Abstract
Background
The aim of this study was to assess the serum zinc levels in patients with common oral mucosal diseases by comparing these to healthy controls.
Material and Methods
A total of 368 patients, which consisted of 156 recurrent aphthous stomatitis (RAS) patients, 57 oral lichen planus (OLP) patients, 55 burning mouth syndrome (BMS) patients, 54 atrophic glossitis (AG) patients, 46 xerostomia patients, and 115 sex-and age-matched healthy control subjects were enrolled in this study. Serum zinc levels were measured in all participants. Statistical analysis was performed using a one-way ANOVA, t-test, and Chi-square test.
Results
The mean serum zinc level in the healthy control group was significantly higher than the levels of all other groups (p < 0.001). No individual in the healthy control group had a serum zinc level less than the minimum normal value. However, up to 24.7% (13/54) of patients with AG presented with zinc deficiency, while 21.2% (33/156) of patients with RAS, 16.4% (9/55) of patients with BMS, 15.2% (7/46) of patients with xerostomia, and 14.0% (8/57) of patients with OLP were zinc deficient. Altogether, the zinc deficiency rate was 19.02% (70/368) in the oral mucosal diseases (OMD) group (all patients with OMD). The difference between the OMD and healthy control group was significant (p <0.001). Gender differences in serum zinc levels were also present, although not statistically significant.
Conclusions
Zinc deficiency may be involved in the pathogenesis of common oral mucosal diseases. Zinc supplementation may be a useful treatment for oral mucosal diseases, but this requires further investigation; the optimal serum level of zinc, for the prevention and treatment of oral mucosal diseases, remains to be determined.
Key words:Oral mucosal diseases, Zinc deficiency, pathogenesis.
Introduction
Oral mucosal diseases (OMD) are common disorders affecting all segments of the general population that represent a series of abnormal alterations in oral mucosal surfaces, which include the labial and buccal mucosae, the alveolar mucosa, all parts of tongue, the floor of the mouth, and the gingival, palatal, and oropharyngeal mucosae (1,2). Although the majority of OMDs are benign, some of them are difficult to manage and usually interfere with important oral functions, systemic health, and quality of life of the patients.
Recurrent aphthous stomatitis (RAS), oral lichen planus (OLP), burning mouth syndrome (BMS), atrophic glossitis (AG), and xerostomia are some of the most prevalent oral mucosal diseases and they typically require multiple visits to specialist outpatient services. However, the etiology and pathogenesis of these disorders is still poorly understood and highly debated (2-5).
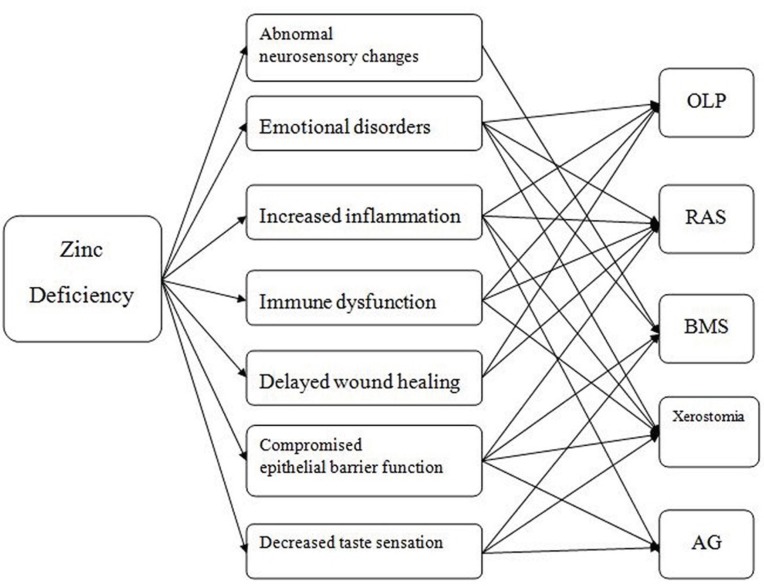
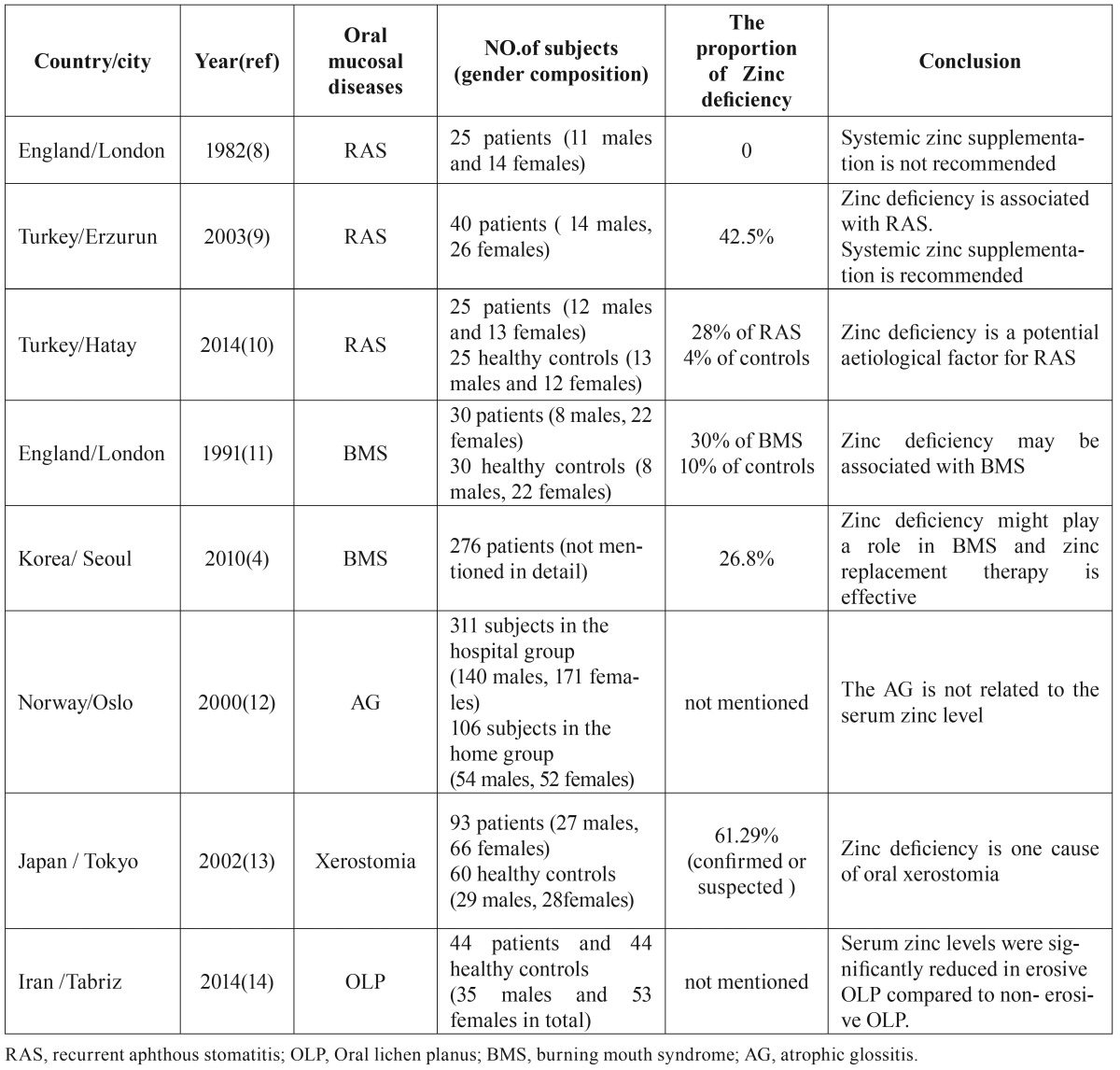
Zinc, one of the most important trace elements within the human body, plays a variety of critical roles in cell growth and reproduction, normal immune functions, collagen synthesis, and wound healing (6,7). And zinc deficiency is related to multiple physiological abnormalities. Therefore, it is plausible that zinc deficiency might be directly involved in the pathogenesis of some oral mucous diseases (Fig. 1). At the present, the available literature concerning serum zinc levels in patients with oral mucosal diseases is severely limited. Moreover, findings from several of these studies are inconsistent (4,8-14) ( Table 1), which demonstrates that further research is needed.
Figure 1.
Zinc deficiency may be involved in the pathogenesis of common oral mucosal diseases through a variety of mechanisms. OLP: oral lichen planus. RAS: Recurrent aphthous stomatitis. BMS: burning mouth síndrome. AG: atrophic glositis.
Table 1. Existing reports of zinc deficiency in oral mucosal diseases in the past three decades.
The aim of this study was to assess the serum zinc levels of patients with some of the most common oral mucosal diseases as compared to healthy controls to determine if zinc deficiency may play a role in the etiology and pathogenesis of oral mucosal disease.
Material and Methods
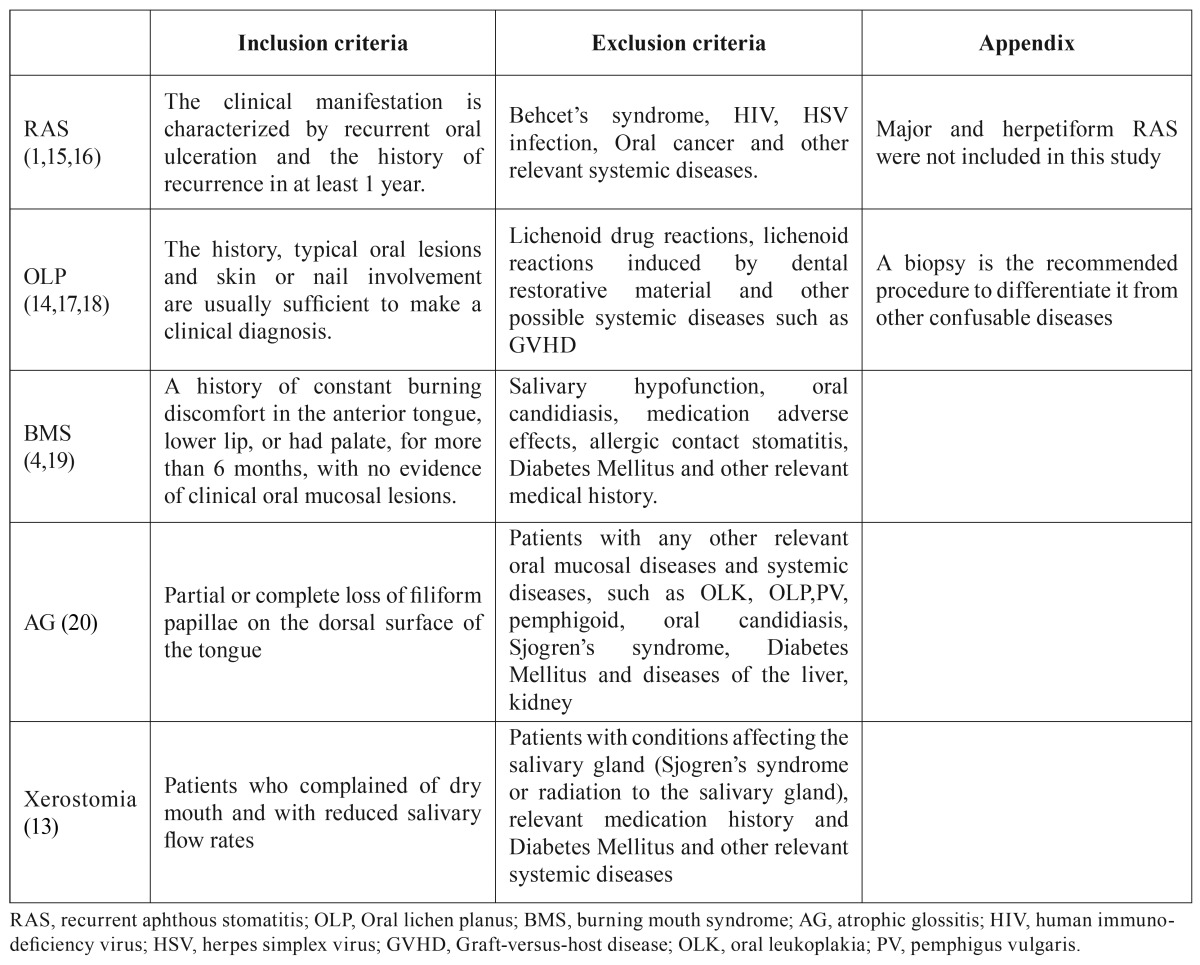
A total of 368 patients, consisting of 156 RAS patients, 57 OLP patients, 55 BMS patients, 54 AG patients and 46 xerostomia patients were enrolled in this study. Sex- and age-matched healthy control subjects were also examined (115 total controls). All participants were evaluated from January 2013 to May 2015 at the Department of Oral Medicine, Shanxi Provincial People’s Hospital, China. An OMD diagnosis was established according to accep-ted criterions (1,4,13-20) ( Table 2). Other less common diseases, such as oral leukoplakia (OLK), discoid lupus erythematosus (DLE), oral acute infection, allergic reactions, and seasonal disorders, such as cheilitis, were not included in this study. None of the control subjects had any oral mucosal lesions or related systemic diseases.
Table 2. Diagnostic criteria for the oral mucosal diseases involved in the present study.
For all participants, blood samples were taken after 12 hours of overnight fasting, medical histories were recorded in detail, and a thorough clinical oral examination was performed by the same professional dentist. The study was approved by the hospital ethics committee and informed consent was obtained from each participant.
Serum zinc levels were measured using a Colorimetric Method Zinc Assay Kit (BSBE, Beijing, China). The accepted normal serum zinc level is 10.7-17.70 μmol/L. Zinc deficiency was defined as a serum Zn level below this cutoff.
Statistical analysis was performed using SPSS version 18.0 software (Chicago, Illinois, USA). Serum zinc levels were compared using a t-test to compare between each OMD group and the healthy control group, while one-way ANOVA was used to compare among different groups. In each group, serum zinc levels were compared between male and female using a t-test. In addition, the sex ratio was compared for all groups by Chi-square test. A p-value < 0.05 was accepted as statistically significant.
Results
- The serum zinc levels
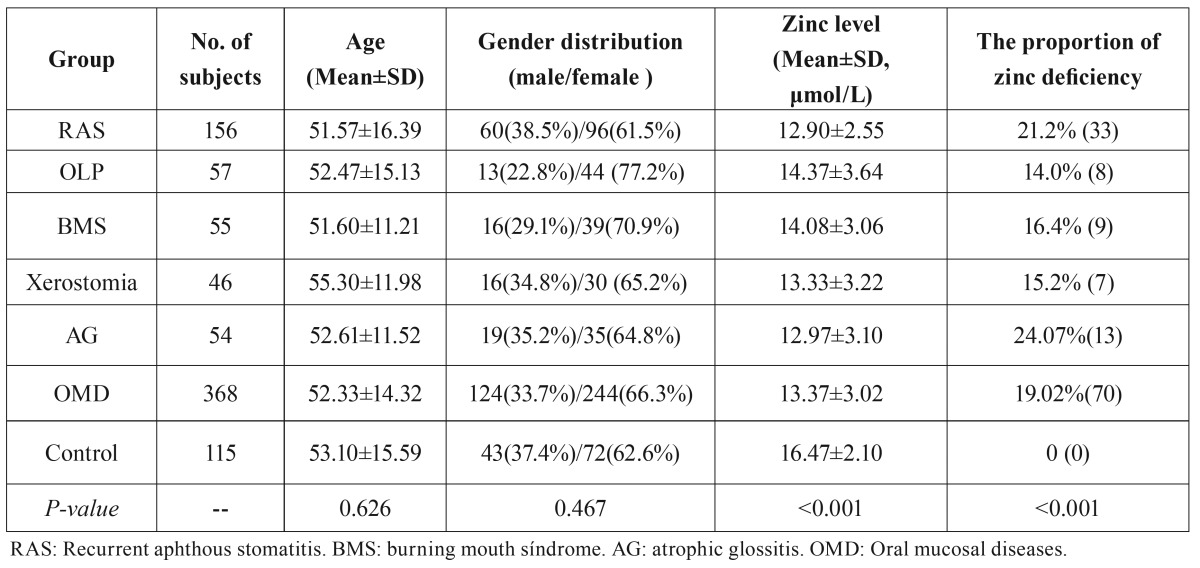
Although the serum zinc levels of some OMD patient groups were still in normative range, the mean serum zinc level of all patients with any OMD was significantly lower than that of the control group (p <0.001, Table 3). Each individual in OMD group had a lower mean serum zinc level than the control group (p <0.001).
Table 3. The basic parameters, zinc levels, and zinc deficiency in each group.
- Zinc deficiency condition
It is worth noting that in the healthy control group, none of the serum zinc levels fell below the minimum normal value. However, the zinc deficiency rate was 19.02% (70/368) for patients with any OMD (all OMD groups collectively). Specifically, 24.07% (13/54) of patients with AG were presented with zinc deficiency, followed by 21.2% (33/156) of patients with RAS, 16.4% (9/55) of patients with BMS, 15.2% (7/46) of patients with xerostomia, and 14.0% (5/57) of patients with OLP. Statistically significant differences were present between all OMD groups and the healthy control group (p <0.001, Table 3).
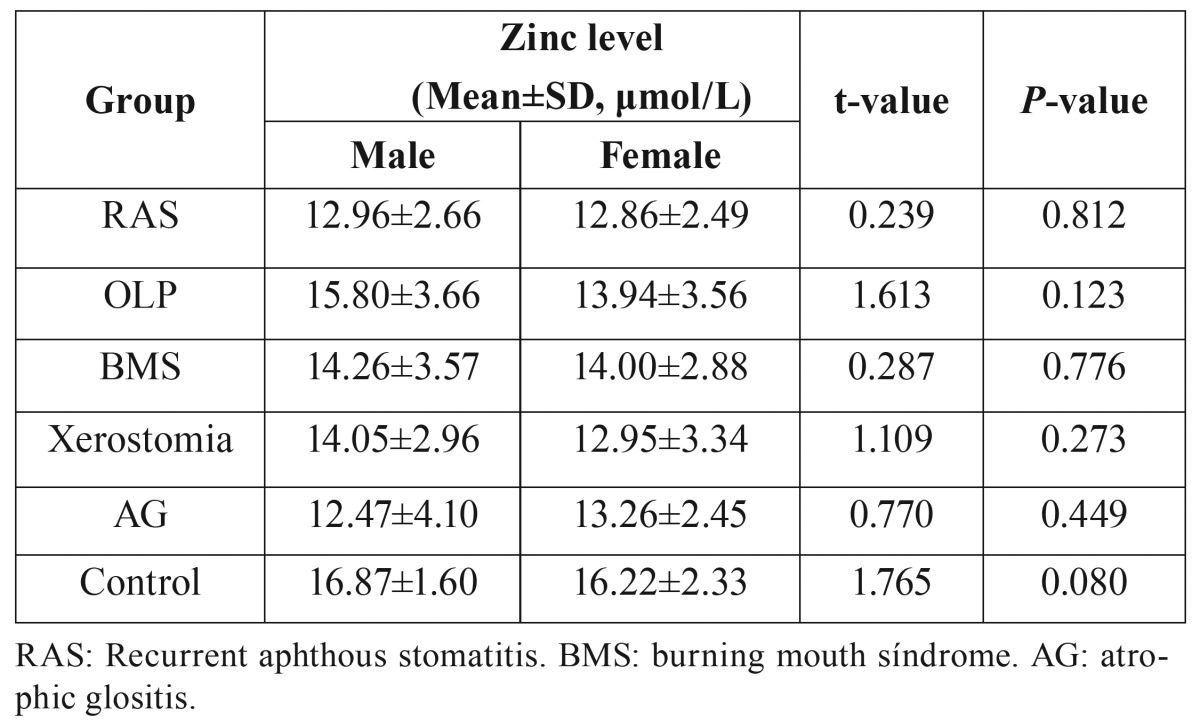
- Gender differences
Interestingly, the serum zinc levels in males were higher than in females for both the control and OMD groups, with the exception of the AG group. However, these differences were not statistically significant ( Table 4).
Table 4. Gender differences in zinc levels in each group.
Discussion
Zinc serves as a cofactor of at least 3000 human proteins, and it participants in various molecular mechanisms, which means that zinc deficiency may be more widely and significantly involved in the pathogenesis of human diseases than generally accepted (6,7,21). Zinc deficiency results in multiple abnormalities, which have a positive correlation with occurrence of the oral mucosal diseases discussed in this paper (Fig. 1). In this study, serum zinc levels in OMD patients were significantly lower than or were at the lower end of the normal range, which is in stark contrast to healthy patients that show no signs of zinc deficiency and have zinc levels that are in higher end of the normal zinc range. This simple observation suggests that serum zinc levels maybe influence the pathogenesis of oral mucosal diseases.
It is widely believed that atrophic glossitis (AG) may be a marker of nutritional deficiency and the significant association of deficiency of iron, vitamin B12 and hemoglobin positivity with AG has been proved (20). In the present study, zinc deficiency was found in nearly a quarter of the AG patients, compared with no deficiency in healthy control. A previous research in Norway revealed that AG was a marker for malnutrition but not related to zinc level (12). However, a more recent and larger scale study in the same Northern Europe country exhibited a relatively high prevalence of zinc deficiency in elderly people and demonstrated the significant association between zinc deficiency and the risk of malnutrition (22). The inconsistent results may be due to the lack of statistical analysis about the prevalence of zinc deficiency of the AG patients in the former study. Similar to our results, the significantly lower serum zinc level or higher prevalence of zinc deficiency was also detected in patients with RAS (9,10), OLP (14), BMS (4,11) and xerostomia (13). Further investigation is required to determine if low zinc levels are secondary to or facilitate disease development. However, we would suspect that low levels of serum zinc are a predisposing factor that may render the individual more susceptible to oral mucosal dysfunction, precipitating the occurrence of OMDs.
The observation that people without OMDs have higher/normal zinc levels, suggests that zinc homeostasis may play a role in OMD prevention and that exogenous zinc could be potentially used as a treatment or preventative measure for OMDs. Despite our findings that zinc deficiency is common in patients with OMD, additional research is needed to determine if modulation of zinc levels would be therapeutically advantageous. We speculate that zinc supplementation could be beneficial for the treatment of oral mucosal diseases. However, this viewpoint is controversial, as many patients exhibit a poor response to exogenous zinc and there may be a high incidence of undesirable side effects with systemic zinc administration. For example, Wray et al. (8) suggested that a routine of zinc supplementation is not advisable/beneficial for patients with RAS. But a more recent article suggested that zinc supplementation may have some positive effects (9). A study in Korea showed that zinc supplementation can be effective against BMS (4), but this view was challenged in a later publication (5). At present, the debate as to the value of zinc supplementation in patients with OMDs continues. Zinc supplementation must be carefully considered, since the optimal level of zinc in the human body is still unknown, and the potential toxic effects of zinc must be considered (21).
We demonstrated that the serum zinc level is likely lower in women than in men. Similar results have been obtained in a large-scale study performed across three European countries (23). Coincidentally, the OMD diseases discussed here are more prevalent in women than man. For example, several studies have denoted that BMS is highly associated with the female gender (24,25). In addition, OLP is twice as common in women as in men (26). Thus, we speculate that the lower level of serum zinc may be one factor that helps explain the increased prevalence of OMD in women.
Our study reveals significant differences in zinc levels between patients with OMD and healthy individuals. Therefore, we postulate that zinc deficiency and lower serum zinc levels are involved in the pathogenesis of oral mucosal diseases and specifically that a decline in a person’s serum zinc level may be predisposing factor for the development of an OMD. However, additional study is necessary to determine the efficiency and safe dosing for zinc supplementation in humans, especially considering the overt controversy surrounding this approach. With convenient accessibility and easy traceability, serum zinc levels might also serve a predictive or diagnostic factor for the occurrence of OMDs. Additional research should help to determine the ideal administration strategy and demonstrate the potential effectiveness of zinc supplementation.
Acknowledgments
This study was supported by Shanxi province Health and family planning commission, Science and Technology Key Project (2014018) and Shanxi Scholarship Council of China. The authors would like to thank the Duoease Scientific Service Center for excellent language editing service.
References
- 1.Stoopler ET, Sollecito TP. Oral mucosal diseases: evaluation and management. Med Clin North Am. 2014;98:1323–52. doi: 10.1016/j.mcna.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Scully C. Clinical practice. Aphthous ulceration. N Engl J Med. 2006;355:165–72. doi: 10.1056/NEJMcp054630. [DOI] [PubMed] [Google Scholar]
- 3.Roopashree MR, Gondhalekar RV, Shashikanth MC, George J, Thippeswamy SH, Shukla A. Pathogenesis of oral lichen planus-a review. J Oral Pathol Med. 2010;39:729–34. doi: 10.1111/j.1600-0714.2010.00946.x. [DOI] [PubMed] [Google Scholar]
- 4.Cho GS, Han MW, Lee B, Roh JL, Choi SH, Cho KJ. Zinc deficiency may be a cause of burning mouth syndrome as zinc replacement therapy has therapeutic effects. J Oral Pathol Med. 2010;39:722–7. doi: 10.1111/j.1600-0714.2010.00914.x. [DOI] [PubMed] [Google Scholar]
- 5.Bhoopathi V, Mascarenhas AK. Zinc-replacement therapy may not reduce oral pain in patients with zinc-deficient burning mouth syndrome (BMS) J Evid Based Dent Pract. 2011;11:189–90. doi: 10.1016/j.jebdp.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 6.Prasad AS. Discovery of human zinc deficiency: its impact on human health and disease. Adv Nutr. 2013;4:176–90. doi: 10.3945/an.112.003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maret W. Zinc and human disease. Met Ions Life Sci. 2013;13:389–414. doi: 10.1007/978-94-007-7500-8_12. [DOI] [PubMed] [Google Scholar]
- 8.Wray D. A double-blind trial of systemic zinc sulfate in recurrent aphthous stomatitis. Oral Surg Oral Med Oral Pathol. 1982;53:469–72. doi: 10.1016/0030-4220(82)90459-5. [DOI] [PubMed] [Google Scholar]
- 9.Orbak R, Cicek Y, Tezel A, Dogru Y. Effects of zinc treatment in patients with recurrent aphthous stomatitis. Dent Mater J. 2003;22:21–9. doi: 10.4012/dmj.22.21. [DOI] [PubMed] [Google Scholar]
- 10.Ozler GS. Zinc deficiency in patients with recurrent aphthous stomatitis: a pilot study. J Laryngol Otol. 2014;128:531–3. doi: 10.1017/S0022215114001078. [DOI] [PubMed] [Google Scholar]
- 11.Maragou P, Ivanyi L. Serum zinc levels in patients with burning mouth syndrome. Oral Surg Oral Med Oral Pathol. 1991;71:447–50. doi: 10.1016/0030-4220(91)90427-e. [DOI] [PubMed] [Google Scholar]
- 12.Bøhmer T, Mowé M. The association between atrophic glossitis and protein-calorie malnutrition in old age. Age Ageing. 2000;29:47–50. doi: 10.1093/ageing/29.1.47. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka M. Secretory function of the salivary gland in patients with taste disorders or xerostomia: correlation with zinc deficiency. ActaOtolaryngol Suppl. 2002;546:134–41. doi: 10.1080/00016480260046526. [DOI] [PubMed] [Google Scholar]
- 14.Gholizadeh N, Mehdipour M, Najafi Sh, Bahramian A, Garjani Sh, Khoeini Poorfar H. Evaluation of the serum zinc level in erosive and non-erosive oral lichen planus. J Dent (Shiraz) 2014;15:52–6. [PMC free article] [PubMed] [Google Scholar]
- 15.Preeti L, Magesh K, Rajkumar K, Karthik R. Recurrent aphthous stomatitis. J Oral Maxillofac Pathol. 2011;15:252–6. doi: 10.4103/0973-029X.86669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jurge S, Kuffer R, Scully C, Porter SR. Mucosal disease series. Number VI. Recurrent aphthous stomatitis. Oral Dis. 2006;12:1–21. doi: 10.1111/j.1601-0825.2005.01143.x. [DOI] [PubMed] [Google Scholar]
- 17.Gupta S, Jawanda MK. Oral Lichen Planus: An Update on Etiology, Pathogenesis, Clinical Presentation, Diagnosis and Management. Indian J Dermatol. 2015;60:222–9. doi: 10.4103/0019-5154.156315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lavanya N, Jayanthi P, Rao UK, Ranganathan K. Oral lichen planus: An update on pathogenesis and treatment. J Oral Maxillofac Pathol. 2011;15:127–32. doi: 10.4103/0973-029X.84474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pekiner FN, Gümrü B, Demirel GY, Ozbayrak S. Burning mouth syndrome and saliva: detection of salivary trace elements and cytokines. J Oral Pathol Med. 2009;38:269–75. doi: 10.1111/j.1600-0714.2008.00734.x. [DOI] [PubMed] [Google Scholar]
- 20.Sun A, Lin HP, Wang YP, Chiang CP. Significant association of deficiency of hemoglobin, iron and vitamin B12, high homocysteine level, and gastric parietal cell antibody positivity with atrophic glossitis. J Oral Pathol Med. 2012;41:500–4. doi: 10.1111/j.1600-0714.2011.01122.x. [DOI] [PubMed] [Google Scholar]
- 21.Skrovanek S, DiGuilio K, Bailey R, Huntington W, Urbas R, Mayilvaganan B. Zinc and gastrointestinal disease. World J Gastrointest Pathophysiol. 2014;5:496–513. doi: 10.4291/wjgp.v5.i4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kvamme JM, Grønli O, Jacobsen BK, Florholmen J. Risk of malnutrition and zinc deficiency in community-living elderly men and women: the Tromsø Study. Public Health Nutr. 2015;18:1907–13. doi: 10.1017/S1368980014002420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnaud J, de Lorgeril M, Akbaraly T, Salen P, Arnout J, Cappuccio FP. Gender differences in copper, zinc and selenium status in diabetic-free metabolic syndrome European population - the IMMIDIET study. Nutr Metab Cardiovasc Dis. 2012;22:517–24. doi: 10.1016/j.numecd.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Kohorst JJ, Bruce AJ, Torgerson RR, Schenck LA, Davis MD. A population-based study of the incidence of burning mouth syndrome. Mayo Clin Proc. 2014;89:1545–52. doi: 10.1016/j.mayocp.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gurvits GE, Tan A. Burning mouth syndrome. World J Gastroenterol. 2013;19:665–72. doi: 10.3748/wjg.v19.i5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scully C, Carrozzo M. Oral mucosal disease: Lichen planus. Br J Oral Maxillofac Surg. 2008;46:15–21. doi: 10.1016/j.bjoms.2007.07.199. [DOI] [PubMed] [Google Scholar]