Abstract
Liver disease is a major cause of illness and death worldwide. In China alone, liver diseases, primarily viral hepatitis (predominantly hepatitis B virus, HBV), nonalcoholic fatty liver disease and alcoholic liver disease affect approximately 300 million people. The establishment of the Expanded Program on Immunization in 1992 has resulted in a substantial decline in the number of newly HBV-infected patients; however, the number of patients with alcoholic and nonalcoholic fatty liver diseases is rising at an alarming rate. Liver cancer, one of the most deadly cancers, is the second most common cancer in China. Approximately 383,000 people die from liver cancer every year in China, which accounts for 51% of the deaths from liver cancer worldwide. Over the past 10 years, China has made some significant efforts to shed its “leader in liver diseases” title by investing large amounts of money in funding research, vaccines and drug development for liver diseases, and by recruiting many Western-trained hepatologists and scientists. Over the last two decades, hepatologists and scientists in China have made significant improvements in liver disease prevention, diagnosis, management and therapy. They have been very active in liver disease research, as shown by the dramatic increase in the number of publications in Hepatology. Nevertheless, many challenges remain that must be tackled collaboratively. In this review, we discuss the epidemiology and characteristics of liver diseases and liver-related research in China.
Introduction
Liver diseases, including hepatitis B virus (HBV) and hepatitis C virus (HCV) infections, alcoholic liver disease (ALD), nonalcoholic fatty liver disease (NAFLD) and associated cirrhosis, liver failure (LF) and hepatocellular carcinoma (HCC), are major causes of illness and death worldwide. In China, liver diseases affect approximately 300 million people, thus having a major impact on the global burden of liver diseases. The Expanded Program on Immunization and the mandatory HCV screening for blood transfusion implemented in China during the early 1990s have largely prevented new HBV and HCV infections, respectively. However, the number of patients with ALD and NAFLD has dramatically increased because of alterations in people’s lifestyles. HBV- and HCV-associated end-stage liver diseases, such as cirrhosis, chronic LF and HCC, have increased in recent years, leading to a high mortality from liver diseases in China. Over the past decade, basic and clinical research on liver diseases in China, which were mainly led by hepatologists and scientists who previously received training in Western countries, has been very active, as shown by a dramatic increase in the number of publications in Hepatology. In this article, we discuss the epidemiology and characteristics of liver diseases, mainly including HBV and HCV, ALD, NAFLD, and end-stage liver diseases, and liver-related research in China.
Epidemiology of liver diseases in China
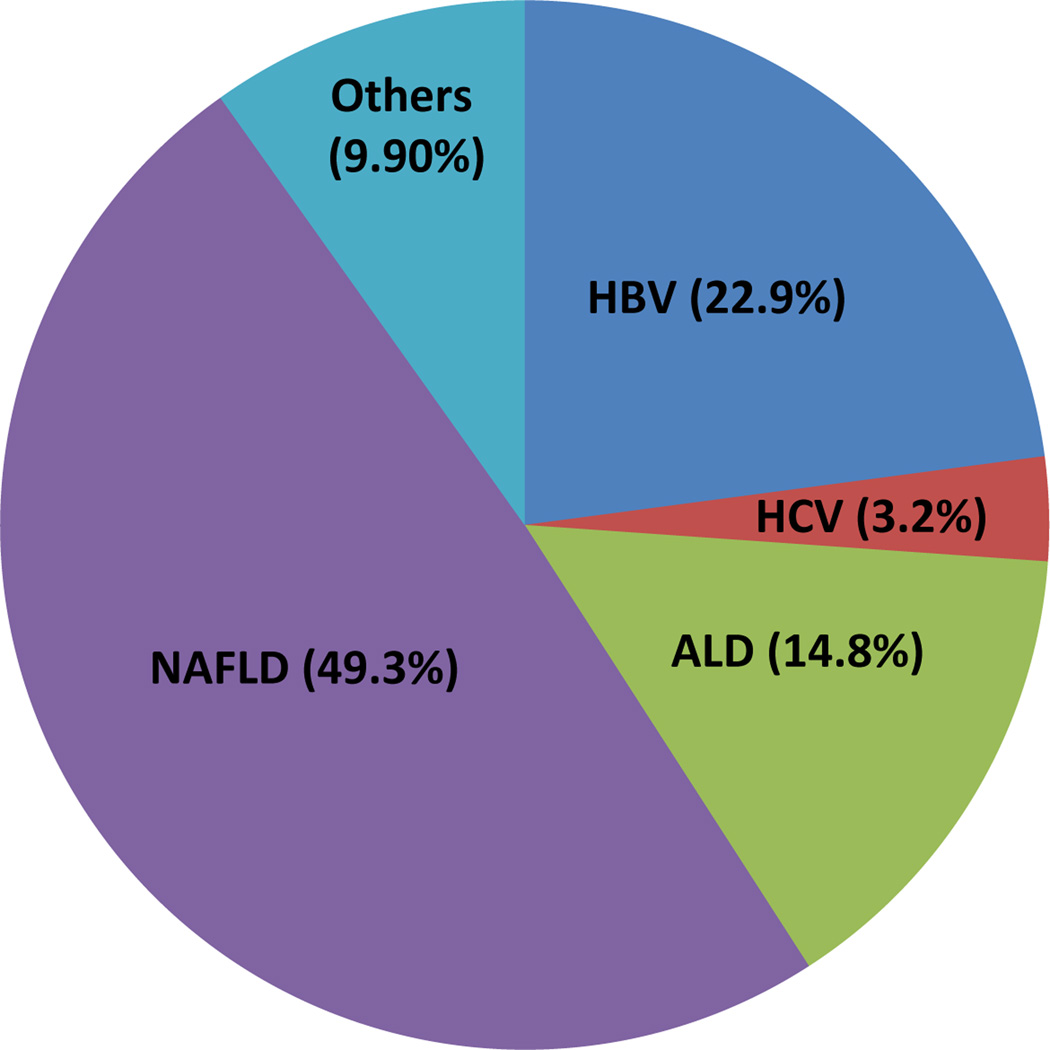
Liver disease causes serious public health problems because of its high prevalence worldwide and poor long-term clinical outcome, including premature deaths from liver decompensation, cirrhosis and HCC. Several types of liver diseases, including chronic HBV and HCV infection, ALD, NAFLD, autoimmune liver disease and drug-induced liver disease (DILI), potentially threaten a large proportion of the global population. This is particularly true in China, where the prevalence of HBV and HCV infection are extremely high, and ALD and NAFLD are emerging as leading causes of chronic liver diseases. For example, HBV infection affects at least 2 billion people worldwide; among these, 350–400 million are chronic HBV carriers.1 In China, although the HBV prevalence was reduced to 7.2% in the general population by 2006, 97 million people are HBV carriers and at least 20 million of them still suffer from active chronic HBV infection, alone or in combination with liver cirrhosis and/or hepatocellular carcinoma.2 HCV infections affect 150 million people worldwide, of which around 10 million are Chinese patients. Both ALD and NAFLD are highly prevalent in developed countries and reported to have prevalence of approximately 7.4% and 20%–33% in the general adult populations, respectively.3, 4 In China, although data on nationwide large-scale epidemiological ALD and NAFLD surveys are unavailable, the prevalence of ALD and NAFLD in some local areas ranges from 2.3% to 6.1% (median 4.5%) and from 6.3% to 27.0% (median 15%), respectively.5 Based on similar populations in Europe and the US (1.2 billion) and in China (1.3 billion), we calculated that the ALD and NAFLD cases in China could possibly account for nearly half of the ALD and NAFLD cases worldwide. The analysis of the above data suggested that liver diseases in China have a major impact on the global burden of diseases (Table 1), and the liver disease profile in China was described in Figure 1. Also, Table 2 shows the number and distribution of inpatients with different types of liver diseases over the last 12 years in the Beijing 302 Hospital.
Table 1.
The etiologies of liver diseases worldwide and in China
| Worldwide | Euro-America | China | |
|---|---|---|---|
| HBV infection history | 2 billion | ||
| HBsAg+ | 350–400 million | < 10 million (< 1%) | 93 million (7.18%) |
| Anti-HCV positive | 200 million (3%) | > 30 million (2–3%) | 13 million (1%) |
| ALD (adult) | > 150 million | 84 million (7.4%) | 60 million (4.5%) |
| NALFD | > 600 million | 400 million (20–33%) | 200 million (15%) |
| Total | > 1300 million | > 500 million | > 400 million |
Figure 1. The distribution of various liver diseases in China.
The number indicates the estimated proportion of each liver disease among global people with liver diseases.
Table 2.
The increased cases with various liver diseases from 2002 to 2013, in Beijing 302 Hospital, China
| Year | HBV | HCV | AIH | ALD | DILI | HCC |
|---|---|---|---|---|---|---|
| 2002 | 3948 | 452 | 34 | 70 | 86 | 300 |
| 2003 | 2901 | 339 | 30 | 106 | 75 | 426 |
| 2004 | 3778 | 556 | 61 | 204 | 118 | 807 |
| 2005 | 4042 | 682 | 81 | 282 | 161 | 753 |
| 2006 | 4171 | 944 | 93 | 319 | 226 | 1148 |
| 2007 | 4502 | 1374 | 147 | 468 | 240 | 1704 |
| 2008 | 4735 | 1683 | 153 | 514 | 362 | 2129 |
| 2009 | 6177 | 2107 | 192 | 664 | 442 | 2874 |
| 2010 | 5885 | 2738 | 188 | 878 | 540 | 4085 |
| 2011 | 5644 | 3324 | 227 | 1117 | 782 | 5655 |
| 2012 | 6023 | 5214 | 239 | 1413 | 882 | 7231 |
| 2013 | 5775 | 5706 | 230 | ND | 1072 | 8621 |
ND, no data
2. Characteristics of liver diseases in China
(1) HBV infection
The HBV carrier rates vary from low (0.1%–2%) in the USA and Western Europe, to intermediate (2%–8%) in Mediterranean countries and Japan, and to high (8%–20%) in sub-Saharan Africa and most parts of Asia.1 In China, a nationwide HBV sero-epidemiological survey conducted in 1992 showed that the HBsAg carrier rate in the entire population was 9.8%. As a result, the Chinese government initiated a universal HBV immunization program in 1992, and a completely free HBV vaccination was provided for all newborns, starting in 2005. These efforts have significantly reduced the HBsAg carrier rates in the general population, from 9.8% in 1992 to 7.2% in 2006.6 In particular, the HBsAg carrier rates in children (< 10 years old) were reduced to 1.5% in 2006.6 Accordingly, the HBs antibody was found in 60% children under 13 years old in 2006.6 Thus, HBV vaccinations have been very successful and has protected, including children, from HBV infection in China over the past 20 years (Table 3).
Table 3.
Impact of HBV vaccination in China over the past 20 years.
| In 1992 | In 2006 | |
|---|---|---|
| HBsAg carrier rates | ||
| General population | 9.75% | 7.18% |
| <4-year-old children | 9.7% | 1% |
| 5–14-year-old children | 10.7% | 2.3% |
| HBsAb carrier rates | ||
| General population | 27.4% | ND |
| 1–13-year-old children | 37.2% (Guangdong) | 60% |
| Anti-HBc prevalence | ||
| < 10-year-old children | Approximately 35% | Approximately 5% |
| 10–19-year-old children | Approximately 47% | Approximately 12% |
ND, no data.
In addition to HBV prevalence, the HBV genotypes observed in China also differ significantly from those in Europe and the USA (Table 4). In Europe and the USA, genotypes A and D are prevalent, while in China, genotypes B and C are more predominant. In addition, genotype B is more frequent in Southern China, while C is found more frequently in Northern China. In some local regions of Northern China, the sub-genotype C2 is predominant, whereas sub-genotype C1 is more prevalent in Southern China.7 Compared with genotype B, HBV genotype C exhibits less replication activity in young patients, but generates higher frequencies of HCC-associated mutations.
Table 4.
Differences in Hepatitis B and C among China, the US and Europe.
| China | US and Europe | |
|---|---|---|
| HBV | ||
| Dominant genotype [2, 8] | B, C | A, D |
| Dominant transmission manner [2, 6, 7] |
Mother-to-infant transmission | Horizontal transmission |
| Dominant infection time [8] | Neonates and Childhood | Adult |
| Immune tolerance phase | Yes | No |
| Clinical features [8] | High chronic infection | High acute infection |
| Therapeutic responses | Low responsiveness in IT phase | High responsiveness |
| HCV | ||
| Dominant genotype [2, 10, 16] | 1b, 2a, 3b, 6 | 3, 4 |
| Dominant transmission manner [9–11, 16] |
Blood transfusions and medical procedures |
Injection drug use |
| IL-28b polymorphism [13, 14, 16] | CC: 80% | 40–60% |
| IFN-α/RBV responsiveness [12, 13, 16] |
70–80% | 40–50% |
Another important difference is the natural history of chronic HBV infections (Table 4). Generally, HBV infection occurs among adults in Europe and the USA, with more than 90% of cases developing into acute resolved HBV infection and less than 5% into chronic HBV infection. In China, because the majority of HBV infection occurs at an early age, chronicity and viral persistence are more easily developed. Indeed, chronic HBV infection persist in 1%–5% of infected adults, 20%–30% of young children and up to 90%–95% of perinatally infected subjects.8 HBsAg prevalence is also characterized by a significant family history in China. It has been reported that 23.2% of HBsAg-positive families contained more than two HBsAg carriers. In 1.4% of families in China, HBsAg carriers account for 50%, or more than four cases in the family.
Although antiviral drugs like NUCs and interferon (IFN)-α are available, there are no data available on the exact number of CHB patients who received antiviral therapy. It is estimated around 10% CHB patients (2 million) in China are undergoing antiviral treatment with follow-up checks every three months. The regimen includes conventional or PEG-IFN-α alone or NUCs (ETV, ADV, LDT, or LAM are available with exception of TDF) alone or in different combinations like ETV plus ADV, or LAV, or ETV plus PEG-IFN-α. NUCs are frequently used in the ETV> ADV > LDT > LAM. In addition, medical insurance only covers the medical cost for in-patients in some provinces. Thus, for example, in 2013, every CHB patient without cirrhosis may pay the average cost of $2000–2500 directly for drugs, hospitalization and three monthly follow-up checks in our hospital. The CHB patient with compensated or decompensated LC pays at least doubles that cost.
(2) HCV infection
In 2005, more than 185 million people were estimated to be HCV-specific antibody positive, representing 2.8% of the world’s population. However, there are marked differences in the HCV prevalence among different countries and regional age- and risk-groups, ranging from 0.1% to 5%. In the US, it is estimated that 3.9 million individuals (or 1.8% of the general population) are infected with HCV. China has been considered a relatively high endemic area for HCV infection in the past. The prevalence of anti-HCV was estimated to average 3.2% in the general population, according to a national epidemiological survey carried out in most regions of China in 1992, with blood or blood product transfusion as the major route of infection. Since 1993, mandatory screening for anti-HCV and other precautions to prevent blood-borne disease transmission have been implemented extensively. New cases of HCV infection have declined dramatically. As shown by a later national survey in 2006, the prevalence of anti-HCV is only 0.43% in China.9 To date, there remain no authoritative data of comprehensive epidemiological surveys in China, However, taking the third generation of anti-HCV tests and HIV/HCV co-infection into account, it has been estimated by some experts that the “true” prevalence of anti-HCV may be approximately 10 million in China (personal communications from some experts in the China CDC).2
HCV genotypes also display substantial differences worldwide (Table 4). In the USA and Europe, genotypes 3 and 4 are predominant, while the predominant HCV genotype in China is genotype 1 (69.6%). In China, genotype 1b accounts for 68.4%, followed by 2a at 19.5%.10 The less common genotypes 3b and 6 (mainly 6a) are observed predominantly in the Southern provinces. Interestingly, subtype 1b strains are more likely to be associated with transmission via blood transfusion and medical procedures, while subtype 6a strains are more likely to be linked to intravenous drug use (IDU) and sexual transmissions.11 This genotypic difference in HCV between western countries and China may be, to some extent, associated with each population’s responsiveness to PEG-IFN-α and ribavirin (PEG-IFN-α/RBV) treatment.12
In addition, single nucleotide polymorphisms near the IL-28B gene region have also been found to be associated with the treatment efficacy of PEG-IFN-α/RBV in HCV-infected patients.13 The frequency of the rs12979860 C allele is higher in Chinese HCV patients than in Caucasian patients.14 Evaluation of a large cohort of Chinese HCV patients showed that the major HCV genotype is 1b (approximately 60%–70%) and the predominant host IL28b genotype of rs12979860 is CC (84%).14 These data suggest that the global differences in the IL-28B allele frequency and HCV genotypes may explain the better response to peg-IFN-α/RBV therapy observed in Chinese patients.
Although a couple of direct antiviral agents (DAAs) are undergoing approval for clinical trials in China, it may at least take 2–3 more years for DAAs to be available for HCV-infected patients. Additionally, because of the extremely high cost and unavailability of DAAs, standard IFN-α/RBV (or PEG-IFN-α/RBV) therapy will remain the first-line therapy in China,15 which can achieve 60%–70% SVR for adult patients and much higher SVRs (up to 98%) for chronic HCV children aged 1–5 years old (unpublished data). Importantly, the Chinese patients who received standard antiviral therapy account for approximately 2% of the 10 million patients with chronic hepatitis C, and around 10 % naïve treatment patients have already been diagnosed with liver cirrhosis (unpublished data). By contrast, most of these recruited DAA-treated patients in previous reports were non-Asian, with genotype 1a and a low fibrosis score. Therefore, the urgent need is to test the DAA treatment for HCV genotype 1b patients who have failed or are ineligible for interferon therapy and have advanced liver fibrosis in China.16 Taken together, we believe that more clinical trials should be conducted in Chinese patients with chronic HCV infection to understand how we can apply these new DAAs.
(3) Alcohol abuse and ALD
Excessive alcohol intake is a major public health challenge worldwide and has been identified as one of the main determinants of a variety of non-communicable diseases, including ALD.3 It has been estimated that in 2010 alone, alcohol-related cirrhosis accounted for 47.9% of all liver cirrhosis deaths and 46.9% of all liver cirrhosis disability adjusted life years (DALYs), representing 0.9% of all global deaths and 0.6% of all global DALYs.3
Alcohol consumption in China has substantially increased over the last three decades.17 In 2007, the prevalence of lifetime alcohol abuse and alcohol dependence in rural Chinese men was 4.8%–11.8% and 8.6%–10.8%, respectively.18 In Beijing, the 12-month prevalence of alcohol abuse and associated alcohol dependence was 13.8% and 1.7%, respectively, and only 2.4% of subjects with alcohol dependence were receiving treatment.19 Alcohol abuse is becoming increasingly severe among the Chinese, especially in Northeast China.20 Unfortunately, nationwide large-scale epidemiological ALD surveys have not been conducted in China. The point prevalence of ALD in some local areas in China has been reported to range from 2.3% to 6.1% (the median prevalence was 4.5% in Chinese people).5 In addition, the prevalence of different stages of ALD was also reported in some surveys. The incidences of alcoholic fatty liver, alcoholic hepatitis and alcoholic cirrhosis were found to be at least 50%, 10% and 10% among heavy alcohol drinkers with a more than 5-year drinking history, respectively.5 From a multicenter study of ALD in China, the annual incidence of ALD among hospitalized patients with liver diseases increased gradually from 2000 to 2004 (2.7%, 2.9%, 3.0%, 3.6% and 4.4%, respectively).5 There has also been a gradual increase in the number of patients with end-stage ALD undergoing liver transplantation (LT) in China over the past 10 years; the proportion of ALD patients receiving LT is around 7.5%. The “6 months rule” for LT is applied in most centers; however, the duration of pre-transplant sobriety for some patients who appeared to have gastrointestinal hemorrhage, hepatic encephalopathy, and acute liver failure was less than 2 months.21
A recently dramatic increase in national alcohol consumption has led to ALD becoming the second most common cause of end-stage liver disease after viral hepatitis in China.17 However, Chinese food culture is lenient towards to alcohol drinking and the drunken state. In addition, because of significant differences in ethanol metabolism, drinking patterns and co-factors between the Chinese and people from Western countries, the pathogenesis and progression of ALD in Chinese patients may be significantly different from those in Western patients. For example, approximately 40–50% of Chinese people are homozygous or heterozygous for the ALDH2*2 allele and have low ALDH2 activity.22 Such people show high blood acetaldehyde concentrations after alcohol consumption; however, how ALDH2 deficiency affects ALD development and progression remains largely unknown. A recent study revealed that ALDH2-deficient mice were more susceptible to alcohol-induced liver inflammation and fibrosis, but were surprisingly resistant to alcohol-induced fatty liver and elevated blood ALT levels.23 This finding suggests that the individuals with inactive ALDH2*2 genes may not have obvious fatty livers or elevated serum ALT levels after moderate or even heavy drinking. However, they may have active hepatitis and liver fibrosis, and should be carefully monitored during follow-up. Because the number of ALD is increased at an alarming rate over the last 20 years, Chinese Society of Hepatology established the fatty liver and ALD study group in 2001 and has published a series of Clinical Practice Guidelines for Diagnosis and Management of ALD since 2003.17 However, in China, cheap price of hard liquor, easy accessibility to alcohol, and alcohol advertisement will make it very difficult to prevent the increase in alcoholic liver disease.
(4) Obesity, type 2 diabetes, metabolic syndrome, and NAFLD
Over the last two decades, the prevalence of overweight and obesity increased remarkably among 7- to 18-year-old students in China. In 2010, it was estimated that 9.9% of Chinese school-aged children and adolescents were overweight and that an additional 5.1% were obese, representing an estimated 30.43 million individuals.24 The situation is similar in Chinese adults, although there are no available data. The age- and sex-adjusted rates of overall obesity and central obesity in the Chinese adult population were 7.5% and 12.3%, respectively.25 Central obesity is thought to be more pathogenic than overall obesity and is significantly correlated with diabetes, metabolic syndrome and NAFLD.26 According to the China Health and Nutrition Survey (CHNS), which was conducted from 1993 to 2009, the age-standardized prevalence of central obesity among Chinese adults with BMI < 25 kg/m2 increased from 11.9% to 21.1%.27 Metabolic syndrome has become an important public health problem in China. The age-standardized prevalence of metabolic syndrome was 9.8% (95% CI 9.0%–10.6%) in men and 17.8% (16.6%–19.0%) in women in China.28 For children ≥ 10 years, the prevalence of metabolic syndrome was 32.3% in the obese group and 8.4% in the overweight group, according to the modified USA National Cholesterol Education Program’s Adult Treatment Panel III report (ATP III) definition.29 The estimated prevalence of diabetes among a representative sample of Chinese adults was 11.6%. Projections based on sample weighting suggest that this prevalence may represent up to 113.9 million Chinese adults with diabetes. Among patients diagnosed with diabetes, only 25.8% (95% CI, 24.9%–26.8%) receive treatment for diabetes, and only 39.7% (95% CI, 37.6–41.8%) of those treated had an adequate level of glycemic control.30 These results indicated that a large proportion of Chinese suffer from obesity, metabolic syndrome, and type 2 diabetes; as a result, these diseases have become important public health concerns in China.
The most common liver disease seen in obesity, metabolic syndrome and diabetes is NAFLD. The presence of metabolic syndrome is a strong predictor for the presence of nonalcoholic steatohepatitis (NASH) in patients with NAFLD. Moreover, type 2 diabetes causes more severe steatohepatitis and advanced fibrosis, and patients with diabetes have an increased risk for cirrhosis and HCC.31 Several population-based epidemiological studies have indicated that the prevalence of NAFLD in China is approximately 15% among adults (6.3%–27.0%) and 1.3% in children and adolescents. The incidence of ultrasound-defined NAFLD in 5,402 nonalcoholic healthy subjects (4,633 men, mean age 37 years) was found to be 6.1% in Shanghai.32 NAFLD has also been found to affect over a quarter of the general population in Hong Kong.33 However, the proportion of patients with advanced fibrosis in NAFLD is low (3.7%) in Hong Kong, and there are no data available on the incidence of NAFLD with fibrosis in Mainland China. In addition, among cases of chronic hepatitis with unknown etiologies, the prevalence of biopsy-proven NASH was 16% (15/97); in patients with morbid obesity, the prevalence was 34% (54/160).34 In 110 biopsy-proven NAFLD patients, simple fatty liver, NASH, and cirrhosis were diagnosed in 45 (40.9%), 63 (57.3%), and 2 (1.8%) cases, respectively.34 These data indicated that NAFLD is possibly replacing hepatitis B as the leading cause of chronic liver disease in China. In addition, NAFLD is highly prevalent in HBV-infected patients, with annual the prevalence increasing between 2002 and 2011 from 8.2% to 31.8%.35 Up to now, there are no specific treatments for the NAFLD; however, government, physicans, and researchers have started to pay attentions on this as demonstrated by listing NAFLD as one of key research projects since 2012, by forming the fatty liver disease interest group under the Chinese Society of Hepatology on 2001, and by organizing biannual meetings for fatty liver disease since 2002.
(5) End-stage liver diseases: LC, chronic LF and HCC
In general, without efficient treatment, all types of chronic hepatitis will finally progress into end-stage liver diseases (ESLD), such as cirrhosis, chronic LF, and HCC. Most ESLD displays a poor clinical outcome. Cirrhosis is the major cause of liver disease-related morbidity and mortality worldwide. In China, the prevalence of HBsAg positivity is more than 70% in cirrhotic patients.36 According to the Management of Clinical Diagnosis and Evaluation for Cirrhosis by Ministry of Science and Technology, nationwide standard screening tests, including Fibroscan and/or Ultrasonic examination, every three months and liver biopsy-proven cirrhosis further confirmed these serological studies, with hepatic HBsAg detected in 79% of cases with cirrhosis.37,51 In addition, serum HBV DNA was detected in 23%–39% of HBsAg-negative cirrhotic patients, suggesting a role for occult HBV infection in the development of cirrhosis.38 In contrast, the prevalence of HCV markers, including anti-HCV and HCV-RNA, in cirrhotic livers varied from 5%–43%. In addition, approximately 7% of cirrhosis cases were attributed to alcohol abuse. In central China, where schistosomiasis was once prevalent, up to 18% of the cases of cirrhosis resulted from this pathogen. These data indicate that chronic HBV and HCV infection, alcohol drinking and parasitic infection are the most important causes of cirrhosis in China, which consequently affect the health of the population significantly. Currently, the Chinese Society of Hepatology is responsible for formulating the diagnosis and treatment guidelines for patients with cirrhosis and decompensated cirrhosis with complications in China. Finally, although a number of centers have used stem cells for the treatment of cirrhosis, this is still on experimental basis and the long-term safety and efficacy need to be investigated in the randomized controlled studies before making any recommendations.
LF is another ESLD. According to the different types of liver disease, precipitating events and progressing conditions, LF is usually categorized into acute (ALF), acute-on-chronic (ACLF) and chronic (CLF).39 In general, ACLF and CLF patients simultaneously suffer from LC. Differing from CLF and ALF, ACLF is a unique clinical entity that is characterized by acute onset, poor prognosis and high short-term mortality.40 However, no consensus has been achieved for ACLF among Western and Eastern experts worldwide on generating a definition to cover all of the diagnostic criteria and clinical features of ACLF.39 One key reason for this absence is that substantial differences in the definition of ACLF exist between Eastern and Western countries. In Western countries, ACLF frequently results from liver cirrhosis with acute decompensation, together with organ failure and complications that include the development of ascites, hepatic encephalopathy, gastrointestinal hemorrhage and bacterial sepsis. The CLIF-SOFA scores are used as a classification system to judge the prognosis of patients (possibility of mortality) and to screen for liver transplant candidates with the most urgent need.41 In contrast, the Eastern definition of ACLF mainly emphasizes acute liver injury with predisposing chronic liver diseases.42 In particular, HBV reactivation is considered to be the most common acute precipitating event that leads to ACLF in patients with either CHB or HBV-associated cirrhosis. Thus, the differences in the Eastern and Western definitions of ACLF, to some extent, reflect the variations in the basic liver disease profiles in Eastern and Western countries, as well as the variations in the pathogenesis of acute deterioration.39
HCC is a major cause of cancer mortality worldwide, and any differences in its global incidence may be explained by differences in the HCV and HBV prevalence and the ages of the patients. In 2013, the WHO reported that primary liver cancer caused 745,517 deaths worldwide, and that HCC represented the major histological type of these liver cancers.43 In another comprehensive review of global mortality, deaths from HCV-related HCC were estimated at 195,700 in 2010. In China, HCC is the second leading cause of cancer mortality, and its annual death rate was 24.15 per 100,000 persons in 2009.44 Approximately 383,203 persons die from liver cancer every year in China, which accounts for 51% of the deaths from liver cancer worldwide.44 Up to 80% of HCC cases in China are attributable to HBV, and approximately 20% of HCC patients test positive for HCV RNA.45 Currently, there are no data recording the contributions of alcoholism and NAFLD to HCC in China, although these entities will likely become leading causes of HCC in the future.
Most Chinese physicians follow the Guidelines from the USA and Europe for the diaganosis and treatment of HCC in China. The Chinese Ministry of Health also generate similar guidelines for the diagnosis and treatment of HCC in China, including initial screening of HCC in clinics by ultrasonic examination and serum AFP, together with monitoring a panel of serum tumor markers. If the positive HCC is found by these tests, MRI and/or CT are further used to identify the location and size of HCC more clearly.
3. Liver transplantation (LT) in China
LT has been well accepted as an effective treatment for end-stage liver diseases in China.46, 47 A total of 20,877 patients had LTs in Mainland China from 1980 to 2011, while in 2005 alone, 2960 patients received LTs. However, the international transplant community has harshly criticized the practices of LT in China in the past because of their commercialization and the use of executed prisoners as a source of donor organs. Over the last decade, many efforts have been made to improve the clinical practices for organ transplantation in China, both ethically and legally. For example, in 2006–2007, two laws, namely the interim Provision on the Administration of Clinical Application of Human Transplant Techniques launched by the Ministry of Health (MOH) and the Human Organ Transplantation Act, were established to guide the medical practices involved in organ transplantation, to safeguard the quality of such grafts, and to protect the safety and legal benefits of the recipients.47 The declaration of the regulation on human organ transplantation in 2007, followed by a series of stringent polices, led to a significant decline in the number of liver transplants in China (2,795 cases in 2006 vs. 1,897 cases in 2011).
Since 2011, the China Organ Transplant Response System (COTRS) under National Health and Family Planning Commission (former MOH) began to generate donor liver allocation. Candidates who are waiting for LT mainly include the chronic LF, ACLF and primary HCC. The criteria for the selection of LT are almost the same as those in the West since the criteria system was established according to western practices. In China, 86 centers have been approved and are eligible for LT. The number of LTs was 20,877 from 1980 to 2011. According to a report by the China Liver Transplant Registry (CLTR) [https://www.cltr.org/], up to 2010, LTs had already been established in nearly all of the provinces of China except Tibet. In particular, Beijing and Shanghai have the highest rates of LT in China. The composition of these candidates differs from their counterparts in Western countries. HBV-related HCC is the most common indication for LT, and approximately 40% of liver grafts were transplanted in Chinese patients with liver cancer. The other indications for LT in China are cirrhosis caused by HBV or HCV infections, alcoholism in adults, and biliary atresia in children. After more than two decades of effort, LT in China has a 90% one-year perioperative survival rate and a three-year graft survival rate of 70%; these rates are comparable to those of Western countries. In general, the total number of liver transplants performed in China each year is second only to the USA. The COTRS is the third largest LT database in the world, behind only the United Network for Organ Sharing and the Euro-Transplant International Foundation.
4. Traditional Chinese Medicine and liver diseases
Many patients with liver diseases in China, especially those who fail to recover under Western medicine treatments, have turned to traditional Chinese medicine (TCM) as a complementary or alternative therapy. Many TCM therapies for hepatitis and cirrhosis, including Chinese herbs, acupuncture, moxibustion, and medicated diets, have been used for centuries in China; however, most of them have not undergone evidence-based clinical trials and are not yet well accepted in Western countries. Fu Zheng Hua Yu, a traditional Chinese medicine compound approved in China for the treatment of liver fibrosis caused by HBV infection, is most likely the first TCM compound that will complete phase II clinical studies for the treatment of HCV-related liver fibrosis in the USA. The results were reported at a satellite symposium during the 2013 annual conference of AASLD and indicated that Fu Zheng Hua Yu tablets are safe, well tolerated and tend to stabilize and improve liver fibrosis in HCV patients with moderate to severe fibrosis.
In China, TCM treatment is quite common. In large or median cities, there are many TCM hospitals and many general hospitals also have TCM departments. It has been estimated that around 25% of in-patients and outpatients receive TCM treatment, and also receive the liver and renal function tests in order to investigate its side events or toxicity. In general, the majority of TCM treatments are safe. However, the hepatotoxicity induced by the widespread use of some Chinese medications should be noted. The common side effects include liver injury, renal dysfunction, fever and an allergic reaction-like skin rash. The most serious side effect is acute liver failure with high mortality.48 Therefore, further clinical trials to test the therapeutic potential and safety of TCMs for liver diseases are needed.
5. Funding, research, and training for hepatology in China
The Chinese economy has grown significantly over the last three decades, which has promoted the development of science and technology. Currently, the Chinese government spends 1.23% of its gross domestic product (GDP) on funding for basic and applied scientific research, and biomedical research has been listed as one of the most important fields for preferential support by the government. Over the past 10 years, funding in the field of life science and medicine, including basic and applied hepatology research, has increased considerably. The major sources of funding for hepatology studies in China are the Ministry of Science and Technology of China (MOST) and the Natural Science Foundation of China (NSFC). The former prefers to support large target-oriented projects, aiming to strengthen fundamental research and “hi-tech” developments in several national programs, including the National Basic Research Program of China (973 Program) and the National High-Tech Program (863 Program). The latter generally supports and encourages individual hepatologists or her/his team to focus on a single key scientific project. For example, two key programs (2007–2012 and 2008–2013) were initiated to investigate the virological and immunological pathogenesis of HBV and HCV infections, respectively. In 2010, the NSFC launched a Major Research Plan (2011–2018) with 200 million RMB to focus on the regulatory network and molecular mechanisms of malignant transformations of non-resolving inflammation. The malignant transformation of hepatitis into liver cancer is an important scientific issue, and is the key research focus of this plan. Overall, liver disease–related research funding at the NSFC has grown from 149 million in 2010 to 352 million RMB in 2013, which included 9.4 million RMB to support research into infectious liver diseases, 12 million for liver fibrosis and cirrhosis research, and 147.6 million for gastrointestinal tumor research. Notably, the Chinese government and industry have recently projected to invest a total of 600 billion RMB ($98.4 billion) to support 16 national key Science and Technology projects by 2020: one of them is the national key project for viral hepatitis, AIDS and other major infectious diseases.49 Two additional 973 projects and several key NFSC funded projects (total 70 million RMB) have also been initialized to focus on NAFLD since 2012. The major purpose of this funding was to promote the generation of new therapeutic regimens to significantly reduce the mortality and morbidity caused by HBV, HCV, and fatty liver-associated liver diseases. In addition, many local governments (eg. provinces and cities) and hosptials also provide research funds for the study of liver diseases.
Hepatology research has been very active in China since 2001, as shown by a steady increase in the number of publications in Hepatology. In 2013, among 388 original papers published in Hepatology, 46, 12, and 4 papers were from Mainland China, Taiwan, and Hong Kong, respectively, ranking the second after the USA. Several main scientific findings concerning HBV and related liver cancers were also originated from Mainland China. In China, most currently active leaders, hepatologists and scientists in the field of hepatology have received training in the USA, Europe or other Western countries. Many young hepatologists who are supported by the government or their hospitals are being sent to Western countries for basic and clinical research training.
Future perspectives
Currently, approximately 300,000 to 400,000 patients die from liver diseases each year in China. The remaining challenges are low awareness of the perniciousness of liver diseases and low treatment rate for patients, especially in the broad rural areas of China. The majority of patients with liver diseases do not see a doctor until they feel uncomfortable and have significant manifestation when they progress to decompensated liver cirrhosis or even HCC. This is why the incidence of liver cirrhosis and HCC has increased during recent years in China. In terms of the number of patients with liver diseases, China is a leader in the global prevalence of liver disease. China has been making significant efforts to shed this title by attempting to control HBV and HCV infections. For example, one of the national goals is to reduce the rate of HBV infection to below 1% by 2050.50 However, unless there is improvement with respect to ALD and NAFLD, China is likely to retain the title of leader in liver disease. Therefore, increased investment is necessary for training young hepatologists and promoting global collaborations in both clinical and basic research related to liver diseases in China.
Acknowledgments
This work was supported by the National Grand Program on Key Infectious Disease (2012ZX10002-007-002; 2013ZX10002001-001-003) and the State Key Development Program for Basic Research of China (2012CB519005; 2012CB517500). ;
Abbreviations
- ADV
adefovir dipivoxil
- ALD
alcoholic liver disease
- China CDC
China Center for Diseases Prevention and Control
- CLIF-SOFA
Chronic liver failure–sequential organ failure assessment
- DAAs
Direct antiviral agents
- DALYs
disability adjusted life years
- DILI
drug-induced liver disease
- ETV
entecavir
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- LAM
lamivudine
- LC
liver cirrhosis
- Ldt
telbivudine
- LF
liver failure
- LT
liver transplantation
- NAFLD
nonalcoholic fatty liver disease
- TCM
traditional Chinese medicine
- TDF
tenofovir
- ESLD
end-stage liver diseases
References
- 1.Lok AS. Prevention of hepatitis B virus-related hepatocellular carcinoma. Gastroenterology. 2004;127:S303–S309. doi: 10.1053/j.gastro.2004.09.045. [DOI] [PubMed] [Google Scholar]
- 2.Cui Y, Jia J. Update on epidemiology of hepatitis B and C in China. J Gastroenterol Hepatol. 2013;28(Suppl 1):7–10. doi: 10.1111/jgh.12220. [DOI] [PubMed] [Google Scholar]
- 3.Rehm J, Samokhvalov AV, Shield KD. Global burden of alcoholic liver diseases. J Hepatol. 2013;59:160–168. doi: 10.1016/j.jhep.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Lazo M, Hernaez R, Bonekamp S, et al. Non-alcoholic fatty liver disease and mortality among US adults: prospective cohort study. BMJ. 2011;343:d6891. doi: 10.1136/bmj.d6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan JG. Epidemiology of alcoholic and nonalcoholic fatty liver disease in China. J Gastroenterol Hepatol. 2013;28(Suppl 1):11–17. doi: 10.1111/jgh.12036. [DOI] [PubMed] [Google Scholar]
- 6.Luo Z, Li L, Ruan B. Impact of the implementation of a vaccination strategy on hepatitis B virus infections in China over a 20-year period. Int J Infect Dis. 2012;16:e82–e88. doi: 10.1016/j.ijid.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Wang HY, Li D, Liu W, et al. Hepatitis B virus subgenotype C2 is the most prevalent subgenotype in northeast China. Clin Microbiol Infect. 2010;16:477–481. doi: 10.1111/j.1469-0691.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 8.Liaw YF, Chu CM. Hepatitis B virus infection. Lancet. 2009;373:582–592. doi: 10.1016/S0140-6736(09)60207-5. [DOI] [PubMed] [Google Scholar]
- 9.Chen YS, Li L, Cui FQ, et al. A sero-epidemiological study on hepatitis C in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2011;32:888–891. [PubMed] [Google Scholar]
- 10.Jia L, Yu J, Yang J, et al. HCV antibody response and genotype distribution in different areas and races of China. Int J Biol Sci. 2009;5:421–427. doi: 10.7150/ijbs.5.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu Y, Wang Y, Xia W, et al. New trends of HCV infection in China revealed by genetic analysis of viral sequences determined from first-time volunteer blood donors. J Viral Hepat. 2011;18:42–52. doi: 10.1111/j.1365-2893.2010.01280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang TJ, Ghany MG. Current and future therapies for hepatitis C virus infection. N Engl J Med. 2013;368:1907–1917. doi: 10.1056/NEJMra1213651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lange CM, Zeuzem S. IL28B single nucleotide polymorphisms in the treatment of hepatitis C. J Hepatol. 2011;55:692–701. doi: 10.1016/j.jhep.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Liao XW, Ling Y, Li XH, et al. Association of genetic variation in IL28B with hepatitis C treatment-induced viral clearance in the Chinese Han population. Antivir Ther. 2011;16:141–147. doi: 10.3851/IMP1703. [DOI] [PubMed] [Google Scholar]
- 15.Hagan LM, Sulkowski MS, Schinazi RF. Cost analysis of sofosbuvir/ribavirin versus sofosbuvir/simeprevir for genotype 1 hepatitis C virus in interferon-ineligible/intolerant individuals. Hepatology. 2014 doi: 10.1002/hep.27151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rao H, Wei L, Lopez-Talavera JC, et al. Distribution and clinical correlates of viral and host genotypes in Chinese patients with chronic hepatitis C virus infection. J Gastroenterol Hepatol. 2014;29:545–553. doi: 10.1111/jgh.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li YM, Fan JG, Wang BY, et al. Guidelines for the diagnosis and management of alcoholic liver disease: update 2010: (published in Chinese on Chinese Journal of Hepatology 2010; 18: 167–170) J Dig Dis. 2011;12:45–50. doi: 10.1111/j.1751-2980.2010.00477.x. [DOI] [PubMed] [Google Scholar]
- 18.Zhou L, Conner KR, Phillips MR, et al. Epidemiology of alcohol abuse and dependence in rural chinese men. Alcohol Clin Exp Res. 2009;33:1770–1776. doi: 10.1111/j.1530-0277.2009.01014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiang YT, Ma X, Lu JY, et al. Alcohol-related disorders in Beijing, China: prevalence, socio-demographic correlates, and unmet need for treatment. Alcohol Clin Exp Res. 2009;33:1111–1118. doi: 10.1111/j.1530-0277.2009.00933.x. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Zhou LY, Meng XW. Genetic polymorphism of two enzymes with alcoholic liver disease in Northeast China. Hepatogastroenterology. 2012;59:204–207. doi: 10.5754/hge10136. [DOI] [PubMed] [Google Scholar]
- 21.Chen GH, Yang Y, Lu MQ, et al. Liver transplantation for end-stage alcoholic liver disease: a single-center experience from mainland China. Alcohol. 2010;44:217–221. doi: 10.1016/j.alcohol.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Eng MY, Luczak SE, Wall TL. ALDH2, ADH1B, and ADH1C genotypes in Asians: a literature review. Alcohol Res Health. 2007;30:22–27. [PMC free article] [PubMed] [Google Scholar]
- 23.Kwon H, Won Y, Park O, et al. Aldehyde dehydrogenase 2 deficiency ameliorates alcoholic fatty liver but worsens liver inflammation and fibrosis in mice. Hepatology. 2014;60:146–157. doi: 10.1002/hep.27036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ji CY, Chen TJ Working Group on Obesity in C. Empirical changes in the prevalence of overweight and obesity among Chinese students from 1985 to 2010 and corresponding preventive strategies. Biomed Environ Sci. 2013;26:1–12. doi: 10.3967/0895-3988.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Xiao Y, Zhao N, Wang H, et al. Association between socioeconomic status and obesity in a Chinese adult population. BMC Public Health. 2013;13:355. doi: 10.1186/1471-2458-13-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan JG, Zhu J, Li XJ, et al. Fatty liver and the metabolic syndrome among Shanghai adults. J Gastroenterol Hepatol. 2005;20:1825–1832. doi: 10.1111/j.1440-1746.2005.04058.x. [DOI] [PubMed] [Google Scholar]
- 27.Du T, Sun X, Yin P, et al. Increasing trends in central obesity among Chinese adults with normal body mass index, 1993–2009. BMC Public Health. 2013;13:327. doi: 10.1186/1471-2458-13-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gu D, Reynolds K, Wu X, et al. Prevalence of the metabolic syndrome and overweight among adults in China. Lancet. 2005;365:1398–1405. doi: 10.1016/S0140-6736(05)66375-1. [DOI] [PubMed] [Google Scholar]
- 29.Wang Q, Yin J, Xu L, et al. Prevalence of metabolic syndrome in a cohort of Chinese schoolchildren: comparison of two definitions and assessment of adipokines as components by factor analysis. BMC Public Health. 2013;13:249. doi: 10.1186/1471-2458-13-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu Y, Wang L, He J, et al. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948–959. doi: 10.1001/jama.2013.168118. [DOI] [PubMed] [Google Scholar]
- 31.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 32.Fan JG, Zhou Q, Wo QH. Effect of body weight mass and its change on the incidence of nonalcoholic fatty liver disease. Zhonghua Gan Zang Bing Za Zhi. 2010;18:676–679. doi: 10.3760/cma.j.issn.1007-3418.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 33.Wong VW, Chu WC, Wong GL, et al. Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: a population study using proton-magnetic resonance spectroscopy and transient elastography. Gut. 2012;61:409–415. doi: 10.1136/gutjnl-2011-300342. [DOI] [PubMed] [Google Scholar]
- 34.Liew PL, Lee WJ, Lee YC, et al. Hepatic histopathology of morbid obesity: concurrence of other forms of chronic liver disease. Obes Surg. 2006;16:1584–1593. doi: 10.1381/096089206779319392. [DOI] [PubMed] [Google Scholar]
- 35.Wang MM, Wang GS, Shen F, et al. Hepatic Steatosis Is Highly Prevalent in Hepatitis B Patients and Negatively Associated with Virological Factors. Dig Dis Sci. 2014 doi: 10.1007/s10620-014-3180-9. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Zhang H, Elizabeth A, et al. Epidemiology of hepatitis B and associated liver diseases in china. Chin Med Sci J. 2013;27:243–248. doi: 10.1016/s1001-9294(13)60009-7. [DOI] [PubMed] [Google Scholar]
- 37.Xuan SY, Xin YN, Chen H, et al. Study on the correlations between HBsAg and hepatitis C virus expression as well as fibrotic staging in hepatocellular carcinoma and pericarcinomatous tissues. Zhonghua Liu Xing Bing Xue Za Zhi. 2006;27:157–160. [PubMed] [Google Scholar]
- 38.Shang QH, Yu JG, Xu CZ, et al. Occult hepatitis B virus infection in chronic viral hepatitis patients with non-A to E hepatitis virus infection. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2008;22:440–442. [PubMed] [Google Scholar]
- 39.Wang FS, Zhang Z. Liver: How can acute-on-chronic liver failure be accurately identified? Nat Rev Gastroenterol Hepatol. 2013;10:390–391. doi: 10.1038/nrgastro.2013.72. [DOI] [PubMed] [Google Scholar]
- 40.Jalan R, Gines P, Olson JC, et al. Acute-on chronic liver failure. J Hepatol. 2012;57:1336–1348. doi: 10.1016/j.jhep.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 41.Moreau R, Jalan R, Gines P, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426–1437. 1437 e1–1437 e9. doi: 10.1053/j.gastro.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 42.Sarin SK, Kumar A, Almeida JA, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL) Hepatol Int. 2009;3:269–282. doi: 10.1007/s12072-008-9106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Globocan. 2012 http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. [Google Scholar]
- 44.Chen W, Zheng R, Zhang S, et al. The incidences and mortalities of major cancers in China, 2009. Chin J Cancer. 2013;32:106–112. doi: 10.5732/cjc.013.10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen VT, Law MG, Dore GJ. Hepatitis B-related hepatocellular carcinoma: epidemiological characteristics and disease burden. J Viral Hepat. 2009;16:453–463. doi: 10.1111/j.1365-2893.2009.01117.x. [DOI] [PubMed] [Google Scholar]
- 46.Rakela J, Fung JJ. Liver transplantation in China. Liver Transpl. 2007;13:182. doi: 10.1002/lt.21079. [DOI] [PubMed] [Google Scholar]
- 47.Chen GH. Liver transplantation in China: retrospect and prospect. Chin Med J (Engl) 2009;122:2229–2230. [PubMed] [Google Scholar]
- 48.Zhao P, Wang C, Liu W, et al. Causes and outcomes of acute liver failure in China. PLoS One. 2013;8:e80991. doi: 10.1371/journal.pone.0080991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.China So. A war that China must win. Spotlight on China. Cell Host & Microbes. 2014;14:5s–12s. [Google Scholar]
- 50.Li X, Xu WF. China's efforts to shed its title of "Leader in liver disease". Drug Discov Ther. 2007;1:84–85. [PubMed] [Google Scholar]