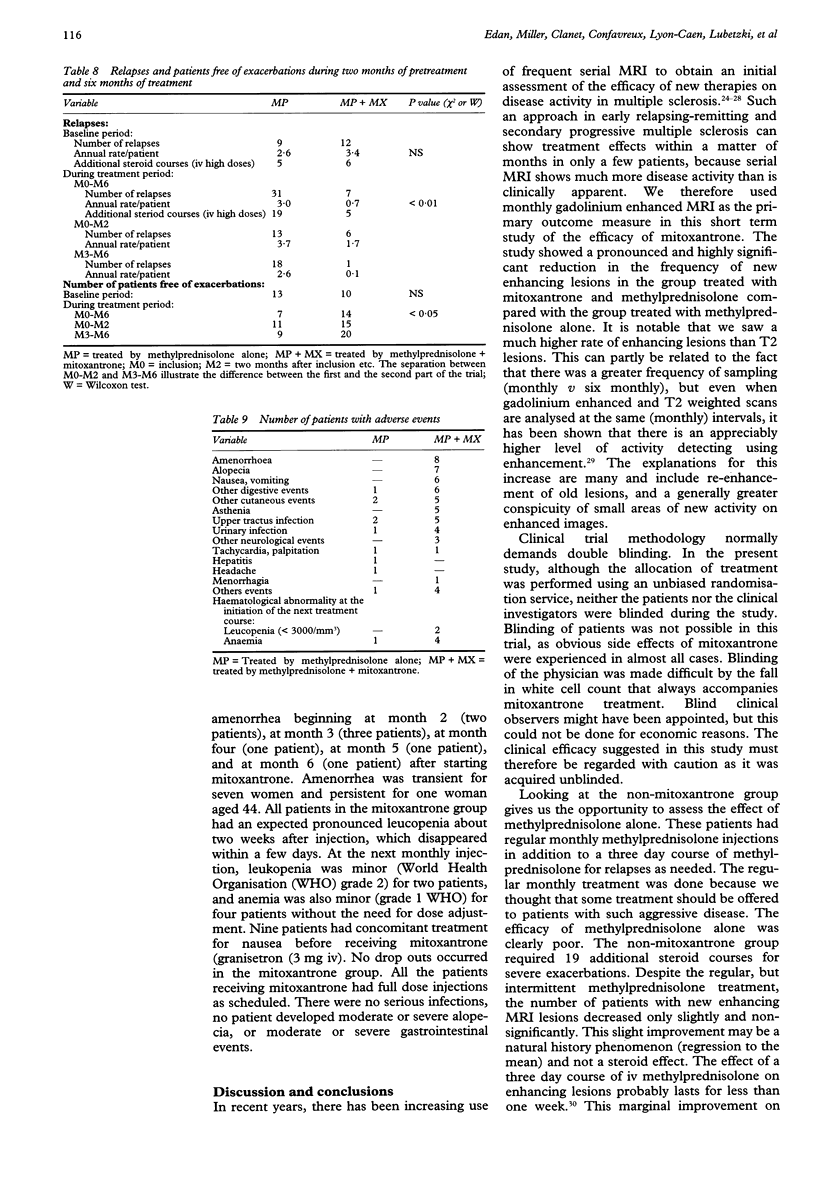
OBJECTIVE: To evaluate the efficiency of mitoxantrone in multiple sclerosis. METHODS: Forty two patients with confirmed multiple sclerosis, selected as having a very active disease on clinical and MRI criteria were randomised to receive either mitoxantrone (20 mg intravenously (IV) monthly) and methylprednisolone (1 g iv monthly) or methylprednisolone alone over six months. In the steroid alone group five patients dropped out due to severe exacerbation. RESULTS: Blinded analysis of MRI data showed significantly more patients with no new enhancing lesions in the mitoxantrone group compared with the steroid alone group, (90% v 31%, P < 0.001). In the mitoxantrone group there was a month by month decrease almost to zero in the number of new enhancing lesions, and in the total number of enhancing lesions, whereas both remained high in the steroid alone group. The differences were significant for both indices at all months from 1-6. Unblinded clinical assessments showed a significant improvement in change in EDSS at months 2-6 in the mitoxantrone group, with a final mean improvement of more than one point (-1.1 v + 0.3; P < 0.001). There was a significant reduction in the number of relapses (7 v 31; P < 0.01), and an increase in the number of patients free of exacerbation (14 v 7; P < 0.05). CONCLUSION: In this selected group of patients with multiple sclerosis with very active disease, mitoxantrone combined with methylprednisolone was effective in improving both clinical and MRI indices of disease activity over a period of six months whereas methylprednisolone alone was not. Further double blinded long term studies are needed to properly evaluate the effect of mitoxantrone on progression in disability.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bastianello S., Pozzilli C., D'Andrea F., Millefiorini E., Trojano M., Morino S., Gasperini C., Bozzao A., Gallucci M., Andreula C. A controlled trial of mitoxantrone in multiple sclerosis: serial MRI evaluation at one year. Can J Neurol Sci. 1994 Aug;21(3):266–270. doi: 10.1017/s0317167100041263. [DOI] [PubMed] [Google Scholar]
- Confavreux C., Compston D. A., Hommes O. R., McDonald W. I., Thompson A. J. EDMUS, a European database for multiple sclerosis. J Neurol Neurosurg Psychiatry. 1992 Aug;55(8):671–676. doi: 10.1136/jnnp.55.8.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley R. J. Clinical safety and tolerance of mitoxantrone. Semin Oncol. 1984 Sep;11(3 Suppl 1):54–58. [PubMed] [Google Scholar]
- De Castro S., Cartoni D., Millefiorini E., Funaro S., Gasperini C., Morino S., Tallarico D., Beni S. Noninvasive assessment of mitoxantrone cardiotoxicity in relapsing remitting multiple sclerosis. J Clin Pharmacol. 1995 Jun;35(6):627–632. doi: 10.1002/j.1552-4604.1995.tb05021.x. [DOI] [PubMed] [Google Scholar]
- Fidler J. M., DeJoy S. Q., Gibbons J. J., Jr Selective immunomodulation by the antineoplastic agent mitoxantrone. I. Suppression of B lymphocyte function. J Immunol. 1986 Jul 15;137(2):727–732. [PubMed] [Google Scholar]
- Fidler J. M., DeJoy S. Q., Smith F. R., 3rd, Gibbons J. J., Jr Selective immunomodulation by the antineoplastic agent mitoxantrone. II. Nonspecific adherent suppressor cells derived from mitoxantrone-treated mice. J Immunol. 1986 Apr 15;136(8):2747–2754. [PubMed] [Google Scholar]
- Goodkin D. E., Bailly R. C., Teetzen M. L., Hertsgaard D., Beatty W. W. The efficacy of azathioprine in relapsing-remitting multiple sclerosis. Neurology. 1991 Jan;41(1):20–25. doi: 10.1212/wnl.41.1.20. [DOI] [PubMed] [Google Scholar]
- Goodkin D. E., Rudick R. A., VanderBrug Medendorp S., Daughtry M. M., Schwetz K. M., Fischer J., Van Dyke C. Low-dose (7.5 mg) oral methotrexate reduces the rate of progression in chronic progressive multiple sclerosis. Ann Neurol. 1995 Jan;37(1):30–40. doi: 10.1002/ana.410370108. [DOI] [PubMed] [Google Scholar]
- Jacobs L. D., Cookfair D. L., Rudick R. A., Herndon R. M., Richert J. R., Salazar A. M., Fischer J. S., Goodkin D. E., Granger C. V., Simon J. H. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG) Ann Neurol. 1996 Mar;39(3):285–294. doi: 10.1002/ana.410390304. [DOI] [PubMed] [Google Scholar]
- Kappos L., Patzold U., Dommasch D., Poser S., Haas J., Krauseneck P., Malin J. P., Fierz W., Graffenried B. U., Gugerli U. S. Cyclosporine versus azathioprine in the long-term treatment of multiple sclerosis--results of the German multicenter study. Ann Neurol. 1988 Jan;23(1):56–63. doi: 10.1002/ana.410230110. [DOI] [PubMed] [Google Scholar]
- Levine S., Saltzman A. Regional suppression, therapy after onset and prevention of relapses in experimental allergic encephalomyelitis by mitoxantrone. J Neuroimmunol. 1986 Dec;13(2):175–181. doi: 10.1016/0165-5728(86)90063-9. [DOI] [PubMed] [Google Scholar]
- Lublin F. D., Lavasa M., Viti C., Knobler R. L. Suppression of acute and relapsing experimental allergic encephalomyelitis with mitoxantrone. Clin Immunol Immunopathol. 1987 Oct;45(1):122–128. doi: 10.1016/0090-1229(87)90118-8. [DOI] [PubMed] [Google Scholar]
- Mauch E., Kornhuber H. H., Krapf H., Fetzer U., Laufen H. Treatment of multiple sclerosis with mitoxantrone. Eur Arch Psychiatry Clin Neurosci. 1992;242(2-3):96–102. doi: 10.1007/BF02191555. [DOI] [PubMed] [Google Scholar]
- McFarland H. F., Frank J. A., Albert P. S., Smith M. E., Martin R., Harris J. O., Patronas N., Maloni H., McFarlin D. E. Using gadolinium-enhanced magnetic resonance imaging lesions to monitor disease activity in multiple sclerosis. Ann Neurol. 1992 Dec;32(6):758–766. doi: 10.1002/ana.410320609. [DOI] [PubMed] [Google Scholar]
- Miller D. H., Albert P. S., Barkhof F., Francis G., Frank J. A., Hodgkinson S., Lublin F. D., Paty D. W., Reingold S. C., Simon J. Guidelines for the use of magnetic resonance techniques in monitoring the treatment of multiple sclerosis. US National MS Society Task Force. Ann Neurol. 1996 Jan;39(1):6–16. doi: 10.1002/ana.410390104. [DOI] [PubMed] [Google Scholar]
- Miller D. H., Barkhof F., Berry I., Kappos L., Scotti G., Thompson A. J. Magnetic resonance imaging in monitoring the treatment of multiple sclerosis: concerted action guidelines. J Neurol Neurosurg Psychiatry. 1991 Aug;54(8):683–688. doi: 10.1136/jnnp.54.8.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. H., Barkhof F., Nauta J. J. Gadolinium enhancement increases the sensitivity of MRI in detecting disease activity in multiple sclerosis. Brain. 1993 Oct;116(Pt 5):1077–1094. doi: 10.1093/brain/116.5.1077. [DOI] [PubMed] [Google Scholar]
- Miller D. H., Thompson A. J., Morrissey S. P., MacManus D. G., Moore S. G., Kendall B. E., Moseley I. F., McDonald W. I. High dose steroids in acute relapses of multiple sclerosis: MRI evidence for a possible mechanism of therapeutic effect. J Neurol Neurosurg Psychiatry. 1992 Jun;55(6):450–453. doi: 10.1136/jnnp.55.6.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau T., Thorpe J., Miller D., Moseley I., Hale G., Waldmann H., Clayton D., Wing M., Scolding N., Compston A. Preliminary evidence from magnetic resonance imaging for reduction in disease activity after lymphocyte depletion in multiple sclerosis. Lancet. 1994 Jul 30;344(8918):298–301. doi: 10.1016/s0140-6736(94)91339-0. [DOI] [PubMed] [Google Scholar]
- Noseworthy J. H., Hopkins M. B., Vandervoort M. K., Karlik S. J., Lee D. H., Penman M., Rice G. P., Grinwich K. D., Cauvier H., Harris B. J. An open-trial evaluation of mitoxantrone in the treatment of progressive MS. Neurology. 1993 Jul;43(7):1401–1406. doi: 10.1212/wnl.43.7.1401. [DOI] [PubMed] [Google Scholar]
- Paty D. W., Li D. K. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. II. MRI analysis results of a multicenter, randomized, double-blind, placebo-controlled trial. UBC MS/MRI Study Group and the IFNB Multiple Sclerosis Study Group. Neurology. 1993 Apr;43(4):662–667. doi: 10.1212/wnl.43.4.662. [DOI] [PubMed] [Google Scholar]
- Posner L. E., Dukart G., Goldberg J., Bernstein T., Cartwright K. Mitoxantrone: an overview of safety and toxicity. Invest New Drugs. 1985;3(2):123–132. doi: 10.1007/BF00174159. [DOI] [PubMed] [Google Scholar]
- Ridge S. C., Sloboda A. E., McReynolds R. A., Levine S., Oronsky A. L., Kerwar S. S. Suppression of experimental allergic encephalomyelitis by mitoxantrone. Clin Immunol Immunopathol. 1985 Apr;35(1):35–42. doi: 10.1016/0090-1229(85)90075-3. [DOI] [PubMed] [Google Scholar]
- Rosenberg L. S., Carvlin M. J., Krugh T. R. The antitumor agent mitoxantrone binds cooperatively to DNA: evidence for heterogeneity in DNA conformation. Biochemistry. 1986 Mar 11;25(5):1002–1008. doi: 10.1021/bi00353a008. [DOI] [PubMed] [Google Scholar]
- Stern Y., Tetrud J. W., Martin W. R., Kutner S. J., Langston J. W. Cognitive change following MPTP exposure. Neurology. 1990 Feb;40(2):261–264. doi: 10.1212/wnl.40.2.261. [DOI] [PubMed] [Google Scholar]
- Stern Y., Tetrud J. W., Martin W. R., Kutner S. J., Langston J. W. Cognitive change following MPTP exposure. Neurology. 1990 Feb;40(2):261–264. doi: 10.1212/wnl.40.2.261. [DOI] [PubMed] [Google Scholar]
- Stone L. A., Frank J. A., Albert P. S., Bash C., Smith M. E., Maloni H., McFarland H. F. The effect of interferon-beta on blood-brain barrier disruptions demonstrated by contrast-enhanced magnetic resonance imaging in relapsing-remitting multiple sclerosis. Ann Neurol. 1995 May;37(5):611–619. doi: 10.1002/ana.410370511. [DOI] [PubMed] [Google Scholar]
- Stone L. A., Smith M. E., Albert P. S., Bash C. N., Maloni H., Frank J. A., McFarland H. F. Blood-brain barrier disruption on contrast-enhanced MRI in patients with mild relapsing-remitting multiple sclerosis: relationship to course, gender, and age. Neurology. 1995 Jun;45(6):1122–1126. doi: 10.1212/wnl.45.6.1122. [DOI] [PubMed] [Google Scholar]
- Weiner H. L., Mackin G. A., Orav E. J., Hafler D. A., Dawson D. M., LaPierre Y., Herndon R., Lehrich J. R., Hauser S. L., Turel A. Intermittent cyclophosphamide pulse therapy in progressive multiple sclerosis: final report of the Northeast Cooperative Multiple Sclerosis Treatment Group. Neurology. 1993 May;43(5):910–918. doi: 10.1212/wnl.43.5.910. [DOI] [PubMed] [Google Scholar]



