Abstract
Patients with head and neck cancer (HNC) receiving intensity-modulated radiation therapy (IMRT) have particularly high rates of fatigue, and pre- and post-radiotherapy fatigue are prognostic factors for pathologic tumor responses and poor survival. Although inflammation has been proposed as one of the potential mechanisms of fatigue in cancer patients, findings have not been consistent, and there is a dearth of longitudinal studies. Accordingly, we conducted a prospective study in 46 HNC patients pre- and one-month post-IMRT. Fatigue was measured by the Multidimensional Fatigue Inventory (MFI)-20 at both time points along with the assessment of peripheral blood inflammatory markers including interleukin (IL)-6, soluble tumor necrosis factor receptor 2, and C-reactive protein (CRP) and gene expression. Generalized estimating equations were used to examine the association between inflammatory markers and fatigue. Gene enrichment analysis using MetaCore software was performed using up-regulated genes that were significantly associated with IMRT and fatigue. Significant associations between fatigue and IL-6 as well as CRP, which were independent of time, were observed. In addition the change in fatigue from pre- to post-IMRT was positively associated with the change in IL-6 and CRP. Analysis of up-regulated gene transcripts as a function of IMRT and fatigue revealed overrepresentation of transcripts related to the defense response and nuclear factor kappa B. In conclusion, our findings support the hypotheses that inflammation is associated with fatigue over time in HNC patients. Future studies on how inflammation contributes to fatigue as well as strategies targeting inflammation to reduce fatigue are warranted.
Keywords: Inflammation, Fatigue, Head and neck cancer, Intensity-modulated radiation therapy
1. Introduction
Compelling evidence demonstrates the importance of cancer-related fatigue to quality of life and survival (Janaki et al., 2010; Fang et al., 2004; Montazeri, 2009). However, little work has been done in patients with head and neck cancer (HNC) Jereczek-Fossa et al., 2007; Sawada et al., 2012; Ackerstaff et al., 2011; Hoskin et al., 2009. The most prevalent HNCs, cancers of the oral cavity and oropharynx, are the 10th most common cancers worldwide (Mehanna et al., 2010). In the US, 55,070 new HNC cases are estimated in 2014, and this number has increased in the past decade (Siegel et al., 2014). New evidence shows that the rise in incidence of HNCs is due to increased human papillomavirus (HPV) infection. If these trends continue, by 2020 HPV-positive HNCs will likely surpass cervical cancer as the most common HPV-associated cancer in the United States (Chaturvedi et al., 2011). HNC patients, usually treated either definitively or adjuvantly with radiotherapy, have particularly high rates of fatigue (Hickok et al., 2005; Gulliford et al., 2012). Intensity-modulated radiation therapy (IMRT) employs multiple intensity levels across each radiation beam allowing for improved conformal and homogenous dose distributions over complex target volumes with sparing of adjacent normal structures. IMRT is also the most frequently used radiation technique for HNC patients. Recent research on IMRT has shown that patients treated with IMRT experience even higher fatigue compared to conventional, 3D conformal-RT (Gulliford et al.,2012; Spratt et al., 2012). IMRT is generally used in combination with concurrent chemotherapy (chemoRT), based on the likely synergistic effect of treatment modalities (Jereczek-Fossa et al., 2007; Bower et al., 2000). Fatigue impacts not only HNC patient’s quality of life, but also long term survival Fang et al., 2004; Ackerstaff et al., 2011). It has been estimated that an increase in baseline fatigue of 10 points (out of 100) yields a 17% reduction in survival in this patient population (Fang et al., 2004). Additionally, our previous findings showed that fatigue was significantly correlated with other common and severe treatment-related symptoms such as mucositis, taste change, and dry mouth, all of which form a symptom cluster with fatigue (Xiao et al., 2013, 2014). However, there is no Food and Drug Administration (FDA)-approved pharmacological agent that reliably prevents or treats cancer-related fatigue (Minton et al., 2010).
Understanding the mechanisms of fatigue is critical to its successful management of HNC patients, and may benefit other cancer or treatment-related symptoms as well. Recent research has shown a potential linkage between fatigue and inflammation that may provide novel insights into the biological mechanisms of cancer-related fatigue (Bower et al., 2011; Collado-Hidalgo et al., 2006; Orre et al., 2009; Barsevick et al., 2010). Studies have found that fatigued cancer patients exhibit higher levels of peripheral inflammatory markers, such as interleukin (IL)-1 receptor antagonist (IL-1ra) Collado-Hidalgo et al., 2006; Orre et al., 2009; Bower et al., 2002; Meyers et al., 2005, IL-6 (Meyers et al., 2005; Costanzo et al., 2005; Wratten et al., 2004), tumor necrosis factor (TNF) Bower et al., 2011; Meyers et al., 2005, and C-reactive protein (CRP) Orre et al., 2009, 2011 compared to those without fatigue. Others, however, report no association between fatigue and inflammation (Bower et al., 2011; Cameron et al., 2012). These conflicting findings emphasize the need for further investigation into the relationship between fatigue and inflammation. Additionally, few studies have examined associations between fatigue and inflammation as assessed by gene expression patterns. For instance, one study found that increased expression of transcripts related to nuclear factor kappa B (NFkB), a key mediator for inflammatory responses, in fatigued breast cancer survivors (Bower et al., 2011). However, this study had only 21 patients and utilized a cross-sectional design. Another study in breast cancer patients found that gene expression changes related to NFkB were only apparent in patients receiving chemotherapy, but not increased in those who did not receive chemotherapy (Torres et al., 2013). Although this study also found patients receiving chemotherapy had worse fatigue (Torres et al., 2013), a direct association between gene expression and fatigue was not examined.
To date, no studies have examined the relationship between inflammation and fatigue in HNC patients. Moreover, since most studies in other cancer types have been cross-sectional (Schubert et al., 2007), exploring this association longitudinally may help clarify conflicting findings. The purpose of this study was to examine the longitudinal association between fatigue and inflammation in patients with HNC receiving IMRT. We explored this association at both the protein and gene expression level in peripheral blood and hypothesized that fatigue would be positively associated with increases in protein concentrations of inflammatory markers including CRP, IL-6 and sTNFR2 (as a measure of TNF activity (Fernandez-Real et al., 1998) and gene transcripts related to NFkB signaling pathways.
2. Methods
This prospective, longitudinal study investigated HNC patients 1 week prior to (baseline) and 1 month post completion of IMRT. The overall length of time between the two assessments was approximately 3 months, including 1.5–2 months for IMRT (or chemoIMRT) and 1 month follow-up after the completion of IMRT (or chemoIMRT). Of note, surgery occurred approximately 1 month before IMRT (or chemoIMRT) and therefore before the baseline assessment. After IRB approval, the principal investigator screened participating radiation oncologists’ schedules for newly diagnosed patients with HNC. Patients’ eligibility was determined by reviewing the electronic medical record. Approximately 80% of screened patients were eligible for this study. Among these patients, 82% were consented to be enrolled into the study. After confirming with the participating radiation oncologist that the patient was eligible and would receive IMRT, the principal investigator or research assistants met with eligible patients to obtain consent before the start of IMRT. All questionnaires were collected at clinic sites, and blood samples were collected by a phlebotomist or certificated nurse on the same day as the questionnaires.
2.1. Sample
The study enrolled patients at the Radiation Oncology Clinics at Emory Clinic and Emory University Hospital Midtown from May, 2012 – December, 2013. Inclusion criteria were: histological proof of squamous cell carcinoma of the head and neck region with no distant metastasis; ≥21 years of age; and no evidence of uncontrolled metabolic, hematologic, cardiovascular, renal, hepatic or neurologic disease. Exclusion criteria were: simultaneous primaries; previous invasive malignancies but disease free for <3 years; pregnancy; and presence of a major psychiatric disorder (e.g. bipolar disorder and schizophrenia) or inability to understand English. Other exclusion criteria that might confound the relationship between fatigue and inflammation included: chronic medical conditions involving the immune system (e.g., HIV, hepatitis B or C) or regular use of immunosuppressive medications (such as glucocorticoids and methotrexate) within 6 months of study entry. Over-the-counter anti-inflammatory medications and antidepressants were allowed.
2.2. Fatigue and social behavioral measures
2.2.1. Fatigue
The Multidimensional Fatigue Inventory (MFI)-20 was used as the primary outcome measure for fatigue. MFI is a 20-item self-report instrument that covers five dimensions of fatigue: general fatigue, physical fatigue, mental fatigue, reduced motivation, and reduced activity (Smets et al., 1995). Each dimension includes four items on a 1- to 5-point scale. The total score, ranging from 20 to 100 (higher scores indicating more fatigue) is calculated as the sum of the five dimensions, and each dimension is the sum of four items. The MFI-20 has well established validity and reliability (α = 0.84) in use with patients with cancer receiving radiation therapy (Schubert et al., 2007; Smets et al., 1995).
2.2.2. Covariates: demographic and clinical variables
Demographic and clinical variables were collected by chart review. Variables included: age, sex, race, education (years), marital status (married/single), body mass index (BMI), antidepressant use (yes/no), tobacco use (yes/no), alcohol use (yes/no), primary cancer site, cancer stage (TNM), radiation dose (Gy), treatment regimen (IMRT/IMRT + chemotherapy/IMRT + chemotherapy + surgery/IMRT + surgery), and chemotherapeutic regimen (cisplatin/carboplatin + paclitaxel/cisplatin switch to carboplatin + paclitaxel). These variables were chosen for their potential influence on fatigue, based on literature reviews and our previous studies (Bower et al., 2011; Wang et al., 2010; Xiao et al., 2011; Mitchell, 2010; Fang et al., 2005).
2.2.3. Covariates: other symptoms
Other symptoms that were investigated as potential cofounders included depressive symptoms, sleep difficulties, cognitive dysfunction, pain, dry mouth, difficulty swallowing, skin burn from radiation, mouth or throat sores, taste change, nausea, and vomiting. These potential confounders were chosen based on literature reviews and our previous studies, which showed significant associations of these symptoms with either the inflammatory markers or fatigue (Xiao et al., 2013; Bower et al., 2011). These symptoms were also included in the core symptoms for patient assessment in those with HNC as determined by the recent National Cancer Institute (NCI) Symptom Management & Quality of Life Steering Committee Clinical Trials Planning Meeting on Patient Reported Outcomes. The Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) was used to measure all above-mentioned symptoms (National Cancer Institute, 2012). Validation studies are being conducted at NCI-designated comprehensive cancer centers and the RTOG (National Cancer Institute, 2010).
Our previous studies have identified two stable and reliable symptom clusters in patients with HNC (Xiao et al., 2013, 2014), and these two symptom clusters were used in this study to group symptoms together with the purpose of reducing the number of covariates. Therefore, the final symptoms and/or symptom clusters defined as covariates were: depressive symptoms, sleep difficulties, cognitive dysfunction, HNC symptom cluster (including symptoms of pain, dry mouth, difficulty swallowing, skin burn from radiation, mouth or throat sores, and taste change), and gastrointestinal (GI) symptom cluster (including symptoms of nausea and vomiting).
2.3. Laboratory methods
Whole blood was collected into chilled EDTA tubes for the isolation of plasma and peripheral blood mononuclear cells (PBMCs). Plasma was separated by centrifugation at 1000g for 10 min at 4 °C, and then aliquoted into siliconized polypropylene tubes and stored at −80 °C until assayed. PBMCs were isolated using density gradient centrifugation and stored in freezing serum (90% fetal bovine serum, 10% DMSO) at −80 °C until mRNA isolation.
2.3.1. Inflammatory markers
Inflammatory markers including IL-6, sTNFR2, and CRP were chosen on the basis of findings from previous research on fatigue and inflammation in cancer patients (Orre et al., 2011; Wang et al., 2010; Lyon et al., 2011; Miller et al., 2008; Reyes-Gibby et al., 2009). Plasma concentrations of IL-6 and sTNFR2 were determined using sandwich ELISA according to manufacturer’s protocol (R & D Systems; Minneapolis, MN). All samples were run in duplicate, and quality control plasma of both low and high inflammatory marker concentrations were included with every assay. The mean inter- and intra-assay coefficients of variation for control samples are reliably 10% or less. CRP was measured using standard turbidimetric assay techniques.
2.3.2. Gene expression
Total RNA was isolated from PBMCs according to manufacturer’s protocol (Qiagen RNeasy Mini Kit: Qiagen; Valencia, CA). RNA integrity was determined initially by 260/280 = 1.9–2.1 and finally by scanning with an Agilent 2100 Bioanalyzer using the RNA 6000 Nano LabChip. Isolated RNAs were kept at −80 C until microarray analysis. The Emory Integrated Genomics Core analyzed RNA samples for gene expression using Illumina Whole Genome BeadChips (HumanHT-12 Expression BeadChip: Illumina; San Diego, CA). Raw probe intensities were corrected for background levels and normalized by the quantile normalization algorithm using GenomeStudio software from Illumina. To restrict our analysis to probes significantly expressed in our PBMC samples, we removed probes that had detection p-values <.05 for fewer than 10% of samples, resulting in a final set of 27,616 probes that were eligible for analysis.
2.4. Data analysis
Descriptive statistics (mean, standard deviation, N, percentage) were used to characterize the sample. A paired t-test was used to examine fatigue and inflammatory marker changes from pre- to post-IMRT. Generalized linear models with the generalized estimating equations (GEE) method and exchangeable within-subject correlation matrix were employed to measure the relationship of fatigue score with predictors (inflammatory markers) and covariates to account for correlated repeated measured outcome data. The first GEE model was constructed to examine the simple association between fatigue and inflammatory markers across time (i.e. prior to and after IMRT) controlling for relevant demographic and clinical covariates including age, smoking status (yes/no), BMI, treatment type, and the use of antidepressants (yes/no). In order to examine whether the association between fatigue and inflammatory markers across time was driven by inflammation-induced changes in depression or other symptoms, a second multivariable GEE analysis was conducted including other symptoms as well as clinical and demographic variables as covariates. Covariates that exhibited a univariate relationship with fatigue at an alpha level of <0.1 were retained in the model. Because the relationship between fatigue and inflammatory markers might differ before versus after IMRT, a third GEE analysis was conducted to examine the interaction effect of biomarkers of inflammation and time on fatigue. For this interaction analysis, inflammatory markers were categorized into three levels. CRP was categorized as low (≤1 mg/L), medium (1–3 mg/L), or high (≥3 mg/L) according to guidelines set forth by the American Heart Association (Ridker, 2003). IL6 and sTNFr2 were categorized based on percentile rank as low (<25th percentile), medium (25–75th percentile) and high (>75th percentile).
Linear regression was used to examine the association between delta inflammatory markers and delta fatigue (1-month post-IMRT – pre-IMRT). To achieve normality, the log value of inflammatory markers were used in data analysis. All analyses were done using SAS 9.3 (SAS Institute, Inc., Cary, North Carolina) with a significance level of 0.05.
To model the associations between gene expression, IMRT, and fatigue, linear regression with fixed effects to account for within-subject correlation in gene expression was used. To identify genes whose expression changed significantly from pre- to post-IMRT, a separate model was fit for each probe where the log expression signal was modeled as a function of IMRT status (pre or post), with covariates included to adjust for subject age and the within-subject fixed effect. To identify genes whose expression predicted changes in fatigue from pre- to post-IMRT, the log expression signal of each probe was modeled as a function of fatigue, adjusting for IMRT status (pre or post), subject age, and the within-subject fixed effect. To establish sets of significant genes, the Benjamini-Hochberg procedure (Benjamini and Hochberg, 1995) was used to control the false discovery rate (FDR) at 1%.
To gain insights into the biological processes involving genes that were significantly related with IMRT and fatigue, we focused on the set of genes that were significantly associated with both IMRT (FDR<.01) and fatigue (FDR<.01). Up-regulated genes associated with IMRT and fatigue were entered into MetaCore software (GeneGo, Inc., St. Joseph, Mich) for enrichment analysis of non-specific functional annotation (Gene Ontology (GO) processes) as well as transcription factor analysis for NFkB-related gene transcripts (Shmelkov et al., 2011). MetaCore software has been shown to be both accurate and comprehensive in identifying transcriptional regulatory networks including those regulated by NFkB (Shmelkov et al., 2011) and has been used by our group in previous studies (Torres et al., 2013).
3. Results
3.1. Demographic and clinical characteristics
All 46 enrolled patients filled out the questionnaires; 43 of them had cytokine data; and 35 had gene expression data at both pre- and 1-month post-IMRT. Demographic and clinical characteristics of the study sample are shown in Table 1. The sample was predominantly middle-aged Caucasian males who were married and had a history of tobacco use. Half of the patients had a history of alcohol use. Most patients were diagnosed with non-laryngeal cancer with advanced disease (stage IV). All participants were treated with IMRT, and the majority received concurrent chemotherapy. Patient receiving definite surgery had lower doses of radiation (average of 66 Gy), and patients receiving concurrent chemoradiation had a higher dose of radiation (average of 70 Gy). For patients having surgery plus concurrent chemoradiation, half of them had either radiation at 66 Gy and the other half had 70 Gy.
Table 1.
Baseline demographic and clinical characteristics of the participants (N = 46).
| Variables | Mean ± SD or N (%) | |
|---|---|---|
| Age (years) | 57.76 ±10.44 | |
| Gender | Male | 39 (85) |
| Female | 7 (15) | |
| Race | White | 39 (85) |
| Non-White | 7 (15) | |
| Marital statusb | Married | 34 (74) |
| Unmarried | 12 (26) | |
| History of tobacco usea |
No | 15 (32) |
| Yes | 28 (61) | |
| History of alcohol usea | No | 21 (46) |
| Yes | 21 (46) | |
| Antidepressant use | No | 39 (85) |
| Yes | 7 (15) | |
| BMI | 26.70 ± 5.62 | |
| Primary cancer site | Non-larynx | 37 (80) |
| Larynx | 9 (20) | |
| Stage | ≤III | 8 (17) |
| IV | 38 (83) | |
| Treatment | IMRT | 1 (2) |
| IMRT + Surgery | 7 (15) | |
| IMRT + Chemo | 34(74) | |
| IMRT + Chemo + Surgery | 4 (9) | |
| Chemotherapy drug | Cisplatin | 25 (66) |
| Carboplatin/Paclitaxel | 8 (21) | |
| Cisplatin/Carboplatin/ Paclitaxel |
4 (11) | |
| Others | 1 (2) | |
| Radiation dose (Gy) | 68.51 ± 3.12 |
Note. IMRT = Intensity-modulated radiation therapy, SD = standard deviation.
Having missing cases: history of tobacco use (3); history of alcohol use (4).
Married includes patients married or living as married; unmarried includes patients single, separated, divorced, or widowed.
3.2. Pre- to post-IMRT changes in fatigue and inflammation
Fatigue was the most commonly reported symptom among 13 patient-reported symptoms in HNC subjects. Ninety-eight percent of patients reported fatigue post-IMRT and 67% reported moderate to very severe fatigue. Additionally, fatigue increased significantly from pre-to post-IMRT (see Table 2). Significant increases in IL6 and sTNFR2 from pre- to post-IMRT were also observed, while the changes in CRP from pre- to post-IMRT were not significant (see Table 2).
Table 2.
Change in fatigue and inflammatory markers from pre- to one-month post-IMRT.
| Sample (N) | Pre-IMRT (Mean ± SD) | 1-month post-IMRT (Mean ± SD) | t-Test | p | |
|---|---|---|---|---|---|
| Fatigue | 46 | 45.70 ±15.41 | 57.07 ±15.91 | 5.38 | <0.0001 |
| IL6 (pg/mL) | 42 | 4.83 ± 4.81 | 8.04 ± 8.67 | 3.46 | 0.0013 |
| sTNFR2 (pg/mL) | 43 | 3.02 ± 1.29 | 4.49 ± 2.37 | 8.02 | <0.0001 |
| CRP (mg/L) | 43 | 10.71 ± 18.06 | 9.59 ± 15.44 | 0.44 | 0.6590 |
Note. CRP = C-reactive protein, IMRT = intensity-modulated radiation therapy, IL6 = interleukin 6, SD = standard deviation, sTNFR2 = soluble tumor necrosis factor receptor 2.
3.3. Association between fatigue and inflammatory markers over time
Controlling for age, smoking, BMI, treatment type, and antidepressant usage, positive associations were found between fatigue and IL6 (estimate = 10.231; p = 0.0210) and CRP (estimate = 5.174; p = 0.0171) across time.
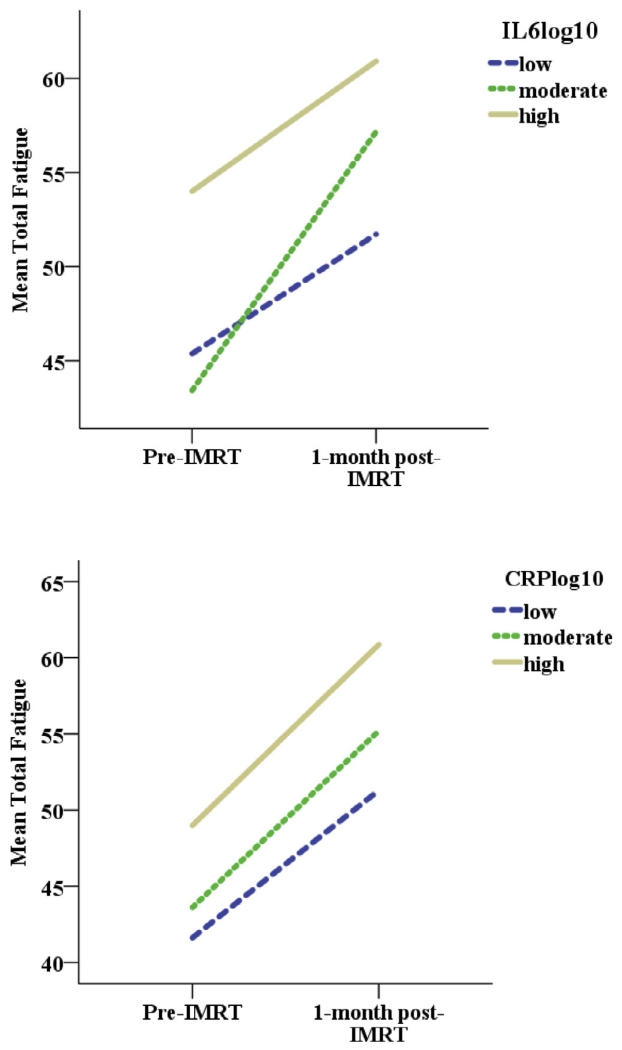
Next, the association between fatigue and inflammatory markers was examined adding depressive symptoms, cognitive dysfunction, and HNC and GI symptom clusters as covariates to the model. Only depressive symptoms, cognitive dysfunction, and the GI symptom cluster, were significantly related to fatigue in the final multivariate GEE model. Controlling for these covariates, inflammatory markers continued to be associated with fatigue as pre- sented in Table 3 for IL6 and Table 4 for CRP. When IL6 increased, fatigue increased significantly (p = 0.0059), after adjusting for significant covariates. A similar result was shown for CRP: when CRP increased, fatigue increased significantly (p = 0.0323), after adjusting for significant covariates. Interestingly, there was no significant interaction effect between IL6 or CRP and time. Indeed, the positive association between IL6 and CRP and fatigue was similar both pre- and post-IMRT (see Fig. 1).
Table 3.
Multivariate GEE analysis for IL6.
| Predictors | Estimates | SE | p |
|---|---|---|---|
| IL6a | 7.75 | 2.81 | 0.0058 |
| Cognitive dysfunction | 9.64 | 1.18 | <0.0001 |
| Depressive symptoms | 4.05 | 1.31 | 0.0020 |
| GI symptom cluster | 1.76 | 0.78 | 0.0237 |
| Timeb | 3.72 | 2.45 | 0.1295 |
Note. GEE = generalized estimating equations, GI = gastrointestinal, IL6 = interleukin 6.
Log10 transformed.
The reference point was pre-intensity-modulated radiation therapy (IMRT). Thus, compared to pre-IMRT, fatigue scores were increased by an average of 3.72 points following IMRT in this model.
Table 4.
Multivariate GEE analysis for CRP.
| Predictors | Estimates | SE | p |
|---|---|---|---|
| CRPa | 3.45 | 1.61 | 0.0323 |
| Cognitive dysfunction | 9.45 | 1.06 | <0.0001 |
| Depressive symptoms | 3.96 | 1.33 | 0.0028 |
| GI symptom cluster | 1.62 | 0.81 | 0.0444 |
| Timeb | 6.01 | 2.46 | 0.0145 |
Note. CRP = C-reactive protein, GEE = generalized estimating equations, GI = gastrointestinal.
Log10 transformed.
The reference point was pre-intensity-modulated radiation therapy (IMRT). Thus, compared to pre-IMRT, fatigue scores were increased by an average of 6.01 points following IMRT in this model.
Fig. 1.
The positive relationship between inflammation and fatigue did not change over time in head and neck cancer patients. The positive association between inflammatory markers (IL6 and CRP) and fatigue was similar at both pre and post-IMRT, because the interaction effect between IL6 and time or CRP and time on fatigue was not significant. Note. CRP = C-reactive protein, IMRT = intensity-modulated radiation therapy, IL 6 = interleukin 6. IL6log10: low: ≤0.42 (25th percentile); moderate: 0.43–0.99; high: ≥0.99 (75th percentile). CRPlog10: low: CRP ≤1; moderate: CRP 1–3; high: CRP ≥3.
Given the potential influence of antidepressants and circadian rhythm on inflammatory markers (Torres et al., 2013; Zhou et al., 2010; Petrovsky et al., 1998; Helmy et al., 2012), the use of antidepressants and the blood collection time were forced into the final model as control variables for data quality. The variables were not significant in the final model, and thus, are not presented in Tables 3 and 4.
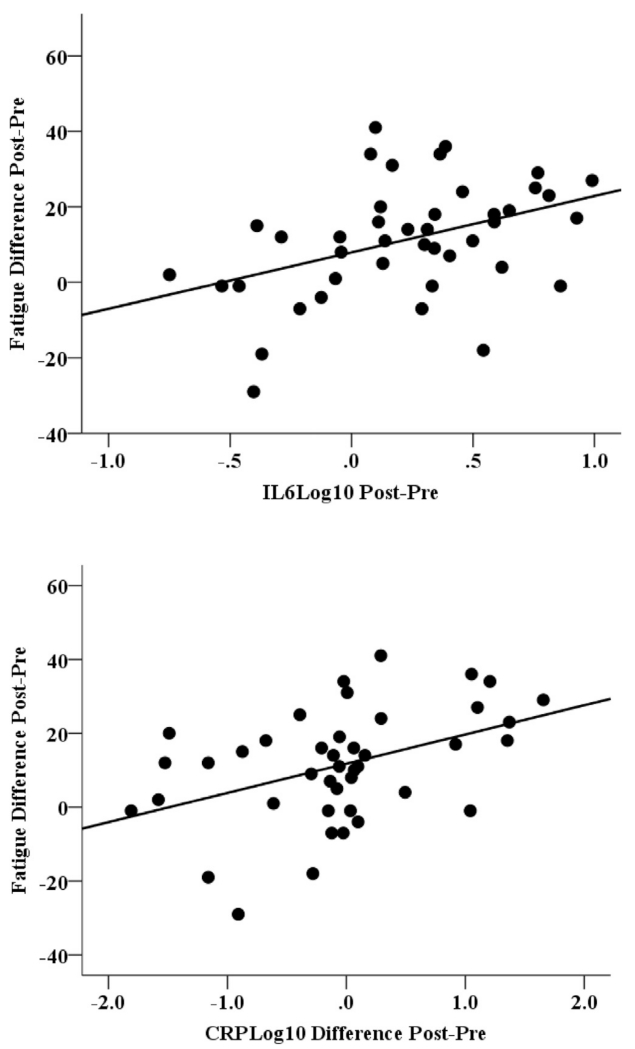
The association between the change scores (delta) in inflammatory markers and fatigue from pre to post was also tested by a linear regression model. Results showed that changes in inflammatory markers, IL6 and CRP, were significantly positively associated with changes in fatigue over time after adjusting for the same significant covariates as described above in GEE models (t = 2.64, p = 0.0121; t = 2.39, p = 0.0224; see Fig. 2).
Fig. 2.
Association of change (post-pre) in IL6 and CRP related to change in fatigue in head and neck cancer patients. Note. CRP = C-reactive protein, IMRT = intensity-modulated radiation therapy, interleukin 6 = IL 6. Changes in inflammatory markers, IL6 and CRP, were significantly positively associated with changes in fatigue over time after adjusting for significant covariates.
3.4. Association between fatigue and gene expression over time
We examined the association between genome-wide gene expression, IMRT, and fatigue via linear regression models with subject-specific fixed effects. 3534 genes demonstrated significant gene expression changes from pre to post-IMRT (FDR<.01, corresponding to p<.0013). 585 genes demonstrated significant association with within-subject changes in fatigue (FDR<.01, corresponding to p<.00021). Of the 543 genes significantly associated with both IMRT and fatigue, 144 (27%) were up-regulated with increased fatigue and 399 (73%) were down-regulated.
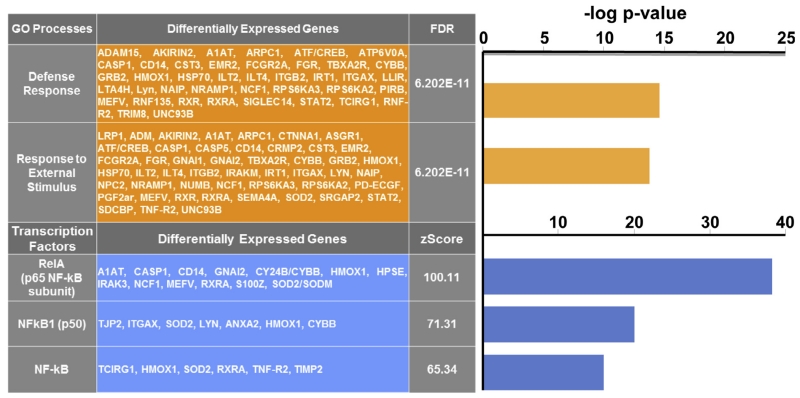
The top 2 biologic processes represented in the GO analysis among the up-regulated genes were the defense response and responses to external stimulus (see Fig. 3). MetaCore analysis of the up-regulated genes that were significantly associated with fatigue revealed an over-representation of genes regulated by NFkB family transcription factors, including RelA (p65 NFkB subunit; z score = 100.11; p = 8.73e-38), NFkB1 (p50; z score = 71.31; p = 2.50e-20), and NFkB (z score = 65.34; p = 1.90e-17; see Fig. 3).
Fig. 3.
Over-represented biologic processes and nuclear factor kappa B (NFkB)-mediated transcription factor networks in up-regulated genes in patients with head and neck cancer treated with intensity-modulated radiation therapy. Note. GO = gene ontology. Fatigue was associated with an over-representation of defense response and response to external stimulus genes and genes regulated by NFkB family transcription factors. Defense response by Metacore: “Reactions, triggered in response to the presence of a foreign body or the occurrence of an injury, which result in restriction of damage to the organism attacked or prevention/recovery from the infection caused by the attack”.
4. Discussion
The major findings of this study include: (1) inflammatory markers were positively associated with fatigue both before and after IMRT in HNC patients; (2) changes in inflammatory markers were associated with changes in fatigue following IMRT; (3) up-regulated gene transcripts associated with fatigue represented transcription factors related to the defense response and NFkB; and (4) although treatment triggered an increase in the inflammatory markers and fatigue, the association between inflammatory markers and fatigue did not rely on IMRT.
Our data support the hypothesis of a positive association between inflammation and fatigue. During the last decade, increasing evidence has identified a promising link between fatigue and inflammation. For instance, increased peripheral levels of IL-1ra (Collado-Hidalgo et al., 2006; Orre et al., 2009; Bower et al., 2002; Meyers et al., 2005), IL-6 (Meyers et al., 2005; Costanzo et al., 2005; Wratten et al., 2004), TNF (Bower et al., 2011; Meyers et al., 2005), CRP (Orre et al., 2009, 2011) appear to be significantly related to severe fatigue in cancer patients across disease trajectories. Other studies, however, have shown weak or non-significant associations among cancer-related fatigue and some of these inflammatory markers (Bower et al., 2011; Cameron et al., 2012; Kwak et al., 2012). Given the cross-sectional design in most studies (Schubert et al., 2007), a longitudinal design helps to elucidate the complex fatigue and inflammation association. Our longitudinal data showed significant predictive effects of IL6 and CRP on fatigue over time, and these effects were independent of other significant covariates, such depressive symptoms and cognitive dysfunction. Interestingly, our data showed that CRP did not increase from pre- to post-treatment but was associated with changes in fatigue, while sTNFR2 increased from pre- to post-treatment but was not associated with changes in fatigue. These data highlight the role of individual variability in inflammatory markers and fatigue responses over time. Overall, these findings support a positive association between inflammation and fatigue before and during cancer and its treatment and indicate that inflammatory markers may represent potential biomarkers of fatigue.
The results from gene expression data analyses further support the positive association between inflammation and fatigue. Consistent with our hypothesis and results from previous studies, three transcription factor networks were related to NFkB in the up-regulated genes that correlated with fatigue. NFkB, a critical transcription factor mediating inflammation and stress-induced responses (Karin and Greten, 2005; Gupta et al., 2011), is usually sequestered in the cytoplasm (Wan and Lenardo, 2010). Activated NF-κB translocates to the nucleus and binds to motifs in target gene-related promoters or enhancers (Wan and Lenardo, 2010; Ho et al., 2005). The subsequent gene expression enhances production of inflammatory cytokines like IL-1, IL-6, and TNF which were elevated in our protein measures (Karin and Greten, 2005). Our findings were consistent with a breast cancer study that showed increased expression of transcripts with response elements for NFkB (Bower et al., 2011). This increased expression of genes regulated by NFkB as found in our data has also been found in our study of breast cancer survivors with fatigue (Torres et al., 2013). Another small study investigating 5 patients with HNC during chemoradiotherapy also found Toll like receptor (TLR)/NFkB signaling pathways might play potential roles in development of treatment-related side effects/symptoms (Sonis et al., 2007).
The effect of treatment (analyzed as “time” in our statistical models) on the inflammatory markers indicates that IMRT increases inflammation in addition to fatigue. These findings are consistent with other studies that have found an association between radiation and inflammation (Multhoff and Radons, 2012; Schaue et al., 2015). Second, our data indicate that the association between inflammation and fatigue does not rely on radiation treatment. Indeed, the results from our study did not show a significant interaction effect between time (pre- vs. post-IMRT) and inflammatory markers and their impact on fatigue. These data are consistent with other studies demonstrating increased fatigue in association with inflammatory markers in patients with other forms of cancer, and are consistent with studies in laboratory animals and humans indicating that tumors themselves can increase inflammatory markers leading to behavioral changes including fatigue in absence of treatment (Schrepf et al., 2015; Pyter et al., 2014).
The mechanisms by which peripheral inflammation contributes to fatigue are not well-documented. Studies have shown that peripheral inflammatory markers likely contribute to fatigue through inhibitory effects on dopaminergic circuits in the basal ganglia (Capuron et al., 2012). The basal ganglia are associated with a variety of functions such as motivation to action and motor activity (Stocco et al., 2010; Arsalidou et al., 2013). Recent studies on patients with chronic fatigue syndrome, multiple sclerosis, and breast cancer have shown that fatigue is associated with dysfunction of certain brain regions including basal ganglia (Menning et al., 2015; Miller et al., 2014; Hanken et al., 2014). Moreover, malignant melanoma patients treated with the inflammatory cytokine, interferon-alpha, were found to exhibit alterations in basal ganglia glucose metabolism as well as dopamine function, which were related to fatigue (Capuron et al., 2007). However, direct association among fatigue, peripheral inflammation, and basal ganglia function in cancer patients has not been established. Future prospective studies measuring fatigue, inflammation, and brain function would help further elucidate the pathophysiology of cancer-related fatigue.
To our knowledge, this is the first study to examine the association between fatigue and inflammation in HNC patients. Other strengths of this study are its longitudinal design and the complementary assessment of protein and gene expression markers of inflammation. Major limitations of the study include the relatively small sample size and the preponderance of Caucasian males, which may challenge generalization of the findings. Nevertheless, the enrolled patient population is similar to those previously reported in HNC clinical trials (Xiao et al., 2013). In addition, use of over-the-counter anti-inflammatory medications were not assessed, which might have influenced the association between fatigue and inflammatory markers. Finally, the data are correlational in nature, and without an intervention to block inflammation, cause and effect inferences cannot be drawn.
Acknowledgments
The study was supported by NIH/NINR K99NR014587, NIH/NCI P30CA138292 and Oncology Nursing Society Foundation. The authors appreciate the support from Emory University School of Nursing, School of Medicine, and Winship Cancer Institute. The authors are also grateful for the support from participants and research staff and students.
References
- Ackerstaff AH, Rasch CR, Balm AJ, et al. Five-year quality of life results of the randomized clinical phase III (RADPLAT) trial, comparing concomitant intra-arterial versus intravenous chemoradiotherapy in locally advanced head and neck cancer. Head Neck. 2011 doi: 10.1002/hed.21851. [DOI] [PubMed] [Google Scholar]
- Arsalidou M, Duerden EG, Taylor MJ. The centre of the brain: topographical model of motor, cognitive, affective, and somatosensory functions of the basal ganglia. Hum. Brain Mapp. 2013;34(11):3031–3054. doi: 10.1002/hbm.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsevick A, Frost M, Zwinderman A, Hall P, Halyard M. I’m so tired: biological and genetic mechanisms of cancer-related fatigue. Qual. Life Res. 2010;19(10):1419–1427. doi: 10.1007/s11136-010-9757-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Roy. Stat. Soc. B. 1995;57(1):289–300. [Google Scholar]
- Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J. Clin. Oncol. 2000;18(4):743–753. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Aziz N, Fahey JL. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom. Med. 2002;64(4):604–611. doi: 10.1097/00006842-200207000-00010. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, Cole SW. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J. Clin. Oncol. 2011;29(26):3517–3522. doi: 10.1200/JCO.2011.36.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Irwin MR, Arevalo JM, Cole SW. Fatigue and gene expression in human leukocytes: increased NF-kappaB and decreased glucocorticoid signaling in breast cancer survivors with persistent fatigue. Brain Behav. Immun. 2011;25(1):147–150. doi: 10.1016/j.bbi.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron BA, Bennett B, Li H, et al. Post-cancer fatigue is not associated with immune activation or altered cytokine production. Ann. Oncol. 2012 doi: 10.1093/annonc/mds108. [DOI] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Demetrashvili MF, et al. Basal ganglia hypermetabolism and symptoms of fatigue during interferon-alpha therapy. Neuropsychopharmacology. 2007;32(11):2384–2392. doi: 10.1038/sj.npp.1301362. [DOI] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Drake DF, et al. Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon-alpha administration. Arch. Gen. Psychiatry. 2012;69(10):1044–1053. doi: 10.1001/archgenpsychiatry.2011.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J. Clin. Oncol. 2011;29(32):4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado-Hidalgo A, Bower JE, Ganz PA, Cole SW, Irwin MR. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin. Cancer Res. 2006;12(9):2759–2766. doi: 10.1158/1078-0432.CCR-05-2398. [DOI] [PubMed] [Google Scholar]
- Costanzo ES, Lutgendorf SK, Sood AK, Anderson B, Sorosky J, Lubaroff DM. Psychosocial factors and interleukin-6 among women with advanced ovarian cancer. Cancer. 2005;104(2):305–313. doi: 10.1002/cncr.21147. [DOI] [PubMed] [Google Scholar]
- Fang FM, Liu YT, Tang Y, Wang CJ, Ko SF. Quality of life as a survival predictor for patients with advanced head and neck carcinoma treated with radiotherapy. Cancer. 2004;100(2):425–432. doi: 10.1002/cncr.20010. [DOI] [PubMed] [Google Scholar]
- Fang FM, Tsai WL, Chien CY, et al. Changing quality of life in patients with advanced head and neck cancer after primary radiotherapy or chemoradiation. Oncology. 2005;68(4-6):405–413. doi: 10.1159/000086982. [DOI] [PubMed] [Google Scholar]
- Fernandez-Real JM, Broch M, Ricart W, et al. Plasma levels of the soluble fraction of tumor necrosis factor receptor 2 and insulin resistance. Diabetes. 1998;47(11):1757–1762. doi: 10.2337/diabetes.47.11.1757. [DOI] [PubMed] [Google Scholar]
- Gulliford SL, Miah AB, Brennan S, et al. Dosimetric explanations of fatigue in head and neck radiotherapy: an analysis from the PARSPORT Phase III trial. Radiother. Oncol. 2012;104(2):205–212. doi: 10.1016/j.radonc.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Gupta SC, Kim JH, Kannappan R, Reuter S, Dougherty PM, Aggarwal BB. Role of nuclear factor kappaB-mediated inflammatory pathways in cancer-related symptoms and their regulation by nutritional agents. Exp. Biol. Med. (Maywood) 2011;236(6):658–671. doi: 10.1258/ebm.2011.011028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanken K, Eling P, Hildebrandt H. The representation of inflammatory signals in the brain – a model for subjective fatigue in multiple sclerosis. Front. Neurol. 2014;5:264. doi: 10.3389/fneur.2014.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmy A, Antoniades CA, Guilfoyle MR, Carpenter KL, Hutchinson PJ. Principal component analysis of the cytokine and chemokine response to human traumatic brain injury. PLoS One. 2012;7(6):e39677. doi: 10.1371/journal.pone.0039677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok JT, Morrow GR, Roscoe JA, Mustian K, Okunieff P. Occurrence, severity, and longitudinal course of twelve common symptoms in 1129 consecutive patients during radiotherapy for cancer. J. Pain Symptom Manage. 2005;30(5):433–442. doi: 10.1016/j.jpainsymman.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Ho WC, Dickson KM, Barker PA. Nuclear factor-kappaB induced by doxorubicin is deficient in phosphorylation and acetylation and represses nuclear factor-kappaB-dependent transcription in cancer cells. Cancer Res. 2005;65(10):4273–4281. doi: 10.1158/0008-5472.CAN-04-3494. [DOI] [PubMed] [Google Scholar]
- Hoskin PJ, Robinson M, Slevin N, Morgan D, Harrington K, Gaffney C. Effect of epoetin alfa on survival and cancer treatment-related anemia and fatigue in patients receiving radical radiotherapy with curative intent for head and neck cancer. J. Clin. Oncol. 2009;27(34):5751–5756. doi: 10.1200/JCO.2009.22.3693. [DOI] [PubMed] [Google Scholar]
- Janaki MG, Kadam AR, Mukesh S, et al. Magnitude of fatigue in cancer patients receiving radiotherapy and its short term effect on quality of life. J. Cancer Res. Ther. 2010;6(1):22–26. doi: 10.4103/0973-1482.63566. [DOI] [PubMed] [Google Scholar]
- Jereczek-Fossa BA, Santoro L, Alterio D, et al. Fatigue during head-and-neck radiotherapy: prospective study on 117 consecutive patients. Int. J. Radiat. Oncol. Biol. Phys. 2007;68(2):403–415. doi: 10.1016/j.ijrobp.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 2005;5(10):749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- Kwak SM, Choi YS, Yoon HM, et al. The relationship between interleukin-6, tumor necrosis factor-alpha, and fatigue in terminally ill cancer patients. Palliat. Med. 2012;26(3):275–282. doi: 10.1177/0269216311406991. [DOI] [PubMed] [Google Scholar]
- Lyon DE, McCain NL, Pickler RH, Munro C, Elswick RK., Jr. Advancing the biobehavioral research of fatigue with genetics and genomics. J. Nurs. Scholarsh. 2011;43(3):274–281. doi: 10.1111/j.1547-5069.2011.01406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehanna H, Paleri V, West CM, Nutting C. Head and neck cancer-Part 1: epidemiology, presentation, and prevention. BMJ. 2010;341:c4684. doi: 10.1136/bmj.c4684. [DOI] [PubMed] [Google Scholar]
- Menning S, de Ruiter MB, Veltman DJ, et al. Multimodal MRI and cognitive function in patients with breast cancer prior to adjuvant treatment- the role of fatigue. NeuroImage Clin. 2015;7:547–554. doi: 10.1016/j.nicl.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers CA, Albitar M, Estey E. Cognitive impairment, fatigue, and cytokine levels in patients with acute myelogenous leukemia or myelodysplastic syndrome. Cancer. 2005;104(4):788–793. doi: 10.1002/cncr.21234. [DOI] [PubMed] [Google Scholar]
- Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J. Clin. Oncol. 2008;26(6):971–982. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Jones JF, Drake DF, Tian H, Unger ER, Pagnoni G. Decreased basal ganglia activation in subjects with chronic fatigue syndrome: association with symptoms of fatigue. PLoS One. 2014;9(5):e98156. doi: 10.1371/journal.pone.0098156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minton O, Richardson A, Sharpe M, Hotopf M, Stone P. Drug therapy for the management of cancer-related fatigue. Cochrane Database Syst. Rev. (Online) 2010;7:CD006704. doi: 10.1002/14651858.CD006704.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SA. Cancer-related fatigue: state of the science. PM & R. 2010;2(5):364–383. doi: 10.1016/j.pmrj.2010.03.024. [DOI] [PubMed] [Google Scholar]
- Montazeri A. Quality of life data as prognostic indicators of survival in cancer patients: an overview of the literature from 1982 to 2008. Health Qual. Life Outcomes. 2009;7:102. doi: 10.1186/1477-7525-7-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multhoff G, Radons J. Radiation, inflammation, and immune responses in cancer. Front. Oncol. 2012;2:58. doi: 10.3389/fonc.2012.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute PRO-CTCAE study overview NCCCP. 2010 https://wiki.nci.nih.gov/display/PROCTCAE/NCCCP+and+PRO-CTCAE.
- National Cancer Institute Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) 2012 http://outcomes.cancer.gov/tools/pro-ctcae.html.
- Orre IJ, Murison R, Dahl AA, Ueland T, Aukrust P, Fossa SD. Levels of circulating interleukin-1 receptor antagonist and C-reactive protein in long-term survivors of testicular cancer with chronic cancer-related fatigue. Brain Behav. Immun. 2009;23(6):868–874. doi: 10.1016/j.bbi.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Orre IJ, Reinertsen KV, Aukrust P, et al. Higher levels of fatigue are associated with higher CRP levels in disease-free breast cancer survivors. J. Psychosom. Res. 2011;71(3):136–141. doi: 10.1016/j.jpsychores.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Petrovsky N, McNair P, Harrison LC. Diurnal rhythms of pro-inflammatory cytokines: regulation by plasma cortisol and therapeutic implications. Cytokine. 1998;10(4):307–312. doi: 10.1006/cyto.1997.0289. [DOI] [PubMed] [Google Scholar]
- Pyter LM, El Mouatassim Bih S, Sattar H, Prendergast BJ. Peripheral tumors alter neuroinflammatory responses to lipopolysaccharide in female rats. Brain Res. 2014;1552:55–63. doi: 10.1016/j.brainres.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Gibby CC, Spitz MR, Yennurajalingam S, et al. Role of inflammation gene polymorphisms on pain severity in lung cancer patients. Cancer Epidemiol. Biomarkers Prev. 2009;18(10):2636–2642. doi: 10.1158/1055-9965.EPI-09-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107(3):363–369. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- Sawada NO, de Paula JM, Sonobe HM, Zago MM, Guerrero GP, Nicolussi AC. Depression, fatigue, and health-related quality of life in head and neck cancer patients: a prospective pilot study. Support Care Cancer. 2012 doi: 10.1007/s00520-012-1390-2. [DOI] [PubMed] [Google Scholar]
- Schaue D, Micewicz ED, Ratikan JA, Xie MW, Cheng G, McBride WH. Radiation and inflammation. Semin. Radiat. Oncol. 2015;25(1):4–10. doi: 10.1016/j.semradonc.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrepf A, Lutgendorf SK, Pyter LM. Pre-treatment effects of peripheral tumors on brain and behavior: neuroinflammatory mechanisms in humans and rodents. Brain Behav. Immun. 2015 doi: 10.1016/j.bbi.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert C, Hong S, Natarajan L, Mills PJ, Dimsdale JE. The association between fatigue and inflammatory marker levels in cancer patients: a quantitative review. Brain Behav. Immun. 2007;21(4):413–427. doi: 10.1016/j.bbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Shmelkov E, Tang Z, Aifantis I, Statnikov A. Assessing quality and completeness of human transcriptional regulatory pathways on a genome-wide scale [serial online] Biol. Direct. 2011;6:15. doi: 10.1186/1745-6150-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J. Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- Smets EM, Garssen B, Bonke B, De Haes JC. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J. Psychosom. Res. 1995;39(3):315–325. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- Sonis S, Haddad R, Posner M, et al. Gene expression changes in peripheral blood cells provide insight into the biological mechanisms associated with regimen-related toxicities in patients being treated for head and neck cancers. Oral Oncol. 2007;43(3):289–300. doi: 10.1016/j.oraloncology.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Spratt DE, Sakae M, Riaz N, et al. Time course and predictors for cancer-related fatigue in a series of oropharyngeal cancer patients treated with chemoradiation therapy. Oncologist. 2012;17(4):569–576. doi: 10.1634/theoncologist.2011-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocco A, Lebiere C, Anderson JR. Conditional routing of information to the cortex: a model of the basal ganglia’s role in cognitive coordination. Psychol. Rev. 2010;117(2):541–574. doi: 10.1037/a0019077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Pace TW, Liu T, et al. Predictors of depression in breast cancer patients treated with radiation: role of prior chemotherapy and nuclear factor kappa B. Cancer. 2013;119(11):1951–1959. doi: 10.1002/cncr.28003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan F, Lenardo MJ. The nuclear signaling of NF-kappaB: current knowledge, new insights, and future perspectives. Cell Res. 2010;20(1):24–33. doi: 10.1038/cr.2009.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XS, Shi Q, Williams LA, et al. Inflammatory cytokines are associated with the development of symptom burden in patients with NSCLC undergoing concurrent chemoradiation therapy. Brain Behav. Immun. 2010;24(6):968–974. doi: 10.1016/j.bbi.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wratten C, Kilmurray J, Nash S, et al. Fatigue during breast radiotherapy and its relationship to biological factors. Int. J. Radiat. Oncol. Biol. Phys. 2004;59(1):160–167. doi: 10.1016/j.ijrobp.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Xiao C. Identifying symptom clusters in patients with head and neck cancer post combined chemoradiation therapy Philadelphia. School of Nursing University of Pennsylvania; 2011. [Google Scholar]
- Xiao C, Hanlon A, Zhang Q, et al. Symptom clusters in patients with head and neck cancer receiving concurrent chemoradiotherapy. Oral Oncol. 2013;49(4):360–366. doi: 10.1016/j.oraloncology.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Hanlon A, Zhang Q, et al. Risk factors for clinician-reported symptom clusters in patients with advanced head and neck cancer in a phase 3 randomized clinical trial: RTOG 0129. Cancer. 2014;120(6):848–854. doi: 10.1002/cncr.28500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Fragala MS, McElhaney JE, Kuchel GA. Conceptual and methodological issues relevant to cytokine and inflammatory marker measurements in clinical research. Curr. Opin. Clin. Nutr. Metab. Care. 2010;13(5):541–547. doi: 10.1097/MCO.0b013e32833cf3bc. [DOI] [PMC free article] [PubMed] [Google Scholar]